Sulfate Radical Technologies as Tertiary Treatment for the Removal of Emerging Contaminants from Wastewater
Abstract
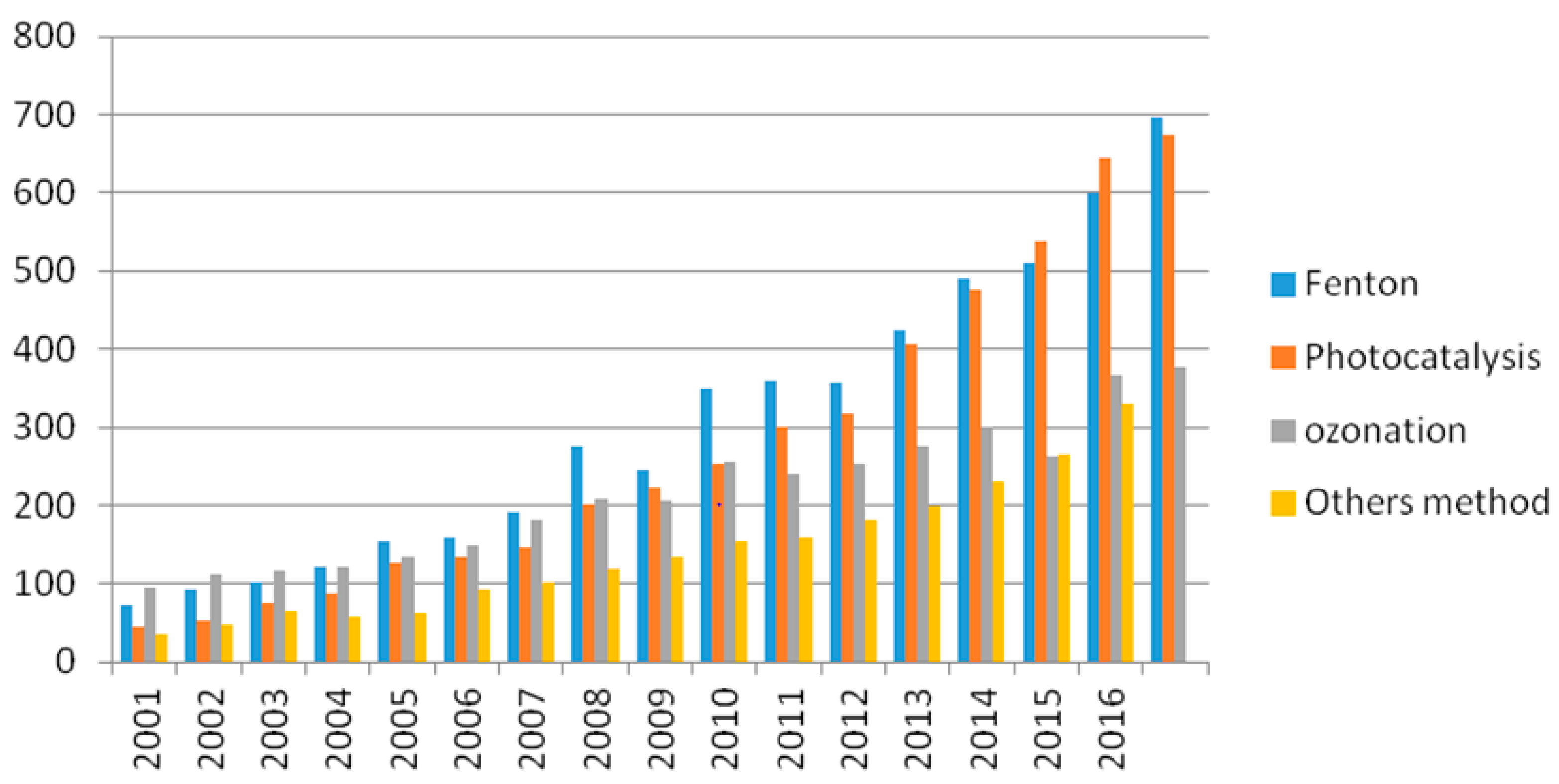
:1. Introduction
2. Sulfate Radicals for the Treatment of Wastewater
3. Ferrous Activation Systems
4. Non-Ferrous Activation Systems
5. Non-Metal Carbon Catalysts
6. Graphenes as Activation Medium for PMS
7. Hybrid Advanced Oxidation Processes Based on Sulfate Radicals
8. Conclusions and Implications for Future Applications
Author Contributions
Conflicts of Interest
References
- Brienza, M.; Mahdi Ahmed, M.; Escande, A.; Plantard, G.; Scrano, L.; Chiron, S.; Bufo, S.A.; Goetz, V. Use of solar advanced oxidation processes for wastewater treatment: Follow up on degradation products, acute toxicity, genotoxicity and estrogenicity. Chemosphere 2016, 148, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Kadokami, K.; Duong, H.T.; Chau, H.T.C. Screening of 1300 organic micro-pollutants in groundwater from Beijing and Tianjin, North China. Chemosphere 2016, 165, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Gómez, C.; Vicente, J.; Echavarri-Erasun, B.; Porte, C.; Lacorte, S. Occurrence of perfluorinated compounds in water, sediment and mussels from the Cantabrian Sea (North Spain). Marine Pollut. Bull. 2011, 62, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Babuponnusami, A.; Muthukumar, K. A review on Fenton and improvements to the Fenton processes for wastewater treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Glaze, W.H.; Kang, J.W.; Chapin, D.H. The chemistry of water treatment involving ozone, hydrogen peroxide and ultraviolet radiation. Ozone Sci. Eng. 1987, 9, 335–352. [Google Scholar] [CrossRef]
- Katsoyiannis, I.A.; Canonica, S.; von Gunten, U. Efficiency and energy requirements for the transformation of organic micropollutants by ozone, O3/H2O2 and UV/H2O2. Water Res. 2011, 45, 3811–3822. [Google Scholar] [CrossRef] [PubMed]
- Bilińska, L.; Gmurek, M.; Ledakowicz, S. Comparison between industrial and simutaled textile wastewater treatment by AOPs-Biodegradability, toxicity and cost assessment. Chem. Eng. J. 2016, 306, 550–559. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P.; Robert, D. Modified TiO2 for environmental photocatalytic applications: A review. Ind. Eng. Chem. Res. 2013, 52, 3581–3599. [Google Scholar] [CrossRef]
- Matta, R.; Tlili, S.; Barbati, S. Removal of carbamazepine from urban wastewater by sulfate radical oxidation. Environ. Chem. Lett. 2011, 9, 347–353. [Google Scholar] [CrossRef]
- Ahmed, M.; Barbati, S.; Doumenq, P.; Chiron, S. Sulfate radical anion oxidation of diclofenac and sulfaméthoxazole for water decontamination. Chem. Eng. J. 2012, 197, 440–447. [Google Scholar] [CrossRef]
- Monteagudo, J.M.; Durán, A.; Latorre, J.; Expósito, A.J. Application of activated persulfate for removal of intermediates from antipyrine wastewater degradation refractory towards hydroxyl radical. J. Hazard. Mater. 2016, 306, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Brienza, M.; Goetz, V.; Chiron, S. Solar photo-Fenton using peroxymonosulfate for orgqnic micropollutqnts removal from domestic wastewater: Comparison whit heretogeneous TiO2 photocatalysis. Chemosphere 2014, 117, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Su, R.; Luo, S.; Spimmey, R.; Cai, M.; Xiao, R.; Wei, Z. Comparison of the reactivity of ibuprofen with sulfate and hydroxyl radicals: an experimental and theorical study. Sci. Total Environ. 2017, 590–591, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Known, M.; Kim, S.; Yoon, Y.; Jung, Y.; Hwang, T.-M.; Lee, J.; Kang, J.-W. Comparative evaluation of ibuprofen removal by UV/H2O2 and UV/S2O82− processes for wastewater treatment. Chem. Eng. J. 2015, 269, 379–390. [Google Scholar]
