Plasma Kinetics of Choline and Choline Metabolites After A Single Dose of SuperbaBoostTM Krill Oil or Choline Bitartrate in Healthy Volunteers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
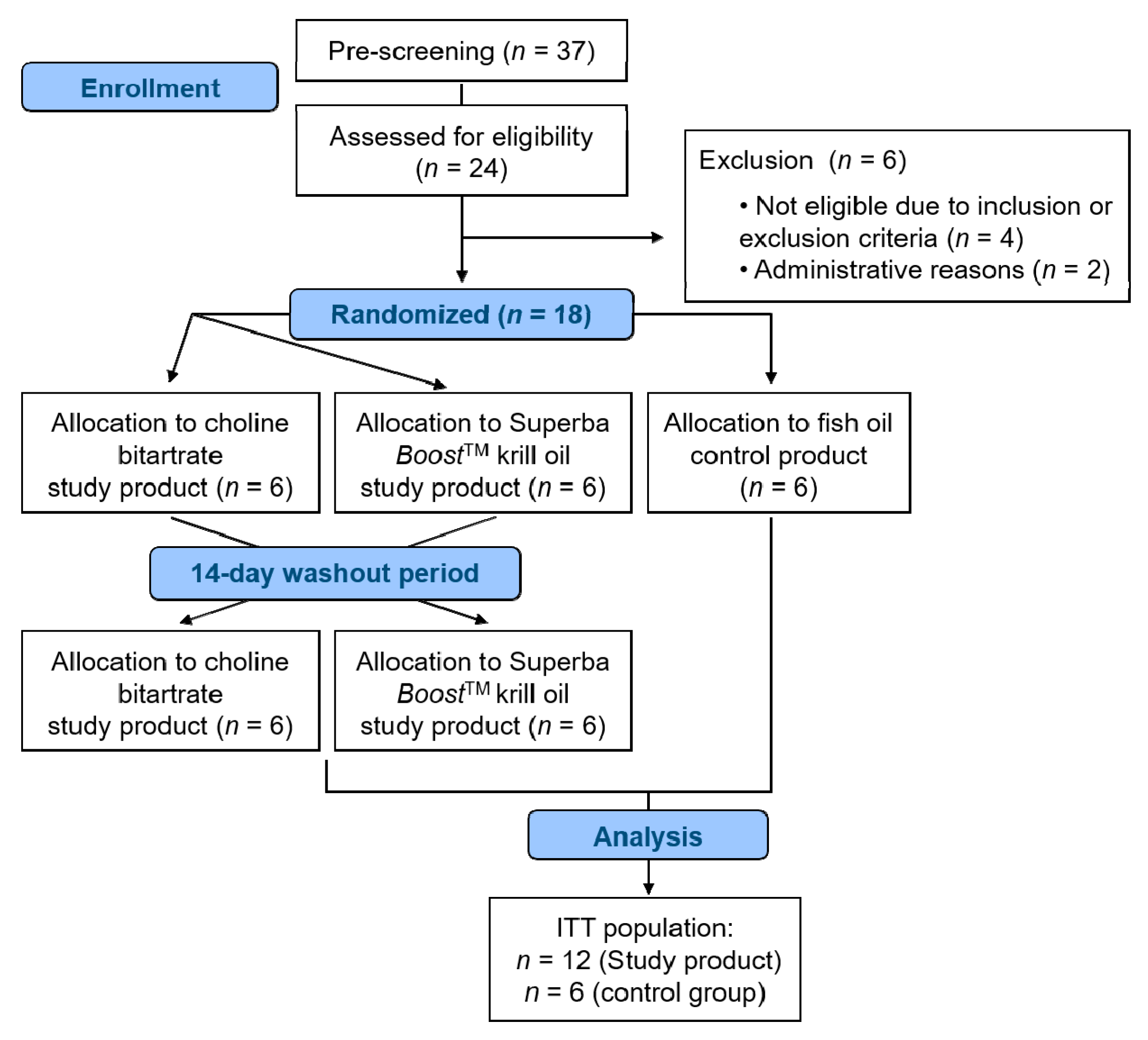
2.2. Study Design
2.3. Intervention
2.4. Sample Collection, Processing and Analysis
2.5. Methods for Safety (Adverse Events, Concomitant Medication and Tolerability)
2.6. Data Analysis and Statistics
3. Results
3.1. Subject Characteristics
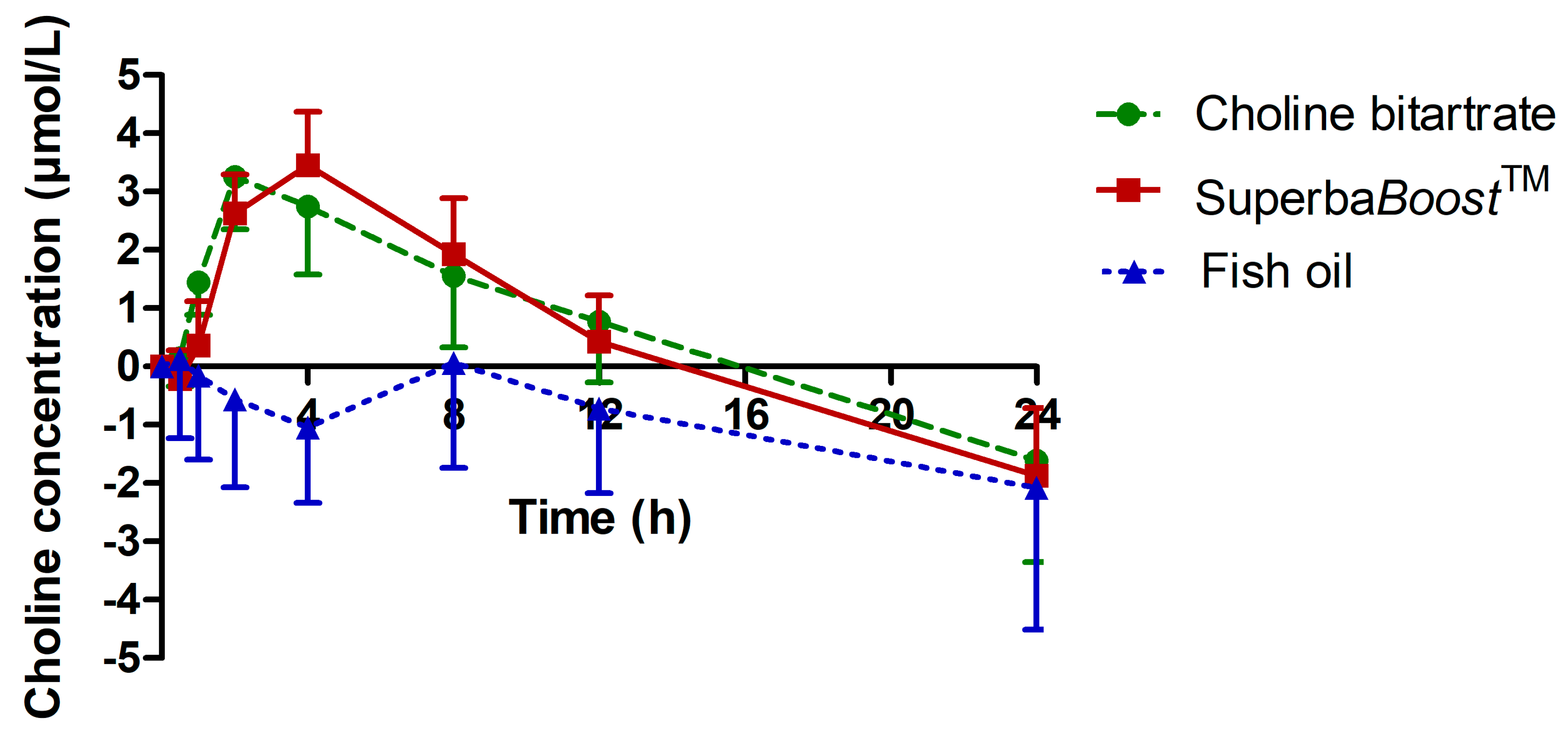
3.2. Choline Pharmacokinetics Upon Intake of The Single-Dosed Choline Sources Phosphatidylcholine and Choline Bitartrate
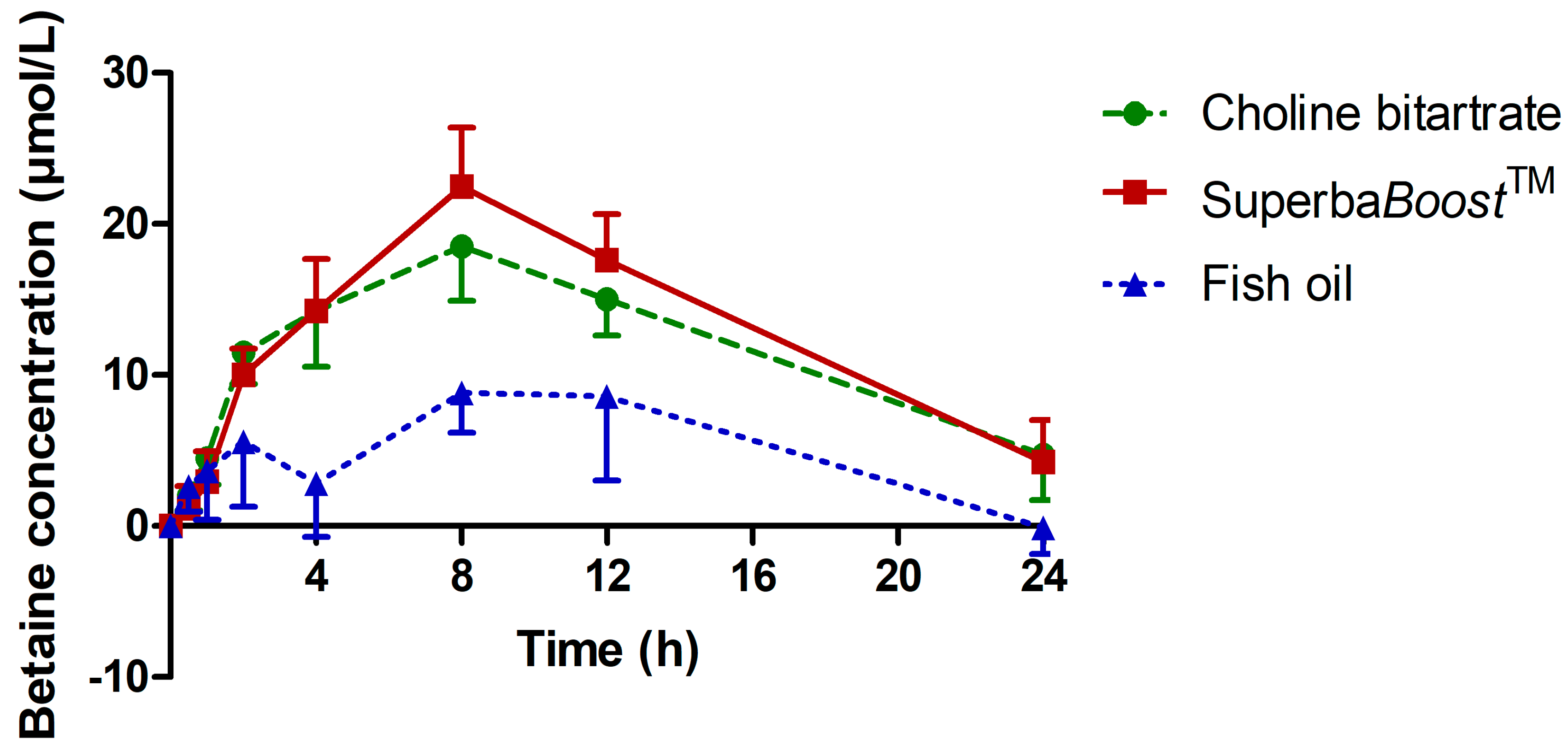
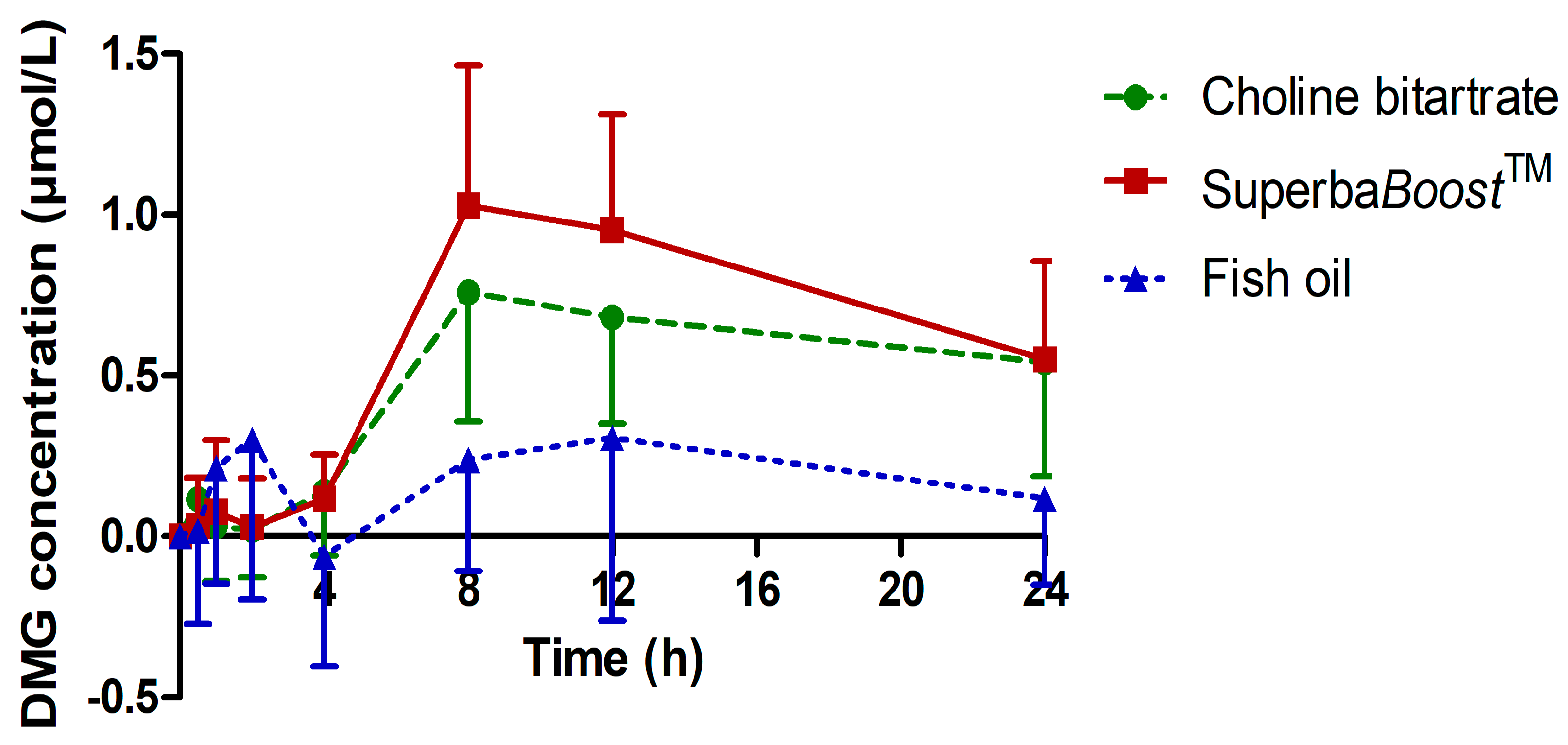
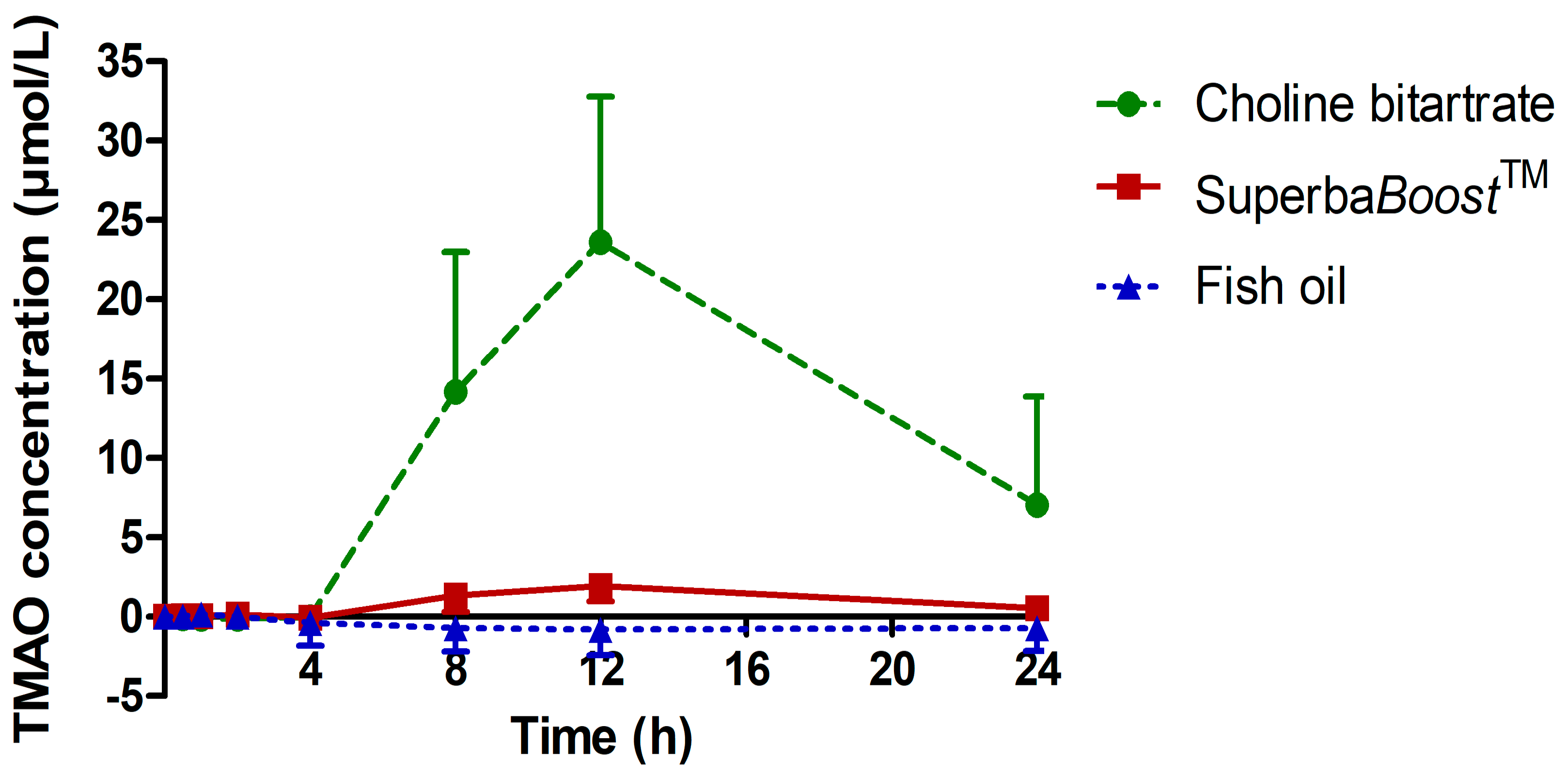
3.3. Pharmacokinetic Characteristics of the Choline Metabolites Betaine, Dimethylglycine (DMG) and Trimethylamine Oxide (TMAO) Upon Choline Intake
3.4. Safety Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zeisel, S.H.; da Costa, K.-A. Choline: An essential nutrient for public health. Nutr. Rev. 2009, 67, 615–623. [Google Scholar] [CrossRef]
- Zeisel, S.H. Choline: An essential nutrient for humans. Nutrition 2000, 16, 669–671. [Google Scholar] [CrossRef]
- Ueland, P.M. Choline and betaine in health and disease. J. Inherit. Metab. Dis. 2011, 34, 3–15. [Google Scholar] [CrossRef]
- Romano, K.A.; Vivas, E.I.; Amador-Noguez, D.; Rey, F.E. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. mBio 2015, 6, e02481. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.C.; Fulgoni, V.L. Assessment of Total Choline Intakes in the United States. J. Am. Coll. Nutr. 2016, 35, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Wiedeman, A.M.; Barr, S.I.; Green, T.J.; Xu, Z.; Innis, S.M.; Kitts, D.D. Dietary Choline Intake: Current State of Knowledge Across the Life Cycle. Nutrients 2018, 10, 1513. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies. Dietary Reference Values for choline. EFSA J. 2016, 14, 158. [Google Scholar] [CrossRef]
- Francis, P.T. The interplay of neurotransmitters in Alzheimer’s disease. CNS Spectr. 2005, 10, 6–9. [Google Scholar] [CrossRef]
