Effects of Unfiltered Coffee and Bioactive Coffee Compounds on the Development of Metabolic Syndrome Components in a High-Fat-/High-Fructose-Fed Rat Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. Experimental Design
2.3. Intervention
2.4. Immunoassay Analyses
2.5. Histological Examinations
2.6. Hyperpolarized-[1-13C]-Pyruvate MR Spectroscopy
2.7. 1H MR Spectroscopy
2.8. Statistical Methods
3. Results
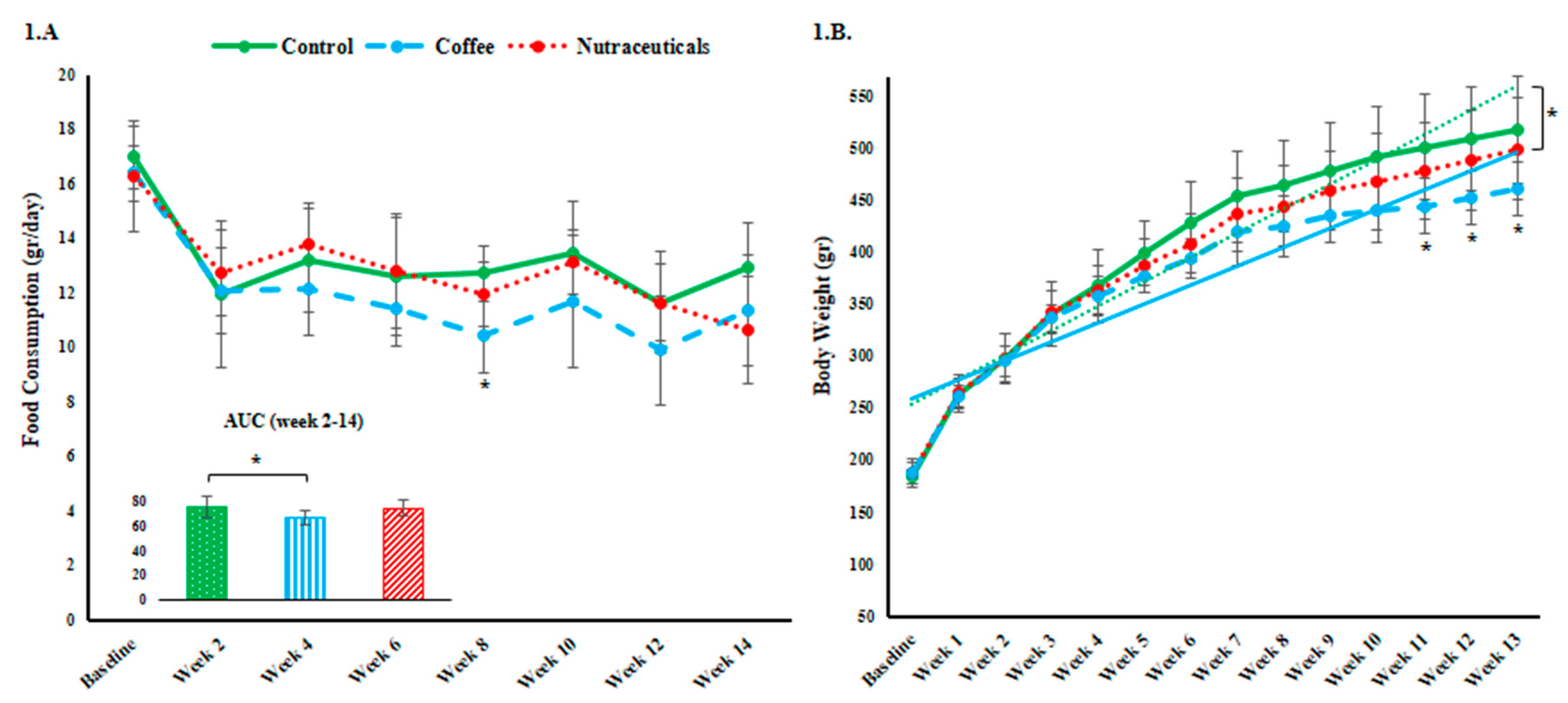
3.1. Food Intake and Body Weight
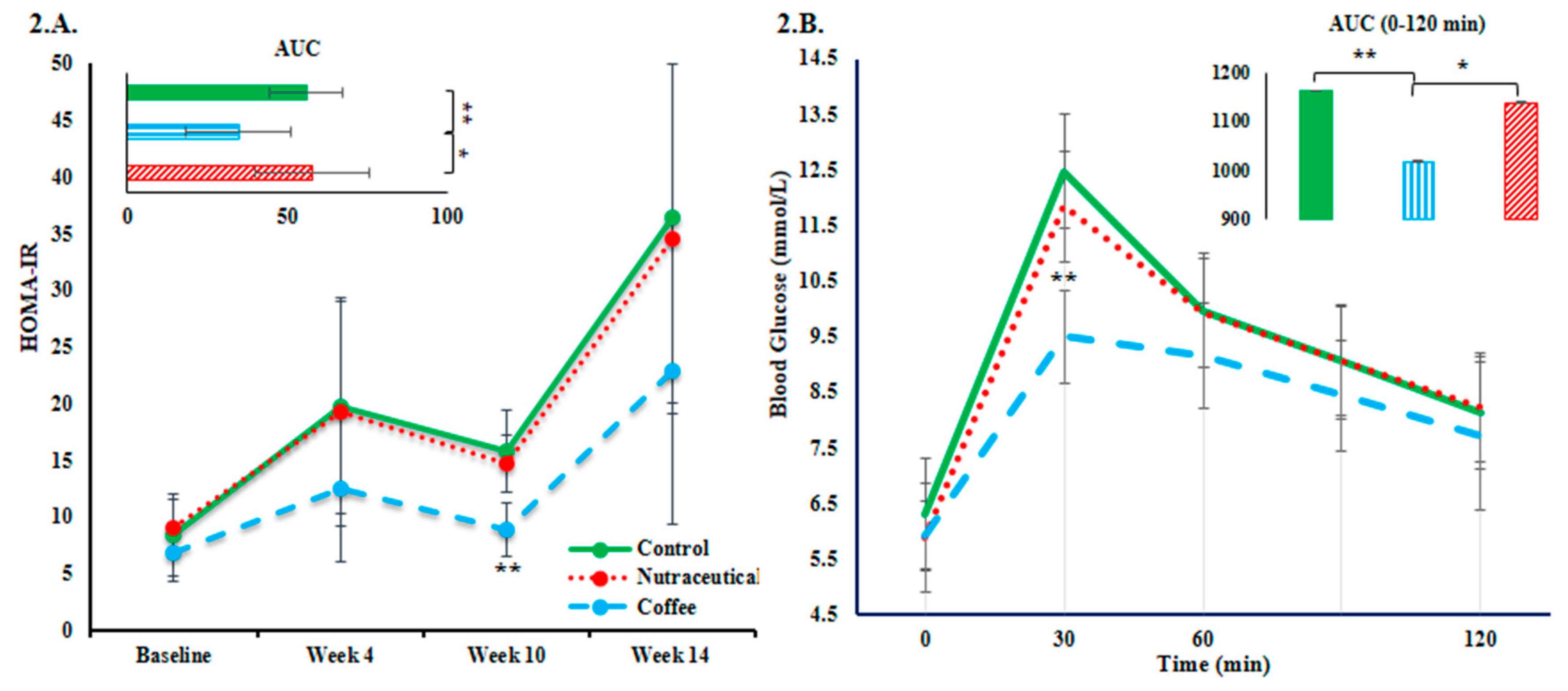
3.2. Plasma Glucose and Insulin Resistance
3.3. Plasma Lipid Profile
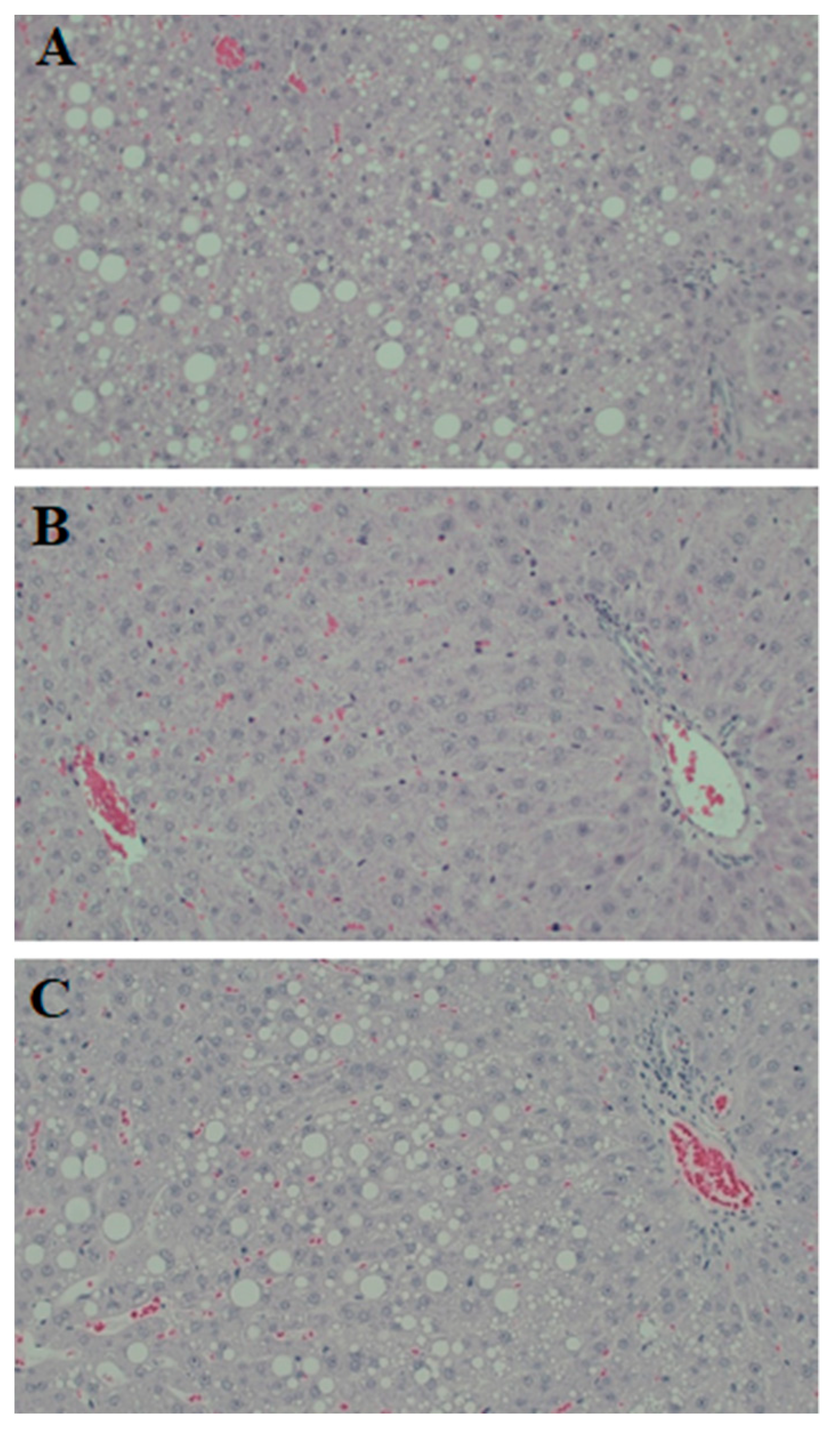
3.4. Hepatic Steatosis
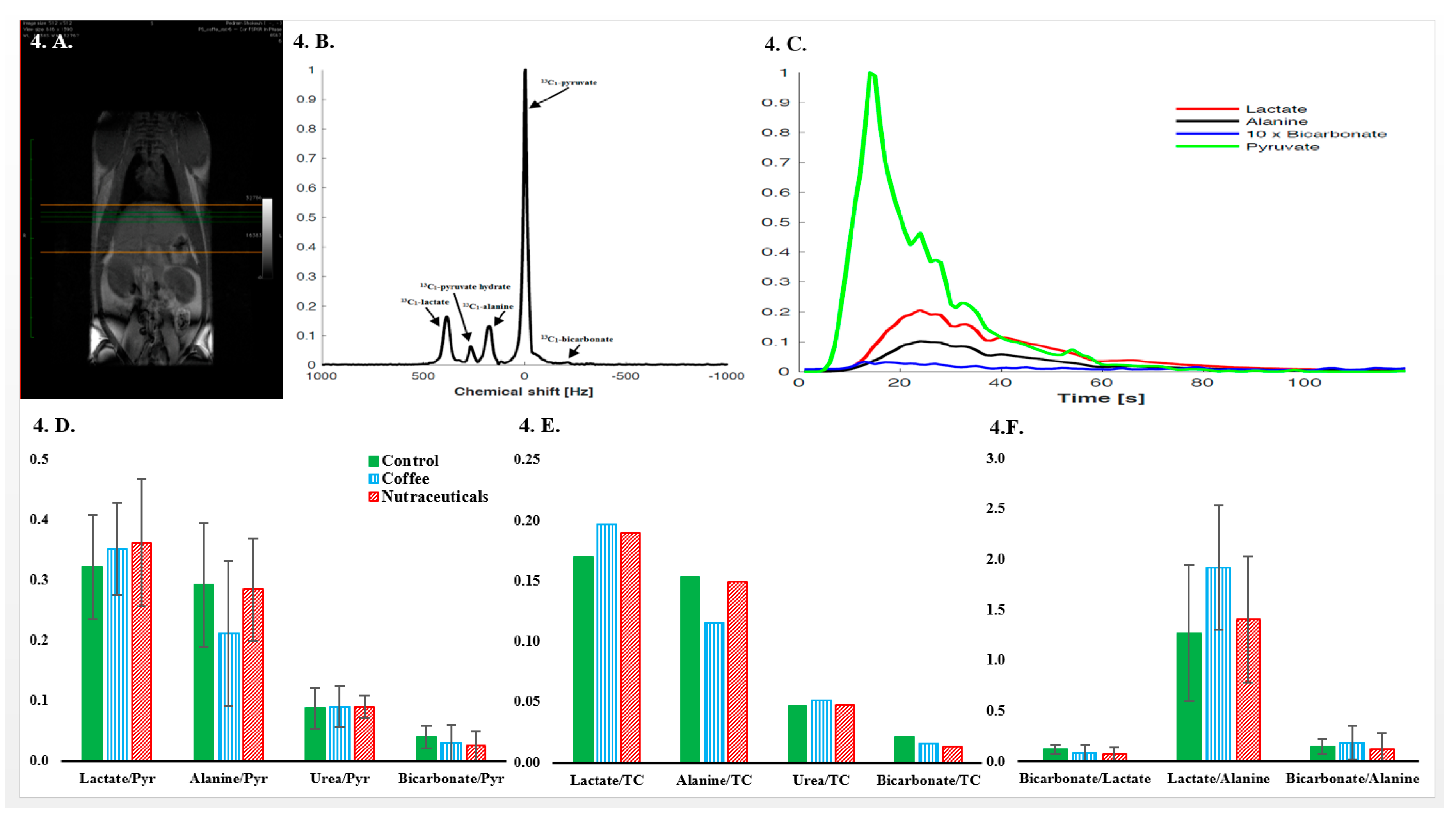
3.5. In Vivo Hyperpolarized-[1-13C]-Pyruvate Metabolic Examinations
3.6. Urine 1H-MR Spectroscopy.
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Ingredients | Gram Percent |
|---|---|
| Casein, 30 Mesh | 25.84 |
| L-Cystine | 0.39 |
| Corn Starch | 0.00 |
| Maltodextrin 10 | 16.15 |
| Sucrose | 8.89 |
| Cellulose | 6.46 |
| Soybean Oil | 32.53 |
| Lard | 31.66 |
| Mineral Mix S10026 | 1.29 |
| DiCalcium Phosphate | 1.68 |
| Calcium Carbonate | 0.71 |
| Potassium Citrate, 1 H2O | 2.13 |
| Vitamin Mix V10001 | 1.29 |
| Choline Bitartrate | 0.26 |
References
- Alberti, K.G.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ruotsalainen, S.; Moilanen, L.; Lepisto, P.; Laakso, M.; Kuusisto, J. The metabolic syndrome predicts cardiovascular mortality: A 13-year follow-up study in elderly non-diabetic Finns. Eur. Heart. J. 2007, 28, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Despres, J.P. Is visceral obesity the cause of the metabolic syndrome? Ann. Med. 2006, 38, 52–63. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, S.; O’Driscoll, L. Metabolic syndrome: A closer look at the growing epidemic and its associated pathologies. Obes. Rev. 2015, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Poole, R.; Kennedy, O.J.; Roderick, P.; Fallowfield, J.A.; Hayes, P.C.; Parkes, J. Coffee consumption and health: Umbrella review of meta-analyses of multiple health outcomes. BMJ 2017, 359, j5024. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, R.M.; Feskens, E.J. Coffee consumption and risk of type 2 diabetes mellitus. Lancet 2002, 360, 1477–1478. [Google Scholar] [CrossRef]