- Epold, I.; Trapido, M.; Dulova, N. Degradation of levofloxacin in aqueous solutions by Fenton, ferrous ion-activated persulfate and combined Fenton/persulfate systems. Chem. Eng. J. 2015, 279, 452–462. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Z.; Li, S.; Liu, L.; Yao, L.; Li, Y.; Zhang, H. Insights into the mechanism of heterogeneous activation of persulfate with a clay/iron-based catalyst under visible LED light irradiation. Appl. Catal. B Environ. 2016, 185, 22–30. [Google Scholar] [CrossRef]
- Liu, H.; Bruton, T.A.; Li, W.; Buren, J.V.; Prasse, C.; Doyle, F.M.; Sedlak, D.L. Oxidation of benzene by persulfate in the presence of Fe(III)- and Mn(IV)-containing oxides: Stoichiometric efficiency and transformation products. Environ. Sci. Technol. 2016, 50, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Anipsitakis, G.P.; Dionysiou, D.D. Radical generation by the interaction of transition metals with common oxidants. Environ. Sci. Technol. 2004, 38, 3705–3712. [Google Scholar] [CrossRef] [PubMed]
- Brienza, M.; Mahdi Ahmed, M.; Escande, A.; Plantard, G.; Scrano, L.; Chiron, S.; Bufo, S.A.; Goetz, V. Relevance of a photo-Fenton like technology based on peroxymonosulphate for 17β-estradiol removal from wastewater. Chem. Eng. J. 2014, 257, 191–199. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, J.; Dai, C.; Zhang, Y.; Zhou, X. Degradation of carbamazepine and toxicity evaluation using the UV/persulfate process in aqueous solution. J. Chem. Technol. Biotechnol. 2014, 90, 701–708. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Tufano, T.P.; Dionysiou, D.D. Chemical and microbial decontamination of pool water using activated potassium peroxymonosulfate. Water Res. 2008, 42, 2899–2910. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhao, R. Advanced oxidation processes (AOPs) in wastewater treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Devi, P.; Das, U.; Dalai, A.K. In situ chemical oxidation: Principle and applications of peroxide and persulfate treatments in wastewater systems. Sci. Total Environ. 2016, 571, 643–657. [Google Scholar] [CrossRef] [PubMed]
- Katsoyiannis, I.A.; Gkotsis, P.; Castellana, M.; Cartechini, F.; Zouboulis, A.I. Production of demineralized water for use in thermal power stations by advanced treatment of secondary wastewater effluent. J. Environ. Manag. 2017, 190, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Köck-Schulmeyer, M.; Villagrasa, M.; de Alda, M.L.; Céspedes-Sànchez, R.; Ventura, F.; Barcélo, D. Occurance and behavior of pesticides in wastewater treatment plants and their environmental impact. Sci. Total Environ. 2013, 458–460, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Huang, W.L.; Fennell, D.E. In situ chemical oxidation of aniline by persulfate with iron(II) activation at ambient temperature. Chin. Chem. Lett. 2010, 21, 911–913. [Google Scholar] [CrossRef]
