Protective Roles of Thymoquinone Nanoformulations: Potential Nanonutraceuticals in Human Diseases
Abstract
:1. Introduction
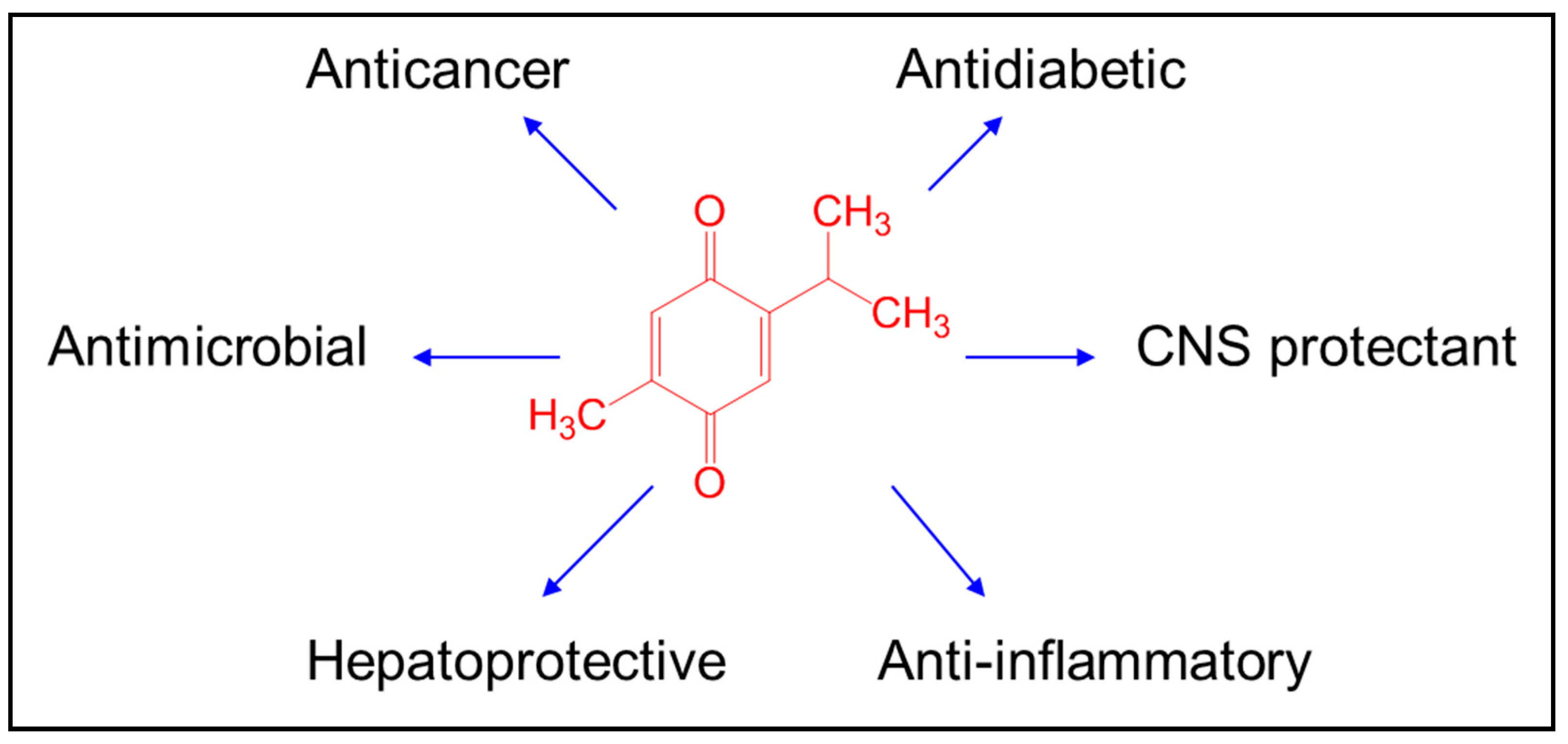
2. Biological Activities of Thymoquinone Nanoformulation
2.1. Anticancer
2.2. Antidiabetic
2.3. Central Nervous System (CNS) Protectant
2.4. Anti-Inflammatory
2.5. Hepatoprotective
2.6. Antimicrobial
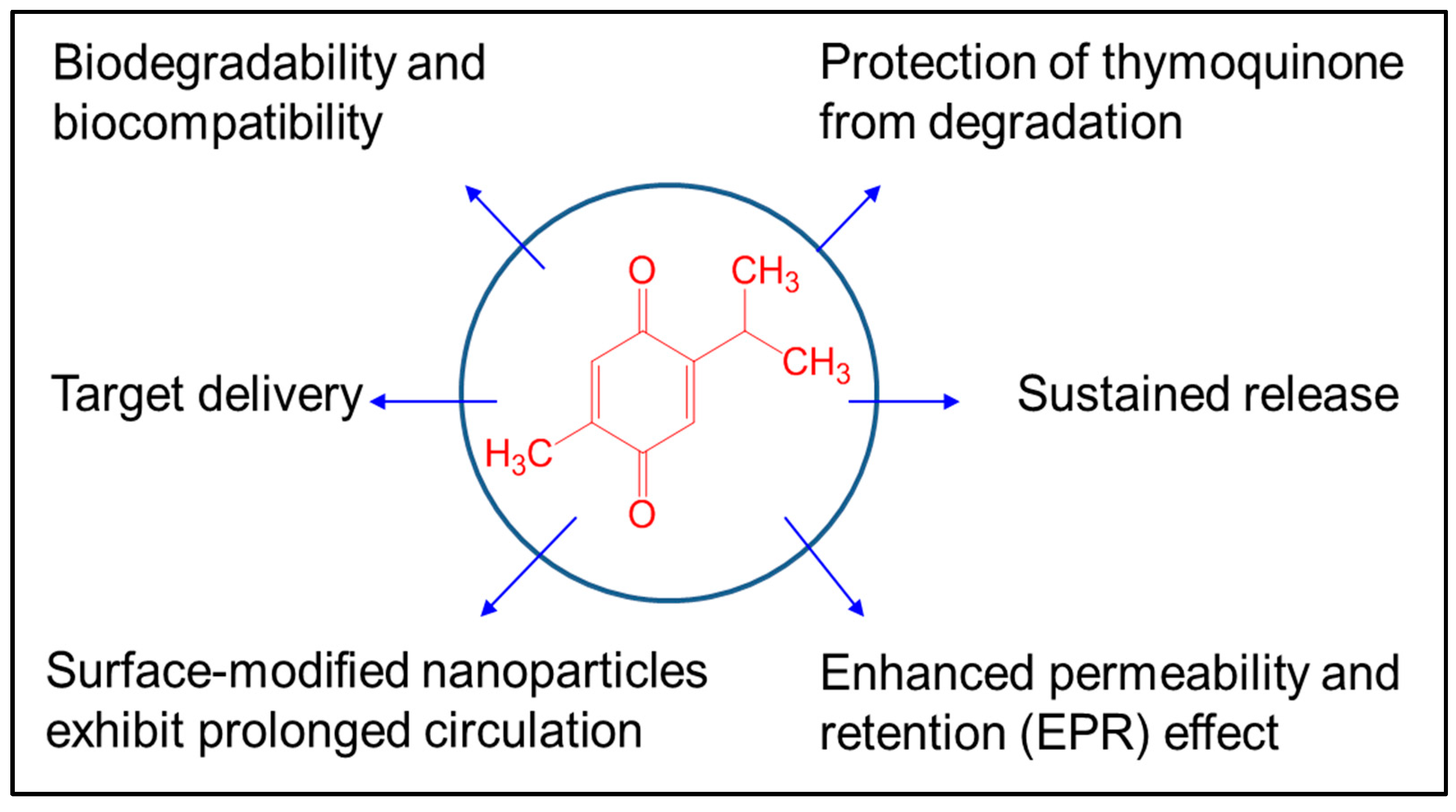
3. Applications of Nano-TQ
3.1. Nano-TQ Cosmetics
3.2. Health and Nutritional Supplement Drinks of Nano-TQ
4. Overcoming the Roadblocks in Clinical Translation of Nano-TQ
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- El-Far, A.; Bazh, E.K.; Moharam, M. Antioxidant and antinematodal effects of Nigella sativa and Zingiber officinale supplementations in ewes. Int. J. Pharm. Sci. Rev. Res. 2014, 26, 222–227. [Google Scholar]
- El-Far, A.H. Thymoquinone anticancer discovery: Possible mechanisms. Curr. Drug Discov. Technol. 2015, 12, 80–89. [Google Scholar] [CrossRef] [PubMed]
- El-Far, A.H.; Korshom, M.A.; Mandour, A.A.; El-Bessoumy, A.A.; El-Sayed, Y.S. Hepatoprotective efficacy of Nigella sativa seeds dietary supplementation against lead acetate-induced oxidative damage in rabbit–purification and characterization of glutathione peroxidase. Biomed. Pharmacother. 2017, 89, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Padhye, S.; Azmi, A.; Wang, Z.W.; Philip, P.A.; Kucuk, O.; Sarkar, F.H.; Mohammad, R.M. Review on molecular and therapeutic potential of thymoquinone in cancer. Nutr. Cancer 2010, 62, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Darakhshan, S.; Pour, A.B.; Colagar, A.H.; Sisakhtnezhad, S. Thymoquinone and its therapeutic potentials. Pharmacol. Res. 2015, 95–96, 138–158. [Google Scholar] [CrossRef] [PubMed]
- Salmani, J.M.; Asghar, S.; Lv, H.X.; Zhou, J.P. Aqueous solubility and degradation kinetics of the phytochemical anticancer thymoquinone; probing the effects of solvents, pH and light. Molecules 2014, 19, 5925–5939. [Google Scholar] [CrossRef] [PubMed]