- Shang, F.; Li, X.; Jiang, X. Coffee consumption and risk of the metabolic syndrome: A meta-analysis. Diabetes Metab. 2016, 42, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Teoh, N.C.; Chitturi, S.; Farrell, G.C. Coffee and non-alcoholic fatty liver disease: Brewing evidence for hepatoprotection? J. Gastroenterol. Hepatol. 2014, 29, 435–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckley, A.J.; Thomas, E.L.; Lessan, N.; Trovato, F.M.; Trovato, G.M.; Taylor-Robinson, S.D. Non-alcoholic fatty liver disease: Relationship with cardiovascular risk markers and clinical endpoints. Diabetes Res. Clin. Pract. 2018, 144, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, I.A.; Clifford, M.N.; Lean, M.E.; Ashihara, H.; Crozier, A. Coffee: Biochemistry and potential impact on health. Food Funct. 2014, 5, 1695–1717. [Google Scholar] [CrossRef] [PubMed]
- Panchal, S.K.; Poudyal, H.; Iyer, A.; Nazer, R.; Alam, M.A.; Diwan, V.; Kauter, K.; Sernia, C.; Campbell, F.; Ward, L.; et al. High-carbohydrate, high-fat diet-induced metabolic syndrome and cardiovascular remodeling in rats. J. Cardiovasc. Pharmacol. 2011, 57, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, R.; Patras, M.A.; Eravuchira, P.J.; Kuhnert, N. Profile and characterization of the chlorogenic acids in green Robusta coffee beans by LC-MS(n): Identification of seven new classes of compounds. J. Agric. Food Chem. 2010, 58, 8722–8737. [Google Scholar] [CrossRef] [PubMed]
- Farah, A.; de Paulis, T.; Trugo, L.C.; Martin, P.R. Effect of roasting on the formation of chlorogenic acid lactones in coffee. J. Agric. Food Chem. 2005, 53, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Stennert, A.; Maier, H.G. Trigonelline in coffee. II. Content of green, roasted and instant coffee. Z. Lebensm. Unters. Forsch. 1994, 199, 198–200. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.M.; Lima, D.R. Coffee consumption, obesity and type 2 diabetes: A mini-review. Eur. J. Nutr. 2016, 55, 1345–1358. [Google Scholar] [CrossRef] [PubMed]