- Igarashi, M.; Ma, K.; Gao, F.; Kim, H.-W.; Rapoport, S.I.; Rao, J.S. Disturbed choline plasmalogen and phospholipid fatty acid concentrations in Alzheimer’s disease prefrontal cortex. J. Alzheimers Dis. 2011, 24, 507–517. [Google Scholar] [CrossRef]
- Sherriff, J.L.; O’Sullivan, T.A.; Properzi, C.; Oddo, J.-L.; Adams, L.A. Choline, Its Potential Role in Nonalcoholic Fatty Liver Disease, and the Case for Human and Bacterial Genes. Adv. Nutr. 2016, 7, 5–13. [Google Scholar] [CrossRef] [Green Version]
- Corbin, K.D.; Zeisel, S.H. Choline metabolism provides novel insights into nonalcoholic fatty liver disease and its progression. Curr. Opin. Gastroenterol. 2012, 28, 159–165. [Google Scholar] [CrossRef] [Green Version]
- Stremmel, W.; Hanemann, A.; Ehehalt, R.; Karner, M.; Braun, A. Phosphatidylcholine (lecithin) and the mucus layer: Evidence of therapeutic efficacy in ulcerative colitis? Dig. Dis. 2010, 28, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Strilakou, A.A.; Lazaris, A.C.; Perelas, A.I.; Mourouzis, I.S.; Douzis, I.C.; Karkalousos, P.L.; Stylianaki, A.T.; Pantos, C.I.; Liapi, C.A. Heart dysfunction induced by choline-deficiency in adult rats: The protective role of L-carnitine. Eur. J. Pharmacol. 2013, 709, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Radziejewska, A.; Chmurzynska, A. Folate and choline absorption and uptake: Their role in fetal development. Biochimie 2019, 158, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Le Grandois, J.; Marchioni, E.; Zhao, M.; Giuffrida, F.; Ennahar, S.; Bindler, F. Investigation of natural phosphatidylcholine sources: Separation and identification by liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS2) of molecular species. J. Agric. Food Chem. 2009, 57, 6014–6020. [Google Scholar] [CrossRef]
- Winther, B.; Hoem, N.; Berge, K.; Reubsaet, L. Elucidation of phosphatidylcholine composition in krill oil extracted from Euphausia superba. Lipids 2011, 46, 25–36. [Google Scholar] [CrossRef]
- Li, Z.; Vance, D.E. Phosphatidylcholine and choline homeostasis. J. Lipid Res. 2008, 49, 1187–1194. [Google Scholar] [CrossRef]
- Zeisel, S.H.; Mar, M.-H.; Howe, J.C.; Holden, J.M. Concentrations of choline-containing compounds and betaine in common foods. J. Nutr. 2003, 133, 1302–1307. [Google Scholar] [CrossRef]
