Metronidazole Degradation via Visible Light-Driven Z-Scheme BiTmDySbO7/BiEuO3 Heterojunction Photocatalyst
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of BiEuO3
2.3. Preparation of BiTmDySbO7
2.4. Preparation of Nitrogen-Doped TiO2
2.5. Preparation of BiTmDySbO7/BiEuO3 Heterojunction Photocatalyst
2.6. Feature Description
2.7. Photoelectrochemical Experiment
2.8. Experimental Setup and Procedure
3. Results and Discussion
3.1. Characterization of Photocatalysts
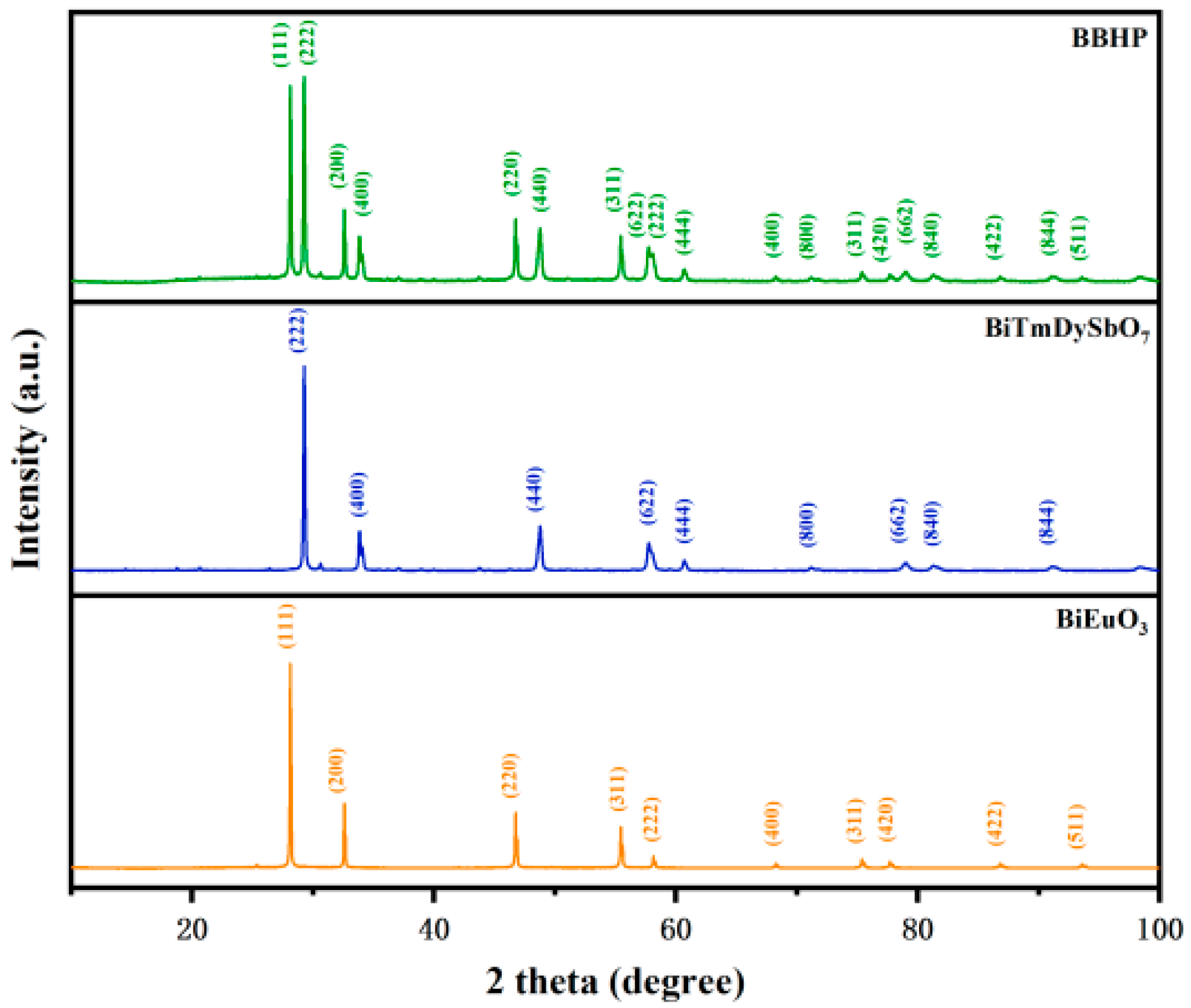
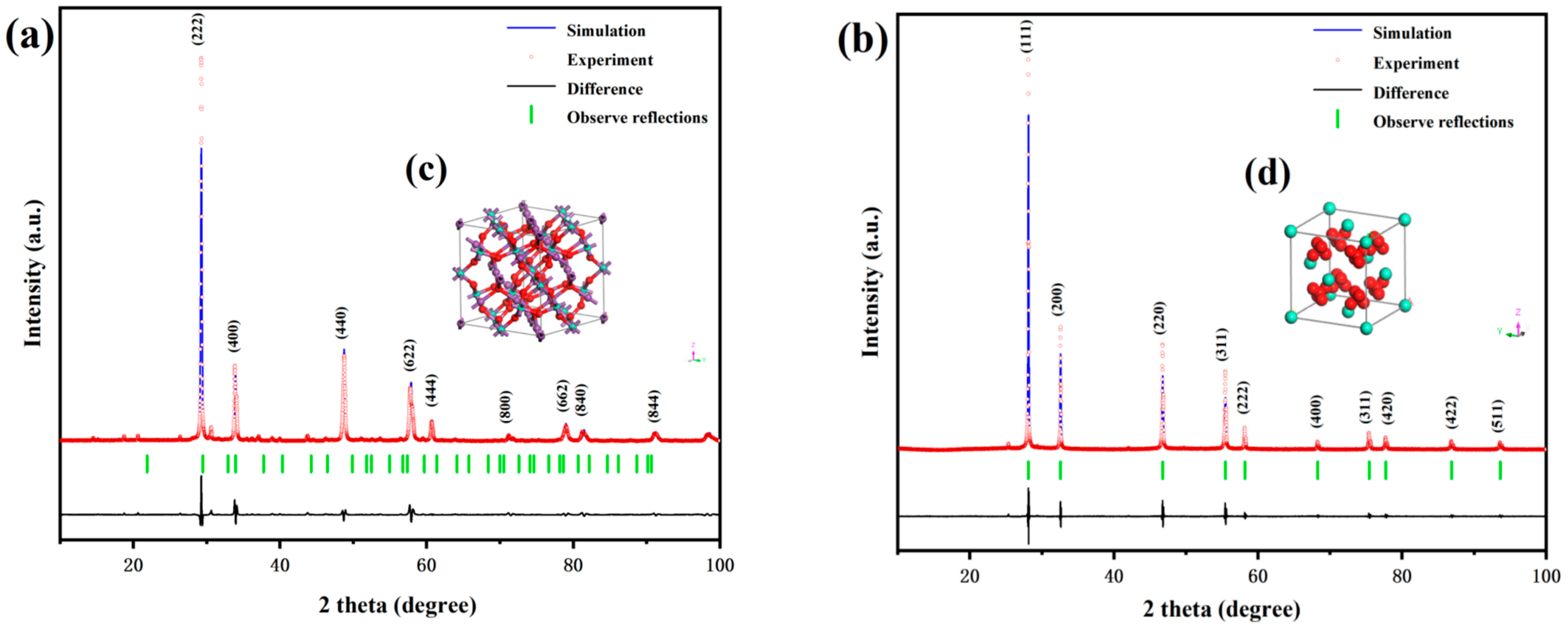
3.1.1. X-Ray Diffraction (XRD) Pattern Analysis