- Mazur, W.M.; Wahala, K.; Rasku, S.; Salakka, A.; Hase, T.; Adlercreutz, H. Lignan and isoflavonoid concentrations in tea and coffee. Br. J. Nutr. 1998, 79, 37–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Pascual-Teresa, S.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Quantitative analysis of flavan-3-ols in Spanish foodstuffs and beverages. J. Agric. Food Chem. 2000, 48, 5331–5337. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Bhupathiraju, S.N.; Chen, M.; van Dam, R.M.; Hu, F.B. Caffeinated and decaffeinated coffee consumption and risk of type 2 diabetes: A systematic review and a dose-response meta-analysis. Diabetes Care 2014, 37, 569–586. [Google Scholar] [CrossRef] [PubMed]
- Huxley, R.; Lee, C.M.; Barzi, F.; Timmermeister, L.; Czernichow, S.; Perkovic, V.; Grobbee, D.E.; Batty, D.; Woodward, M. Coffee, decaffeinated coffee, and tea consumption in relation to incident type 2 diabetes mellitus: A systematic review with meta-analysis. Arch. Intern. Med. 2009, 169, 2053–2063. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.; Varghese, M.; Wang, J.; Zhao, W.; Chen, F.; Knable, L.A.; Ferruzzi, M.; Pasinetti, G.M. Dietary supplementation with decaffeinated green coffee improves diet-induced insulin resistance and brain energy metabolism in mice. Nutr. Neurosci. 2012, 15, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Song, S.J.; Choi, S.; Park, T. Decaffeinated green coffee bean extract attenuates diet-induced obesity and insulin resistance in mice. Evid. Based Complement. Alternat. Med. 2014, 2014, 718379. [Google Scholar] [CrossRef] [PubMed]
- Scientific Opinion on the safety of caffeine. EFSA J. 2015, 13, 4102. [CrossRef] [Green Version]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Haycock, G.B.; Schwartz, G.J.; Wisotsky, D.H. Geometric method for measuring body surface area: A height-weight formula validated in infants, children, and adults. J. Pediatr. 1978, 93, 62–66. [Google Scholar] [CrossRef]
- Jouihan, H. Measurement of Liver Triglyceride Content. Bio. Protoc. 2012, 2, e223. [Google Scholar] [CrossRef]
- Laustsen, C.; Nielsen, P.M.; Norlinger, T.S.; Qi, H.; Pedersen, U.K.; Bertelsen, L.B.; Ostergaard, J.A.; Flyvbjerg, A.; Ardenkjaer-Larsen, J.H.; Palm, F.; et al. Antioxidant treatment attenuates lactate production in diabetic nephropathy. Am. J. Physiol. Renal. Physiol. 2017, 312, F192–F199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wishart, D.S.; Tzur, D.; Knox, C.; Eisner, R.; Guo, A.C.; Young, N.; Cheng, D.; Jewell, K.; Arndt, D.; Sawhney, S.; et al. HMDB: The Human Metabolome Database. Nucleic Acids Res. 2007, 35, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Hayter, A.J. The maximum familywise error rate of Fisher’s least significant difference test. J. Am. Stat. Assoc. 1986, 81, 1000–1004. [Google Scholar] [CrossRef]
