Low Diversity of Human Milk Oligosaccharides is Associated with Necrotising Enterocolitis in Extremely Low Birth Weight Infants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Clinical Outcomes
2.3. Purification of Human Milk Oligosaccharides
2.4. Analysis of Human Milk Oligosaccharides
2.5. Statistical Analyses
3. Results
3.1. Description of the Study Population
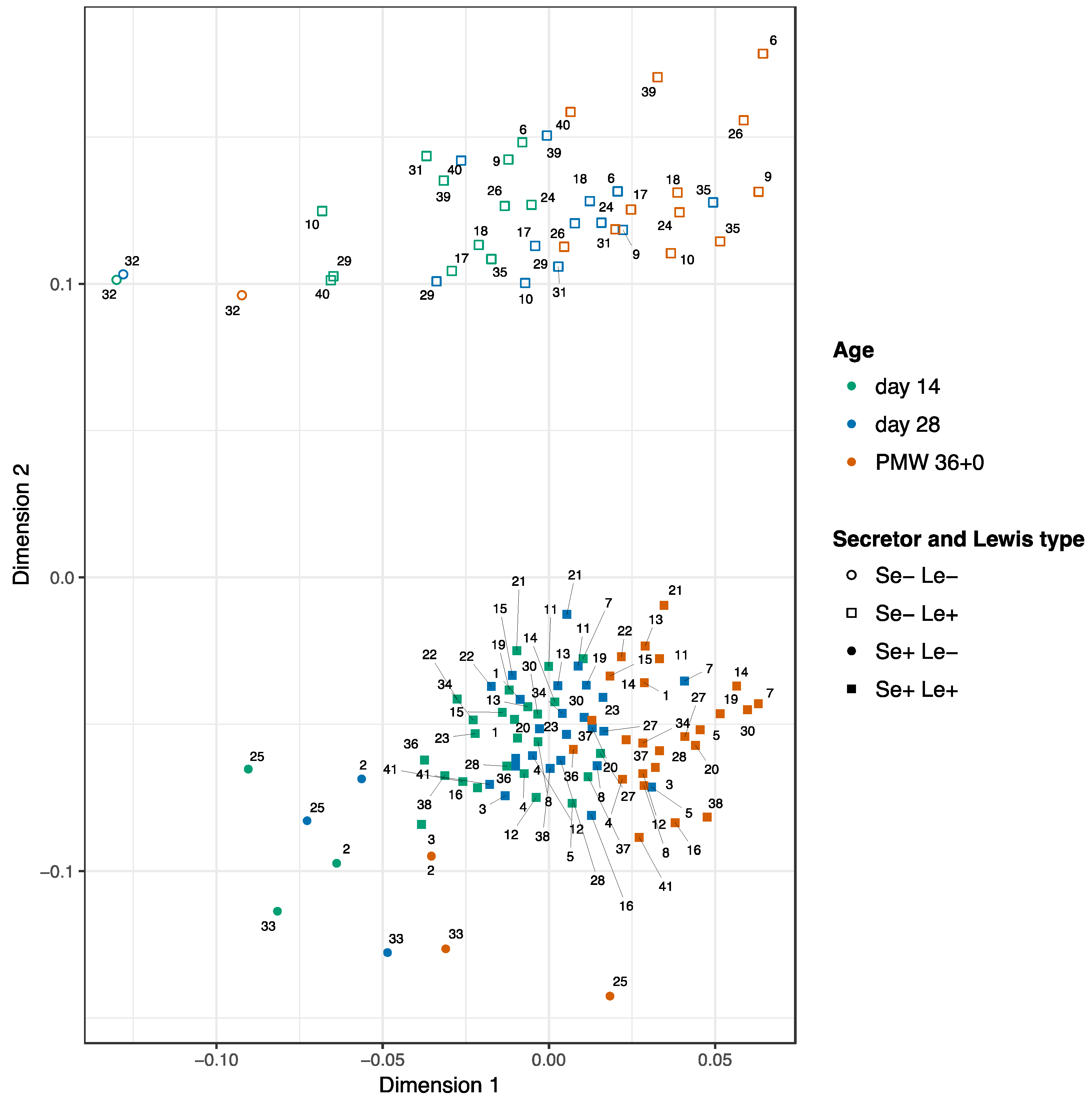
3.2. Development of the HMOs Over Time
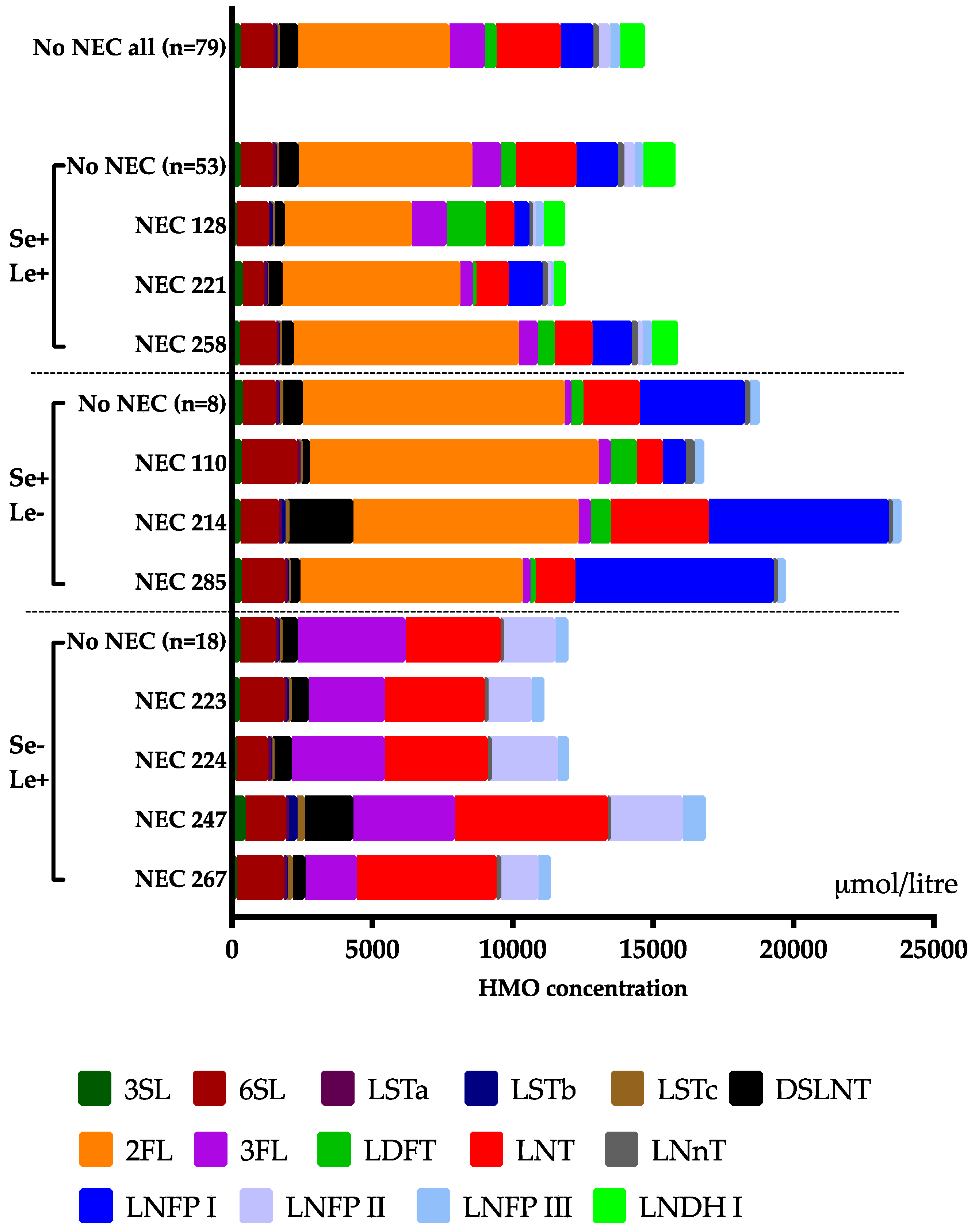
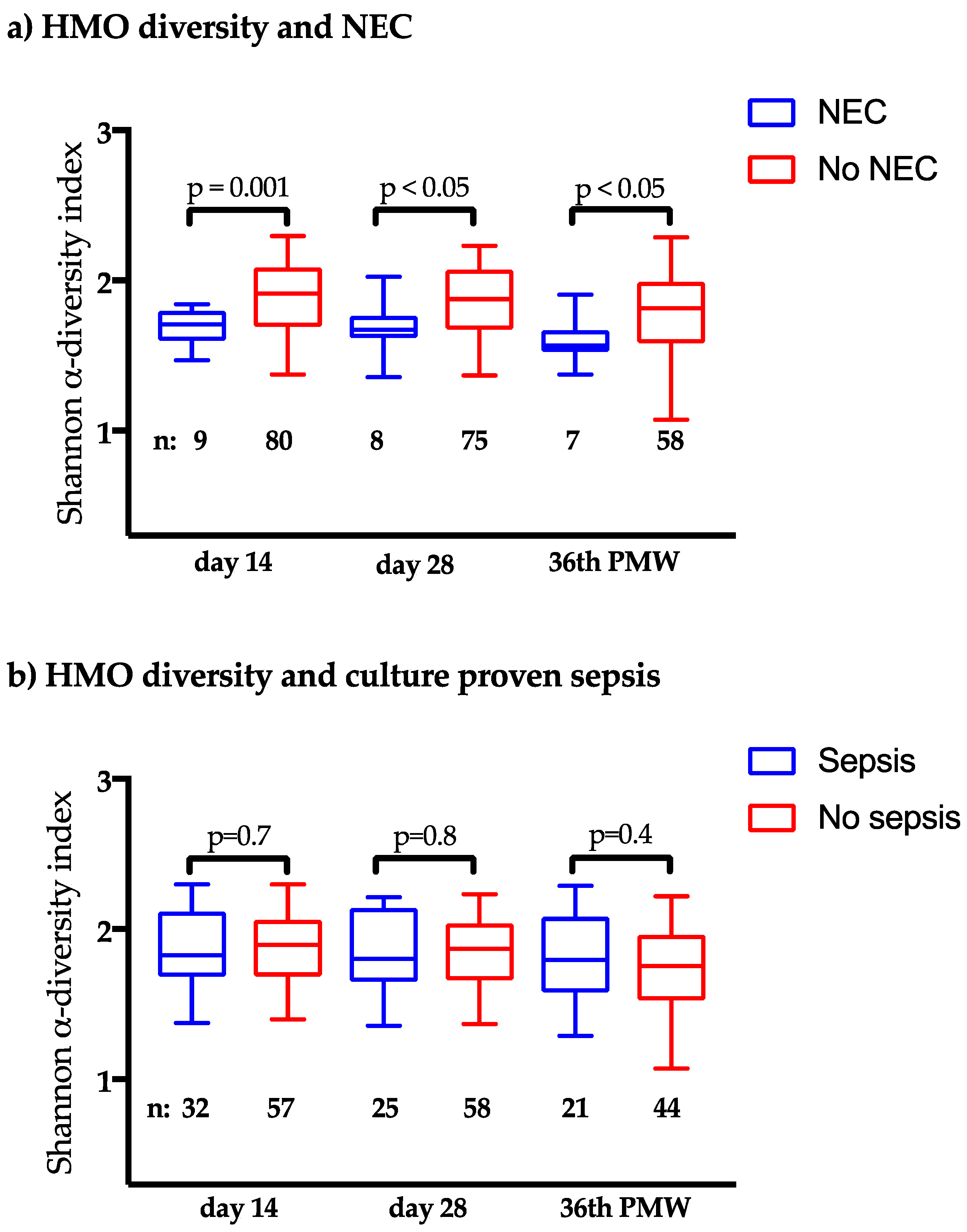
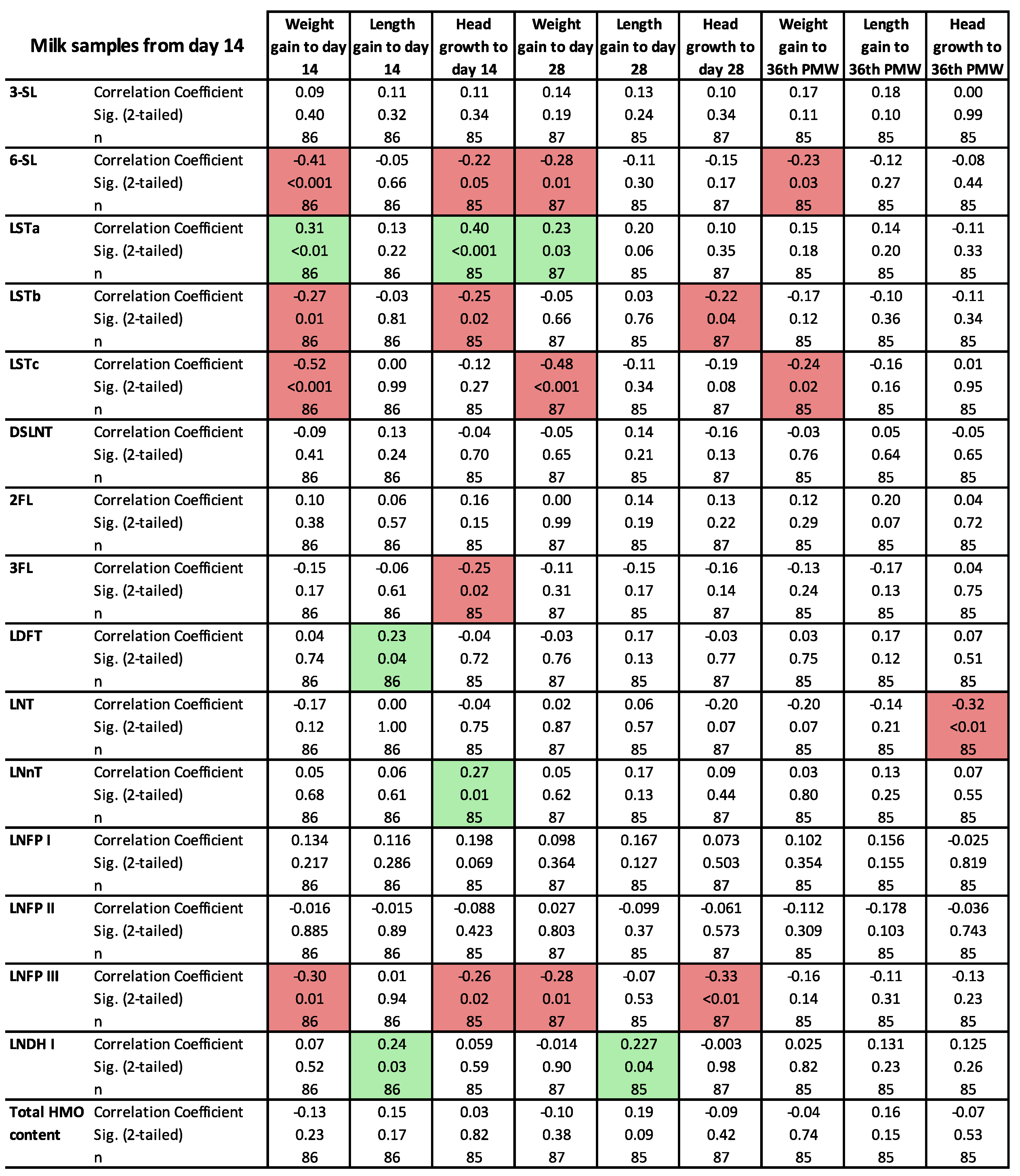
3.3. Clinical Outcomes in Relation to the HMO Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- EXPRESS Group; Fellman, V.; Hellström-Westas, L.; Norman, M.; Westgren, M.; Källén, K.; Lagercrantz, H.; Marsál, K.; Serenius, F.; Wennergren, M. One-year survival of extremely preterm infants after active perinatal care in sweden. JAMA 2009, 301, 2225–2233. [Google Scholar] [CrossRef] [PubMed]
- Boyd, C.A.; Quigley, M.A.; Brocklehurst, P. Donor breast milk versus infant formula for preterm infants: Systematic review and meta-analysis. Arch. Dis. Child.-Fetal Neonatal Ed. 2007, 92, F169–F175. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.J.; Ternberg, J.L.; Feigin, R.D.; Keating, J.P.; Marshall, R.; Barton, L.; Brotherton, T. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann. Surg. 1978, 187, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.W.; Stoll, B.J. Necrotising enterocolitis. Lancet 2006, 368, 1271–1283. [Google Scholar] [CrossRef]
- Thomas, J.P.; Raine, T.; Reddy, S.; Belteki, G. Probiotics for the prevention of necrotising enterocolitis in very low-birth-weight infants: A meta-analysis and systematic review. Acta. Paediatr. 2017, 106, 1729–1741. [Google Scholar] [CrossRef] [PubMed]
- Cristofalo, E.A.; Schanler, R.J.; Blanco, C.L.; Sullivan, S.; Trawoeger, R.; Kiechl-Kohlendorfer, U.; Dudell, G.; Rechtman, D.J.; Lee, M.L.; Lucas, A.; et al. Randomized trial of exclusive human milk versus preterm formula diets in extremely premature infants. J. Pediatr. 2013, 163, 1592–1595. [Google Scholar] [CrossRef] [PubMed]