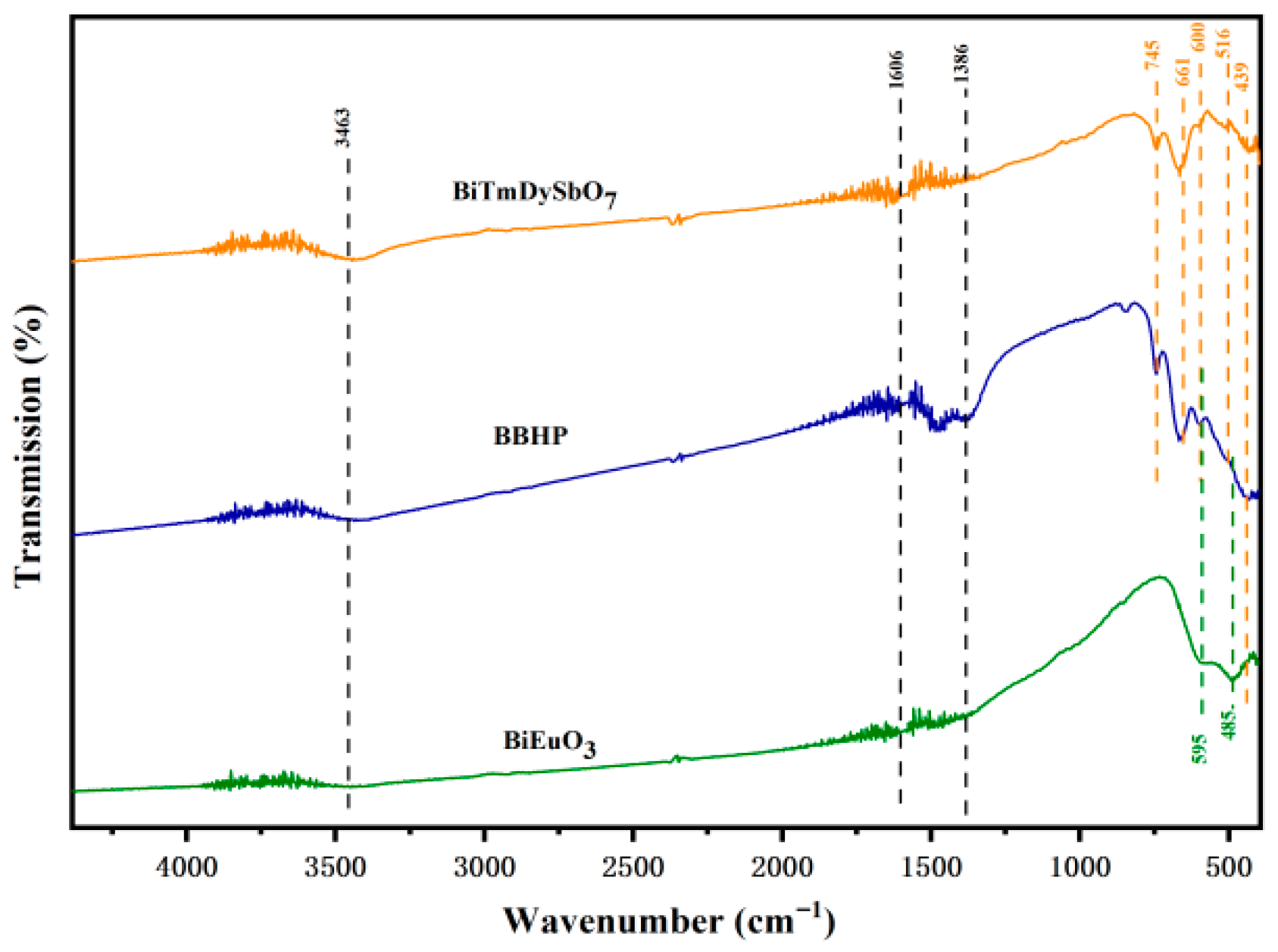
3.1.2. Fourier Transform Infrared Spectroscopy Analysis
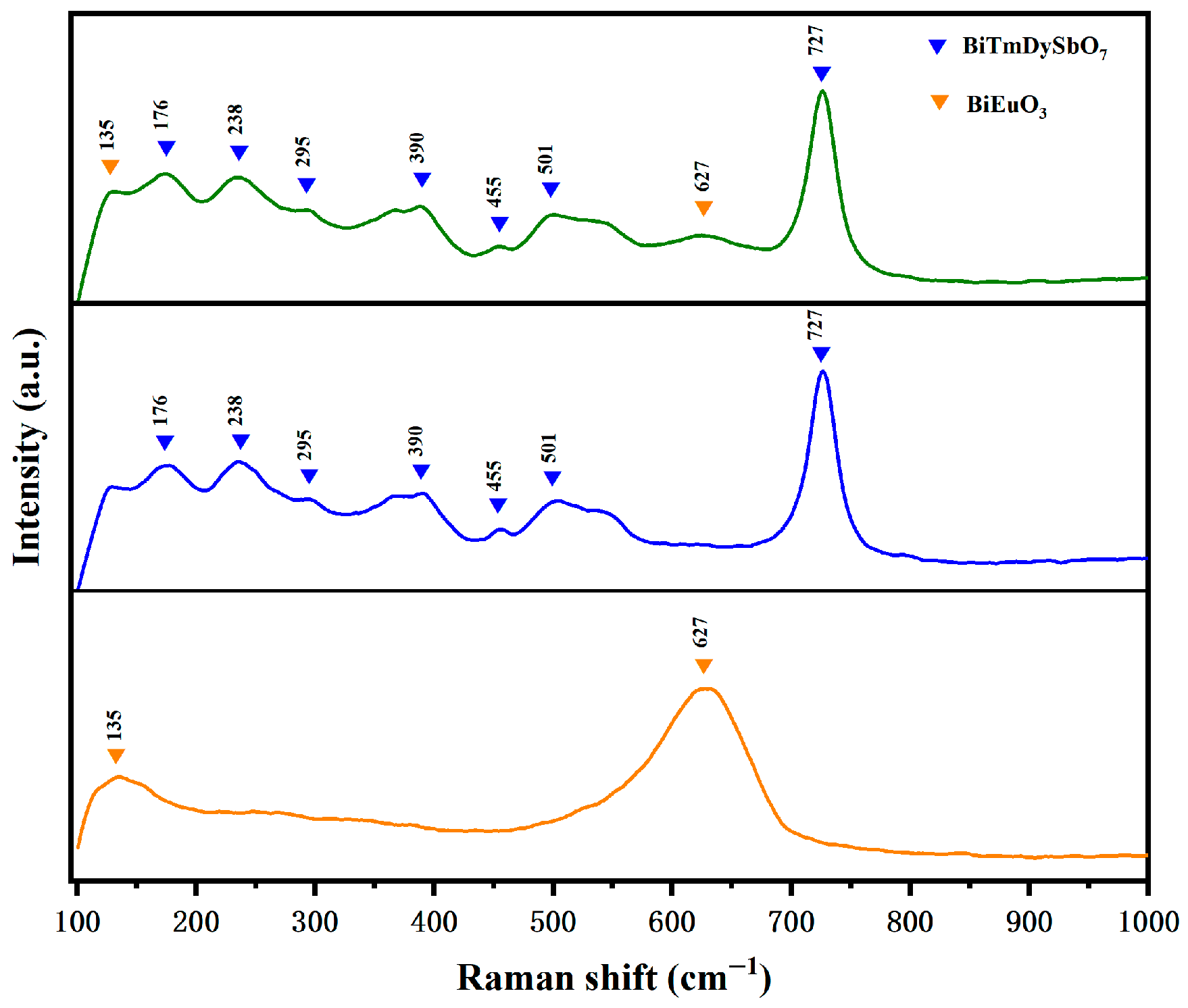
3.1.3. Raman Spectroscopy Analysis
