Substantial Increase in Compliance with Saturated Fatty Acid Intake Recommendations after One Year Following the American Heart Association Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. American Heart Association Dietary Counselling
2.3. Dietary Assessment
2.4. Statistical Analyses
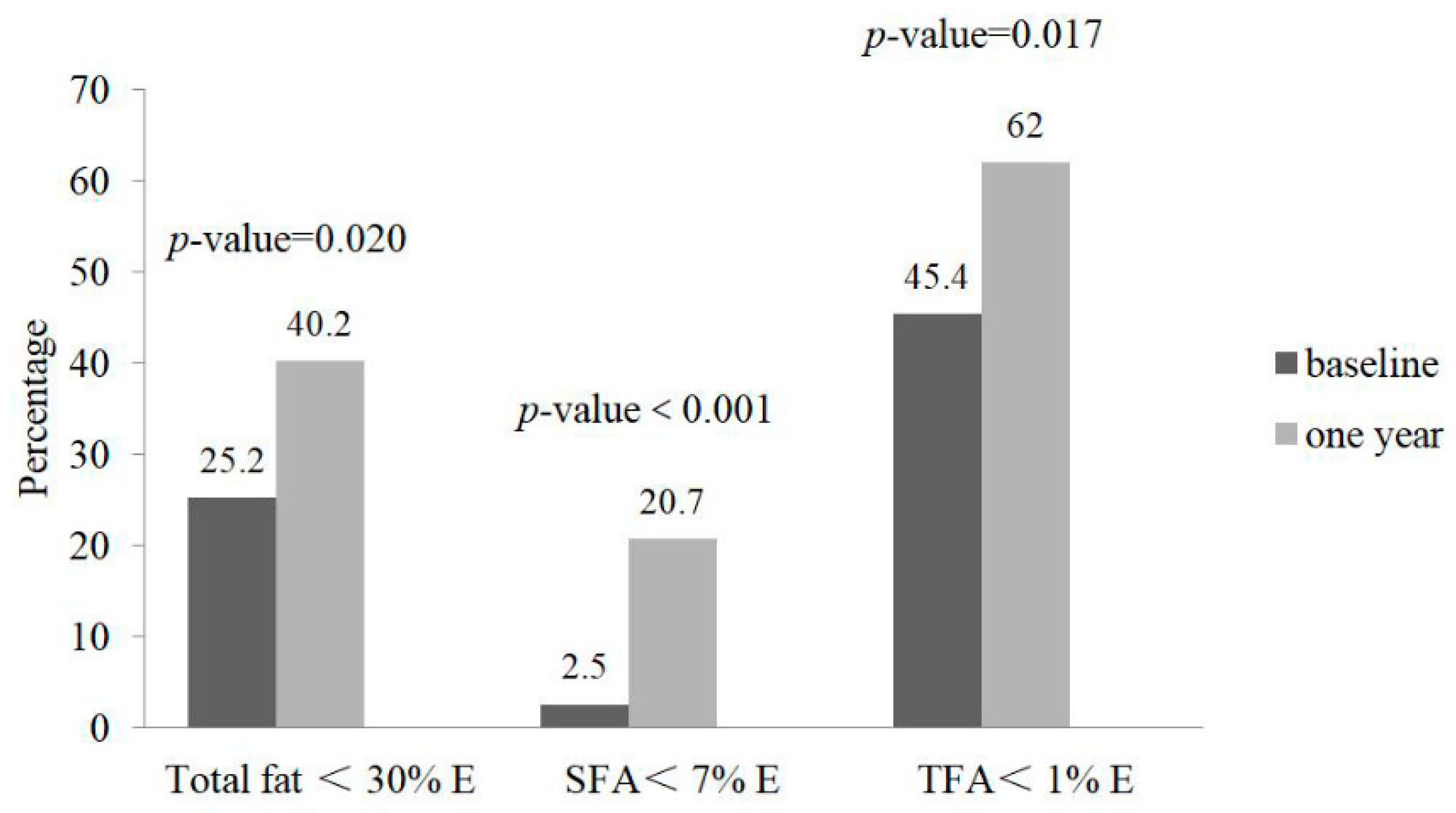
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Angell, S.Y.; Cobb, L.K.; Curtis, C.J.; Konty, K.J.; Silver, L.D. Change in trans fatty acid content of fast food purchases associated with New York City’s restaurant regulation: A pre-post study. Ann. Intern. Med. 2012, 157, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Núñez, B.; Dijck-Brouwer, D.A.; Muskiet, F.A. The relation of saturated fatty acids with low-grade inflammation and cardiovascular disease. J. Nutr. Biochem. 2016, 36, 1–20. [Google Scholar] [CrossRef] [PubMed]
- De Souza, R.J.; Mente, A.; Maroleanu, A.; Cozma, A.I.; Ha, V.; Kishibe, T.; Uleryk, E.; Budylowski, P.; Schünemann, H.; Beyene, J.; et al. Intake of saturated and trans unsaturated fatty acids and risk ofall cause mortality, cardiovascular disease, and type 2 diabetes: Systematic review and meta-analysis of observational studies. BMJ 2015, 351, h3978. [Google Scholar] [CrossRef] [PubMed]
- Nishida, C.; Uauy, R. WHO Scientific Update on health consequences of trans fatty acids: Introduction. Eur. J. Clin. Nutr. 2009, 63, S1. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Stampfer, M.J. Removing industrial trans fat from foods. BMJ 2010, 340, c1826. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Micha, R.; Wallace, S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: A systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2010, 7, e1000252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hooper, L.; Martin, N.; Abdelhamid, A.; Davey Smith, G. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst. Rev. 2015, 6, CD011737. [Google Scholar] [CrossRef] [PubMed]
- Bjermo, H.; Iggman, D.; Kullberg, J.; Dahlman, I.; Johansson, L.; Persson, L.; Berglund, J.; Pulkki, K.; Basu, S.; Uusitupa, M.; et al. Effects of n-6 PUFAs compared with SFAs on liver fat, lipoproteins, and inflammation in abdominal obesity: A randomized controlled trial. Am. J. Clin. Nutr. 2012, 95, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Farvid, M.S.; Ding, M.; Pan, A.; Sun, Q.; Chiuve, S.E.; Steffen, L.M.; Willett, W.C.; Hu, F.B. Dietary linoleic acid and risk of coronary heart disease: A systematic review and meta-analysis of prospective cohort studies. Circulation 2014, 130, 1568–1578. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Babio, N.; Martínez-González, M.A.; Corella, D.; Ros, E.; Martín-Peláez, S.; Estruch, R.; Arós, F.; Gómez-Gracia, E.; Fiol, M.; et al. PREDIMED Study Investigators Dietary fat intake and risk of cardiovascular disease and all-cause mortality in a population at high risk of cardiovascular disease. Am. J. Clin. Nutr. 2015, 102, 1563–1573. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hruby, A.; Bernstein, A.M.; Ley, S.H.; Wang, D.D.; Chiuve, S.E.; Sampson, L.; Rexrode, K.M.; Rimm, E.B.; Willett, W.C.; et al. Saturated fats compared with unsaturated fats and sources of carbohydrates in relation to risk of coronary heart disease: A prospective cohort study. J. Am. Coll. Cardiol. 2015, 66, 1538–1548. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, M.U.; O’Reilly, E.J.; Heitmann, B.L.; Pereira, M.A.; Bälter, K.; Fraser, G.E.; Goldbourt, U.; Hallmans, G.; Knekt, P.; Liu, S.; et al. Major types of dietary fat and risk of coronary heart disease: A pooled analysis of 11 cohort studies. Am. J. Clin. Nutr. 2009, 89, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Eckel, R.H.; Jakicic, J.M.; Ard, J.D.; de Jesus, J.M.; Houston-Miller, N.; Hubbard, V.S.; Lee, I.M.; Lichtenstein, A.H.; Loria, C.M.; Millen, B.E.; et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129, S76–S99. [Google Scholar] [CrossRef] [PubMed]
- Dietary Guidelines Advisory Committee. Scientific Report of the 2015 Dietary Guidelines Advisory Committee. Available online: https://www.health.gov/dietaryguidelines/2015-scientific-report/ (accessed on 15 February 2015).
- Jacobson, T.A.; Maki, K.C.; Orringer, C.E.; Jones, P.H.; Kris-Etherton, P.; Sikand, G.; La Forge, R.; Daniels, S.R.; Wilson, D.P.; Morris, P.B.; et al. National Lipid Association Recommendations for Patient Centered Management of Dyslipidemia: Part 2. J. Clin. Lipidol. 2015, 9, S1–S22. [Google Scholar] [CrossRef] [PubMed]
- Rehm, C.D.; Peñalvo, J.L.; Afshin, A.; Mozaffarian, D. Dietary intake among US adults, 1999–2012. JAMA 2016, 315, 2542–2553. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Qiao, Q.H.; Zhang, S.C.; Chen, Y.H.; Chao, G.Q.; Fang, L.Z. Metabolic syndrome and gallstone disease. World J. Gastroenterol. 2012, 18, 4215–4220. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.M.; Khan, S.A.; Jackson, R.T.; Duane, M. Prevalence of the Metabolic Syndrome in Central and South American Immigrant Residents of the Washington, DC, Area. J. Nutr. Metab. 2017, 2017, 9531964. [Google Scholar] [CrossRef] [PubMed]
- Lovre, D.; Mauvais-Jarvis, F. Trends in Prevalence of the Metabolic Syndrome. JAMA 2015, 314, 950. [Google Scholar] [CrossRef] [PubMed]
- Khalfa, A.; Tiali, A.; Zemour, L.; Fatah, A.; Mekki, K. Prevalence of metabolic syndrome and its association with lifestyle and cardiovascular biomarkers among postmenopausal women in western Algeria. Int. J. Gynaecol. Obstet. 2017, 138, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Nupponen, M.; Pahkala, K.; Juonala, M.; Magnussen, C.G.; Niinikoski, H.; Rönnemaa, T.; Viikari, J.S.; Saarinen, M.; Lagström, H.; Jula, A.; et al. Metabolic syndrome from adolescence to early adulthood: Effect of infancy-onset dietary counseling of low saturated fat: The Special Turku Coronary Risk Factor Intervention Project (STRIP). Circulation 2015, 131, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Micha, R.; Mozaffarian, D. Trans fatty acids: Effects on metabolic syndrome, heart disease and diabetes. Nat. Rev. Endocrinol. 2009, 5, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Nettleton, J.A.; Jebb, S.; Risérus, U.; Koletzko, B.; Fleming, J. Role of dietary fats in the prevention and treatment of the metabolic syndrome. Ann. Nutr. Metab. 2014, 64, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Olendzki, B.C.; Wedick, N.M.; Persuitte, G.M.; Culver, A.L.; Li, W.; Merriam, P.A.; Carmody, J.; Fang, H.; Zhang, Z.; et al. Challenges in sodium intake reduction and meal consumption patterns among participants with metabolic syndrome in a dietary trial. Nutr. J. 2013, 12, 163. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [PubMed]