- Roze, E.; Ta, B.D.; van der Ree, M.H.; Tanis, J.C.; van Braeckel, K.N.; Hulscher, J.B.; Bos, A.F. Functional impairments at school age of children with necrotizing enterocolitis or spontaneous intestinal perforation. Pediatr. Res. 2011, 70, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Lönnerdal, B. Bioactive proteins in human milk: Health, nutrition, and implications for infant formulas. J. Pediatr. 2016. [Google Scholar] [CrossRef] [PubMed]
- Manti, S.; Lougaris, V.; Cuppari, C.; Tardino, L.; Dipasquale, V.; Arrigo, T.; Salpietro, C.; Leonardi, S. Breastfeeding and il-10 levels in children affected by cow’s milk protein allergy: A restrospective study. Immunobiology 2017, 222, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Musilova, S.; Rada, V.; Vlkova, E.; Bunesova, V. Beneficial effects of human milk oligosaccharides on gut microbiota. Benef. Microbes 2014, 5, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Manthey, C.F.; Autran, C.A.; Eckmann, L.; Bode, L. Human milk oligosaccharides protect against enteropathogenic escherichia coli attachment in vitro and epec colonization in suckling mice. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 165–168. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Liu, S.; Leone, S.; Newburg, D.S. Human colostrum oligosaccharides modulate major immunologic pathways of immature human intestine. Mucosal. Immunol. 2014, 7, 1326–1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newburg, D.S.; Ruiz-Palacios, G.M.; Morrow, A.L. Human milk glycans protect infants against enteric pathogens. Annu. Rev. Nutr. 2005, 25, 37–58. [Google Scholar] [CrossRef] [PubMed]
- Jantscher-Krenn, E.; Zherebtsov, M.; Nissan, C.; Goth, K.; Guner, Y.S.; Naidu, N.; Choudhury, B.; Grishin, A.V.; Ford, H.R.; Bode, L. The human milk oligosaccharide disialyllacto-n-tetraose prevents necrotising enterocolitis in neonatal rats. Gut 2012, 61, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Autran, C.A.; Schoterman, M.H.; Jantscher-Krenn, E.; Kamerling, J.P.; Bode, L. Sialylated galacto-oligosaccharides and 2′-fucosyllactose reduce necrotising enterocolitis in neonatal rats. Br. J. Nutr. 2016, 116, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Autran, C.A.; Kellman, B.P.; Kim, J.H.; Asztalos, E.; Blood, A.B.; Spence, E.C.H.; Patel, A.L.; Hou, J.; Lewis, N.E.; Bode, L. Human milk oligosaccharide composition predicts risk of necrotising enterocolitis in preterm infants. Gut 2018, 67, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- McGuire, M.K.; Meehan, C.L.; McGuire, M.A.; Williams, J.E.; Foster, J.; Sellen, D.W.; Kamau-Mbuthia, E.W.; Kamundia, E.W.; Mbugua, S.; Moore, S.E.; et