- Ueland, P.M.; Holm, P.I.; Hustad, S. Betaine: A key modulator of one-carbon metabolism and homocysteine status. Clin. Chem. Lab. Med. 2005, 43, 1069–1075. [Google Scholar] [CrossRef]
- Baker, J.R.; Chaykin, S. The biosynthesis of trimethylamine-N-oxide. J. Biol. Chem. 1962, 237, 1309–1313. [Google Scholar] [CrossRef]
- Zeisel, S.H.; Wishnok, J.S.; Blusztajn, J.K. Formation of methylamines from ingested choline and lecithin. J. Pharmacol. Exp. Ther. 1983, 225, 320–324. [Google Scholar] [PubMed]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, W.H.W.; Wang, Z.; Kennedy, D.J.; Wu, Y.; Buffa, J.A.; Agatisa-Boyle, B.; Li, X.S.; Levison, B.S.; Hazen, S.L. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ. Res. 2015, 116, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Kaysen, G.A.; Johansen, K.L.; Chertow, G.M.; Dalrymple, L.S.; Kornak, J.; Grimes, B.; Dwyer, T.; Chassy, A.W.; Fiehn, O. Associations of Trimethylamine N-Oxide with Nutritional and Inflammatory Biomarkers and Cardiovascular Outcomes in Patients New to Dialysis. J. Ren. Nutr. 2015, 25, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Trøseid, M.; Ueland, T.; Hov, J.R.; Svardal, A.; Gregersen, I.; Dahl, C.P.; Aakhus, S.; Gude, E.; Bjørndal, B.; Halvorsen, B.; et al. Microbiota-dependent metabolite trimethylamine-N-oxide is associated with disease severity and survival of patients with chronic heart failure. J. Intern. Med. 2015, 277, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tang, W.H.W.; Buffa, J.A.; Fu, X.; Britt, E.B.; Koeth, R.A.; Levison, B.S.; Fan, Y.; Wu, Y.; Hazen, S.L. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur. Heart J. 2014, 35, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.W.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Hazen, S.L. Microbiome, trimethylamine N-oxide, and cardiometabolic disease. Transl. Res. 2017, 179, 108–115. [Google Scholar] [CrossRef]
- Heianza, Y.; Ma, W.; Manson, J.E.; Rexrode, K.M.; Qi, L. Gut Microbiota Metabolites and Risk of Major Adverse Cardiovascular Disease Events and Death: A Systematic Review and Meta-Analysis of Prospective Studies. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef]