- Khan, J.A.; He, X.; Khan, H.M.; Shah, N.S.; Dionysiou, D.D. Oxidative degradation of atrazine in aqueous solution by UV/H2O2/Fe2+, UV/S2O82−/Fe2+ and UV/HSO5−/Fe2+ processes: A comparative study. Chem. Eng. J. 2013, 281, 376–383. [Google Scholar] [CrossRef]
- Asha, T.T.; Gandhimathi, R.; Ramesh, S.T.; Nidheesh, P.V. Treatment of stabilized leachate by ferrous-activated persulfate oxidative system. J. Hazard. Toxic Radioact. Waste 2017, 21. [Google Scholar] [CrossRef]
- Rastogi, A.; Al-Abed, S.R.; Dionysiou, D.D. Effect of inorganic, synthetic and naturally occurring chelating agents on Fe(II) mediated advanced oxidation of chlorophenols. Water Res. 2009, 43, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, A.; Al-Abed, S.R.; Dionysiou, D.D. Sulfate radical-based ferrous-peroxymonosulfate oxidative system for PCBs degradation in aqueous and sediment systems. Appl. Catal. B Environ. 2009, 85, 171–179. [Google Scholar] [CrossRef]
- Liang, C.; Bruell, C.J.; Marley, M.C.; Sperry, K.L. Perulfate oxidation for in situ remediation of TCE.II. Activated by chelated ferrous ion. Chemosphere 2004, 55, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.S.; Sun, C.; Sun, J.Q.; Zhou, R. Kinetic modeling and efficiency of sulfate radical-based oxidation to remove p-nitroaniline from wastewater by persulfate/Fe3O4 nanoparticles process. Sep. Purif. Technol. 2015, 142, 182–188. [Google Scholar] [CrossRef]
- Dong, H.; He, Q.; Zeng, G.; Tang, L.; Zhang, L.; Xie, Y.; Zeng, Y.; Zhao, F. Degradation of thrichloroethene by nanoscale zero-valent iron (nZVI) and nZVI activated persulfate in the absence and presence of EDTA. Chem. Eng. J. 2017, 316, 410–418. [Google Scholar] [CrossRef]
- Oh, S.-Y.; Kim, H.-W.; Park, J.-M.; Park, H.-S.; Yoon, C. Oxidation of polyvinyl alcohol by persulfate activated whit heat, Fe2+, and zero-valent iron. J. Hazard. Mater. 2009, 168, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Shao, Y.; Gao, N.; Chu, W.; Deng, J.; Shen, X.; Lu, X.; Zhu, Y.; Wei, X. Degradation of alachlor with zero-valent iron activating persulfate oxidation. J. Taiwan Inst. Chem. Eng. 2016, 63, 379–385. [Google Scholar] [CrossRef]
- Wei, X.; Gao, N.; Li, C.; Deng, Y.; Zhou, S.; Li, L. Zero-valent iron (ZVI) activation of persulfate (PS) for oxidation of bentazon in water. Chem. Eng. J. 2016, 285, 660–670. [Google Scholar] [CrossRef]
- Zeng, J.; Hu, L.; Tan, X.; He, C.; He, Z.; Pan, W.; Hou, Y.; Shu, D. Elimination of methyl mercaptan in ZVI-S2O82-system activated with in-situ generated ferrous ions from zero valent iron. Catal. Today 2017, 281, 520–526. [Google Scholar] [CrossRef]
- Kambhu, A.; Gren, M.; Tang, W.; Comfort, S.; Harris, C.E. Remediating 1,4-dioxane-contaminated water with slow-release persulfate and zerovalent iron. Chemosphere 2017, 175, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.; Li, J.; Zheng, X. Enhaced removal of pentachlorophenol by a novel composite: Nanoscale zero valent iron immobilized on organobentonite. Environ. Pollut. 2011, 159, 3744–3749. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Fang, Z.; Liang, B.; Gu, F.; Xu, Z. Degradation of decabromodiphenyl ether by nano zero valent iron immobilized in mesoporous silica microspheres. J. Hazard. Mater. 2011, 193, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lin, S.; Chen, Z.L.; Megharaj, M.; Naidu, R. Kaolinite-supported nanoscale zero-valent iron for removal of Pb2+ from aqueous solution: Reactivity, characterizzation and mechanism. Water Res. 2011, 45, 3481–3488. [Google Scholar] [CrossRef] [PubMed]