al. What’s normal? Oligosaccharide concentrations and profiles in milk produced by healthy women vary geographically. Am. J. Clin. Nutr. 2017, 105, 1086–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charbonneau, M.R.; O’Donnell, D.; Blanton, L.V.; Totten, S.M.; Davis, J.C.; Barratt, M.J.; Cheng, J.; Guruge, J.; Talcott, M.; Bain, J.R.; et al. Sialylated milk oligosaccharides promote microbiota-dependent growth in models of infant undernutrition. Cell 2016, 164, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Monaco, M.H.; Wang, M.; Comstock, S.S.; Kuhlenschmidt, T.B.; Fahey, G.C., Jr.; Miller, M.J.; Kuhlenschmidt, M.S.; Donovan, S.M. Human milk oligosaccharides shorten rotavirus-induced diarrhea and modulate piglet mucosal immunity and colonic microbiota. ISME J. 2014, 8, 1609–1620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, A.E.; Autran, C.A.; Szyszka, A.; Escajadillo, T.; Huang, M.; Godula, K.; Prudden, A.R.; Boons, G.J.; Lewis, A.L.; Doran, K.S.; et al. Human milk oligosaccharides inhibit growth of group b streptococcus. J. Biol. Chem. 2017, 292, 11243–11249. [Google Scholar] [CrossRef] [PubMed]
- Wejryd, E.; Marchini, G.; Frimmel, V.; Jonsson, B.; Abrahamsson, T. Probiotics promoted head growth in extremely low birthweight infants in a double-blind placebo-controlled trial. Acta. Paediatr. 2018, 35, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Niklasson, A.; Albertsson-Wikland, K. Continuous growth reference from 24th week of gestation to 24 months by gender. BMC Pediatr. 2008, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Gabrielli, O.; Zampini, L.; Galeazzi, T.; Padella, L.; Santoro, L.; Peila, C.; Giuliani, F.; Bertino, E.; Fabris, C.; Coppa, G.V. Preterm milk oligosaccharides during the first month of lactation. Pediatrics 2011, 128, e1520–e1531. [Google Scholar] [CrossRef] [PubMed]
- Nakhla, T.; Fu, D.; Zopf, D.; Brodsky, N.L.; Hurt, H. Neutral oligosaccharide content of preterm human milk. Br. J. Nutr. 1999, 82, 361–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thurl, S.; Muller-Werner, B.; Sawatzki, G. Quantification of individual oligosaccharide compounds from human milk using high-ph anion-exchange chromatography. Anal. Biochem. 1996, 235, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Landberg, E.; Lundblad, A.; Påhlsson, P. Temperature effects in high-performance anion-exchange chromatography of oligosaccharides. J. Chromatogr. A 1998, 814, 97–104. [Google Scholar] [CrossRef]