- Alkharfy, K.M.; Ahmad, A.; Khan, R.M.; Al-Shagha, W.M. Pharmacokinetic plasma behaviors of intravenous and oral bioavailability of thymoquinone in a rabbit model. Eur. J. Drug Metab. Pharmacokinet. 2015, 40, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Schneider-Stock, R.; Fakhoury, I.H.; Zaki, A.M.; El-Baba, C.O.; Gali-Muhtasib, H.U. Thymoquinone: Fifty years of success in the battle against cancer models. Drug Discov. Today 2014, 19, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Ballout, F.; Habli, Z.; Rahal, O.N.; Fatfat, M.; Gali-Muhtasib, H. Thymoquinone-based nanotechnology for cancer therapy: Promises and challenges. Drug Discov. Today 2018, 23, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Ghosh, K.K. Thymoquinone. In Nutraceuticals: Efficacy, Safety and Toxicity; Gupta, R.C., Ed.; Elsevier: Boston, MA, USA, 2016; pp. 541–550. [Google Scholar]
- Imran, M.; Rauf, A.; Khan, I.A.; Shahbaz, M.; Qaisrani, T.B.; Fatmawati, S.; Abu-Izneid, T.; Imran, A.; Ur Rahman, K.; Gondal, T.A. Thymoquinone: A novel strategy to combat cancer: A review. Biomed. Pharmacother. 2018, 106, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Khader, M.; Eckl, P.M. Thymoquinone: An emerging natural drug with a wide range of medical applications. Iran J. Basic Med. Sci. 2014, 17, 950–957. [Google Scholar] [PubMed]
- Srinivas, P.R.; Philbert, M.; Vu, T.Q.; Huang, Q.; Kokini, J.L.; Saltos, E.; Chen, H.; Peterson, C.M.; Friedl, K.E.; McDade-Ngutter, C.; et al. Nanotechnology research: Applications in nutritional sciences. J. Nutr. 2010, 140, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yalcin, M.; Lin, Q.; Ardawi, M.M.; Mousa, S.A. Self-assembly of green tea catechin derivatives in nanoparticles for oral lycopene delivery. J. Control. Release 2017, 248, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.; Takhistov, P.; McClements, D.J. Functional materials in food nanotechnology. J. Food Sci. 2006, 71, R107–R116. [Google Scholar] [CrossRef]