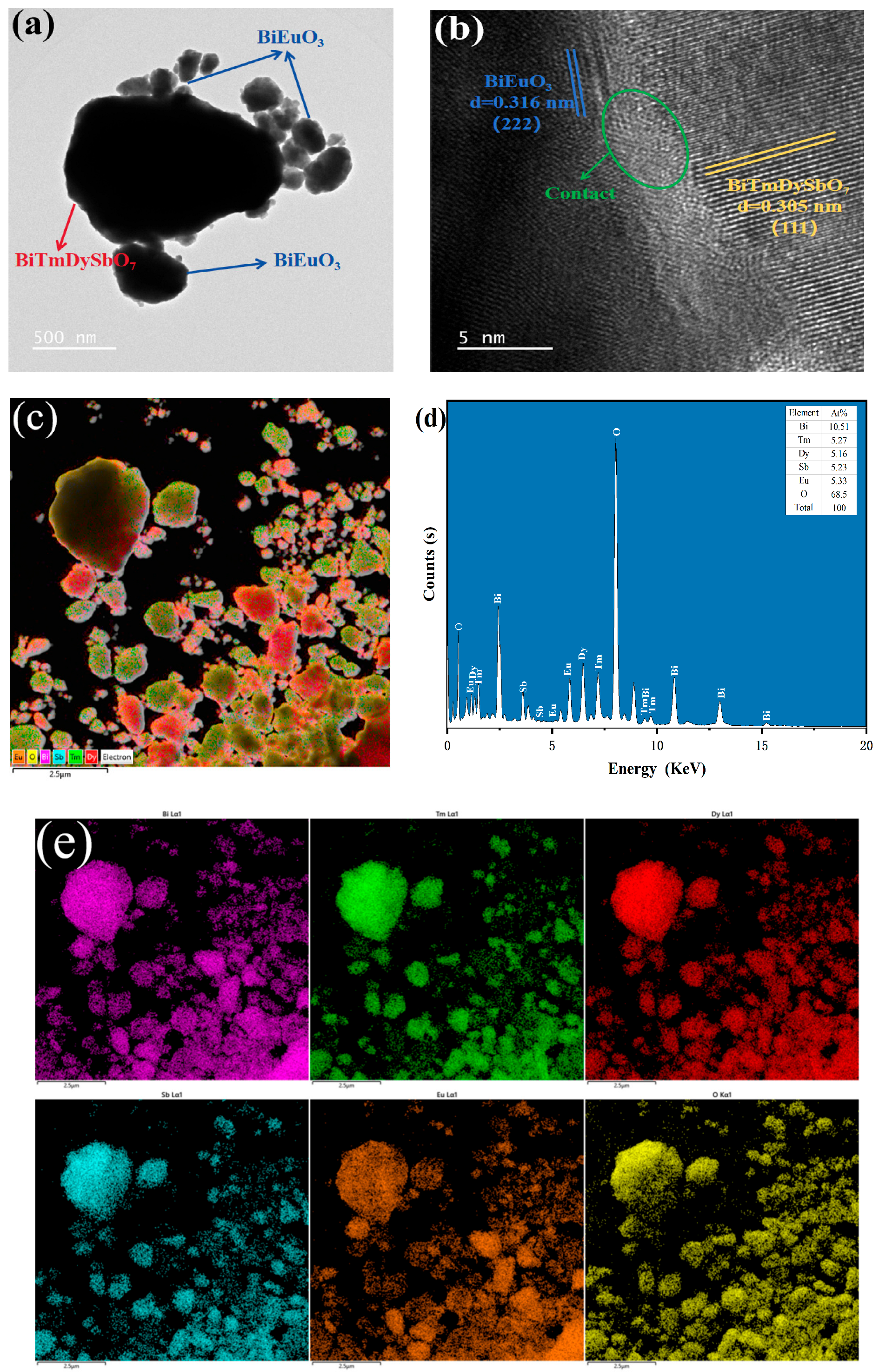
3.1.4. Morphology and Elemental Analysis
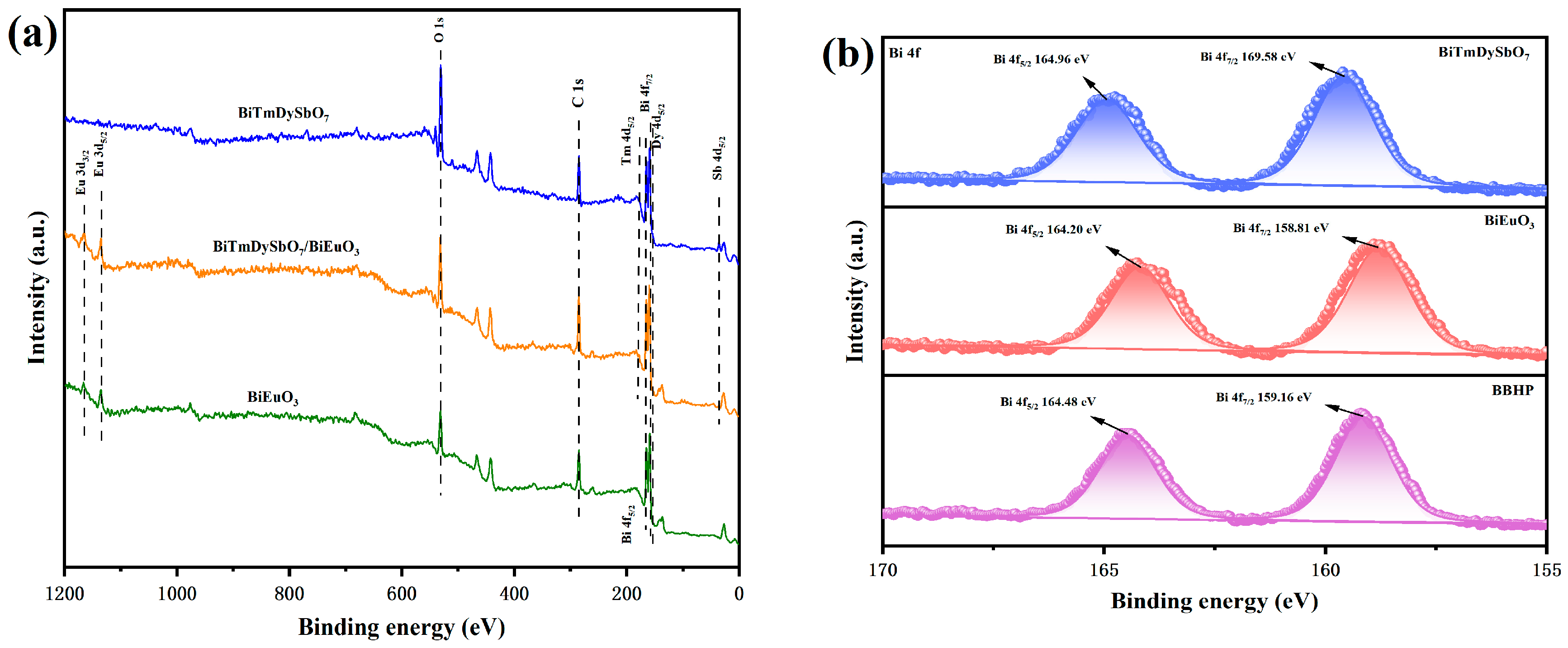
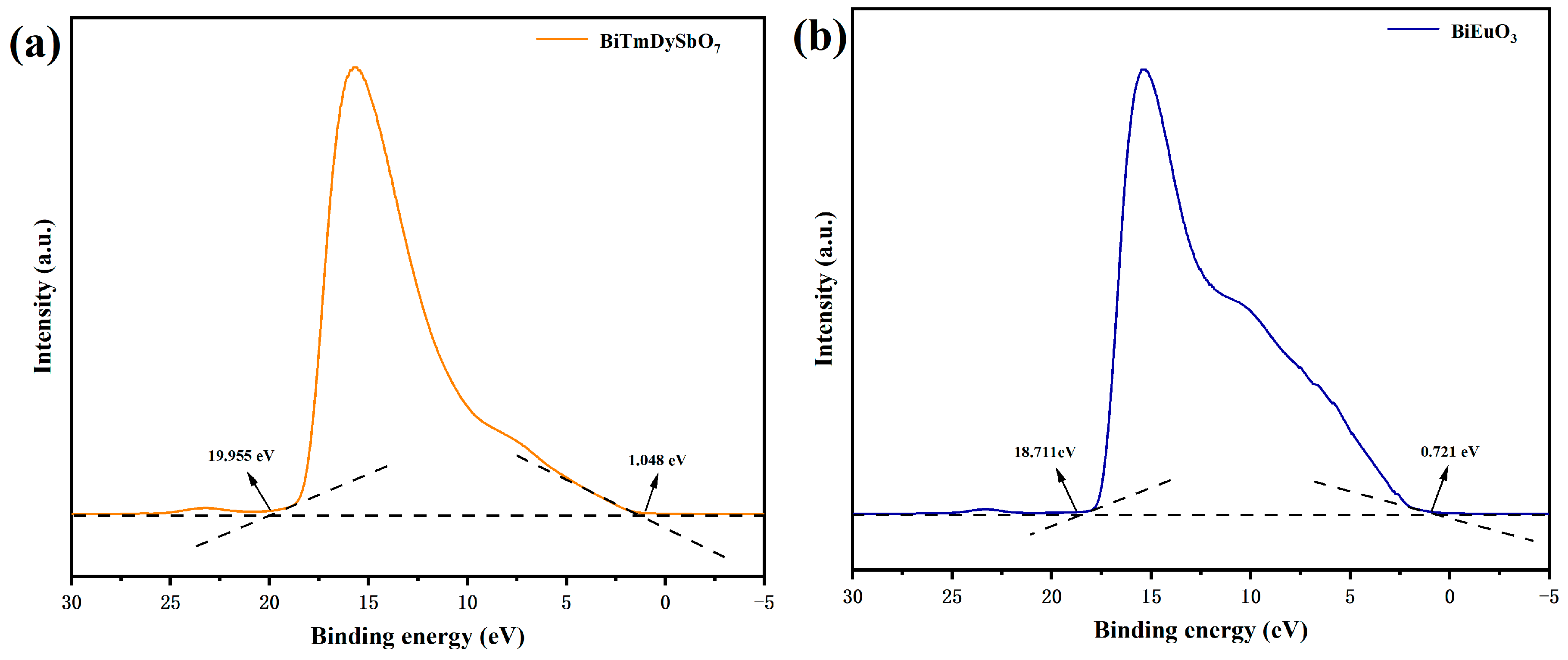