- Panchal, S.K.; Brown, L. Rodent models for metabolic syndrome research. J. Biomed. Biotechnol. 2011, 2011, 351982. [Google Scholar] [CrossRef] [PubMed]
- Crescenzo, R.; Bianco, F.; Coppola, P.; Mazzoli, A.; Tussellino, M.; Carotenuto, R.; Liverini, G.; Iossa, S. Fructose supplementation worsens the deleterious effects of short-term high-fat feeding on hepatic steatosis and lipid metabolism in adult rats. Exp. Physiol. 2014, 99, 1203–1213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cowan, T.E.; Palmnas, M.S.; Yang, J.; Bomhof, M.R.; Ardell, K.L.; Reimer, R.A.; Vogel, H.J.; Shearer, J. Chronic coffee consumption in the diet-induced obese rat: Impact on gut microbiota and serum metabolomics. J. Nutr. Biochem. 2014, 25, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Aw, W.; Egashira, K.; Takahashi, S.; Aoyama, S.; Saito, K.; Kishimoto, Y.; Kato, H. Coffee intake mitigated inflammation and obesity-induced insulin resistance in skeletal muscle of high-fat diet-induced obese mice. Genes Nutr. 2014, 9, 389. [Google Scholar] [CrossRef] [PubMed]
- Rustenbeck, I.; Lier-Glaubitz, V.; Willenborg, M.; Eggert, F.; Engelhardt, U.; Jorns, A. Effect of chronic coffee consumption on weight gain and glycaemia in a mouse model of obesity and type 2 diabetes. Nutr. Diabetes 2014, 4, e123. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, Y.; Kobayashi, M.; Yamauchi, R.; Ojika, M.; Hiramitsu, M.; Inoue, T.; Katagiri, T.; Murai, A.; Horio, F. Coffee and caffeine improve insulin sensitivity and glucose tolerance in C57BL/6J mice fed a high-fat diet. Biosci. Biotechnol. Biochem. 2011, 75, 2309–2315. [Google Scholar] [CrossRef] [PubMed]
- Panchal, S.K.; Poudyal, H.; Waanders, J.; Brown, L. Coffee extract attenuates changes in cardiovascular and hepatic structure and function without decreasing obesity in high-carbohydrate, high-fat diet-fed male rats. J. Nutr. 2012, 142, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Panchal, S.K.; Wong, W.Y.; Kauter, K.; Ward, L.C.; Brown, L. Caffeine attenuates metabolic syndrome in diet-induced obese rats. Nutrition 2012, 28, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Jang, J.S.; Hong, S.M. Long-term consumption of