The Acute Effect of Oleic- or Linoleic Acid-Containing Meals on Appetite and Metabolic Markers; A Pilot Study in Overweight or Obese Individuals
Abstract
:1. Introduction
2. Materials and Methods
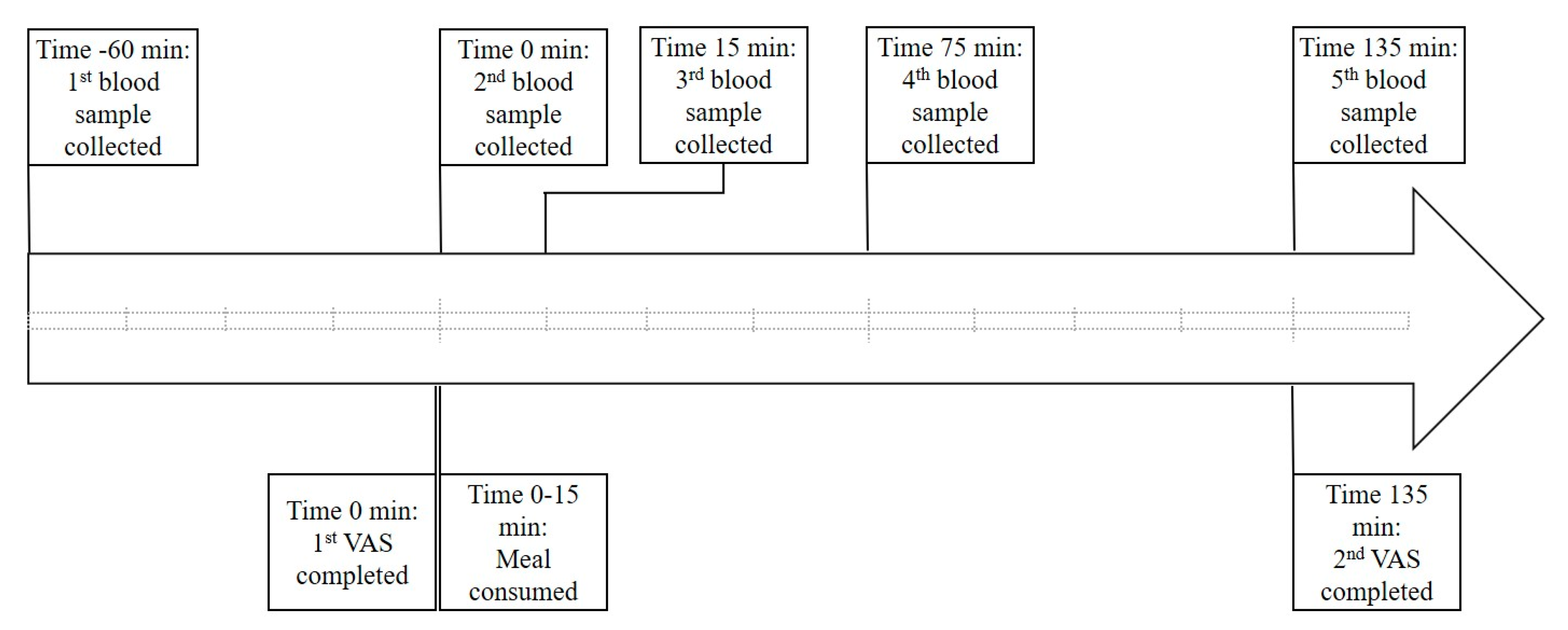
2.1. Study Design
2.2. Test Meal Compositions
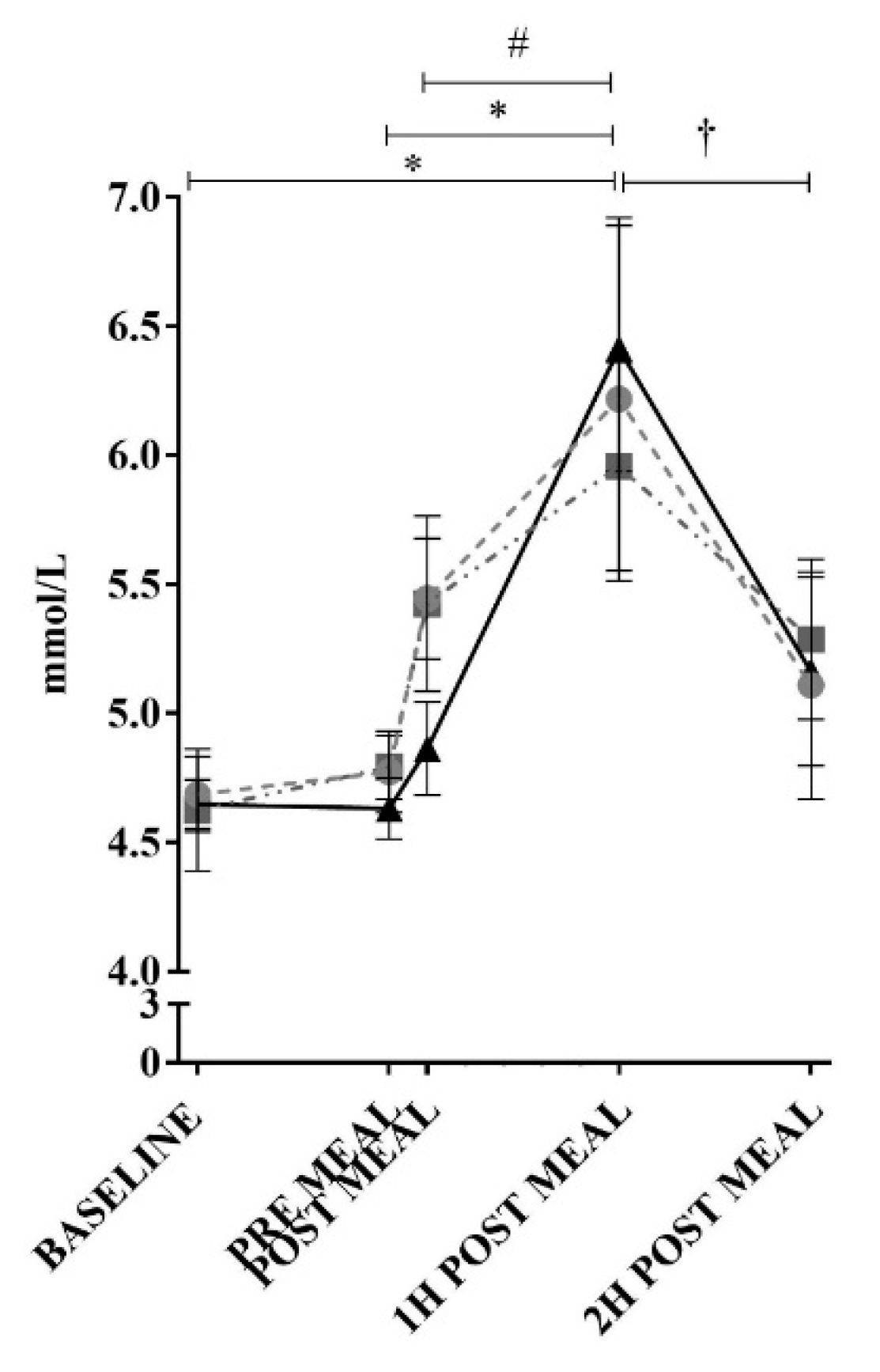
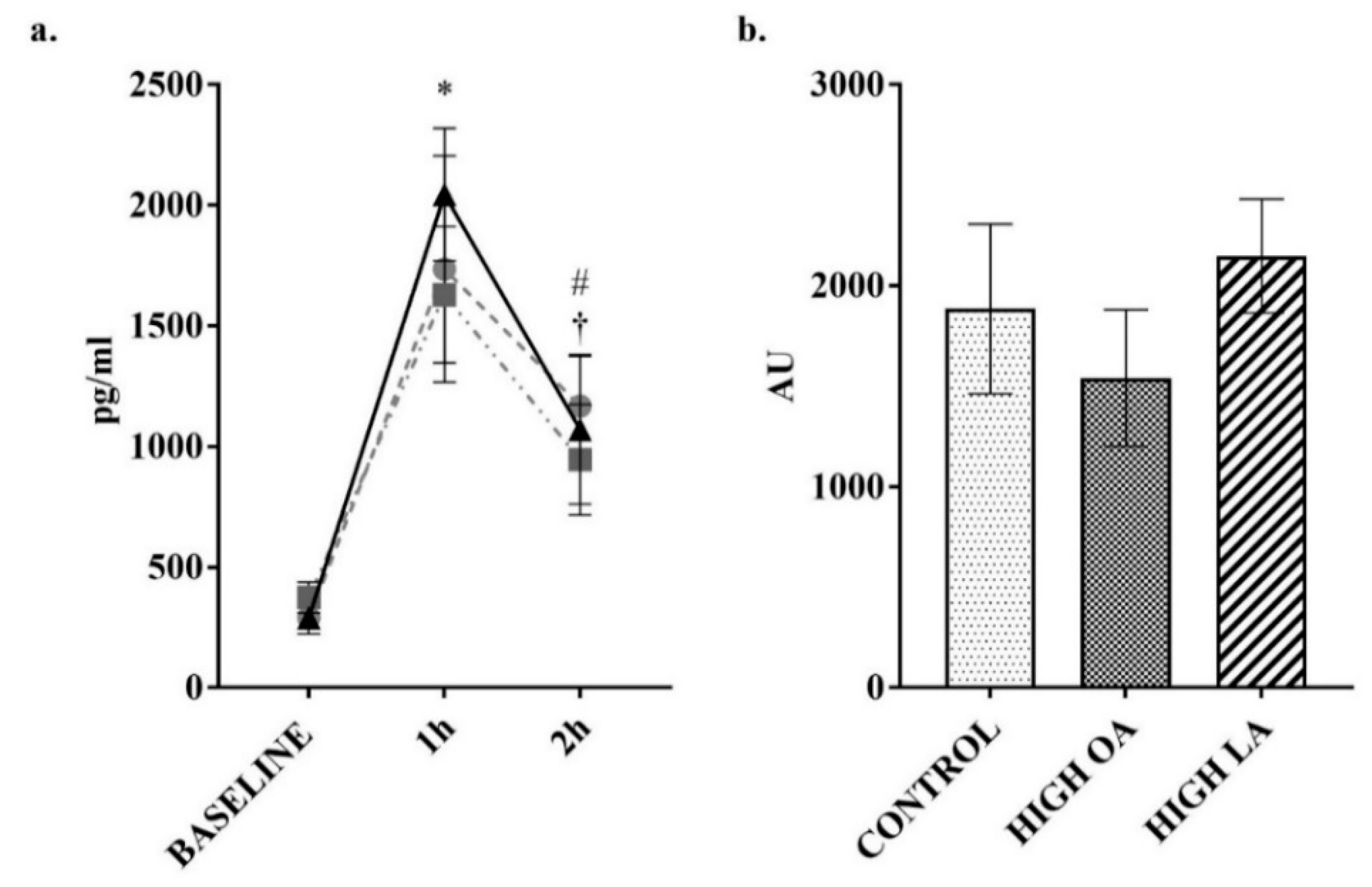
2.3. Blood Collection and Analysis
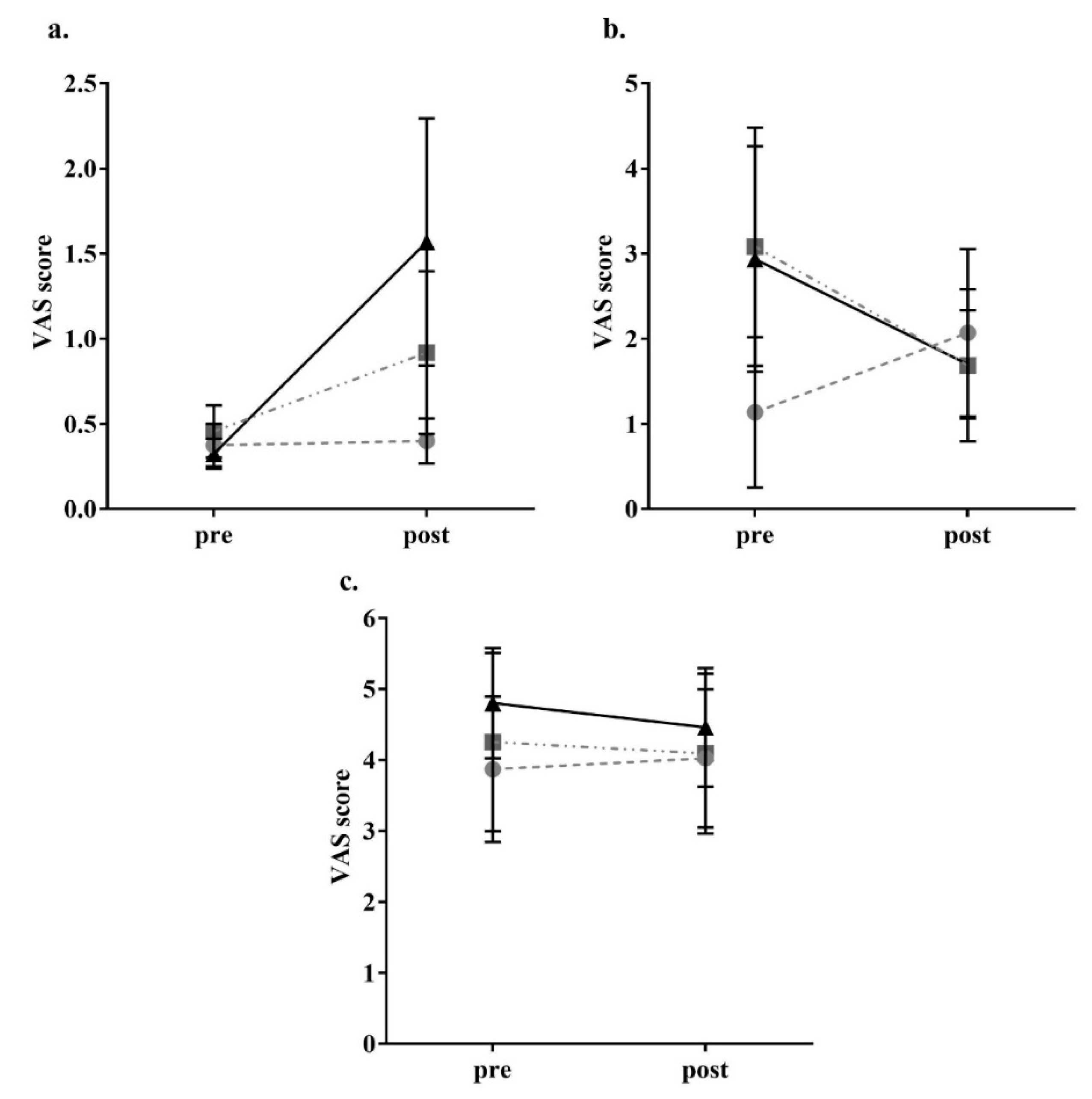
2.4. Appetite Assessment
2.5. Statistical Analysis
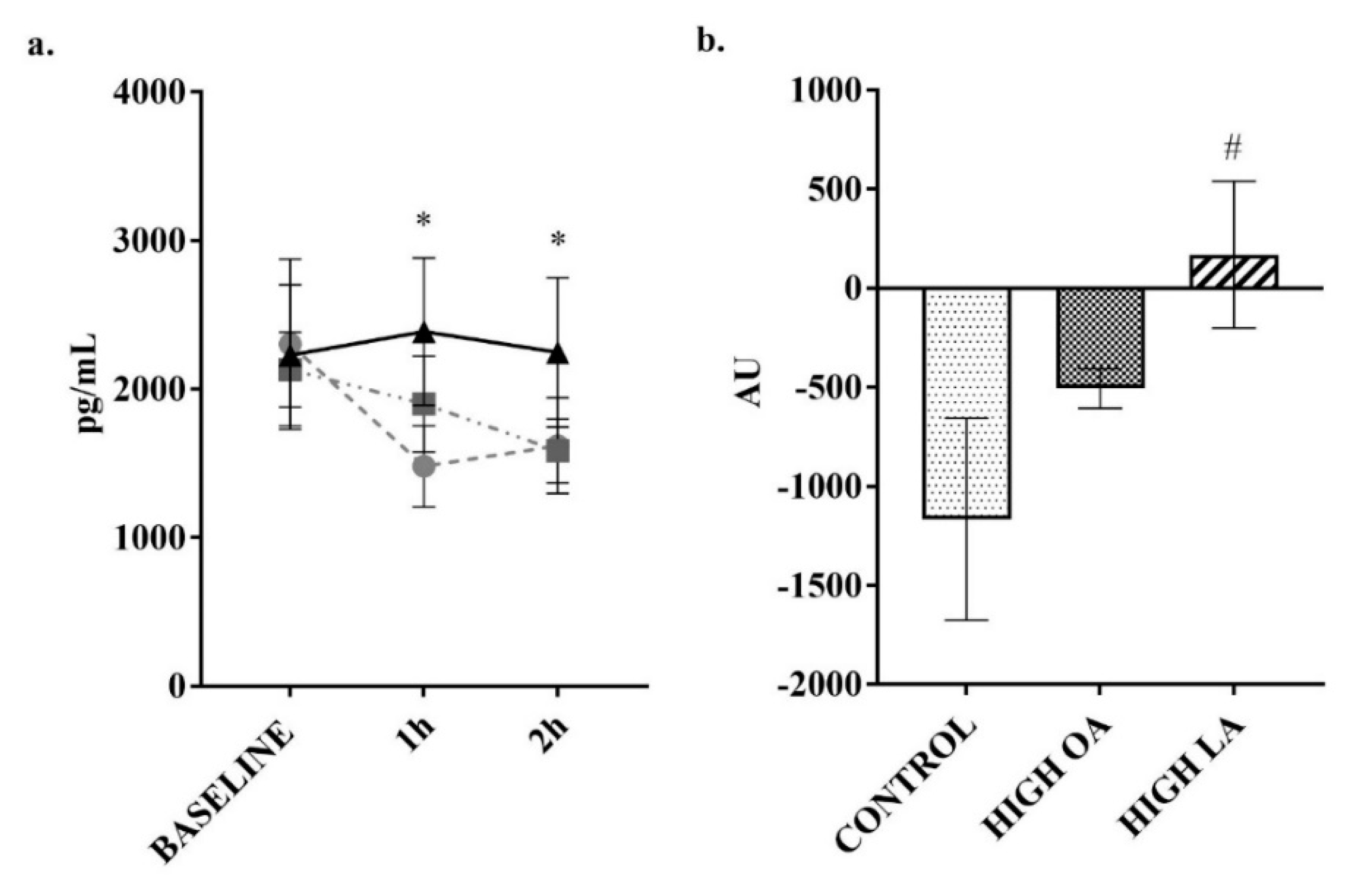
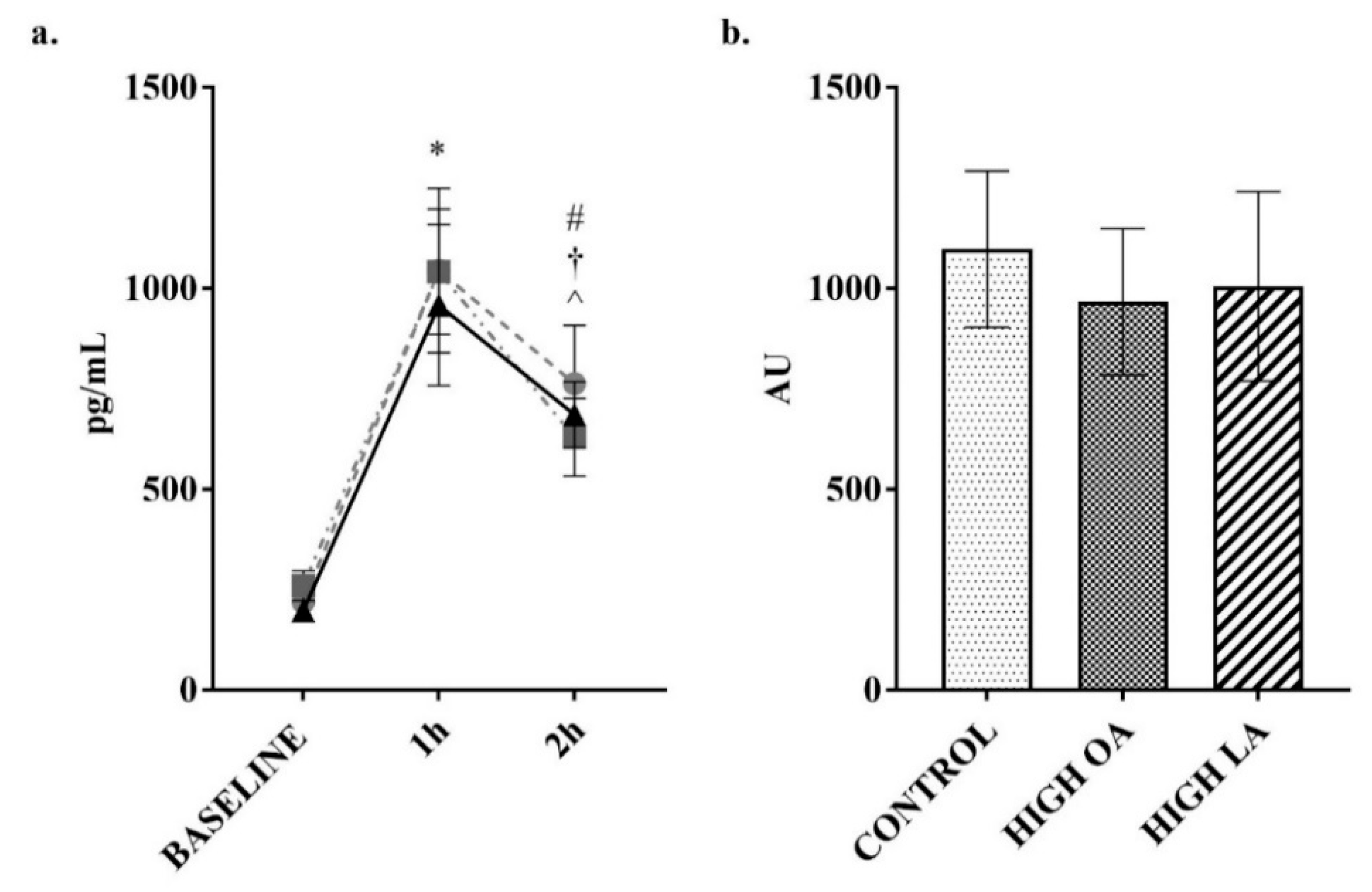
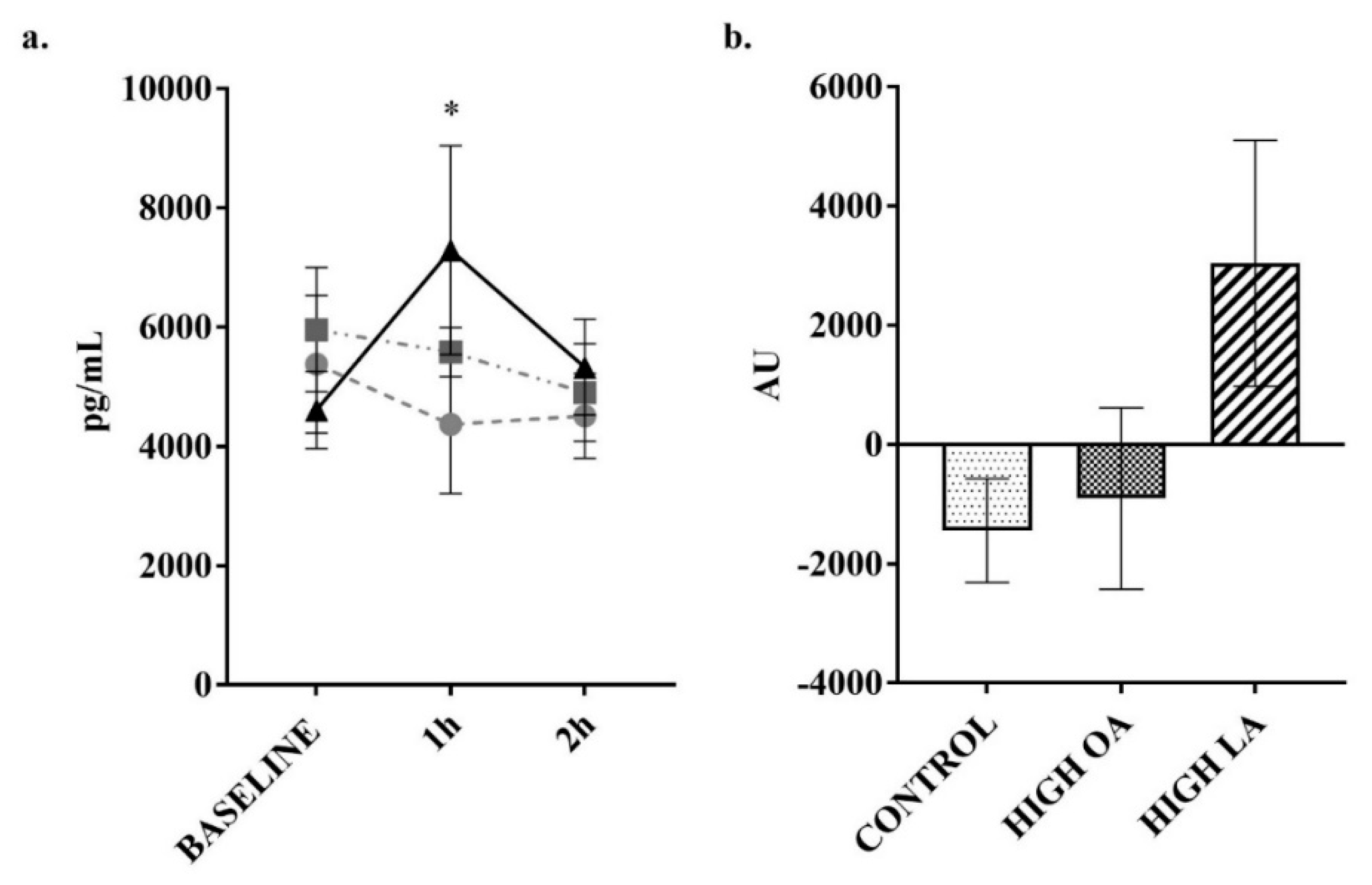
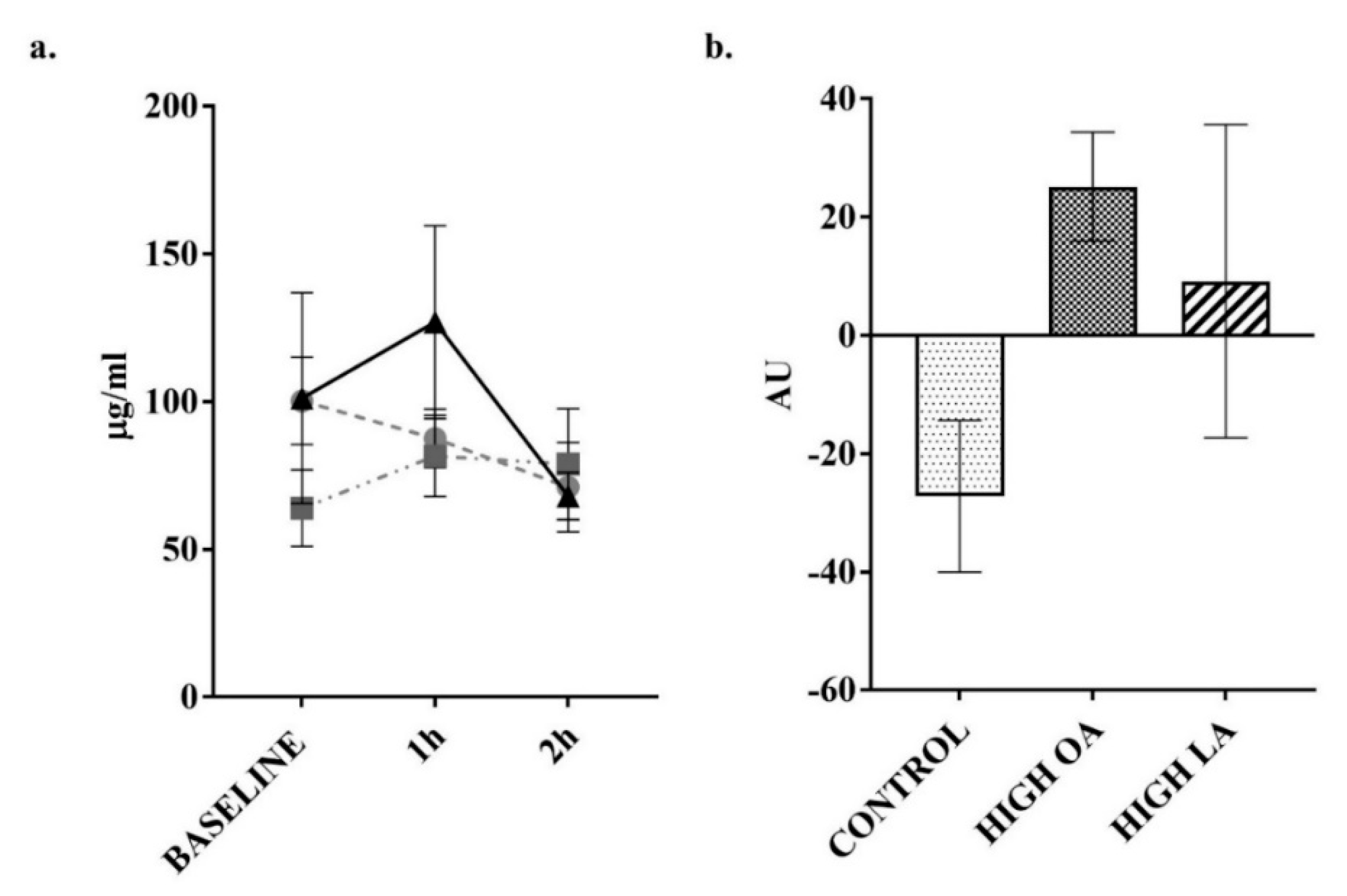
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Morton, G.J.; Cummings, D.E.; Baskin, D.G.; Barsh, G.S.; Schwartz, M.W. Central nervous system control of food intake and body weight. Nature 2006, 443, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Neary, N.M.; Goldstone, A.P.; Bloom, S.R. Appetite regulation: From the gut to the hypothalamus. Clin. Endocrinol. 2004, 60, 153–160. [Google Scholar] [CrossRef]
- Blundell, J.E.; Macdiarmid, J.I. Fat as a risk factor for overconsumption: Satiation, satiety, and patterns of eating. J. Am. Diet. Assoc. 1997, 97, S63–S69. [Google Scholar] [CrossRef]
- Bray, G.A.; Popkin, B.M. Dietary fat intake does affect obesity! Am. J. Clin. Nutr. 1998, 68, 1157–1173. [Google Scholar] [CrossRef] [PubMed]
- Duca, F.A.; Sakar, Y.; Covasa, M. The modulatory role of high fat feeding on gastrointestinal signals in obesity. J. Nutr. Biochem. 2013, 24, 1663–1677. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Miranda, J.; Marin, C. Dietary, physiological, and genetic impacts on postprandial lipid metabolism. In Fat Detection: Taste, Texture, and Post Ingestive Effects; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Rolls, B.J. Carbohydrates, fats, and satiety. Am. J. Clin. Nutr. 1995, 61, 960S–967S. [Google Scholar] [CrossRef]