- Nihei, T.; Suzuki, H.; Aoki, A.; Yuminoki, K.; Hashimoto, N.; Sato, H.; Seto, Y.; Onoue, S. Development of a novel nanoparticle formulation of thymoquinone with a cold wet-milling system and its pharmacokinetic analysis. Int. J. Pharm. 2016, 511, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, W.C. Concept and clinical evaluation of carrier-mediated anticancer agents. Oncologist 2008, 13, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Soni, P.; Kaur, J.; Tikoo, K. Dual drug-loaded paclitaxel–thymoquinone nanoparticles for effective breast cancer therapy. J. Nanopart. Res. 2015, 17, 18. [Google Scholar] [CrossRef]
- Elmowafy, M.; Samy, A.; Raslan, M.A.; Salama, A.; Said, R.A.; Abdelaziz, A.E.; El-Eraky, W.; Awdan, S.E.; Viitala, T. Enhancement of bioavailability and pharmacodynamic effects of thymoquinone via nanostructured lipid carrier (NLC) formulation. AAPS PharmSciTech 2015, 17, 663–672. [Google Scholar] [CrossRef] [PubMed]
- El-Ashmawy, N.E.; Khedr, E.G.; Ebeid, E.-Z.M.; Salem, M.L.; Zidan, A.-A.A.; Mosalam, E.M. Enhanced anticancer effect and reduced toxicity of doxorubicin in combination with thymoquinone released from poly-N-acetyl glucosamine nanomatrix in mice bearing solid ehrlish carcinoma. Eur. J. Pharm. Sci. 2017, 109, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Ahir, M.; Patra, P.; Mukherjee, S.; Ghosh, S.; Mazumdar, M.; Chattopadhyay, S.; Das, T.; Chattopadhyay, D.; Adhikary, A. PEGylated-thymoquinone-nanoparticle mediated retardation of breast cancer cell migration by deregulation of cytoskeletal actin polymerization through miR-34a. Biomaterials 2015, 51, 91–107. [Google Scholar] [CrossRef] [PubMed]
- Rani, R.; Dahiya, S.; Dhingra, D.; Dilbaghi, N.; Kim, K.-H.; Kumar, S. Improvement of antihyperglycemic activity of nano-thymoquinone in rat model of type-2 diabetes. Chem. Biol. Interact. 2018. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Khan, Z.I.; Mustafa, G.; Kumar, M.; Islam, F.; Bhatnagar, A.; Ahmad, F.J. Development and evaluation of thymoquinone-encapsulated chitosan nanoparticles for nose-to-brain targeting: A pharmacoscintigraphic study. Int. J. Nanomed. 2012, 7, 5705–5718. [Google Scholar] [CrossRef] [PubMed]