- Clarke, K.R.; Green, H.R. Statistical design and analysis for a ‘biological effects’ study. Mar. Ecol. Prog. Ser. 1988, 46, 213–226. [Google Scholar] [CrossRef]
- Minchin, P.R.; Peter, R. An evaluation of relative robustness of techniques for ecological ordinations. Vegetation 1987, 69, 89–107. [Google Scholar] [CrossRef]
- R-Core-Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Good, M.; Sodhi, C.P.; Yamaguchi, Y.; Jia, H.; Lu, P.; Fulton, W.B.; Martin, L.Y.; Prindle, T.; Nino, D.F.; Zhou, Q.; et al. The human milk oligosaccharide 2′-fucosyllactose attenuates the severity of experimental necrotising enterocolitis by enhancing mesenteric perfusion in the neonatal intestine. Br. J. Nutr. 2016, 116, 1175–1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreas, N.J.; Al-Khalidi, A.; Jaiteh, M.; Clarke, E.; Hyde, M.J.; Modi, N.; Holmes, E.; Kampmann, B.; Mehring Le Doare, K. Role of human milk oligosaccharides in group b streptococcus colonisation. Clin. Transl. Immunol. 2016, 5, e99. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, B.S.; Philippot, L. Insights into the resistance and resilience of the soil microbial community. FEMS Microbiol. Rev. 2013, 37, 112–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMurtry, V.E.; Gupta, R.W.; Tran, L.; Blanchard, E.E.t.; Penn, D.; Taylor, C.M.; Ferris, M.J. Bacterial diversity and clostridia abundance decrease with increasing severity of necrotizing enterocolitis. Microbiome 2015, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, T.R.; Jakobsson, H.E.; Andersson, A.F.; Björkstén, B.; Engstrand, L.; Jenmalm, M.C. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin. Exp. Allergy 2014, 44, 842–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eaton, S.; Rees, C.M.; Hall, N.J. Current research on the epidemiology, pathogenesis, and management of necrotizing enterocolitis. Neonatology 2017, 111, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Marseglia, L.; D’Angelo, G.; Manti, S.; Aversa, S.; Reiter, R.J.; Antonuccio, P.; Centorrino, A.; Romeo, C.; Impellizzeri, P.; Gitto, E. Oxidative stress-mediated damage in newborns with necrotizing enterocolitis: A possible role of melatonin. Am. J. Perinatol. 2015, 32, 905–909. [Google Scholar] [PubMed]