- Udayasoorian, C.; Ramalingam, P.; Jayabalakrishnan, R.M.; Vinoth Kumar, K. Carbon supported zero valent iron nanoparticles for treating PCP in pulp and paper effluent. Res. J. Chem. Environ. 2013, 17, 97–108. [Google Scholar]
- Chen, Z.X.; Jin, X.Y.; Chen, Z.L.; Megharaj, M.; Naidu, R. Removal of methyl orange from aqueous solution using bentonite-supported nanoscale zero-valent iron. J. Colloid Interface Sci. 2011, 363, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Diao, Z.-H.; Xu, X.-R.; Jiang, D.; Kong, L.-J.; Sun, Y.-X.; hu, Y.-X.; Hao, Q.-W.; Chen, H. Bentoninte-supported nanoscale zero-valent iron/persulfate system for the simultaneous removal of Cr(VI) and phenol from aqueous solutions. Chem. Eng. J. 2016, 302, 213–222. [Google Scholar] [CrossRef]
- Liang, C.; Liang, C.P.; Chen, C.C. pH dependence of persulfate activation by EDTA/Fe (III) for defradation of trichloroethylene. J. Contam. Hydrol. 2009, 106, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.J.; Lin, Y.T.; Shih, W.H. Treatment of trichloroethylene by adsorption and persulfate oxidation in batch studies. Ind. Eng. Chem. Res. 2009, 48, 8373–8380. [Google Scholar] [CrossRef]
- Dulova, N.; Kattel, E.; Trapido, M. Degradation of naproxen by ferrous ion-activated hydrogen proxide, persulfate and combined hydrogen peroxide/persulfate processes: The effect of citric acid addition. Chem. Eng. J. 2017, 318, 254–263. [Google Scholar] [CrossRef]
- Katsoyiannis, I.A.; Ruettimann, T.; Hug, S.J. pH dependence of Fenton reagent generation and As(III) oxidation and removal by corrosion of zero valent iron in aerated water. Environ. Sci. Technol. 2008, 42, 7424–7430. [Google Scholar] [CrossRef] [PubMed]
- Funai, D.H.; Didier, F.; Giménez, J.; Esplugas, S.; Marco, P.; Machulek Junior, A. Photo-Fenton treatment of valproate under UVC, UVA and simulated solar radiation. J. Hazard. Mater. 2017, 323, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Mohseni, M.; Vahabzadeh, F. Study on trend of biodegradability of phenolic compounds during photo-fenton advanced oxidation process. Iran. J. Chem. Eng. 2008, 5, 23–32. [Google Scholar]
- Spuhler, D.; Rengifo-Herrera, J.; Pulgarin, C. The effect of Fe2+, Fe3+, H2O2 and the photo-Fenton reagent at near neutral pH on the solar disinfection (SODIS) at low temperatures of water containing Escherichia coli K12. Appl. Catal. B 2010, 96, 126–141. [Google Scholar] [CrossRef]
- Oller, I.; Malato, S.; Sánchez-Pérez, J.A. Combination of advanced oxidation processes and biological treatment for wastewater decontamination—A review. Sci. Total Environ. 2011, 409, 4114–4166. [Google Scholar] [CrossRef] [PubMed]
- Bernabeua, A.; Verchera, R.F.; Santos-Juanesa, L.; Simónb, P.J.; Lardínb, C.; Martínezc, M.A.; Vicentec, J.A.; Gonzálezc, R.; Llosác, C.; Arquesa, A.; et al. Solar photocatalysis as tertiary treatment to remove emerging pollutants from wastewater treatment plant effluents. Catal. Today 2011, 161, 235–240. [Google Scholar] [CrossRef]
- Klamerth, N.; Malato, S.; Maldonado, M.I.; Agüera, A.; Fernandez-Alba, A.R. Application of Photo-Fenton as a tertiary treatment of emerging contaminants in municipal wastewater. Environ. Sci. Technol. 2010, 44, 1792–1798. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Rodríguez, L.; Spasiano, D.; Oller, I.; Fernández-Calderero, I.; Agüera, A.; Malato, S. Solar photo-Fenton optimization for the treatment of MWTP effluents containing emerging contaminants. Catal. Today 2013, 209, 188–194. [Google Scholar] [CrossRef]