- Odeh, F.; Ismail, S.I.; Abu-Dahab, R.; Mahmoud, I.S.; Al Bawab, A. Thymoquinone in liposomes: A study of loading efficiency and biological activity towards breast cancer. Drug Deliv. 2012, 19, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Ganea, G.M.; Fakayode, S.O.; Losso, J.N.; van Nostrum, C.F.; Sabliov, C.M.; Warner, I.M. Delivery of phytochemical thymoquinone using molecular micelle modified poly(d,l-lactide-co-glycolide) (PLGA) nanoparticles. Nanotechnology 2010, 21, 285104. [Google Scholar] [CrossRef] [PubMed]
- Tubesha, Z.; Abu Bakar, Z.; Ismail, M. Characterization and stability evaluation of thymoquinone nanoemulsions prepared by high-pressure homogenization. J. Nanomater. 2013, 2013. [Google Scholar] [CrossRef]
- Verma, D.; Thakur, P.S.; Padhi, S.; Khuroo, T.; Talegaonkar, S.; Iqbal, Z. Design expert assisted nanoformulation design for co-delivery of topotecan and thymoquinone: Optimization, in vitro characterization and stability assessment. J. Mol. Liq. 2017, 242, 382–394. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2009, 33, S62–S69. [Google Scholar]
- AbuKhader, M.M. Thymoquinone: A promising antidiabetic agent. Int. J. Diabetes Dev. Ctries. 2012, 32, 65–68. [Google Scholar] [CrossRef]
- Atta, M.; Almadaly, E.; El-Far, A.; Saleh, R.M.; Assar, D.H.; Al Jaouni, S.K.; Mousa, S.A. Thymoquinone defeats diabetes-induced testicular damage in rats targeting antioxidant, inflammatory and aromatase expression. Int. J. Mol. Sci. 2017, 18, 919. [Google Scholar] [CrossRef] [PubMed]
- Abulfadl, Y.S.; El-Maraghy, N.N.; Ahmed, A.E.; Nofal, S.; Abdel-Mottaleb, Y.; Badary, O.A. Thymoquinone alleviates the experimentally induced Alzheimer’s disease inflammation by modulation of TLRs signaling. Hum. Exp. Toxicol. 2018, 37, 1092–1104. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, F.A.; Hossain, M.K.; Mostofa, A.G.M.; Akbor, M.M.; Bin Sayeed, M.S. Therapeutic potential of thymoquinone in glioblastoma treatment: Targeting major gliomagenesis signaling pathways. Biomed. Res. Int. 2018, 2018, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, S.S.; Oryan, S.; Izadpanah, E.; Hassanzadeh, K. Thymoquinone exerts neuroprotective effect in animal model of parkinson’s disease. Toxicol. Lett. 2017, 276, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Najmi, A.K.; Ahmad, I.; Ahmad, F.J.; Akhtar, M.J.; Imam, S.S.; Akhtar, M. Formulation and evaluation of nano lipid formulation containing CNS acting drug: Molecular docking, in-vitro assessment and bioactivity detail in rats. Artif. Cells Nanomed. Biotechnol. 2018, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.; Ismail, M.; Azmi, N.H.; Bakar, M.F.A.; Yida, Z.; Stanslas, J.; Sani, D.; Basri, H.; Abdullah, M.A. Beneficial effects of TQRF and TQ nano- and conventional emulsions on memory deficit, lipid peroxidation, total antioxidant status, antioxidants genes expression and soluble Aβ levels in high fat-cholesterol diet-induced rats. Chem. Biol. Interact. 2017, 275, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Rifaioglu, M.M.; Nacar, A.; Yuksel, R.; Yonden, Z.; Karcioglu, M.; Zorba, O.U.; Davarci, I.; Sefil, N.K. Antioxidative and anti-inflammatory effect of thymoquinone in an acute Pseudomonas prostatitis rat model. Urol. Int. 2013, 91, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Pooladanda, V.; Bulbake, U.; Doppalapudi, S.; Rafeeqi, T.A.; Godugu, C.; Khan, W. Liposphere mediated topical delivery of thymoquinone in the treatment of psoriasis. Nanomedicine 2017, 13, 2251–2262. [Google Scholar] [CrossRef] [PubMed]
- Farkhondeh, T.; Noorbakhsh, M.-F.; Hayati, F.; Samarghandian, S.; Shaterzadeh-Yazdi, H. An overview on hepatoprotective effects of thymoquinone. Recent Pat. Food Nutr. Agric. 2018, 9, 14–22. [Google Scholar]
- Kalam, M.A.; Raish, M.; Ahmed, A.; Alkharfy, K.M.; Mohsin, K.; Alshamsan, A.; Al-Jenoobi, F.I.; Al-Mohizea, A.M.; Shakeel, F. Oral bioavailability enhancement and hepatoprotective effects of thymoquinone by self-nanoemulsifying drug delivery system. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Ahmad, I.; Akhter, S.; Jain, G.K.; Iqbal, Z.; Talegaonkar, S.; Ahmad, F.J. Nanocarrier based formulation of thymoquinone improves oral delivery: Stability assessment, in vitro and in vivo studies. Colloids Surf. B Biointerfaces 2013, 102, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Bert, F.; Nicolas-Chanoine, M.-H. The challenges of multi-drug-resistance in hepatology. J. Hepatol. 2016, 65, 1043–1054. [Google Scholar] [CrossRef] [PubMed]
- Bakal, S.N.; Bereswill, S.; Heimesaat, M.M. Finding novel antibiotic substances from medicinal plants-Antimicrobial properties of Nigella sativa directed against multidrug-resistant bacteria. Eur. J. Microbiol. Immunol. 2017, 7, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, M.A.; Gondal, M.A.; Al-Zahrani, A.-H.J.; Rashid, S.G.; Ali, A. Synthesis, morphology and antifungal activity of nano-particulated amphotericin-b, ketoconazole and thymoquinone against Candida albicans yeasts and Candida biofilm. J. Environ. Sci. Health Part A Toxic Hazard. Subst. Environ. Eng. 2015, 50, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Lohani, A.; Verma, A.; Joshi, H.; Yadav, N.; Karki, N. Nanotechnology-based cosmeceuticals. ISRN Dermatol. 2014, 2014, 843687. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Sprando, R.L. Application of nanotechnology in cosmetics. Pharm. Res. 2010, 27, 1746–1749. [Google Scholar] [CrossRef] [PubMed]
- Raj, S.; Jose, S.; Sumod, U.S.; Sabitha, M. Nanotechnology in cosmetics: Opportunities and challenges. J. Pharm. Bioallied Sci. 2012, 4, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Shahroudi, M.J.; Mehri, S.; Hosseinzadeh, H. Anti-aging effect of Nigella sativa fixed oil on D-galactose-induced aging in mice. J. Pharmacopunct. 2017, 20, 29–35. [Google Scholar]
- Al-Haj, N.A.; Shamsudin, M.N.; Alipiah, N.M.; Zamri, H.F.; Bustamam, A.; Ibrahim, S.; Abdullah, R. Characterization of Nigella sativa L. Essential oil-loaded solid lipid nanoparticles. Am. J. Pharmacol. Toxicol. 2010, 5, 52–57. [Google Scholar] [CrossRef]