- American Heart Association Nutrition Committee; Lichtenstein, A.H.; Appel, L.J.; Brands, M.; Carnethon, M.; Daniels, S.; Franch, H.A.; Franklin, B.; Kris-Etherton, P.; Harris, W.S.; et al. Diet and lifestyle recommendations revision 2006: A scientific statement from the American Heart Association Nutrition Committee. Circulation 2006, 114, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Olendzki, B.C.; Wang, J.; Persuitte, G.M.; Li, W.; Fang, H.; Merriam, P.A.; Wedick, N.M.; Ockene, I.S.; Culver, A.L.; et al. Single-component versus multicomponent dietary goals for the metabolic syndrome: A randomized trial. Ann. Intern. Med. 2015, 162, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Schakel, S.F. Maintaining a nutrient database in a changing marketplace: Keeping pace with changing food products-a research perspective. J. Food Comp. Anal. 2001, 14, 315–322. [Google Scholar] [CrossRef]
- Corwin, R.L.; Hartman, T.J.; Maczuga, S.A.; Graubard, B.I. Dietary saturated fat intake is inversely associated with bone density in humans: Analysis of NHANES III. J. Nutr. 2006, 136, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Boyd, N.F.; Stone, J.; Vogt, K.N.; Connelly, B.S.; Martin, L.J.; Minkin, S. Dietary fat and breast cancer risk revisited: A meta-analysis of the published literature. Br. J. Cancer 2003, 89, 1672–1685. [Google Scholar] [CrossRef] [PubMed]
- Lof, M.; Weiderpass, E. Impact of diet on breast cancer risk. Curr. Opin. Obstet. Gynecol. 2009, 21, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Lin, O.S. Acquired risk factors for colorectal cancer. Methods Mol. Biol. 2009, 472, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Crowe, F.L.; Allen, N.E.; Appleby, P.N.; Overvad, K.; Aardestrup, I.V.; Johnsen, N.F.; Tjønneland, A.; Linseisen, J.; Kaaks, R.; Boeing, H.; et al. Fatty acid composition of plasma phospholipids and risk of prostate cancer in a case-control analysis nested within the European Prospective Investigation into Cancer and Nutrition. Am. J. Clin. Nutr. 2008, 88, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Kurahashi, N.; Inoue, M.; Iwasaki, M.; Sasazuki, S.; Tsugane, A.S. Japan Public Health Center-Based Prospective Study Group. Dairy product, saturated fatty acid, and calcium intake and prostate cancer in a prospective cohort of Japanese men. Cancer Epidemiol. Biomark. Prev. 2008, 17, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Farinós, N.; Dal Re Saavedra, M.Á.; Villar Villalba, C.; Robledo de Dios, T. Trans-fatty acid content of food products in Spain in 2015. Gac. Sanit. 2016, 30, 379–382. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Revealing Trans Fats. Available online: https://www.highbeam.com/doc/1G1-109906683.html (accessed on 1 September 2003).
- Rubinstein, A.; Elorriaga, N.; Garay, O.U.; Poggio, R.; Caporale, J.; Matta, M.G.; Augustovski, F.; Pichon-Riviere, A.; Mozaffarian, D. Eliminating artificial trans fatty acids in Argentina: Estimated effects on the burden of coronary heart disease and costs. Bull. World Health Organ. 2015, 93, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Allen, K. Partially Hydrogenated Oils and Trans Fats Information for Consumers. Available online: https://digitalcommons.usu.edu/extension_curall/749 (accessed on 18 June 2015).
- US Food and Drug Administration. Final Determination Regarding Partially Hydrogenated Oils (Removing Trans Fat). Available online: https://www.fda.gov/food/ingredientspackaginglabeling/foodadditivesingredients/ucm449162.htm (accessed on 18 May 2018).