- Leshuk, T.; Wong, T.; Linley, S.; Peru, K.M.; Headley, J.V.; Gu, F. Solar photocatalytic degradation of naphthenic acids in oil sands process-effected water. Chemosphere 2016, 144, 1854–1861. [Google Scholar] [CrossRef] [PubMed]
- Malato, S.; Maldonado, M.I.; Fernández-Ibáñez, P.; Oller, I.; Polo, I.; Sánchez-Moreno, R. Decontamination and disinfection of water by solar photocatalysis: The pilot plants of the Plataforma Solar de Almeria. Mater. Sci. Semicond. Process. 2016, 42, 15–23. [Google Scholar] [CrossRef]
- Dhatshanamurthi, P.; Shanthi, M.; Swaminathan, M. An efficient pilot scale solar treatment method for dye industry effluent using nano-ZnO. J. Water Processes Eng. 2017, 16, 28–34. [Google Scholar] [CrossRef]
- Khandarkhaeva, M.; Batoeva, A.; Aseev, D.; Sizykh, M.; Tsydenova, O. Oxidation of atrazine in aqueous media by solar-enhaced Fenton like process involving persulfate and ferrous ion. Ecotoxicol. Environ. Saf. 2017, 137, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Wu, M.-S. Degradation of ciprofloxacin by UV/S2O82-process in a large photoreactor. J. Photochem. Photobiol. A 2014, 285, 1–6. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Stathatos, E.; Dionysiou, D.D. Heterogenuous activation of Ocone using Co3O4. J. Phys. Chem. B 2005, 109, 13052–13055. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Long, M. Cobalt-catalyzed sulfate radical-based advanced oxidation: A review on heterogeneous catalysts and applications. Appl. Catal. B Environ. 2016, 181, 103–117. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, M.; And Zhu, L. Activation of persulfate by Co3O4 nanoparticles for organge G degradation. RSC Adv. 2016, 5, 758–768. [Google Scholar] [CrossRef]
- Deng, J.; Shao, Y.; Gao, N.; Tan, C.; Zhou, S.; Hu, X. CoFe2O4 magnetic nanoparticles as highly active heretogeneuous catalyst of oxone for degradation of diclofenac in water. J. Hazard. Mater. 2013, 262, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.; Sun, H.; Wang, S.; Ang, H.M.; Tadé, M.O. Co-SBA-15 for heterogeneous oxidation of phenol with sulphate radical for wastewater treatment. Catal. Today 2011, 175, 380–385. [Google Scholar] [CrossRef]
- Shi, P.; Su, R.; Wan, F.; Zhu, M.; Li, D.; Xu, S. Co3O4 nanocrystals on graphene oxide as a synergistic catalyst for degradation of Orange II in water y advanced oxidation technology based on sulfate radiclas. Appl. Catal. B Environ. 2011, 123–124, 265–272. [Google Scholar] [CrossRef]
- Saputra, E.; Muhammad, S.; Sun, H.; Ang, H.; Tadè, M.O.; Wang, S. Manganese oxides at different oxidation states for heterogeneous activation of peroxymonosulfate for phenol degradation in aqueous solutions. Appl. Catal. B Environ. 2013, 142–143, 729–735. [Google Scholar] [CrossRef]
- Liu, C.S.; Shih, K.; Sun, C.X.; Wang, F. Oxidative degradation of propachlor by ferrous and copper ion activated persulfate. Sci. Total Environ. 2012, 416, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.; Wu, W.; Liu, C.; Dionysioum, D.D.; Deng, Y.; Zhou, D. Activation of persulfate with vanadium species for PCBs degradation: A mechanistic study. Appl. Catal. B Environ. 2017, 202, 1–11. [Google Scholar] [CrossRef]