caffeine improves glucose homeostasis by enhancing insulinotropic action through islet insulin/insulin-like growth factor 1 signaling in diabetic rats. Metabolism 2007, 56, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Davoodi, S.H.; Hajimiresmaiel, S.J.; Ajami, M.; Mohseni-Bandpei, A.; Ayatollahi, S.A.; Dowlatshahi, K.; Javedan, G.; Pazoki-Toroudi, H. Caffeine treatment prevented from weight regain after calorie shifting diet induced weight loss. Iran. J. Pharm. Res. 2014, 13, 707–718. [Google Scholar] [PubMed]
- Onakpoya, I.; Terry, R.; Ernst, E. The use of green coffee extract as a weight loss supplement: A systematic review and meta-analysis of randomised clinical trials. Gastroenterol. Res. Pract. 2011, 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.S.; Jeon, S.M.; Kim, M.J.; Yeo, J.; Seo, K.I.; Choi, M.S.; Lee, M.K. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem. Toxicol. 2010, 48, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Gao, M.; Liu, D. Chlorogenic acid improves high fat diet-induced hepatic steatosis and insulin resistance in mice. Pharm. Res. 2015, 32, 1200–1209. [Google Scholar] [CrossRef] [PubMed]
- Murase, T.; Misawa, K.; Minegishi, Y.; Aoki, M.; Ominami, H.; Suzuki, Y.; Shibuya, Y.; Hase, T. Coffee polyphenols suppress diet-induced body fat accumulation by downregulating SREBP-1c and related molecules in C57BL/6J mice. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E122–E133. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.J.; Zhu, Q.; Zhong, Y.L.; Xu, S.H.; Wang, Z. Chlorogenic Acid Maintains Glucose Homeostasis through Modulating the Expression of SGLT-1, GLUT-2, and PLG in Different Intestinal Segments of Sprague-Dawley Rats Fed a High-Fat Diet. Biomed. Environ. Sci. 2015, 28, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Tung, Y.C.; Yang, G.; Li, S.; Ho, C.T. Molecular mechanisms of the anti-obesity effect of bioactive compounds in tea and coffee. Food. Funct. 2016, 7, 4481–4491. [Google Scholar] [CrossRef] [PubMed]
- Maki, C.; Funakoshi-Tago, M.; Aoyagi, R.; Ueda, F.; Kimura, M.; Kobata, K.; Tago, K.; Tamura, H. Coffee extract inhibits adipogenesis in 3T3-L1 preadipocyes by interrupting insulin signaling through the downregulation of IRS1. PLoS ONE 2017, 12, e0173264. [Google Scholar] [CrossRef] [PubMed]