- Zolnik, B.S.; González-Fernández, A.F.; Sadrieh, N.; Dobrovolskaia, M.A. Minireview: Nanoparticles and the immune system. Endocrinology 2010, 151, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Jazieh, A.R.; Al Sudairy, R.; Abulkhair, O.; Alaskar, A.; Al Safi, F.; Sheblaq, N.; Young, S.; Issa, M.; Tamim, H. Use of complementary and alternative medicine by patients with cancer in Saudi Arabia. J. Altern. Complement. Med. 2012, 18, 1045–1049. [Google Scholar] [CrossRef] [PubMed]
- Van de Waterbeemd, H.; Gifford, E. Admet in silico modelling: Towards prediction paradise? Nat. Rev. Drug Discov. 2003, 2, 192–204. [Google Scholar] [PubMed]
- Pacwa-Plociniczak, M.; Plaza, G.A.; Piotrowska-Seget, Z.; Cameotra, S.S. Environmental applications of biosurfactants: Recent advances. Int. J. Mol. Sci. 2011, 12, 633–654. [Google Scholar] [CrossRef] [PubMed]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Barenholz, Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Desai, N. Challenges in development of nanoparticle-based therapeutics. AAPS J. 2012, 14, 282–295. [Google Scholar] [CrossRef] [PubMed]
- Bareford, L.M.; Swaan, P.W. Endocytic mechanisms for targeted drug delivery. Adv. Drug Deliv. Rev. 2007, 59, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Dufour, G.; Evrard, B.; de Tullio, P. 2D-Cosy NMR spectroscopy as a quantitative tool in biological matrix: Application to cyclodextrins. AAPS J. 2015, 17, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, H.; Fontana, F.; Hirvonen, J.T.; Santos, H.A. Current developments and applications of microfluidic technology toward clinical translation of nanomedicines. Adv. Drug Deliv. Rev. 2018, 128, 54–83. [Google Scholar] [CrossRef] [PubMed]
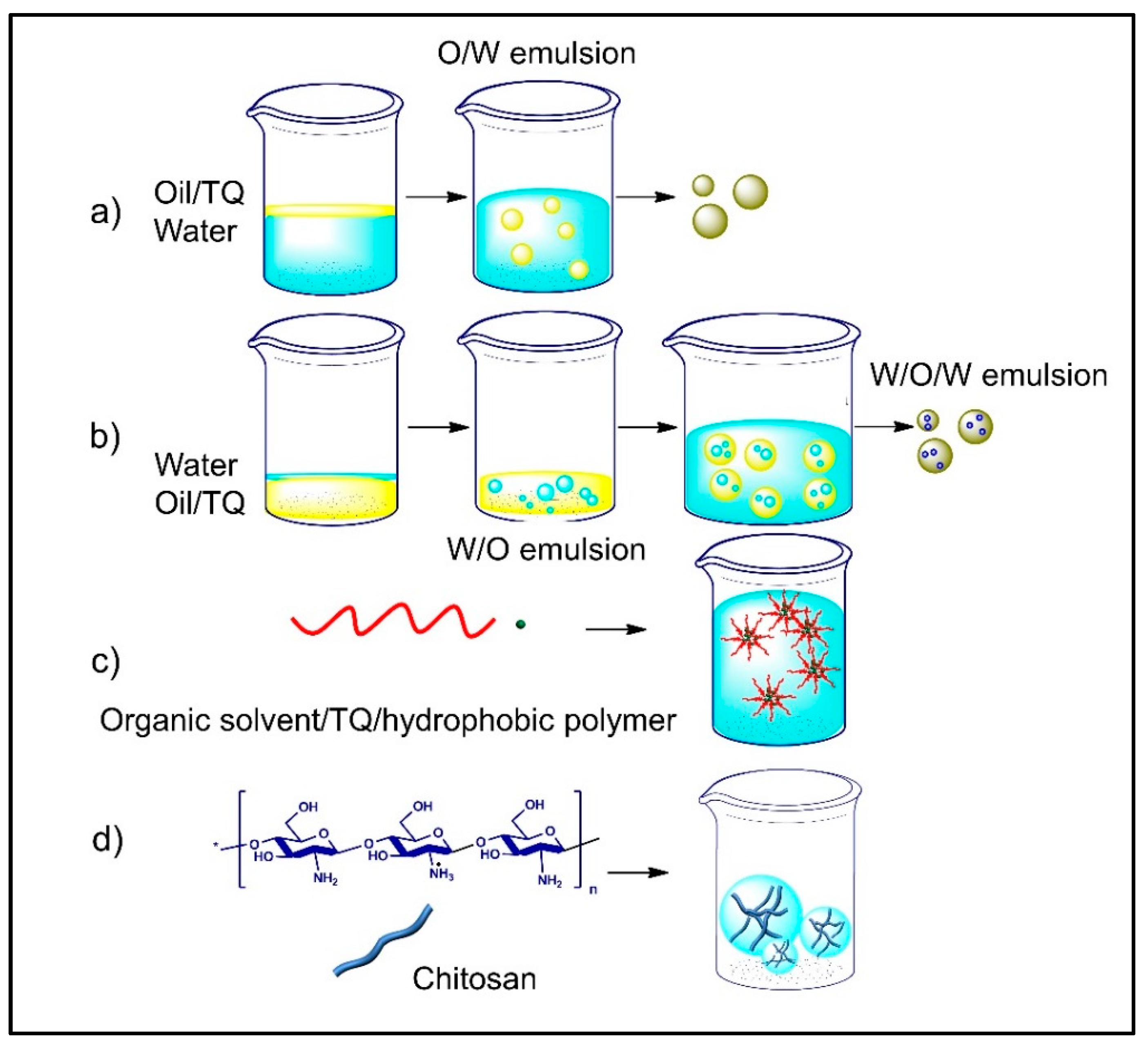

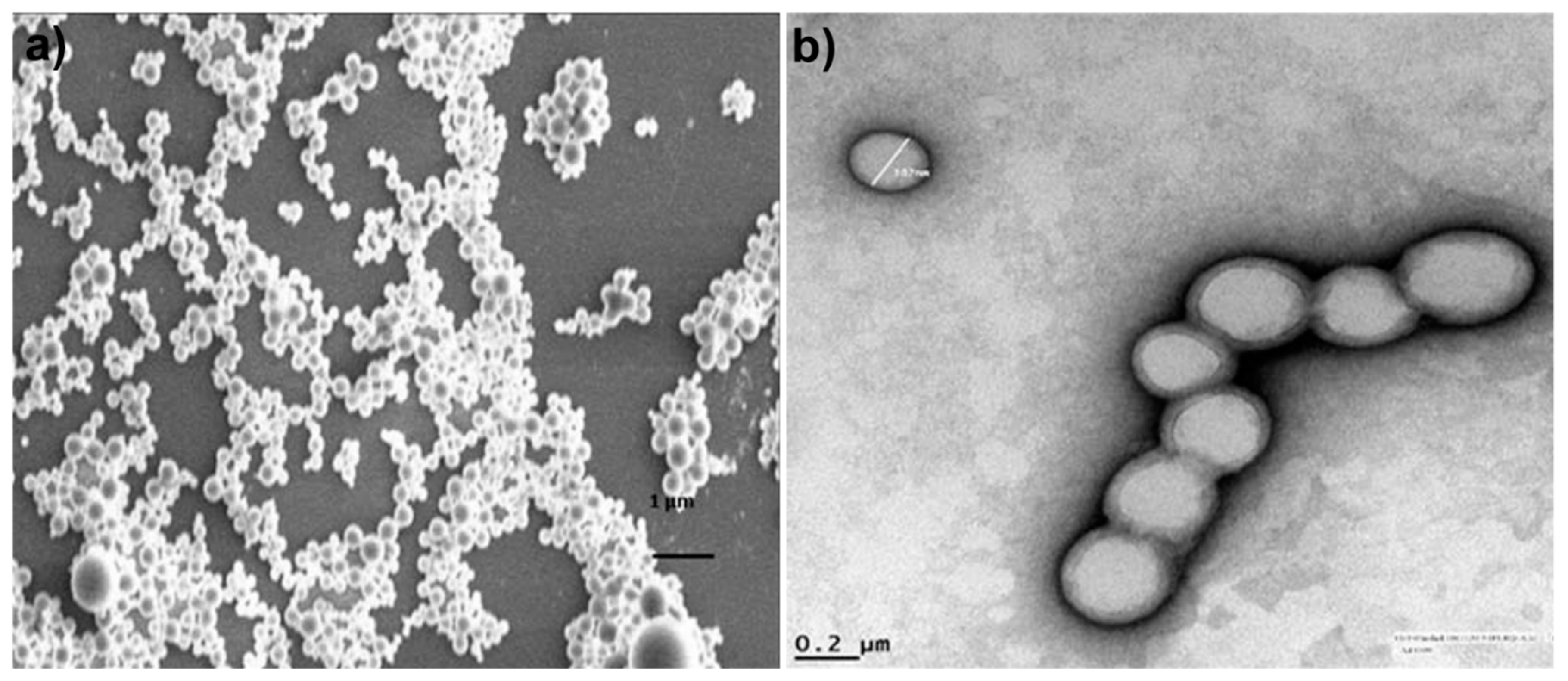

| Method | Materials, Stabilizer | Size (nm) | Animal, Dose | Therapeutic Effect | Reference |
|---|---|---|---|---|---|
| Single emulsion | PLGA | 200~300 | Enhanced anticancer activity | [18] | |
| Compritol ATO 888, gelucire | ~200 | Rats, 20 mg/kg, oral | 4-Fold enhancement of oral bioavailability | [19] | |