- Tortosa-Caparrós, E.; Navas-Carrillo, D.; Marín, F.; Orenes-Piñero, E. Anti-inflammatory effects of omega 3 and omega 6 polyunsaturated fatty acids in cardiovascular disease and metabolic syndrome. Crit. Rev. Food Sci. Nutr. 2017, 57, 3421–3429. [Google Scholar] [CrossRef] [PubMed]
- Balk, E.M.; Lichtenstein, A.H. Omega-3 Fatty Acids and Cardiovascular Disease: Summary of the 2016 Agency of Healthcare Research and Quality Evidence Review. Nutrients 2017, 9, 865. [Google Scholar] [CrossRef] [PubMed]
- Tørris, C.; Småstuen, M.C.; Molin, M. Nutrients in Fish and Possible Associations with Cardiovascular Disease Risk Factors in Metabolic Syndrome. Nutrients 2018, 10, 952. [Google Scholar] [CrossRef] [PubMed]
- Gillingham, L.G.; Harris-Janz, S.; Jones, P.J. Dietary monounsaturated fatty acids are protective against metabolic syndrome and cardiovascular disease risk factors. Lipids 2011, 46, 209–228. [Google Scholar] [CrossRef] [PubMed]
- Ros, E. Dietary cis-monounsaturated fatty acids and metabolic control in type 2 diabetes. Am. J. Clin. Nutr. 2003, 78, 617S–625S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kris-Etherton, P.M. AHA Science Advisory. Monounsaturated fatty acids and risk of cardiovascular disease. Circulation 1999, 100, 1253–1258. [Google Scholar] [CrossRef] [PubMed]
- Mente, A.; de Koning, L.; Shannon, H.S.; Anand, S.S. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch. Intern. Med. 2009, 169, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M.; Lichtenstein, A.H.; Wu, J.H.Y.; Appel, L.J.; Creager, M.A.; Kris-Etherton, P.M.; Miller, M.; Rimm, E.B.; Rudel, L.L.; Robinson, J.G.; et al. Dietary Fats and Cardiovascular Disease: A Presidential Advisory from the American Heart Association. Circulation 2017, 136, e1–e23. [Google Scholar] [CrossRef] [PubMed]
- Olendzki, B.; Procter-Gray, E.; Magee, M.F.; Youssef, G.; Kane, K.; Churchill, L.; Ockene, J.; Li, W. Racial Differences in Misclassification of Healthy Eating Based on Food Frequency Questionnaire and 24 Hour Dietary Recalls. J. Nutr. Health Aging 2017, 21, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Micha, R.; Mozaffarian, D. Saturated fat and cardiometabolic risk factors, coronary heart disease, stroke, and diabetes: A fresh look at the evidence. Lipids 2010, 45, 893–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natarajan, L.; Pu, M.; Messer, K. Exact statistical tests for the intersection of independent lists of genes. Ann. Appl. Stat. 2012, 6, 521–541. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.E.; Zhang, J.; Kris-Etherton, P.M. Cardiovascular disease risk of dietary stearic acid compared with trans, other saturated, and unsaturated fatty acids: A systematic review. Am. J. Clin. Nutr. 2010, 91, 46–63. [Google Scholar] [CrossRef] [PubMed]