- Bae, S.; Ulrich, C.M.; Neuhouser, M.L.; Malysheva, O.; Bailey, L.B.; Xiao, L.; Brown, E.C.; Cushing-Haugen, K.L.; Zheng, Y.; Cheng, T.-Y.D.; et al. Plasma choline metabolites and colorectal cancer risk in the Women’s Health Initiative Observational Study. Cancer Res. 2014, 74, 7442–7452. [Google Scholar] [CrossRef]
- Janeiro, M.H.; Ramírez, M.J.; Milagro, F.I.; Martínez, J.A.; Solas, M. Implication of Trimethylamine N-Oxide (TMAO) in Disease: Potential Biomarker or New Therapeutic Target. Nutrients 2018, 10, 1398. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Yang, X.; Li, J.; Chen, X.; Zhao, X.; Chen, Y.; Wen, Y. Trimethylamine N-oxide prime NLRP3 inflammasome via inhibiting ATG16L1-induced autophagy in colonic epithelial cells. Biochem. Biophys. Res. Commun. 2017, 490, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Jiao, X.; Ma, Y.; Liu, Y.; Zhang, L.; He, Y.; Chen, Y. Trimethylamine N-oxide induces inflammation and endothelial dysfunction in human umbilical vein endothelial cells via activating ROS-TXNIP-NLRP3 inflammasome. Biochem. Biophys. Res. Commun. 2016, 481, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-L.; Zhu, X.-H.; Ran, L.; Lang, H.-D.; Yi, L.; Mi, M.-T. Trimethylamine-N-Oxide Induces Vascular Inflammation by Activating the NLRP3 Inflammasome Through the SIRT3-SOD2-mtROS Signaling Pathway. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Rohrmann, S.; Linseisen, J.; Allenspach, M.; von Eckardstein, A.; Müller, D. Plasma Concentrations of Trimethylamine-N-oxide Are Directly Associated with Dairy Food Consumption and Low-Grade Inflammation in a German Adult Population. J. Nutr. 2016, 146, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Velasquez, M.T.; Ramezani, A.; Manal, A.; Raj, D.S. Trimethylamine N-Oxide: The Good, the Bad and the Unknown. Toxins 2016, 8, 326. [Google Scholar] [CrossRef]
- Li, T.; Chen, Y.; Gua, C.; Li, X. Elevated Circulating Trimethylamine N-Oxide Levels Contribute to Endothelial Dysfunction in Aged Rats through Vascular Inflammation and Oxidative Stress. Front. Physiol. 2017, 8, 350. [Google Scholar] [CrossRef]