| Double emulsion | Poly-N-acetyl glucosamine, PVA | 185 | Mice, ~150 mg/kg, subcutaneous injection | Inhibited tumor growth 43%, 31% | [20] |
| Nano-precipitate | PVP, PEG200, PEG4000, P123 | 20~40 | Mice, 5 mg/kg, subcutaneous injection | Reduced tumor and increased lifespan | [21] |
| Gum rosin, oleic acid, PVA, polysorbate 80 | 50–90 | Wistar female albino rats, 20, 40, 80 mg/kg, oral | Significantly decreased blood glucose level and glycated hemoglobin | [22] | |
| Ionic gelation | Chitosan, TPP | 150 | Male Wistar rats, 2.52 mg/kg, intranasal | 15-Fold higher brain targeting efficiency | [23] |
| Film rehydration | Liposomes | 100 | More potent anti-proliferative activity | [24] | |
| Cold wet-milling | HPC-SSL | 143 | Male Sprague-Dawley rats, 2 mg/kg, oral | 6-Fold enhancement of oral bioavailability | [16] |
| Thermal Stress | Complexity | Scale-Up Principle | Organic Solvent | |
|---|---|---|---|---|
| Ball Mill Emax (wet milling) | Yes | >50 cycles | Top-down | No |
| Microfluidizer® | No | 5–6 cycles | Bottom-up (single emulsion) | Yes |
| NanoAssemblr™ | No | 1-step | Bottom-up (nano-precipitate) | Yes |
| MicroJet Reactor (MJR®) | No | 1-step | Bottom-up (nano-precipitate) | Yes |
| Nano Spray Dryer B-90 (spray-drying) | No | 1-step | Bottom-up (freeze-dry) | Yes |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Far, A.H.; Al Jaouni, S.K.; Li, W.; Mousa, S.A. Protective Roles of Thymoquinone Nanoformulations: Potential Nanonutraceuticals in Human Diseases. Nutrients 2018, 10, 1369. https://doi.org/10.3390/nu10101369
El-Far AH, Al Jaouni SK, Li W, Mousa SA. Protective Roles of Thymoquinone Nanoformulations: Potential Nanonutraceuticals in Human Diseases. Nutrients. 2018; 10(10):1369. https://doi.org/10.3390/nu10101369
Chicago/Turabian StyleEl-Far, Ali H., Soad K. Al Jaouni, Weikun Li, and Shaker A. Mousa. 2018. "Protective Roles of Thymoquinone Nanoformulations: Potential Nanonutraceuticals in Human Diseases" Nutrients 10, no. 10: 1369. https://doi.org/10.3390/nu10101369