- Duan, X.; Su, C.; Zhou, L.; Sun, H.; Suvorova, A.; Odedairo, T.; Zhu, Z.; Shao, Z.; Wang, S. Surface controlled generation of reactive radicals from persulfate by carbocatalysis on nanodiamonds. Appl. Catal. B Environ. 2016, 194, 7–15. [Google Scholar] [CrossRef]
- Indrawirawan, S.; Sun, H.; Duan, X.; Wang, S. Nanocarbons in different structural dimensions (0–3D) for phenol adsorption and metal-free catalytic oxidation. Appl. Catal. B 2015, 179, 352–362. [Google Scholar] [CrossRef]
- Kimura, M.; Miyamoto, I. Discovery of the activated carbon radical AC+ and the novel oxidation reactions comprising the AC/AC+ cycle as a catalyst in an aqueous solution. Bull. Chem. Soc. Jpn. 1994, 67, 2357–2360. [Google Scholar] [CrossRef]
- Muhammad, S.; Shukla, P.R.; Tade, M.O.; Wang, S. Heterogeneous activation of peroxymonosulphate by supported ruthenium catalysts for phenol degradation in water. J. Hazard. Mater. 2012, 215–216, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Xiao, T.; Zhang, J.; Chen, Y.; Li, L. Activated carbon fiber as heterogeneous catalyst of peroxymonosulfate activation for efficient degradation of Acid Orange 7 in aqueous solution. Sep. Purif. Technol. 2015, 143, 19–26. [Google Scholar] [CrossRef]
- Zhang, J.; Shao, X.; Shi, C.; Yang, S. Decolorizaton of acid Orange 7 with peroxymonosulfate oxidation catalyzed by granular activated carbon. Chem. Eng. J. 2013, 232, 259–265. [Google Scholar] [CrossRef]
- Peng, W.; Liu, S.; Sun, H.; Yao, Y.; Zhic, L.; Wang, S. Synthesis of porous reduced graphene oxide as metal-free carbon for adsorption and catalytic oxidation of organics in water. J. Mater. Chem. A 2013, 1, 5854–5859. [Google Scholar] [CrossRef]
- Duan, X.; Ao, Z.; Zhou, L.; Sun, H.; Wang, G.; Wang, S. Occurrence of radical and nonradical pathways from carbocatalysts for aqueous and non aqueous catalytic oxidation. Appl. Catal. B Environ. 2016, 188, 98–105. [Google Scholar] [CrossRef]
- Chen, X.; Wang, W.; Xiao, H.; Hong, C.; Zhu, F.; Yao, Y.; Xue, Z. Accelerated TiO2 photocatalytic degradation of Acid Orange 7 under visible light mediated by peroxymonosulfate. Chem. Eng. J. 2012, 193–194, 290–295. [Google Scholar] [CrossRef]
- Kuriechen, S.K.; Murugesan, S.; Raj, S.P.; Maruthamuthu, P. Visible light assisted photocatalytic mineralization of Reactive Red 180 using colloidal TiO2 and oxone. Chem. Eng. J. 2011, 174, 530–538. [Google Scholar] [CrossRef]
- Pang, Y.; Lei, H. Degradation of p-nitrophenol through microwave-assisted heterogeneous activation of peroxymonosulfate by manganese ferrite. Chem. Eng. J. 2016, 287, 585–592. [Google Scholar] [CrossRef]
- Zhao, L.; Ji, Y.; Kong, D.; Lu, J.; Zhou, Q.; Yin, X. Simultaneous removal of bisphenol A and phosphate in zero-valent iron activated persulfate oxidation processes. Chem. Eng. J. 2016, 303, 458–466. [Google Scholar] [CrossRef]
- Li, H.; Wan, J.; Ma, Y.; Wang, Y. Reaction pathway and oxidation mechanisms of dibutyl phtalate by persulfate actovated with zero-valent iron. Sci. Total Environ. 2016, 303, 458–466. [Google Scholar]