- Diep, T.A.; Madsen, A.N.; Holst, B.; Kristiansen, M.M.; Wellner, N.; Hansen, S.H.; Hansen, H.S. Dietary fat decreases intestinal levels of the anorectic lipids through a fat sensor. FASEB J. 2011, 25, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Blasbalg, T.L.; Hibbeln, J.R.; Ramsden, C.E.; Majchrzak, S.F.; Rawlings, R.R. Changes in consumption of omega-3 and omega-6 fatty acids in the united states during the 20th century. Am. J. Clin. Nutr. 2011, 93, 950–962. [Google Scholar] [CrossRef]
- Naughton, S.; Mathai, M.L.; Hryciw, D.H.; McAinch, A.J. Australia’s nutrition transition 1961–2009: A focus on fats. Br. J. Nutr. 2015, 114, 337–346. [Google Scholar] [CrossRef]
- Sheehy, T.; Sharma, S. The nutrition transition in the republic of Ireland: Trends in energy and nutrient supply from 1961 to 2007 using food and agriculture organization food balance sheets. Br. J. Nutr. 2011, 106, 1078–1089. [Google Scholar] [CrossRef]
- Sheehy, T.; Sharma, S. The nutrition transition in Barbados: Trends in macronutrient supply from 1961 to 2003. Br. J. Nutr. 2010, 104, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Guerra, F.; Paccaud, F.; Marques-Vidal, P. Trends in food availability in Switzerland, 1961–2007. Eur. J. Clin. Nutr. 2012, 66, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Balanza, R.; García-Lorda, P.; Pérez-Rodrigo, C.; Aranceta, J.; Bonet, M.B.; Salas-Salvadó, J. Trends in food availability determined by the food and agriculture organization’s food balance sheets in Mediterranean Europe in comparison with other European areas. Public Health Nutr. 2007, 10, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B. The nutrition transition and obesity in the developing world. J. Nutr. 2001, 131, 871S–873S. [Google Scholar] [CrossRef] [PubMed]