- Bode, L.; Kunz, C.; Muhly-Reinholz, M.; Mayer, K.; Seeger, W.; Rudloff, S. Inhibition of monocyte, lymphocyte, and neutrophil adhesion to endothelial cells by human milk oligosaccharides. J. Thromb. Haemost. 2004, 92, 1402–1410. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Liu, S.; Kling, D.E.; Leone, S.; Lawlor, N.T.; Huang, Y.; Feinberg, S.B.; Hill, D.R.; Newburg, D.S. The human milk oligosaccharide 2′-fucosyllactose modulates cd14 expression in human enterocytes, thereby attenuating lps-induced inflammation. Gut 2016, 65, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Bienenstock, J.; Buck, R.H.; Linke, H.; Forsythe, P.; Stanisz, A.M.; Kunze, W.A. Fucosylated but not sialylated milk oligosaccharides diminish colon motor contractions. PLoS ONE 2013, 8, e76236. [Google Scholar] [CrossRef] [PubMed]
- Coppa, G.V.; Zampini, L.; Galeazzi, T.; Facinelli, B.; Ferrante, L.; Capretti, R.; Orazio, G. Human milk oligosaccharides inhibit the adhesion to caco-2 cells of diarrheal pathogens: Escherichia coli, vibrio cholerae, and salmonella fyris. Pediatr. Res. 2006, 59, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Pammi, M.; Cope, J.; Tarr, P.I.; Warner, B.B.; Morrow, A.L.; Mai, V.; Gregory, K.E.; Kroll, J.S.; McMurtry, V.; Ferris, M.J.; et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: A systematic review and meta-analysis. Microbiome 2017, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.T.; Chen, C.; Newburg, D.S. Utilization of major fucosylated and sialylated human milk oligosaccharides by isolated human gut microbes. Glycobiology 2013, 23, 1281–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Underwood, M.A.; Gaerlan, S.; De Leoz, M.L.; Dimapasoc, L.; Kalanetra, K.M.; Lemay, D.G.; German, J.B.; Mills, D.A.; Lebrilla, C.B. Human milk oligosaccharides in premature infants: Absorption, excretion, and influence on the intestinal microbiota. Pediatr. Res. 2015, 78, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Marseglia, L.; Manti, S.; D’Angelo, G.; Cuppari, C.; Salpietro, V.; Filippelli, M.; Trovato, A.; Gitto, E.; Salpietro, C.; Arrigo, T. Obesity and breastfeeding: The strength of association. Women Birth 2015, 28, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.C.; Lewis, Z.T.; Krishnan, S.; Bernstein, R.M.; Moore, S.E.; Prentice, A.M.; Mills, D.A.; Lebrilla, C.B.; Zivkovic, A.M. Growth and morbidity of gambian infants are influenced by maternal milk oligosaccharides and infant gut microbiota. Sci. Rep. 2017, 7, 40466. [Google Scholar] [CrossRef] [PubMed]