- Randrianarisoa, E.; Lehn-Stefan, A.; Wang, X.; Hoene, M.; Peter, A.; Heinzmann, S.S.; Zhao, X.; Königsrainer, I.; Königsrainer, A.; Balletshofer, B.; et al. Relationship of Serum Trimethylamine N-Oxide (TMAO) Levels with early Atherosclerosis in Humans. Sci. Rep. 2016, 6, 26745. [Google Scholar] [CrossRef] [Green Version]
- Boutagy, N.E.; Neilson, A.P.; Osterberg, K.L.; Smithson, A.T.; Englund, T.R.; Davy, B.M.; Hulver, M.W.; Davy, K.P. Short-term high-fat diet increases postprandial trimethylamine-N-oxide in humans. Nutr. Res. 2015, 35, 858–864. [Google Scholar] [CrossRef]
- Sun, G.; Yin, Z.; Liu, N.; Bian, X.; Yu, R.; Su, X.; Zhang, B.; Wang, Y. Gut microbial metabolite TMAO contributes to renal dysfunction in a mouse model of diet-induced obesity. Biochem. Biophys. Res. Commun. 2017, 493, 964–970. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed]
- de La Huerga, J.; Popper, H. Urinary excretion of choline metabolites following choline administration in normals and patients with hepatobiliary diseases. J. Clin. Investig. 1951, 30, 463–470. [Google Scholar] [CrossRef] [PubMed]
- De La Huerga, J.; Popper, H. Factors influencing choline absorption in the intestinal tract. J. Clin. Investig. 1952, 31, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-Y.; Choi, S.-U.; Lee, K.-H.; Yang, J.-B.; Cho, S.-W.; Ro, J.; Kim, H.; Khadka, P.; Lee, J.; Cho, C.-W. Discriminative Measurement and Pharmacokinetic Evaluation of Choline Alphoscerate against Endogenous Choline in Human. Bull. Korean Chem. Soc. 2015, 36, 2089–2094. [Google Scholar] [CrossRef]
- Holm, P.I.; Ueland, P.M.; Kvalheim, G.; Lien, E.A. Determination of choline, betaine, and dimethylglycine in plasma by a high-throughput method based on normal-phase chromatography-tandem mass spectrometry. Clin. Chem. 2003, 49, 286–294. [Google Scholar] [CrossRef]
- Lever, M.; Sizeland, P.C.M.; Frampton, C.M.; Chambers, S.T. Short and long-term variation of plasma glycine betaine concentrations in humans. Clin. Biochem. 2004, 37, 184–190. [Google Scholar] [CrossRef]