- Naughton, S.S.; Mathai, M.L.; Hryciw, D.H.; McAinch, A.J. Fatty acid modulation of the endocannabinoid system and the effect on food intake and metabolism. Int. J. Endocrinol. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Engeli, S.; Bohnke, J.; Feldpausch, M.; Gorzelniak, K.; Janke, J.; Batkai, S.; Pacher, P.; Harvey-White, J.; Luft, F.; Sharma, A.; et al. Activation of the peripheral endocannabinoid system in human obesity. Diabetes 2005, 54, 2838–2843. [Google Scholar] [CrossRef] [PubMed]
- Galli, C.; Calder, P.C. Effects of fat and fatty acid intake on inflammatory and immune responses: A critical review. Ann. Nutr. Metab. 2009, 55, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Naughton, S.S.; Mathai, M.L.; Hryciw, D.H.; McAinch, A.J. Linoleic acid and the pathogenesis of obesity. Prostaglandins Other Lipid Mediat. 2016, 125, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Lambert, E.V.; Goedecke, J.H.; Bluett, K.; Heggie, K.; Claassen, A.; Rae, D.E.; West, S.; Dugas, J.; Dugas, L.; Meltzeri, S.; et al. Conjugated linoleic acid versus high-oleic acid sunflower oil: Effects on energy metabolism, glucose tolerance, blood lipids, appetite and body composition in regularly exercising individuals. Br. J. Nutr. 2007, 97, 1001–1011. [Google Scholar] [CrossRef]
- Schwinkendorf, D.R.; Tsatsos, N.G.; Gosnell, B.A.; Mashek, D.G. Effects of central administration of distinct fatty acids on hypothalamic neuropeptide expression and energy metabolism. Int. J. Obes. (Lond) 2011, 35, 336–344. [Google Scholar] [CrossRef]
- Flint, A.; Helt, B.; Raben, A.; Toubro, S.; Astrup, A. Effects of different dietary fat types on postprandial appetite and energy expenditure. Obes. Res. 2003, 11, 1449–1455. [Google Scholar] [CrossRef]