- Qin, W.; Fang, G.; Wang, Y.; Wu, T.; Zhu, C.; Zhou, D. Efficient transformation of DDT by persoxymonosulfate activated by colbalt in aqueous systems: Kinetics, products, and reactive species identification. Chemosphere 2016, 148, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wan, J.; Ma, Y.; Wang, Y.; Chen, X.; Guan, Z. Degradation of refractory dibutyl phthalate by peroxymonosulfate activated whit novel catalysts cobalt metal-organic frameworks: Mechanism, performance, and stability. J. Hazard. Mater. 2016, 318, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.-Y.; Zhang, Y.-Q.; Huang, S.-B.; Hussain, I. Oxidative degradation of p-chloroaniline by copper oxidate activated persulfate. Chem. Eng. J. 2013, 218, 348–391. [Google Scholar] [CrossRef]
- Ji, Q.; Li, J.; Xiong, Z.; Lai, B. Enhanced reactivity of microscale Fe/Cu bimetallic particles (mFe/Cu) with persulfate (PS) for p-nitrophenol (PNP) removal in aqueous solution. Chemosphere 2017, 172, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Guo, J.; Yang, L.; Lan, Y. Degradation of methyl orange by sodium persulfate activated with zero-valent zinc. Sep. Purif. Technol. 2014, 132, 168–173. [Google Scholar] [CrossRef]
- Liang, S.X.; Jia, Z.; Zhang, W.C.; Wang, W.M.; Zhang, L.C. Rapid malachite green degradation using Fe73.5Si13.5B9Cu1Nb3 metallic glass for activation of persulfate under UV-Vis light. Mater. Des. 2017, 119, 244–253. [Google Scholar] [CrossRef]
- Jiang, M.; Lu, J.; Ji, Y.; Kong, D. Bicarbonate-activated persulfate oxidation of acetamoniphen. Water Res. 2017, 116, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zeng, Z.; Li, Y.; Huang, Z.; Yang, J. Catalytic oxidation of 4-chlorophenol on in-situ sulphur-doped activated carbon with sulphate radicals. Sep. Purif. Technol. 2017, 179, 257–264. [Google Scholar] [CrossRef]
- Lee, H.; Lee, H.-J.; Jeong, J.; Lee, J.; Park, N.-B.; Lee, C. Activation of persulfates by carbon nanotubes: Oxidation of organic compounds by nonradical mechanisms. Chem. Eng. J. 2015, 266, 28–33. [Google Scholar] [CrossRef]
- Sun, H.; Kwan, C.; Suvorova, A.; Ang, H.M.; Tadé, M.O.; Wang, S. Catalytic oxidation of organic pollutants on pristine and surface nitrogen-modified carbon nanotubes with sulfate radicals. Appl. Catal. B Environ. 2014, 154–155, 134–141. [Google Scholar] [CrossRef]
- Kang, J.; Duan, X.; Zhou, L.; Sun, H.; Tadé, M.O.; Wang, S. Carbocatalytic activation of persulfate for removal of antibiotics in water solutions. Chem. Eng. J. 2016, 288, 399–405. [Google Scholar] [CrossRef]
- Duan, X.; Sun, H.; Kang, J.; Wang, Y.; Indrawirawan, S.; Wang, S. Insights into heterogeneous catalysis of persulfate activation on dimensional-structured nanocarbons. ACS Catal. 2015, 5, 4629–4636. [Google Scholar] [CrossRef]
- Jafari, A.J.; Kakavandi, B.; Jaafarzadeh, N.; Kalantary, R.R.; Ahmadi, M.; Barbaei, A.A. Fenton-like catalytic oxidation of tetracycline by AC@Fe3O4 as a heterogeneous persulfate activator: adsorption and degradation studies. J. Ind. Eng. Chem. 2017, 45, 323–333. [Google Scholar] [CrossRef]
- Andrew Lin, K.Y.; Zhang, Z.-Y. α-Sulfur as a metal-free catalyst to active peroxymonosulfate under visible light irradiation for decolorization. RSC Adv. 2016, 6, 15027–15034. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, Y.; Sun, H.; Xiao, J.; Cao, H.; Wang, S. 2D/2D nano-hybrids of γ-MnO2 on reduced graphene oxide for catalytic ozonation and coupling peroxymonosulfate activation. J. Hazard. Mater. 2016, 301, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Zhang, H.; Zhong, X.; Hou, L. Ultrasound enhanced heterogeneous activation of peroxymonosulfate by a bimetallic Fe-Co/SBA-15 catalyst for degradation of Orange II in water. J. Hazard. Mater. 2015, 283, 70–79. [Google Scholar] [CrossRef] [PubMed]