- Flock, M.R.; Kris-Etherton, P.M. Diverse physiological effects of long-chain saturated fatty acids: Implications for cardiovascular disease. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Warensjö, E.; Jansson, J.H.; Cederholm, T.; Boman, K.; Eliasson, M.; Hallmans, G.; Johansson, I.; Sjögren, P. Biomarkers of milk fat and the risk of myocardial infarction in men and women: A prospective, matched case-control study. Am. J. Clin. Nutr. 2010, 92, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Soedamah-Muthu, S.S.; Ding, E.L.; Al-Delaimy, W.K.; Hu, F.B.; Engberink, M.F.; Willett, W.C.; Geleijnse, J.M. Milk and dairy consumption and incidence of cardiovascular diseases and all-cause mortality: Dose-response meta-analysis of prospective cohort studies. Am. J. Clin. Nutr. 2011, 93, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Azadbakht, L.; Mirmiran, P.; Esmaillzadeh, A.; Azizi, F. Dairy consumption is inversely associated with the prevalence of the metabolic syndrome in Tehranian adults. Am. J. Clin. Nutr. 2005, 82, 523–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, R.A.; Makrides, M.; Smithers, L.G.; Voevodin, M.; Sinclair, A.J. The effect of dairy foods on CHD: A systematic review of prospective cohort studies. Br. J. Nutr. 2009, 102, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
| Month/Session | Topic |
|---|---|
| Month 1/group 1 | Orientation/Getting Started with AHA Eating |
| Month 1/individual visit #1 | Individual Consultation |
| Month 2/group 2 | Hunger, Satiety, and Appetite |
| Month 3/group 3 | AHA Meal Planning: Focus on Whole Grains |
| Month 3/group 4 | AHA Meal Planning: Focus on Proteins |
| Month 4/group 5 | AHA Meal Planning: Focus on Vegetables |
| Month 5/group 6 | AHA Meal Planning: Focus on Fats and Oils |
| Month 6/group 7 | AHA Meal Planning: Focus on Fruits |
| Month 7/group 8 | Four keys to AHA Eating Out |
| Month 8/group 9 | The Science of Taste |
| Month 9/group 10 | Motivation and Long-Term Changes |
| Month 10/group 11 | How to Improve Dietary Quality |
| Month 11/group 12 | Savvy Super Marketing: Supermarket Tour |
| Month 12/individual visit #2 | Individual Consultation |
| Variable | n (%) | SFA Intake | TFA Intake | ||||||
|---|---|---|---|---|---|---|---|---|---|
| g/day | p-Value | %E | p-Value | g/day | p-Value | %E | p-Value | ||
| Gender | |||||||||
| Male | 34 (28.6) | 32.18 (26.35 to 38.00) | 0.001 | 11.86 (10.77 to 12.94) | 0.471 | 3.14 (2.37 to 3.91) | 0.078 | 1.13 (0.98 to 1.28) | 0.524 |
| Female | 85 (71.4) | 24.09 (21.97 to 26.21) | 11.44 (10.86 to 12.02) | 2.50 (2.17 to 2.84) | 1.20 (1.07 to 1.34) | ||||
| Age group | |||||||||
| 20–40 | 12 (10.1) | 32.87 (26.01 to 39.74) | 0.296 | 11.28 (9.88 to 12.68) | 0.771 | 3.41 (1.78 to 5.03) | 0.24 | 1.08 (0.78 to 1.37) | 0.424 |
| 41–60 | 35 (29.4) | 24.91 (21.59 to 28.24) | 11.20 (10.31 to 12.09) | 2.44 (2.01 to 2.86) | 1.14 (0.94 to 1.35) | ||||
| 51–60 | 46 (38.7) | 26.26 (21.62 to 30.90) | 11.81 (10.87 to 12.74) | 2.89 (2.25 to 3.52) | 1.29 (1.10 to 1.48) | ||||
| 61–70 | 26 (21.8) | 25.66 (21.34 to 29.97) | 11.74 (10.62 to 12.86) | 2.33 (1.86 to 2.81) | 1.09 (0.90 to 1.28) | ||||
| Race/ethnicity | |||||||||
| Caucasian | 108 (90.8) | 27.17 (24.74 to 29.59) | 0.038 | 11.75 (11.21 to 12.29) | 0.020 | 2.73 (2.39 to 3.06) | 0.406 | 1.20 (1.08 to 1.31) | 0.380 |
| Others | 11 (9.2) | 18.86 (12.57 to 25.16) | 9.69 (8.36 to 11.02) | 2.26 (0.84 to 3.68) | 1.03 (0.74 to 1.33) | ||||
| Highest education level | |||||||||
| High school diploma or less | 13 (11.0) | 30.98 (20.35 to 41.62) | 0.335 | 12.47 (10.67 to 14.28) | 0.48 | 2.59 (1.36 to 3.82) | 0.873 | 1.08 (0.72 to 1.43) | 0.777 |