- Strik, C.M.; Lithander, F.E.; McGill, A.-T.; MacGibbon, A.K.; McArdle, B.H.; Poppitt, S.D. No evidence of differential effects of SFA, MUFA or PUFA on post-ingestive satiety and energy intake: A randomised trial of fatty acid saturation. Nutr. J. 2010, 9. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.H.; Marklund, M.; Imamura, F.; Tintle, N.; Korat, A.V.A.; De Goede, J.; Zhou, X.; Yang, W.-S.; de Oliveira Otto, M.C.; Kröger, J. Omega-6 fatty acid biomarkers and incident type 2 diabetes: Pooled analysis of individual-level data for 39,740 adults from 20 prospective cohort studies. Lancet Diabetes Endocrinol. 2017, 5, 965–974. [Google Scholar] [CrossRef]
- Forouhi, N.G.; Krauss, R.M.; Taubes, G.; Willett, W. Dietary fat and cardiometabolic health: Evidence, controversies, and consensus for guidance. BMJ 2018, 361. [Google Scholar] [CrossRef] [PubMed]
- Poreba, M.; Rostoff, P.; Siniarski, A.; Mostowik, M.; Golebiowska-Wiatrak, R.; Nessler, J.; Undas, A.; Gajos, G. Relationship between polyunsaturated fatty acid composition in serum phospholipids, systemic low-grade inflammation, and glycemic control in patients with type 2 diabetes and atherosclerotic cardiovascular disease. Cardiovasc. Diabetol. 2018, 17. [Google Scholar] [CrossRef] [PubMed]
- Henderson, G.; Crofts, C.; Schofield, G. Linoleic acid and diabetes prevention. Lancet Diabetes Endocrinol. 2018, 6, 12–13. [Google Scholar] [CrossRef]
- Kaviani, S.; Cooper, J. Appetite responses to high-fat meals or diets of varying fatty acid composition: A comprehensive review. Eur. J. Clin. Nutr. 2017, 71, 1154–1165. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.A.; Sturm, K.; MacIntosh, C.G.; Feinle, C.; Horowitz, M.; Chapman, I.M. Relation between food intake and visual analogue scale ratings of appetite and other sensations in healthy older and young subjects. Eur. J. Clin. Nutr. 2004, 58, 212–218. [Google Scholar] [CrossRef] [Green Version]