3.1.5. X-Ray Photoelectron Spectroscopy Analysis
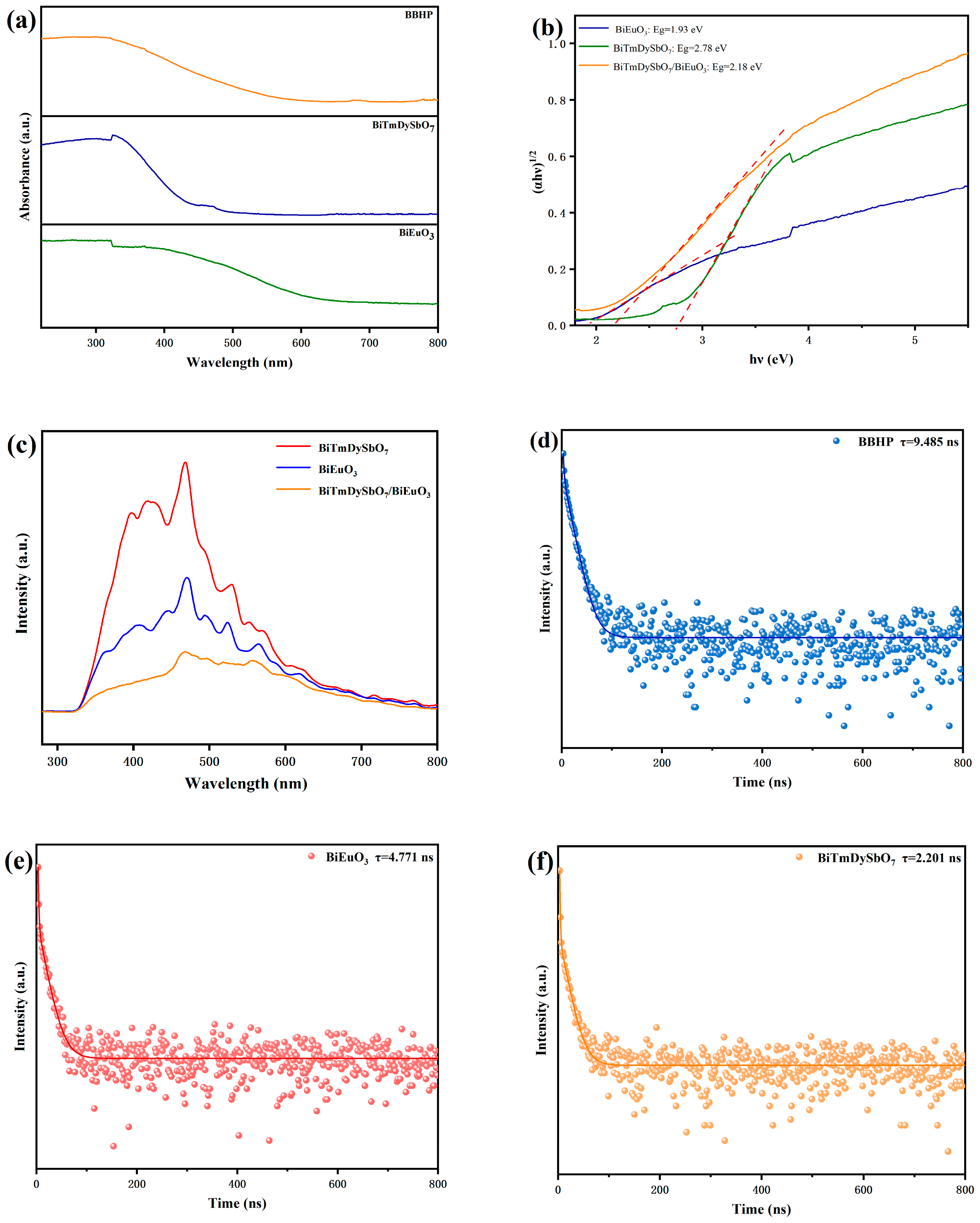
3.1.6. Optical Properties Analysis