| Bachelor’s degree or less | 71 (60.2) | 26.23 (23.46 to 29.00) | 11.49 (10.87 to 12.11) | 2.75 (2.35 to 3.16) | 1.19 (1.06 to 1.32) | ||||
| Graduate/professional | 34 (28.8) | 24.86 (20.48 to 29.25) | 11.43 (10.34 to 12.52) | 2.57 (1.92 to 3.23) | 1.21 (0.98 to 1.44) | ||||
| Household income | |||||||||
| $0–$30,000 | 12 (10.1) | 33.45 (22.53 to 44.37) | 0.177 | 11.67 (9.48 to 13.87) | 0.385 | 4.21 (2.08 to 6.33) | 0.028 | 1.32 (0.93 to 1.72) | 0.447 |
| $30,000–$50,000 | 19 (16.0) | 26.60 (21.42 to 31.78) | 12.34 (10.98 to 13.71) | 2.72 (1.99 to 3.45) | 1.35 (0.93 to 1.76) | ||||
| $50,000–$75,000 | 20 (16.8) | 25.14 (17.08 to 33.20) | 11.31 (9.63 to 12.99) | 2.46 (1.96 to 2.95) | 1.18 (1.01 to 1.36) | ||||
| More than $75,000 | 43 (36.1) | 27.13 (23.85 to 30.42) | 11.79 (11.03 to 12.55) | 2.61 (2.16 to 3.06) | 1.14 (0.98 to 1.30) | ||||
| Unclear | 25 (21.0) | 22.61 (18.73 to 26.50) | 10.72 (9.80 to 11.65) | 2.25 (1.56 to 2.94) | 1.05 (0.85 to 1.26) | ||||
| Components of MetS | |||||||||
| 3 | 58 (48.7) | 24.37 (21.49 to 27.25) | 0.03 | 11.26 (10.47 to 12.06) | 0.532 | 2.61 (2.14 to 3.08) | 0.497 | 1.20 (1.04 to 1.35) | 0.783 |
| 4 | 41 (34.5) | 30.59 (25.62 to 35.57) | 11.82 (11.02 to 12.62) | 2.93 (2.31 to 3.55) | 1.13 (1.00 to 1.27) | ||||
| 5 | 20 (16.8) | 23.69 (20.31 to 27.08) | 11.90 (10.61 to 13.18) | 2.40 (1.76 to 3.03) | 1.24 (0.87 to 1.60) | ||||
| Baseline (n = 119) | One Year (n = 92) | Change (n = 92) | p-Value | |
|---|---|---|---|---|
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | ||
| Total energy (kcal/day) | 1957.7 (1829.1 to 2086.3) | 1500.9 (1386.8 to 1615.0) | −489.4 (−604.30 to −374.50) | <0.001 |
| Total Fat | ||||
| g/day | 74.76 (68.95 to 80.58) | 54.36 (48.89 to 59.83) | −21.95 (−27.50 to −16.39) | <0.001 |
| %E | 33.08 (32.06 to 34.09) | 31.06 (29.45 to 32.68) | −2.15 (−3.74 to −0.56) | 0.009 |
| SFA, total | ||||
| g/day | 26.40 (24.10 to 28.70) | 16.69 (15.00 to 18.38) | −10.51 (−12.95 to −8.08) | <0.001 |
| %E | 11.56 (11.05 to 12.07) | 9.67 (8.99 to 10.34) | −2.06 (−2.84 to −1.29) | <0.001 |
| TFA, total | ||||
| g/day | 2.69 (2.36 to 3.01) | 1.58 (1.32 to 1.84) | −1.19 (−1.59 to −0.80) | <0.001 |
| %E | 1.18 (1.08 to 1.29) | 0.89 (0.79 to 1.00) | −0.32 (−0.47 to −0.16) | <0.001 |
| MUFA, total | ||||
| g/day | 26.30 (24.29 to 28.30) | 19.88 (17.75 to 22.01) | −7.01 (−8.96 to −5.07) | <0.001 |
| %E | 11.77 (11.29 to 12.24) | 11.35 (10.61 to 12.08) | −0.50 (−1.22 to 0.22) | 0.17 |
| Oleic acid | ||||
| g/day | 24.52 (22.61 to 26.43) | 18.63 (16.59 to 20.67) | −6.49 (−8.35 to −4.64) | <0.001 |
| %E | 11.28 (10.76 to 11.81) | 10.98 (10.25 to 11.72) | −0.41 (−1.13 to 0.31) | 0.259 |
| PUFA, total | ||||
| g/day | 16.01 (14.40 to 17.62) | 13.15 (11.41 to 14.88) | −2.90 (−4.53 to −1.27) | 0.001 |
| %E | 7.05 (6.63 to 7.48) | 7.34 (6.73 to 7.94) | 0.39 (−0.27 to 1.05) | 0.245 |
| LA | ||||
| g/day | 14.05 (12.58 to 15.53) | 11.50 (9.89 to 13.12) | −2.62 (−4.12 to −1.13) | 0.001 |
| %E | 6.32 (5.92 to 6.71) | 6.70 (6.10 to 7.30) | 0.40 (−0.24 to 1.03) | 0.216 |
| ALA | ||||
| g/day | 1.52 (1.36 to 1.67) | 1.26 (1.10 to 1.41) | −0.26 (−0.46 to −0.05) | 0.014 |
| %E | 0.70 (0.64 to 0.76) | 0.76 (0.69 to 0.83) | 0.06 (−0.04 to 0.17) | 0.22 |