- Purslow, L.R.; Sandhu, M.S.; Forouhi, N.; Young, E.H.; Luben, R.N.; Welch, A.A.; Khaw, K.T.; Bingham, S.A.; Wareham, N.J. Energy intake at breakfast and weight change: Prospective study of 6764 middle-aged men and women. Am. J. Epidemiol. 2008, 167, 188–192. [Google Scholar] [CrossRef]
- Winkler, G.; Doring, A.; Keil, U. Meal patterns in middle-aged men in southern Germany: Results from the Monica Augsburg dietary survey 1984/85. Appetite 1999, 32, 33–37. [Google Scholar] [CrossRef]
- Frankenfield, D.; Roth-Yousey, L.; Compher, C. Comparison of predictive equations for resting metabolic rate in healthy nonobese and obese adults: A systematic review. J. Am. Diet. Assoc. 2005, 105, 775–789. [Google Scholar] [CrossRef]
- Mifflin, M.D.; St Jeor, S.T.; Hill, L.A.; Scott, B.J.; Daugherty, S.A.; Koh, Y.O. A new predictive equation for resting energy expenditure in healthy individuals. Am. J. Clin. Nutr. 1990, 51, 241–247. [Google Scholar] [CrossRef]
- Volpe, S.; Bernier Sabelawski, S.; Mohr, C.R. Fitness Nutrition for Special Dietary Needs; Human Kinetics: Champaign, IL, USA, 2007; p. 186. [Google Scholar]
- Beaudoin, M.S.; Robinson, L.E.; Graham, T.E. An oral lipid challenge and acute intake of caffeinated coffee additively decrease glucose tolerance in healthy men. J. Nutr. 2011, 141, 574–581. [Google Scholar] [CrossRef]
- Flint, A.; Raben, A.; Blundell, J.E.; Astrup, A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 38–48. [Google Scholar] [CrossRef] [Green Version]