- Cho, C.E.; Taesuwan, S.; Malysheva, O.V.; Bender, E.; Yan, J.; Caudill, M.A. Choline and one-carbon metabolite response to egg, beef and fish among healthy young men: A short-term randomized clinical study. Clin. Nutr. Exp. 2016, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Midttun, Ø.; Kvalheim, G.; Ueland, P.M. High-throughput, low-volume, multianalyte quantification of plasma metabolites related to one-carbon metabolism using HPLC-MS/MS. Anal. Bioanal. Chem. 2013, 405, 2009–2017. [Google Scholar] [CrossRef]
- Zeisel, S.H.; Growdon, J.H.; Wurtman, R.J.; Magil, S.G.; Logue, M. Normal plasma choline responses to ingested lecithin. Neurology 1980, 30, 1226–1229. [Google Scholar] [CrossRef]
- Wurtman, R.J.; Hirsch, M.J.; Growdon, J.H. Lecithin consumption raises serum-free-choline levels. Lancet 1977, 2, 68–69. [Google Scholar] [CrossRef]
- Jäger, R.; Purpura, M.; Kingsley, M. Phospholipids and sports performance. J. Int. Soc. Sports Nutr. 2007, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Compher, C.W.; Kinosian, B.P.; Stoner, N.E.; Lentine, D.C.; Buzby, G.P. Choline and vitamin B12 deficiencies are interrelated in folate-replete long-term total parenteral nutrition patients. J. Parenter. Enter. Nutr. 2002, 26, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.I.; Miller, J.W.; Da Costa, K.A.; Nadeau, M.; Smith, D.; Selhub, J.; Zeisel, S.H.; Mason, J.B. Severe folate deficiency causes secondary depletion of choline and phosphocholine in rat liver. J. Nutr. 1994, 124, 2197–2203. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, M.D.; Zeisel, S.H. Diet, methyl donors and DNA methylation: Interactions between dietary folate, methionine and choline. J. Nutr. 2002, 132, S2333–S2335. [Google Scholar] [CrossRef] [PubMed]
- Jacob, R.A.; Jenden, D.J.; Allman-Farinelli, M.A.; Swendseid, M.E. Folate nutriture alters choline status of women and men fed low choline diets. J. Nutr. 1999, 129, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.H.; Stabler, S.P.; Savage, D.G.; Lindenbaum, J. Metabolic abnormalities in cobalamin (vitamin B12) and folate deficiency. FASEB J. 1993, 7, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Bjørndal, B.; Bruheim, I.; Lysne, V.; Ramsvik, M.S.; Ueland, P.M.; Nordrehaug, J.E.; Nygård, O.K.; Berge, R.K. Plasma choline, homocysteine and vitamin status in healthy adults supplemented with krill oil: A pilot study. Scand. J. Clin. Lab. Investig. 2018, 78, 527–532. [Google Scholar] [CrossRef]