- Kunz, C.; Meyer, C.; Collado, M.C.; Geiger, L.; Garcia-Mantrana, I.; Bertua-Rios, B.; Martinez-Costa, C.; Borsch, C.; Rudloff, S. Influence of gestational age, secretor, and lewis blood group status on the oligosaccharide content of human milk. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 789–798. [Google Scholar] [CrossRef] [PubMed]
| Name | Type | Secreted by | Day 14 n = 78 Median (IQR) | Day 28 n = 71 Median (IQR) | 36th PMW N = 56 Median (IQR) |
|---|---|---|---|---|---|
| 3′-sialyllactose (3SL) | Sialylated | All | 328 (255–395) | 292 (226–336) | 207 (161–280) |
| 6′-sialyllactose (6SL) | Sialylated | All | 1197 (935–1587) | 821 (602–1019) | 291 (191–488) |
| Sialyl-lacto-N-tetraose a (LSTa) | Sialylated | All | 9 (5–14) | 4 (3–8) | 3 (2–5) |
| Sialyl-lacto-N-tetraose b (LSTb) | Sialylated | All | 69 (42–127) | 102 (45–142) | 75 (33–108) |
| Sialyl-lacto-N-neotetraosec (LSTc) | Sialylated | All | 130 (86–197) | 79 (48–107) | 20 (12–34) |
| Disialyl-lacto-N-tetraose (DSLNT) | Sialylated | All | 657 (475–1049) | 669 (394–910) | 389 (206–516) |
| 2′-fucosyllactose (2FL) | Neutral | Se+ | 5390 (0–7383) | 4720 (0–7072) | 4379 (1964–5840) |
| 3′-fucosyllactose (3FL) | Neutral | All | 1241 (569–2005) | 1486 (795–2680) | 1803 (1061–3003) |
| Lacto-difucotetraose (LDFT) | Neutral | Se+ | 394 (0–685) | 388 (0–717) | 466 (38–645) |
| Lacto-N-tetraose (LNT) | Neutral | All | 2294 (1708–3138) | 2205 (1640–2897) | 1529 (1112–2189) |
| Lacto-N-neotetraose (LNnT) | Neutral | All | 180 (96–263) | 151 (86–220) | 155 (90–271) |
| Lacto-N-fucopentaose I (LNFP I) | Neutral | Se+ | 1163 (0–1852) | 819 (0–1714) | 536 (95–1126) |
| Lacto-N-fucopentaose II (LNFP II) | Neutral | Le+ | 401 (147–948) | 384 (152–826) | 324 (177–685) |
| Lacto-N-fucopentaose III (LNFP III) | Neutral | All | 362 (261–496) | 402 (301–503) | 423 (320–518) |
| Lacto-N-difucohexaose I (LNDH I) | Neutral | Se+ Le+ | 652 (0–1176) | 726 (0–1173) | 454 (0–968) |
| Σ analyzed HMO | 15770 (12694–17393) | 14992 (12803–17655) | 11676 (10157–13703) |