- Brennan, I.M.; Luscombe-Marsh, N.D.; Seimon, R.V.; Otto, B.; Horowitz, M.; Wishart, J.M.; Feinle-Bisset, C. Effects of fat, protein, and carbohydrate and protein load on appetite, plasma cholecystokinin, peptide yy, and ghrelin, and energy intake in lean and obese men. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G129–G140. [Google Scholar] [CrossRef] [PubMed]
- Little, T.J.; Feltrin, K.L.; Horowitz, M.; Smout, A.J.; Rades, T.; Meyer, J.H.; Pilichiewicz, A.N.; Wishart, J.; Feinle-Bisset, C. Dose-related effects of lauric acid on antropyloroduodenal motility, gastrointestinal hormone release, appetite, and energy intake in healthy men. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R1090–R1098. [Google Scholar] [CrossRef] [Green Version]
- Parker, B.A.; Ludher, A.K.; Loon, T.K.; Horowitz, M.; Chapman, I.M. Relationships of ratings of appetite to food intake in healthy older men and women. Appetite 2004, 43, 227–233. [Google Scholar] [CrossRef]
- López, S.; Bermúdez, B.; Pacheco, Y.M.; Villar, J.; Abia, R.; Muriana, F.J. Distinctive postprandial modulation of β cell function and insulin sensitivity by dietary fats: Monounsaturated compared with saturated fatty acids. Am. J. Clin. Nutr. 2008, 88, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Seino, Y.; Fukushima, M.; Yabe, D. Gip and glp-1, the two incretin hormones: Similarities and differences. J. Diabetes Investig. 2010, 1, 8–23. [Google Scholar] [CrossRef]
- Colagiuri, S.; Davies, D.; Girgis, S.; Colagiuri, R. National Evidence Based Guidelines for Case Detection and Diagnosis of Type 2 Diabetes. Available online: https://www.google.com.tw/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=2ahUKEwjh0dzfjsndAhVIQd4KHQyICS4QFjAAegQIABAC&url=http%3A%2F%2Fstatic.diabetesaustralia.com.au%2Fs%2Ffileassets%2Fdiabetes-australia%2Faf2389ea-8f61-4c54-82d6-77ab07f03597.pdf&usg=AOvVaw3tYcJsm3defIXiwmqT1Fyl (accessed on 20 November 2017).