- Berge, R.K.; Ramsvik, M.S.; Bohov, P.; Svardal, A.; Nordrehaug, J.E.; Rostrup, E.; Bruheim, I.; Bjørndal, B. Krill oil reduces plasma triacylglycerol level and improves related lipoprotein particle concentration, fatty acid composition and redox status in healthy young adults—A pilot study. Lipids Health Dis. 2015, 14, 163. [Google Scholar] [CrossRef]
- Qi, J.; You, T.; Li, J.; Pan, T.; Xiang, L.; Han, Y.; Zhu, L. Circulating trimethylamine N-oxide and the risk of cardiovascular diseases: A systematic review and meta-analysis of 11 prospective cohort studies. J. Cell. Mol. Med. 2018, 22, 185–194. [Google Scholar] [CrossRef]
- Burri, L.; Hoem, N.; Monakhova, Y.B.; Diehl, B.W.K. Fingerprinting Krill Oil by 31 P, 1 H and 13 C NMR Spectroscopies. J. Am. Oil Chem. Soc. 2016, 93, 1037–1049. [Google Scholar] [CrossRef]
| Variable | Mean | 95% CI |
|---|---|---|
| Age (years) | 43.3 | 34.0–52.6 |
| BMI (kg/m2) | 23.6 | 21.8–25.3 |
| Systolic BP (mmHg) | 128.5 | 117.6–139.4 |
| Diastolic BP (mmHg) | 78.5 | 72.6–84.4 |
| Hemoglobin (g/dL) | 14.2 | 13.4–14.9 |
| Cholesterol (mg/dL) | 180.5 | 155.1–205.9 |
| HDL (mg/dL) | 60 | 51.6–68 4 |
| LDL (mg/dL) | 112 2 | 86.3–138 |
| AST (U/L) | 21.6 | 19.6–23.6 |
| ALT (U/L) | 24.2 | 20.3–28 |
| Choline (baseline) (µmol/L) | 9.6 | 8.5–10.6 |
| Betaine (baseline) (µmol/L) | 33.8 | 28.5–39.1 |
| DMG (baseline) (µmol/L) | 3.4 | 3–3.8 |
| TMAO (baseline) (µmol/L) | 2.1 | 1.4–2.8 |
| Variable | Mean 1 | 95% CI 1 | p-Value 2 |
|---|---|---|---|
| AUC0–12 h ((µmol/L)*h) (SB) | 26.07 | 19.72–32.42 | 0.2966 |
| AUC0–12 h ((µmol/L)*h) (CB) | 23.28 | 13.61–32.95 | |
| AUC0–24 h ((µmol/L)*h) (SB) | 29.57 | 21.26–37.87 | 0.4144 |
| AUC0–24 h ((µmol/L)*h) (CB) | 30.36 | 12.88–47.85 | |
| Cmax (µmol/L) (SB) | 4.21 | 3.43–4.99 | 0.2690 |
| Cmax (µmol/L) (CB) | 3.67 | 2.82–4.51 | |
| Tmax (h) (SB) | 4.17 | 2.9–5.43 | 0.0076 |
| Tmax (h) (CB) | 2.67 | 2.04–3.29 |
| Variable | Mean | 95% CI | p-Value 1 |
|---|---|---|---|
| AUC0–12 h ((µmol/L)*h) (SB) | 185.8 | 154–217.6 | 0.0641 |