| No NEC (n = 96) | NEC (n = 10) | p * | |
|---|---|---|---|
| Gestational age, weeks + days, mean (SD) | 25 + 4 (9 days) | 25 + 1 (9 days) | 0.3 |
| Birth weight, g, mean (SD) | 749 (136) | 681 (123) | 0.1 |
| Birth weight zscore, mean (SD) | −1.1 (1.2) | −1.4 (1.6) | 0.6 |
| Birth length, cm, mean (SD) | 32.7 (2.5) | 32.0 (2.2) | 0.4 |
| Birth length z-score, mean (SD) | −1.5 (1.8) | −1.4 (2.0) | 0.9 |
| Birth head circumference, cm, mean (SD) | 23.0 (1.5) | 22.8 (1.2) | 0.5 |
| Birth head circumference z-score, mean (SD) | −0.8 (0.8) | −0.6 (0.7) | 0.4 |
| Small for gestational age, n (%) | 22 (23) | 2 (20) | 0.8 |
| Caesarean section, n (%) | 62 (65) | 6 (60) | 0.8 |
| Apgar score 5 min, mean (SD) | 6.2 (2.6) | 7.0 (2.4) | 0.3 |
| Apgar score 10 min, mean (SD) | 7.8 (1.9) | 8.4 (1.6) | 0.3 |
| Male, n (%) | 49 (51) | 9 (90) | <0.01 |
| Infants from multiple pregnancy, n (%) | 35 (36) | 4 (40) | 0.8 |
| Maternal smoking, n (%) | 2 (2) | 1 (10) | 0.5 |
| Maternal preeclampsia, n (%) | 10 (10) | 1 (10) | 1.0 |
| Preterm premature rupture of membranes, n (%) | 30 (30) | 4 (40) | 0.6 |
| Maternal chorioamnionitis, n (%) | 20 (21) | 3 (30) | 0.6 |
| Maternal prepartal antibiotics, n (%) | 51 (53) | 5 (50) | 0.9 |
| Prenatal steroids, n (%) | 94 (81) | 10 (100) | 0.2 |
| Received surfactant, n (%) | 78 (81) | 9 (90) | 0.4 |
| Antibiotics during first week, n (%) | 95 (99) | 10 (100) | 1.0 |
| Antibiotics during second week, n (%) | 76 (79) | 10 (100) | 0.2 |
| Probiotic supplementation, n (%) | 50 (52) | 5 (50) | 1.0 |
| Patent ductus arteriosus treated, n (%) | 70 (73) | 7 (70) | 1.0 |
| Secreted by | NEC (n = 9) Median (IQR) | No NEC (n = 80) Median (IQR) | p * | |||
|---|---|---|---|---|---|---|
| 3SL | All | 318 | (231–376) | 321 | (254–395) | 0.8 |
| 6SL | All | 1437 | (1220–1635) | 1159 | (919–1585) | 0.2 |
| LSTa | All | 9 | (7–17) | 10 | (5–14) | 0.6 |
| LSTb | All | 49 | (29–144) | 68 | (42–128) | 0.4 |
| LSTc | All | 138 | (103–196) | 130 | (85–198) | 0.7 |
| DSLNT | All | 572 | (401–1193) | 674 | (498–1038) | 0.4 |
| 2FL | Se+ | 6331 | (0–8026) | 5390 | (3374–7223) | 0.9 |
| 3FL | All | 675 | (436–3004) | 1255 | (575–1841) | 0.6 |
| LDFT | Se+ | 45 | (0–651) | 410 | (28–686) | 0.2 |
| LNT | All | 3507 | (1277–4320) | 2294 | (1710–2990) | 0.7 |
| LNnT | All | 129 | (90–208) | 187 | (111–273) | 0.2 |