- Weber, M.A.; Schiffrin, E.L.; White, W.B.; Mann, S.; Lindholm, L.H.; Kenerson, J.G.; Flack, J.M.; Carter, B.L.; Materson, B.J.; Ram, C.V.S. Clinical practice guidelines for the management of hypertension in the community. J. Clin. Hypertens. 2014, 16, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Pencharz, P.; Clandinin, M. Absorption of 13c-labeled stearic, oleic, and linoleic acids in humans: Application to breath tests. J. Lab. Clin. Med. 1985, 105, 647–652. [Google Scholar] [PubMed]
- Schwingshackl, L.; Strasser, B.; Hoffmann, G. Effects of monounsaturated fatty acids on glycaemic control in patients with abnormal glucose metabolism: A systematic review and meta-analysis. Ann. Nutr. Metab. 2011, 58, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; Korat, A.A.; Malik, V.; Hu, F.B. Metabolic effects of monounsaturated fatty acid-enriched diets compared with carbohydrate or polyunsaturated fatty acid-enriched diets in patients with type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Diabetes Care 2016, 39, 1448–1457. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.; McInerney, D.; Owens, D.; Collins, P.; Johnson, A.; Tomkin, G.H. Diabetes and the Mediterranean diet: A beneficial effect of oleic acid on insulin sensitivity, adipocyte glucose transport and endothelium-dependent vasoreactivity. QJM 2000, 93, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Adams-Huet, B.; Brinkley, L.; Grundy, S.M.; Garg, A. Lipid, glycemic, and insulin responses to meals rich in saturated, cis-monounsaturated, and polyunsaturated (n-3 and n-6) fatty acids in subjects with type 2 diabetes. Diabetes Care 2007, 30, 2993–2998. [Google Scholar] [CrossRef]
- Abate, N.; S Sallam, H.; Rizzo, M.; Nikolic, D.; Obradovic, M.; Bjelogrlic, P.; R Isenovic, E. Resistin: An inflammatory cytokine. Role in cardiovascular diseases, diabetes and the metabolic syndrome. Curr. Pharm. Des. 2014, 20, 4961–4969. [Google Scholar] [CrossRef]
- Lee, S.; Lee, H.-C.; Kwon, Y.-W.; Lee, S.E.; Cho, Y.; Kim, J.; Lee, S.; Kim, J.-Y.; Lee, J.; Yang, H.-M. Adenylyl cyclase-associated protein 1 is a receptor for human resistin and mediates inflammatory actions of human monocytes. Cell Metab. 2014, 19, 484–497. [Google Scholar] [CrossRef]
- Granados, N.; Amengual, J.; Ribot, J.; Palou, A.; Bonet, M.L. Distinct effects of oleic acid and its trans-isomer elaidic acid on the expression of myokines and adipokines in cell models. Br. J. Nutr. 2011, 105, 1226–1234. [Google Scholar] [CrossRef] [Green Version]
- Haugen, F.; Zahid, N.; Dalen, K.T.; Hollung, K.; Nebb, H.I.; Drevon, C.A. Resistin expression in 3t3-L1 adipocytes is reduced by arachidonic acid. J. Lipid Res. 2005, 46, 143–153. [Google Scholar] [CrossRef]
- Park, H.K.; Kwak, M.K.; Kim, H.J.; Ahima, R.S. Linking resistin, inflammation, and cardiometabolic diseases. Korean J. Intern. Med. 2017, 32, 239–247. [Google Scholar] [CrossRef]
- Degawa-Yamauchi, M.; Bovenkerk, J.E.; Juliar, B.E.; Watson, W.; Kerr, K.; Jones, R.; Zhu, Q.; Considine, R.V. Serum resistin (FIZZ3) protein is increased in obese humans. J. Clin. Endocrinol. Metab. 2003, 88, 5452–5455. [Google Scholar] [CrossRef] [PubMed]
- Heilbronn, L.K.; Rood, J.; Janderova, L.; Albu, J.B.; Kelley, D.E.; Ravussin, E.; Smith, S.R. Relationship between serum resistin concentrations and insulin resistance in nonobese, obese, and obese diabetic subjects. J. Clin. Endocrinol. Metab. 2004, 89, 1844–1848. [Google Scholar] [CrossRef] [PubMed]
- Owecki, M.; Miczke, A.; Nikisch, E.; Pupek-Musialik, D.; Sowiński, J. Serum resistin concentrations are higher in human obesity but independent from insulin resistance. Exp. Clin. Endocrinol. Diabetes 2011, 119, 117–121. [Google Scholar] [CrossRef] [PubMed]
- De León, A.C.; González, D.A.; Hernández, A.G.; Coello, S.D.; Marrugat, J.; Sánchez, J.J.A.; Díaz, B.B.; Rodríguez, I.M.; Pérez, M.d.C.R. Relationships between serum resistin and fat intake, serum lipid concentrations and adiposity in the general population. J. Atheroscler. Thromb. 2014, 21, 454–462. [Google Scholar] [CrossRef]
- Silha, J.V.; Krsek, M.; Skrha, J.V.; Sucharda, P.; Nyomba, B.; Murphy, L.J. Plasma resistin, adiponectin and leptin levels in lean and obese subjects: Correlations with insulin resistance. Eur. J. Endocrinol. 2003, 149, 331–335. [Google Scholar] [CrossRef]
- Scoditti, E.; Massaro, M.; Carluccio, M.A.; Pellegrino, M.; Wabitsch, M.; Calabriso, N.; Storelli, C.; De Caterina, R. Additive regulation of adiponectin expression by the mediterranean diet olive oil components oleic acid and hydroxytyrosol in human adipocytes. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- Mantzoros, C.S.; Williams, C.J.; Manson, J.E.; Meigs, J.B.; Hu, F.B. Adherence to the mediterranean dietary pattern is positively associated with plasma adiponectin concentrations in diabetic women. Am. J. Clin. Nutr. 2006, 84, 328–335. [Google Scholar] [CrossRef]
- Esposito, K.; Di Palo, C.; Maiorino, M.I.; Petrizzo, M.; Bellastella, G.; Siniscalchi, I.; Giugliano, D. Long-term effect of Mediterranean-style diet and calorie restriction on biomarkers of longevity and oxidative stress in overweight men. Cardiol. Res. Pract. 2010, 2011. [Google Scholar] [CrossRef]
- Esposito, K.; Giugliano, D. Lifestyle and adiponectin level: Four-year follow-up of controlled trials. Arch. Intern. Med. 2010, 170, 1270–1271. [Google Scholar] [CrossRef]