| AUC0–12 h ((µmol/L)*h) (CB) | 168.4 | 137.4–199.4 | |
| AUC0–24 h ((µmol/L)*h) (SB) | 317.1 | 255.3–378.9 | 0.2394 |
| AUC0–24 h ((µmol/L)*h) (CB) | 286.9 | 229.0–344.8 | |
| Cmax (µmol/L) (SB) | 22.55 | 18.62–26.48 | 0.0122 |
| Cmax (µmol/L) (CB) | 18.87 | 15.31–22.42 | |
| Tmax (h) (SB) | 7.67 | 6.93–8.40 | 1.0 |
| Tmax (h) (CB) | 7.67 | 6.36–8.98 |
| Variable | Mean | 95% CI | p-Value 1 |
|---|---|---|---|
| AUC0–12 h ((µmol/L)*h) (SB) | 6.75 | 3.99–9.51 | 0.0890 |
| AUC0–12 h ((µmol/L)*h) (CB) | 5.17 | 2.55–7.78 | |
| AUC0–24 h ((µmol/L)*h) (SB) | 15.79 | 10.11–21.46 | 0.1546 |
| AUC0–24 h ((µmol/L)*h) (CB) | 12.74 | 7.13–18.35 | |
| Cmax (µmol/L) (SB) | 1.22 | 0.81–1.61 | 0.1614 |
| Cmax (µmol/L) (CB) | 0.95 | 0.64–1.27 | |
| Tmax (h) (SB) | 12.0 | 8.25–15.75 | 0.9336 |
| Tmax (h) (CB) | 13.33 | 8.21–18.45 |
| Variable | Mean | 95% CI | p-Value 1 |
|---|---|---|---|
| AUC0–12 h ((µmol/L)*h) (SB) | 10.06 | 4.19–15.92 | <0.0001 |
| AUC0–12 h ((µmol/L)*h) (CB) | 104.3 | 59.8–148.8 | |
| AUC0–24 h ((µmol/L)*h) (SB) | 25.41 | 11.76–39.06 | <0.0001 |
| AUC0–24 h ((µmol/L)*h) (CB) | 288.3 | 231.8–362.8 | |
| Cmax (µmol/L) (SB) | 2.24 | 1.17–3.30 | <0.0001 |
| Cmax (µmol/L) (CB) | 28.99 | 20.36–37.63 | |
| Tmax (h) (SB) | 12.0 | 9.35–14.65 | 0.7884 |
| Tmax (h) (CB) | 11.67 | 8.91–14.42 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mödinger, Y.; Schön, C.; Wilhelm, M.; Hals, P.-A. Plasma Kinetics of Choline and Choline Metabolites After A Single Dose of SuperbaBoostTM Krill Oil or Choline Bitartrate in Healthy Volunteers. Nutrients 2019, 11, 2548. https://doi.org/10.3390/nu11102548
Mödinger Y, Schön C, Wilhelm M, Hals P-A. Plasma Kinetics of Choline and Choline Metabolites After A Single Dose of SuperbaBoostTM Krill Oil or Choline Bitartrate in Healthy Volunteers. Nutrients. 2019; 11(10):2548. https://doi.org/10.3390/nu11102548
Chicago/Turabian StyleMödinger, Yvonne, Christiane Schön, Manfred Wilhelm, and Petter-Arnt Hals. 2019. "Plasma Kinetics of Choline and Choline Metabolites After A Single Dose of SuperbaBoostTM Krill Oil or Choline Bitartrate in Healthy Volunteers" Nutrients 11, no. 10: 2548. https://doi.org/10.3390/nu11102548