| LNFP I | Se+ | 797 | (0–3903) | 1165 | (441–1789) | 0.5 |
| LNFP II | Le+ | 124 | (0–1996) | 413 | (191–880) | 0.6 |
| LNFP III | All | 363 | (332–437) | 351 | (267–526) | 0.8 |
| LNDH I | Se+ Le+ | 0 | (0–213) | 882 | (0–1279) | <0.01 |
| Σ analyzed HMO | 15889 | (11623–18311) | 15770 | (13405–17274) | 0.8 | |
| Secretedby | Sepsis (n = 32) Median (IQR) | No Sepsis (n = 57) Median (IQR) | p * | |||
|---|---|---|---|---|---|---|
| 3-SL | All | 283 | (236–336) | 331 | (268–399) | 0.2 |
| 6-SL | All | 1313 | (1088–1647) | 1142 | (876–1503) | <0.05 |
| LSTa | All | 7 | (4–12) | 11 | (7–14) | 0.1 |
| LSTb | All | 62 | (34–154) | 72 | (42–114) | 0.5 |
| LSTc | All | 134 | (102–174) | 127 | (79–199) | 0.8 |
| DSLNT | All | 622 | (426–941) | 644 | (502–1093) | 0.2 |
| 2FL | Se+ | 4829 | (0–7674) | 5944 | (3429–7467) | 0.3 |
| 3FL | All | 1367 | (595–2983) | 1227 | (524–1833) | 0.4 |
| LDFT | Se+ | 386 | (0–664) | 404 | (22–690) | 0.7 |
| LNT | All | 2459 | (1481–3630) | 2194 | (1768–2710) | 0.5 |
| LNnT | All | 202 | (100–273) | 177 | (110–257) | 0.9 |
| LNFP I | Se+ | 957 | (0–1625) | 1490 | (475–2042) | 0.2 |
| LNFP II | Le+ | 494 | (171–1348) | 372 | (123–749) | 0.3 |
| LNFP III | All | 363 | (269–468) | 351 | (255–531) | 0.8 |
| LNDH I | Se+ Le+ | 632 | (0–1194) | 821 | (0–1231) | 0.5 |
| Σ analyzed HMO | 15723 | (13137–17176) | 15972 | (13008–17512) | 0.8 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wejryd, E.; Martí, M.; Marchini, G.; Werme, A.; Jonsson, B.; Landberg, E.; Abrahamsson, T.R. Low Diversity of Human Milk Oligosaccharides is Associated with Necrotising Enterocolitis in Extremely Low Birth Weight Infants. Nutrients 2018, 10, 1556. https://doi.org/10.3390/nu10101556
Wejryd E, Martí M, Marchini G, Werme A, Jonsson B, Landberg E, Abrahamsson TR. Low Diversity of Human Milk Oligosaccharides is Associated with Necrotising Enterocolitis in Extremely Low Birth Weight Infants. Nutrients. 2018; 10(10):1556. https://doi.org/10.3390/nu10101556
Chicago/Turabian StyleWejryd, Erik, Magalí Martí, Giovanna Marchini, Anna Werme, Baldvin Jonsson, Eva Landberg, and Thomas R. Abrahamsson. 2018. "Low Diversity of Human Milk Oligosaccharides is Associated with Necrotising Enterocolitis in Extremely Low Birth Weight Infants" Nutrients 10, no. 10: 1556. https://doi.org/10.3390/nu10101556