| AOP | Advantages | Disadvantages |
|---|---|---|
| Fenton’s Reaction |
|
|
| TiO2 catalyzed UV oxidation |
|
|
| H2O2/O3 |
|
|
| O3/UV |
|
|
| H2O2/UV |
|
|
| Sonication |
|
|
| Targeted Compound | k SR | k HR | Reference |
|---|---|---|---|
| Sulfamethoxazole | 54.12 ± 0.1 × 10−2 min−1 | 3.12 ± 0.02 × 10−2 min−1 | [12] |
| Bifenthrin | 28.65 ± 0.06 × 10−2 min−1 | 1.65 ± 0.08 × 10−2 min−1 | |
| Mesotrione | 21.32 ± 0.08 × 10−2 min−1 | 1.45 ± 0.07 × 10−2 min−1 | |
| Carbamazepine | 18.69 ± 0.03 × 10−2 min−1 | 2.96 ± 0.01 × 10−2 min−1 | |
| Diclofenac | 58.19 ± 0.4 × 10−2 min−1 | 3.65 ± 0.06 × 10−2 min−1 | |
| Clothianidin | 29.54 ± 0.1 × 10−2 min−1 | 1.20 ± 0.09 × 10−2 min−1 | |
| Ibuprofen | 1.66 ± 0.12 × 109 M−1 s−1 | 3.43 ± 0.06 × 109 M−1 s−1 | [13] |
| 1.32 × 109 M−1 s−1 | 5.57 × 109 M−1 s−1 | [14] | |
| Levofloxacin | 0.93 ± 0.02 × 10−2 min−1 | 20.81 ± 0.7 × 10−2 min−1 | [15] |
| Target Pollutant | Performance | Reference | |
|---|---|---|---|
| Metal Catalyst | |||
| Fe(II) | Aniline |
| [26] |
| Atrazine and TOC |
| [27] | |
| Fe(III)/chelating agent | Iopamidol |
| [33] |
| ZVI | Bispenol and phosphate |
| [81] |
| Dibutyl phthalate |
| [82] | |
| Cobalt | DDT |
| [83] |
| Co-based metal organic frameworks (MOFs) | Dibutyl phthalate |
| [84] |
| Copper oxidate | p-chloroaniline [PCA] |
| [85] |
| Copper ion | Propachlor |
| [68] |
| Fe/Cu | p-nitrophenol (PNP) |
| [86] |
| Manganite | Phenol |
| [87] |
| Vanadium | 2,4,4-trichlorobiphenyl [PCB28] |
| [69] |
| Zn0 | Methyl orange (MO) |
| [39] |
| Metallic glass (Fe73.5Si13.5B9Cu1Nb3) | Malachite green (MG) |
| [88] |
| Metal-free catalyst | |||
| Bicarbonate | Acetaminophen |
| [89] |
| Powder activated carbon (PAC) | Phenol |
| [67] |
| S-doped activated carbon (ACS) | 4-chlorophenol (4CP) |
| [90] |
| Carbon nanotubes (CNTs) | Phenol |
| [91] |
| Nitrogen-CNT | Phenol |
| [92] |
| Nitrogen doped Reduced Graphene Oxide (N-rGO) | sulfachloropyridazine (SCP) |
| [93] |
| Annealed Nanodiamonds (ANDs) | Phenol, catechol, benzoic acid, sulfachloropyridazine, methylene blue |
| [77] |
| Hybrid AOP | |||
| Kaolinite-supported iron oxide/PS/Vis LED | Rhodamine B (RhB) |
| [94] |
| Microwave (MW) irradiation/MnFe2O4 | 4-nitrophenol |
| [80] |
| Ferro-ferric oxide coated on activated carbon (AC@Fe3O4) | Tetracycline (TC) |
| [95] |
| TiO2/UV-vis | Acid Orange 7 (AO7) |
| [78] |
| α-sulfur/UV-vis | Rhodamine B |
| [96] |
| γ-MnO2/rGO under ozonation | 4-nitrophenol |
| [97] |
| Fe-Co/SBA-15 with ultrasonic irradiation | Orange II |
| [98] |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brienza, M.; Katsoyiannis, I.A. Sulfate Radical Technologies as Tertiary Treatment for the Removal of Emerging Contaminants from Wastewater. Sustainability 2017, 9, 1604. https://doi.org/10.3390/su9091604
Brienza M, Katsoyiannis IA. Sulfate Radical Technologies as Tertiary Treatment for the Removal of Emerging Contaminants from Wastewater. Sustainability. 2017; 9(9):1604. https://doi.org/10.3390/su9091604
Chicago/Turabian StyleBrienza, Monica, and Ioannis A. Katsoyiannis. 2017. "Sulfate Radical Technologies as Tertiary Treatment for the Removal of Emerging Contaminants from Wastewater" Sustainability 9, no. 9: 1604. https://doi.org/10.3390/su9091604