| AA | ||||
| g/day | 0.13 (0.12 to 0.15) | 0.11 (0.10 to 0 13) | −0.01 (−0.03 to 0.01) | 0.308 |
| %E | 0.06 (0.06 to 0.07) | 0.07 (0.06 to 0.08) | 0.01 (0.00 to 0.02) | 0.021 |
| EPA | ||||
| g/day | 0.05 (0.03 to 0.07) | 0.05 (0.03 to 0.07) | 0.00 (−0.02 to 0.02) | 0.774 |
| DPA | ||||
| g/day | 0.03 (0.02 to 0.04) | 0.02 (0.02 to 0.03) | 0.00 (−0.01 to 0.01) | 0.855 |
| DHA | ||||
| g/day | 0.12 (0.08 to 0.17) | 0.12 (0.08 to 0.17) | 0.01 (−0.03 to 0.06) | 0.559 |
| Location | Breakfast | Lunch | Dinner | Snack | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline | One-Year | Baseline | One-Year | Baseline | One-Year | Baseline | One-Year | |
| At home | ||||||||
| n = 123 | n = 146 | n = 93 | n = 91 | n = 194 | n = 176 | n = 346 | n = 262 | |
| SFA (g) | 4.54 (3.49–5.59) 2 | 2.48 (1.50–3.45) 1,2 | 7.53 (6.35–8.72) 2 | 5.13 (3.93–6.33) 1 | 10.05 (9.19–10.91) | 6.51 (5.61–7.41) 1 | 4.03 (3.33–4.73) 2 | 2.09 (1.31–2.87) 1,2 |
| SFA (%E) | 9.63 (7.97–11.28) | 7.68 (6.14–9.21) | 11.25 (9.36–13.13) | 9.80 (7.91–11.69) | 10.45 (9.03–11.87) | 9.43 (8.00–10.86) | 10.37 (9.28–11.46) | 7.95 (6.72–9.19) 1 |
| Away from home | ||||||||
| n = 30 | n = 24 | n = 55 | n = 64 | n = 7 | n = 10 | n = 131 | n = 109 | |
| SFA (g) | 4.82 (2.80–6.84) | 3.64 (1.38–5.89) | 6.11 (4.60–7.62) | 4.35 (2.94–5.76) | 7.99 (3.88–12.11) | 5.38 (1.93–8.83) | 2.83 (1.80–3.86) 2 | 1.54 (0.42–2.67) 2 |
| SFA (%E) | 11.03 (7.83–14.23) | 10.21 (6.62–13.79) | 8.54 (6.14–10.93) | 7.61 (5.38–9.84) | 8.67 (2.11–15.22) | 8.63 (3.14–14.13) | 11.13 (9.50–12.76) | 7.06 (5.27–8.86) 1 |
| Restaurant/fast food | ||||||||
| n = 15 | n = 5 | n = 33 | n = 23 | n = 37 | n = 24 | n = 21 | n = 4 | |
| SFA (g) | 8.81 (5.98–11.63) 3 | 9.90 (5.05–14.76) 3 | 10.24 (8.31–12.16) 3 | 6.53 (4.21–8.84) 1,2 | 11.63 (9.80–13.45) | 10.60 (8.36–12.85) 3 | 5.83 (3.41–8.25) 2 | 5.62 (0.20–11.04) |
| SFA (%E) | 12.75 (8.25–17.24) | 11.18 (3.45–18.91) | 9.09 (5.99–12.18) | 9.55 (5.87–13.24) | 9.11 (6.13–12.08) | 8.88 (5.26–12.49) | 13.72 (9.88–17.56) | 14.41 (5.78–23.04) |
| Weekdays | ||||||||
| n = 102 | n = 124 | n = 115 | n = 123 | n = 148 | n = 144 | n = 332 | n = 266 | |
| SFA (g) | 4.63 (3.49–5.76) 2 | 2.58 (1.54–3.62) 1,2 | 6.46 (5.38–7.53) 2 | 5.08 (4.03–6.12) 2 | 9.33 (8.38–10.29) | 7.00 (6.03–7.98) 1 | 3.34 (2.64–4.05) 2 | 2.09 (1.32–2.86) 1,2 |
| SFA (%E) | 9.73 (7.94–11.52) | 7.43 (5.79–9.07) 2 | 9.48 (7.78–11.18) | 9.16 (7.51–10.80) | 10.01 (8.44–11.57) | 9.70 (8.14–11.25) | 11.08 (9.97–12.19) | 7.50 (6.27–8.73) 2 |
| Weekend days | ||||||||
| n = 66 | n = 51 | n = 66 | n = 55 | n = 90 | n = 66 | n = 166 | n = 109 | |
| SFA (g) | 5.58 (4.20–6.95) 2 | 3.78 (2.22–5.33) 2 | 9.64 (8.26–11.02) 2,4 | 5.18 (3.68–6.68) 1 | 11.73 (10.54–12.92) 4 | 6.91 (5.53–8.29) 1 | 4.71 (3.80–5.63) 2,4 | 1.87 (0.75–2.98) 1,2 |
| SFA (%E) | 10.83 (8.66–13.01) | 9.91 (7.45–12.38) | 11.02 (8.80–13.23) | 8.66 (6.28–11.05) | 10.49 (8.52–12.46) | 8.56 (6.36–10.76) | 10.04 (8.60–11.49) | 8.52 (6.75–10.28) |
| Location | Breakfast | Lunch | Dinner | Snack | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline | One-Year | Baseline | One-Year | Baseline | One-Year | Baseline | One-Year | |
| At home | ||||||||