- Mellbye, F.B.; Jeppesen, P.B.; Hermansen, K.; Gregersen, S. Cafestol, a bioactive substance in coffee, stimulates insulin secretion and increases glucose uptake in muscle cells: Studies in vitro. J. Nat. Prod. 2015, 78, 2447–2451. [Google Scholar] [CrossRef] [PubMed]
- Mellbye, F.B.; Jeppesen, P.B.; Shokouh, P.; Laustsen, C.; Hermansen, K.; Gregersen, S. Cafestol, a Bioactive Substance in Coffee, Has Antidiabetic Properties in KKAy Mice. J. Nat. Prod. 2017, 80, 2353–2359. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Ju, J.; Wang, K.; Gu, C.; Feng, Y. Evaluation of hypoglycemic activity of total lignans from Fructus Arctii in the spontaneously diabetic Goto-Kakizaki rats. J. Ethnopharmacol. 2014, 151, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Xue, W.; Liang, S.; Zhao, J.; Zhang, X. Acute caffeine ingestion reduces insulin sensitivity in healthy subjects: A systematic review and meta-analysis. Nutr. J. 2016, 15, 103. [Google Scholar] [CrossRef] [PubMed]
- Selvin, E.; Steffes, M.W.; Zhu, H.; Matsushita, K.; Wagenknecht, L.; Pankow, J.; Coresh, J.; Brancati, F.L. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N. Engl. J. Med. 2010, 362, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Ma, D.; Zhang, Y.; Liu, Z.; Wang, P. The effect of coffee consumption on serum lipids: A meta-analysis of randomized controlled trials. Eur. J. Clin. Nutr. 2012, 66, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Cao, J.; Feng, Q.; Peng, J.; Hu, Y. Roles of chlorogenic Acid on regulating glucose and lipids metabolism: A review. Evid. Based Complement. Alternat. Med. 2013, 2013, 801457. [Google Scholar] [CrossRef] [PubMed]
- Vitaglione, P.; Morisco, F.; Mazzone, G.; Amoruso, D.C.; Ribecco, M.T.; Romano, A.; Fogliano, V.; Caporaso, N.; D’argenio, G. Coffee reduces liver damage in a rat model of steatohepatitis: The underlying mechanisms and the role of polyphenols and melanoidins. Hepatology 2010, 52, 1652–1661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beynen, A.C.; Weusten-Van Der Wouw, M.P.; De Roos, B.; Katan, M.B. Boiled coffee fails to raise serum cholesterol in hamsters and rats. Br. J. Nutr. 1996, 76, 755–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Liu, Y.; Hua, Z.; Ren, L.; Li, Q.; Li, N. Animal models for the atherosclerosis research: A review. Protein Cell 2011, 2, 189–201. [Google Scholar] [CrossRef]