- Callahan, H.S.; Cummings, D.E.; Pepe, M.S.; Breen, P.A.; Matthys, C.C.; Weigle, D.S. Postprandial suppression of plasma ghrelin level is proportional to ingested caloric load but does not predict intermeal interval in humans. J. Clin. Endocrinol. Metab. 2004, 89, 1319–1324. [Google Scholar] [CrossRef]
- Meier, U.; Gressner, A.M. Endocrine regulation of energy metabolism: Review of pathobiochemical and clinical chemical aspects of leptin, ghrelin, adiponectin, and resistin. Clin. Chem. 2004, 50, 1511–1525. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, J.L.; Paton, C.M.; Cooper, J.A. Hunger and satiety responses to high-fat meals after a high polyunsaturated fat diet: A randomized trial. Nutrition 2017, 41, 14–23. [Google Scholar] [CrossRef]
- Tschöp, M.; Weyer, C.; Tataranni, P.A.; Devanarayan, V.; Ravussin, E.; Heiman, M.L. Circulating ghrelin levels are decreased in human obesity. Diabetes 2001, 50, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, P.; Piscitelli, F.; Scognamiglio, P.; Monteleone, A.M.; Canestrelli, B.; Di Marzo, V.; Maj, M. Hedonic eating is associated with increased peripheral levels of ghrelin and the endocannabinoid 2-arachidonoyl-glycerol in healthy humans: A pilot study. J. Clin. Endocrinol. Metab. 2012. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, A.; de la Cour, C.D.; Stegmark, A.; Hakanson, R.; Erlanson-Albertsson, C. Overeating of palatable food is associated with blunted leptin and ghrelin responses. Regul. Pept. 2005, 130, 123–132. [Google Scholar] [CrossRef]
- Xiao, C.; Giacca, A.; Carpentier, A.; Lewis, G. Differential effects of monounsaturated, polyunsaturated and saturated fat ingestion on glucose-stimulated insulin secretion, sensitivity and clearance in overweight and obese, non-diabetic humans. Diabetologia 2006, 49, 1371–1379. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Kanthimathi, M.S.; Tan, A.T.-B.; Nesaretnam, K.; Teng, K.-T. The amount and types of fatty acids acutely affect insulin, glycemic and gastrointestinal peptide responses but not satiety in metabolic syndrome subjects. Eur. J. Nutr. 2018, 57, 1–12. [Google Scholar] [CrossRef]
- Lardinois, C.K.; Starich, G.; Mazzaferri, E.L. The postprandial response of gastric inhibitory polypeptide to various dietary fats in man. J. Am. Coll. Nutr. 1988, 7, 241–247. [Google Scholar] [CrossRef]
- Gutierrez-Aguilar, R.; Woods, S.C. Nutrition and L and K-enteroendocrine cells. Curr. Opin. Endocrinol. Diabetes Obes. 2011, 18. [Google Scholar] [CrossRef]
- Mennella, I.; Savarese, M.; Ferracane, R.; Sacchi, R.; Vitaglione, P. Oleic acid content of a meal promotes oleoylethanolamide response and reduces subsequent energy intake in humans. Food Funct. 2015, 6, 203–209. [Google Scholar] [CrossRef]
- Casas-Agustench, P.; López-Uriarte, P.; Bulló, M.; Ros, E.; Gómez-Flores, A.; Salas-Salvadó, J. Acute effects of three high-fat meals with different fat saturations on energy expenditure, substrate oxidation and satiety. Clin. Nutr. 2009, 28, 39–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacIntosh, B.A.; Ramsden, C.E.; Faurot, K.R.; Zamora, D.; Mangan, M.; Hibbeln, J.R.; Mann, J.D. Low-n-6 and low-n-6 plus high-n-3 diets for use in clinical research. Br. J. Nutr. 2013, 110, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Maljaars, J.; Romeyn, E.A.; Haddeman, E.; Peters, H.P.; Masclee, A.A. Effect of fat saturation on satiety, hormone release, and food intake. Am. J. Clin. Nutr. 2009, 89, 1019–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Nutrient | Control | High-OA | High-LA |
|---|---|---|---|
| Carbohydrate (g) | 50.9 | 33.7 | 33.7 |
| Carbohydrate (% E) | 73.46 | 39.74 | 39.74 |
| Protein (g) | 4.8 | 5.8 | 5.8 |
| Protein (% E) | 6.91 | 6.84 | 6.84 |
| Fat (g) | 7.3 | 21.5 | 21.5 |
| Fat (% E) | 23.12 | 55.13 | 55.13 |
| Saturated (g) | 2.5 | 3.1 | 3.1 |
| MUFA (g) | 2.5 | 15.1 | 3.1 |
| Oleic Acid (g) | 2.4 | 14.8 | 2.9 |
| PUFA (g) | 2.3 | 3.2 | 15.2 |
| Linoleic Acid (g) | 2.2 | 3.0 | 14.9 |
| Fiber (g) | 3.2 | 4.0 | 4.0 |
| Omega 6:Omega 3 | 16.7 | 13.1 | 77.2 |
| Parameter | Average ± SEM | |
|---|---|---|
| Age (years) | 45.8 ± 3.6 | |
| Height (cm) | 169.5 ± 2.2 | |
| Weight (kg) | 92.2 ± 4.5 | |
| BMI (kg/m2) | 32.0 ± 1.3 | |
| Waist Circumference (cm) | Female | 102.4 ± 7.1 |
| Male | 108.6 ± 7.9 | |
| Hip Circumference (cm) | Female | 119.6 ± 4.1 |
| Male | 111.3 ± 5.7 | |
| Waist-To-Hip Ratio | Female | 0.83 ± 0.03 |
| Male | 0.97 ± 0.01 | |
| Fat Mass (% of Total Mass) | 37.4 ± 2.9 | |
| Lean Mass (% of Total Mass) | 55.7 ± 2.8 | |
| Systolic Blood Pressure (mmHg) | 128.4 ± 4.1 | |
| Diastolic Blood Pressure (mmHg) | 82.3 ± 3.5 | |
| Heart Rate (bpm) | 73 ± 4.4 | |
| Fasting Serum Glucose (mmol/L) | 4.6 ± 0.1 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naughton, S.S.; Hanson, E.D.; Mathai, M.L.; McAinch, A.J. The Acute Effect of Oleic- or Linoleic Acid-Containing Meals on Appetite and Metabolic Markers; A Pilot Study in Overweight or Obese Individuals. Nutrients 2018, 10, 1376. https://doi.org/10.3390/nu10101376
Naughton SS, Hanson ED, Mathai ML, McAinch AJ. The Acute Effect of Oleic- or Linoleic Acid-Containing Meals on Appetite and Metabolic Markers; A Pilot Study in Overweight or Obese Individuals. Nutrients. 2018; 10(10):1376. https://doi.org/10.3390/nu10101376
Chicago/Turabian StyleNaughton, Shaan S., Erik D. Hanson, Michael L. Mathai, and Andrew J. McAinch. 2018. "The Acute Effect of Oleic- or Linoleic Acid-Containing Meals on Appetite and Metabolic Markers; A Pilot Study in Overweight or Obese Individuals" Nutrients 10, no. 10: 1376. https://doi.org/10.3390/nu10101376