| n = 123 | n = 146 | n = 93 | n = 91 | n = 194 | n = 176 | n = 346 | n = 262 | |
| TFA (g) | 0.36 (0.17–0.56) 2 | 0.21 (0.03–0.39) 2 | 0.68 (0.46–0.90) | 0.38 (0.16–0.61) | 0.90 (0.75–1.06) | 0.60 (0.43–0.76) 1 | 0.42 (0.30–0.54) 2 | 0.17 (0.03–0.30) 1,2 |
| TFA (%E) | 0.79 (0.54–1.05) | 0.68 (0.45–0.92) | 1.09 (0.80–1.38) | 0.81 (0.51–1.10) | 0.83 (0.61–1.05) | 0.80 (0.58–1.02) | 0.95 (0.78–1.12) | 0.94 (0.75–1.13) |
| Away from home | ||||||||
| n = 30 | n = 24 | n = 55 | n = 64 | n = 7 | n = 10 | n = 131 | n = 109 | |
| TFA (g) | 0.40 (0.01–0.78) | 0.47 (0.04–0.90) | 0.84 (0.55–1.12) | 0.52 (0.26–0.79) | 1.10 (0.31–1.90) | 0.36 (−0.30–1.03) | 0.32 (0.13–0.51) | 0.11 (−0.10–0.32) |
| TFA (%E) | 0.90 (0.40–1.40) | 1.15 (0.60–1.71) | 1.00 (0.63–1.37) | 0.78 (0.43–1.12) | 1.04 (0.02–2.06) | 0.43 (−0.43–1.29) | 0.96 (0.70–1.22) | 0.64 (0.34–0.94) |
| Restaurant/ fast food | ||||||||
| n = 15 | n = 5 | n = 33 | n = 23 | n = 37 | n = 24 | n = 21 | n = 4 | |
| TFA (g) | 1.12 (0.58–1.66) 3 | 1.61 (0.67–2.54) 3 | 1.81 (1.45–2.18) 3 | 0.95 (0.51–1.39) 1,2,3 | 1.47 (1.12–1.81) 3 | 2.10 (1.67–2.53) 3 | 0.60 (0.14–1.06) 2 | 1.51 (0.46–2.55) 3 |
| TFA (%E) | 1.64 (0.94–2.34) 3 | 1.87 (0.66–3.08) | 1.97 (1.49–2.45) 2,3 | 1.21 (0.64–1.78) | 1.18 (0.72–1.64) | 1.62 (1.06–2.19) 3 | 1.38 (0.79–1.98) | 1.55 (0.20–2.90) |
| Weekdays | ||||||||
| n = 102 | n = 124 | n = 115 | n = 123 | n = 148 | n = 144 | n = 332 | n = 266 | |
| TFA (g) | 0.42 (0.20–0.63) 2 | 0.26 (0.07–0.46) 2 | 0.84 (0.63–1.04) | 0.52 (0.32–0.71) | 0.97 (0.79–1.15) | 0.76 (0.57–0.94) | 0.36 (0.24–0.49) 2 | 0.17 (0.03–0.31) 2 |
| TFA (%E) | 0.90 (0.62–1.18) | 0.73 (0.47–0.98) | 1.18 (0.92–1.44) | 0.91 (0.66–1.17) | 0.89 (0.65–1.13) | 0.86 (0.62–1.10) | 0.94 (0.76–1.11) | 0.74 (0.54–0.93) |
| Weekend days | ||||||||
| n = 66 | n = 51 | n = 66 | n = 55 | n = 90 | n = 66 | n = 166 | n = 109 | |
| TFA (g) | 0.48 (0.21–0.74) 2 | 0.38 (0.08–0.68) 2 | 1.12 (0.85–1.38) | 0.52 (0.23–0.81) 1 | 1.05 (0.82–1.28) | 0.78 (0.52–1.05) | 0.49 (0.32–0.66) 2 | 0.19 (−0.02–0.39) 2 |
| TFA (%E) | 0.88 (0.54–1.22) | 0.96 (0.58–1.35) | 1.25 (0.91–1.60) 2 | 0.72 (0.35–1.09) | 0.80 (0.50–1.11) | 0.88 (0.54–1.22) | 1.09 (0.86–1.32) | 1.25 (0.97–1.54) 4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Chiriboga, D.; Olendzki, B.; Xie, B.; Li, Y.; McGonigal, L.J.; Maldonado-Contreras, A.; Ma, Y. Substantial Increase in Compliance with Saturated Fatty Acid Intake Recommendations after One Year Following the American Heart Association Diet. Nutrients 2018, 10, 1486. https://doi.org/10.3390/nu10101486
Zhao M, Chiriboga D, Olendzki B, Xie B, Li Y, McGonigal LJ, Maldonado-Contreras A, Ma Y. Substantial Increase in Compliance with Saturated Fatty Acid Intake Recommendations after One Year Following the American Heart Association Diet. Nutrients. 2018; 10(10):1486. https://doi.org/10.3390/nu10101486
Chicago/Turabian StyleZhao, Miaomiao, David Chiriboga, Barbara Olendzki, Bin Xie, Yawen Li, Lisa Jo McGonigal, Ana Maldonado-Contreras, and Yunsheng Ma. 2018. "Substantial Increase in Compliance with Saturated Fatty Acid Intake Recommendations after One Year Following the American Heart Association Diet" Nutrients 10, no. 10: 1486. https://doi.org/10.3390/nu10101486