- Salomone, F.; Li Volti, G.; Vitaglione, P.; Morisco, F.; Fogliano, V.; Zappala, A.; Palmigiano, A.; Garozzo, D.; Caporaso, N.; D’Argenio, G.; et al. Coffee enhances the expression of chaperones and antioxidant proteins in rats with nonalcoholic fatty liver disease. Transl. Res. 2014, 163, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Comment, A.; Merritt, M.E. Hyperpolarized magnetic resonance as a sensitive detector of metabolic function. Biochemistry 2014, 53, 7333–7357. [Google Scholar] [CrossRef] [PubMed]
- Moon, C.M.; Oh, C.H.; Ahn, K.Y.; Yang, J.S.; Kim, J.Y.; Shin, S.S.; Lim, H.S.; Heo, S.H.; Seon, H.J.; Kim, J.W.; et al. Metabolic biomarkers for non-alcoholic fatty liver disease induced by high-fat diet: In vivo magnetic resonance spectroscopy of hyperpolarized [1-13C] pyruvate. Biochem. Biophys. Res. Commun. 2017, 482, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.J.; Miller, J.J.; McCallum, C.; Rider, O.J.; Neubauer, S.; Heather, L.C.; Tyler, D.J. Assessment of Metformin-Induced Changes in Cardiac and Hepatic Redox State Using Hyperpolarized[1-13C]Pyruvate. Diabetes 2016, 65, 3544–3551. [Google Scholar] [CrossRef] [PubMed]
- Erk, T. Bioavailability of Coffee Polyphenols: Focus on Dose-and structure Response. Ph.D. Thesis, Technische Universität Kaiserslautern, Kaiserslautern, Germany, July 2014. [Google Scholar]
| Mean (SD) | Control | Coffee | Nutraceuticals | Between Group p-Value |
|---|---|---|---|---|
| Fasting plasma glucose (mmol/L) | ||||
| Baseline | 7.69 (0.75) | 7.35 (0.42) | 7.60 (0.82) | 0.588 |
| Week 4 | 8.24 (1.09) | 7.93 (1.03) | 8.43 (0.63) | 0.560 |
| Week 10 | 8.17 (1.92) | 7.51 (0.61) | 7.70 (0.92) | 0.588 |
| Week 14 | 10.00 (1.48) | 9.09 (1.50) | 9.46 (1.55) | 0.491 |
| Glycated hemoglobin (mM/M) | ||||
| Week 14 | 22.88 (1.25) | 21.25 (1.04) * | 23.67 (2.16) †† | 0.018 |
| Fasting plasma Insulin (mU/L) | ||||
| Baseline | 24.47 (8.79) | 23.50 (7.88) | 26.94 (5.58) | 0.647 |
| Week 4 | 52.55 (21.29) | 34.26 (13.49) | 52.85 (23.56) | 0.126 |
| Week 10 | 41.21 (13.82) | 26.49 (6.14) | 43.31 (8.88) †† | 0.008 |
| Week 14 | 80.73 (33.83) | 53.11 (29.25) | 76.07 (27.91) | 0.211 |
| AUC | 146.38 (49.54) | 100.79 (34.09) * | 133.16 (41.80) † | 0.049 |
| Mean (SD) | Control Group | Coffee Group | Chemicals Group | Between Group p-Value |
|---|---|---|---|---|
| Plasma | ||||
| Lipids (mmol/L) | ||||
| Total Cholesterol | 1.650 (0.118) | 1.728 (0.099) | 1.558 (0.125) | 0.604 |
| NonHDL-C | 0.544 (0.045) | 0.535 (0.092) | 0.488 (0.029) | 0.588 |
| LDL-C | 0.250 (0.023) | 0.336 (0.036) | 0.257 (0.011) | 0.156 |
| HDL-C | 1.106 (0.085) | 1.193 (0.098) | 1.080 (0.104) | 0.690 |
| Triglycerides | 0.649 (0.041) | 0.380 (0.053) ** | 0.535 (0.082) | 0.010 |
| Nonesterified fatty acids | 0.279 (0.039) | 0.295 (0.025) | 0.288 (0.039) | 0.941 |
| Adiponectine (μg/mL) | 5.694 (0.371) | 5.188 (0.618) | 4.701 (0.573) | 0.474 |
| Total antioxidant capacity (Trolox equivalent, mmol/L) | 102.323 (1.743) | 97.228 (2.168) | 102.272 (2.344) | 0.152 |
| Alanine transaminase (U/L) | 18.838 (1.280) | 19.875 (1.612) | 17.983 (1.061) | 0.718 |
| Liver triglycerides content (mmol/L) | 2.806 (0.384) | 1.379 (0.108) **,† | 2.386 (0.432) | 0.010 |
| Urine (relative units) | ||||
| Trigonelline | 0.0002 (0.000) | 0.038 (0.015) | 0.025 (0.019) | 0.597 ¥ |
| Ethanol | 1.355 (0.829) | 1.378 (0.257) | 1.404 (0.262) | 0.991 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shokouh, P.; Jeppesen, P.B.; Hermansen, K.; Laustsen, C.; Stødkilde-Jørgensen, H.; Hamilton-Dutoit, S.J.; Søndergaard Schmedes, M.; Qi, H.; Stokholm Nørlinger, T.; Gregersen, S. Effects of Unfiltered Coffee and Bioactive Coffee Compounds on the Development of Metabolic Syndrome Components in a High-Fat-/High-Fructose-Fed Rat Model. Nutrients 2018, 10, 1547. https://doi.org/10.3390/nu10101547
Shokouh P, Jeppesen PB, Hermansen K, Laustsen C, Stødkilde-Jørgensen H, Hamilton-Dutoit SJ, Søndergaard Schmedes M, Qi H, Stokholm Nørlinger T, Gregersen S. Effects of Unfiltered Coffee and Bioactive Coffee Compounds on the Development of Metabolic Syndrome Components in a High-Fat-/High-Fructose-Fed Rat Model. Nutrients. 2018; 10(10):1547. https://doi.org/10.3390/nu10101547
Chicago/Turabian StyleShokouh, Pedram, Per Bendix Jeppesen, Kjeld Hermansen, Christoffer Laustsen, Hans Stødkilde-Jørgensen, Stephen Jacques Hamilton-Dutoit, Mette Søndergaard Schmedes, Haiyun Qi, Thomas Stokholm Nørlinger, and Søren Gregersen. 2018. "Effects of Unfiltered Coffee and Bioactive Coffee Compounds on the Development of Metabolic Syndrome Components in a High-Fat-/High-Fructose-Fed Rat Model" Nutrients 10, no. 10: 1547. https://doi.org/10.3390/nu10101547