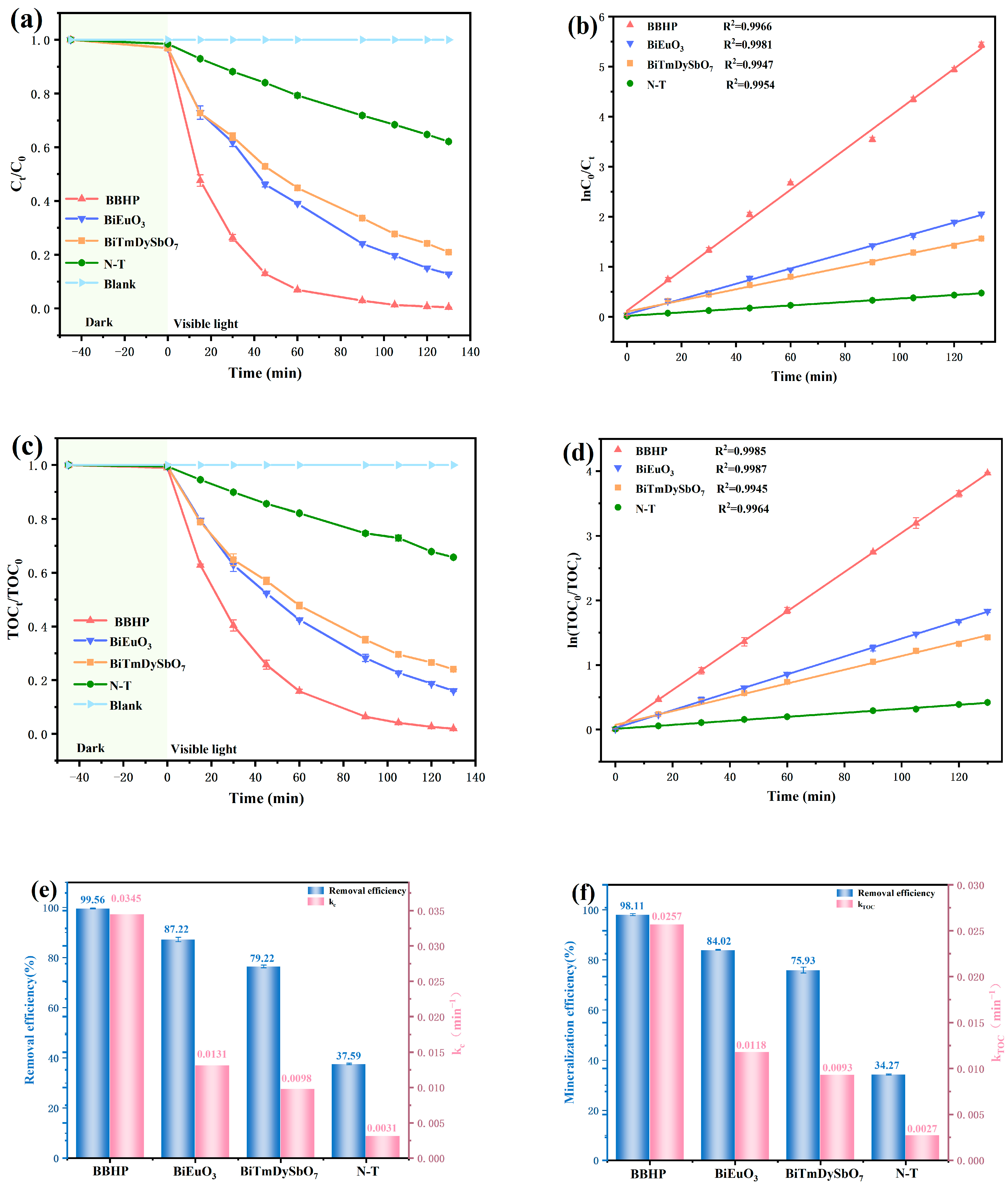
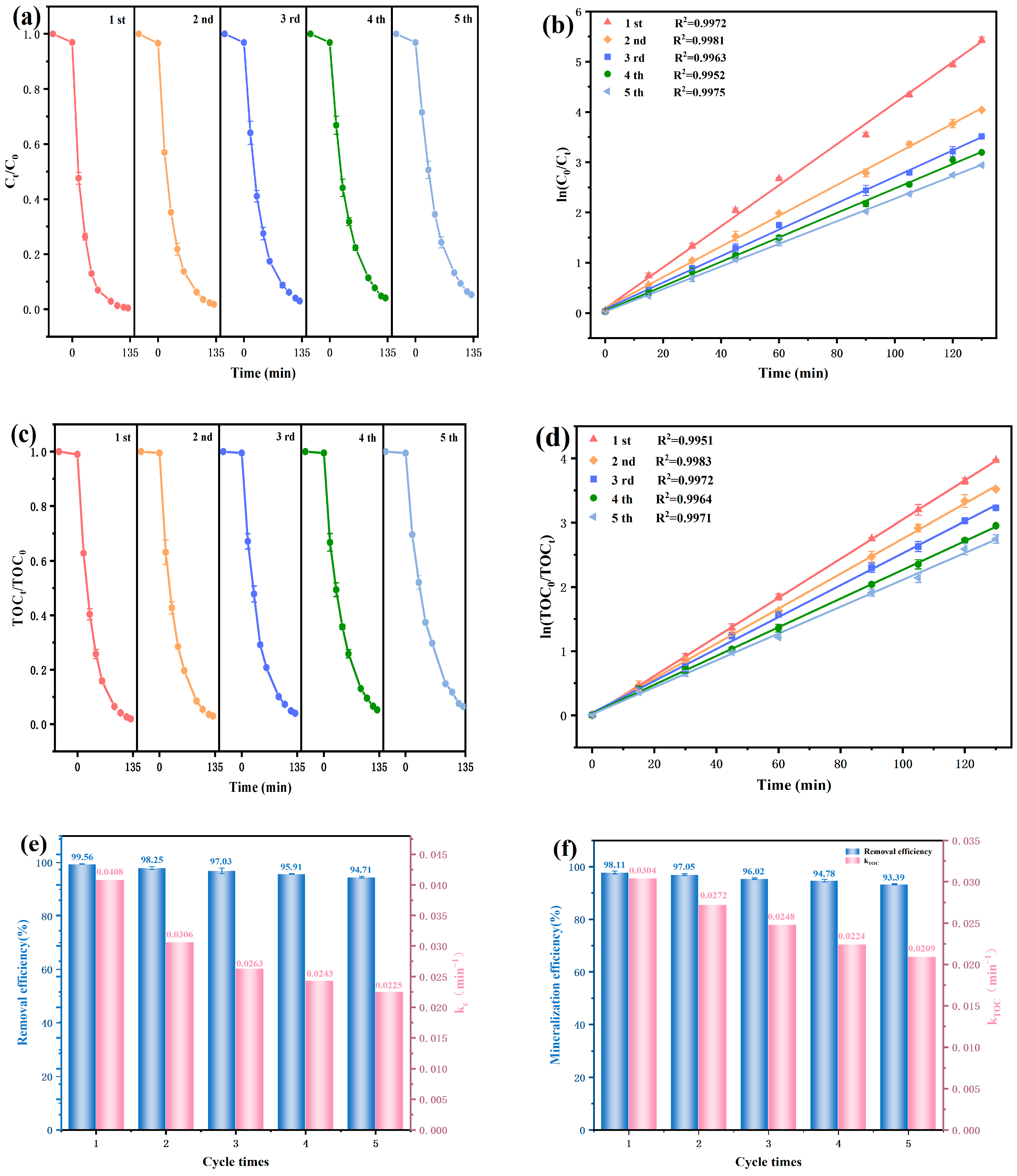
3.2. Examination of Photocatalytic Efficiency
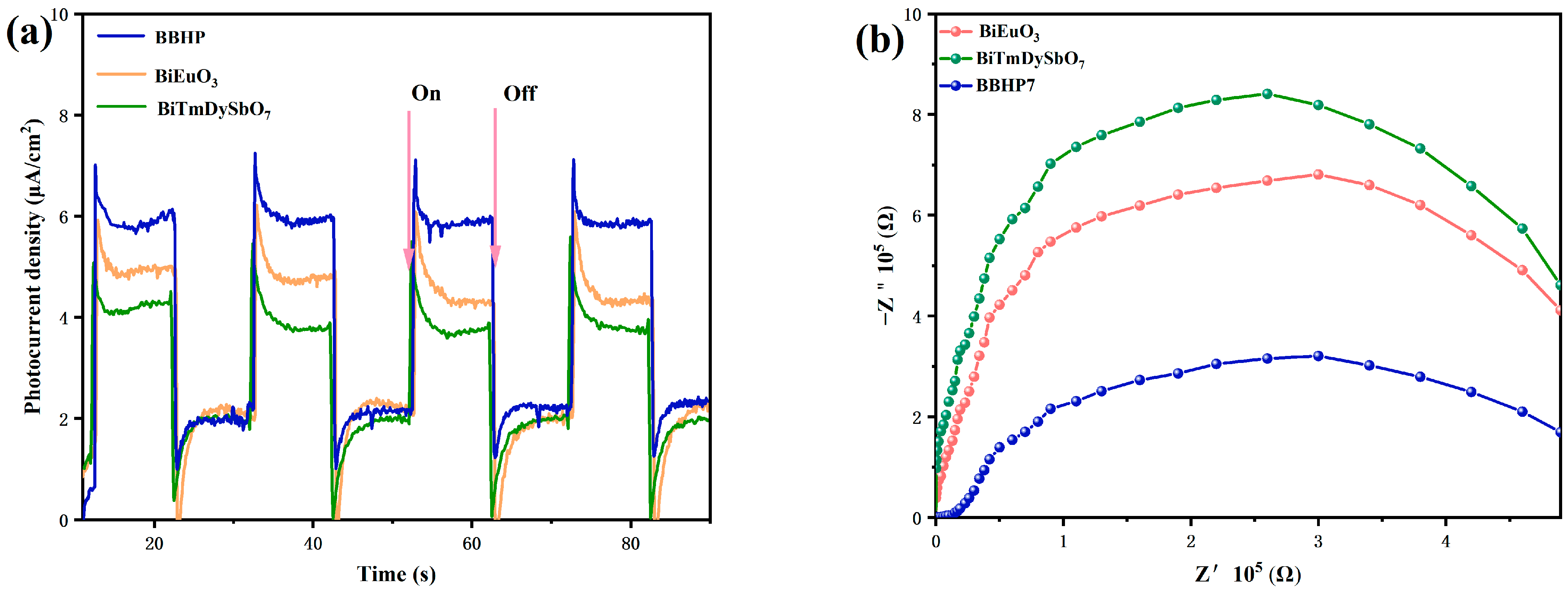
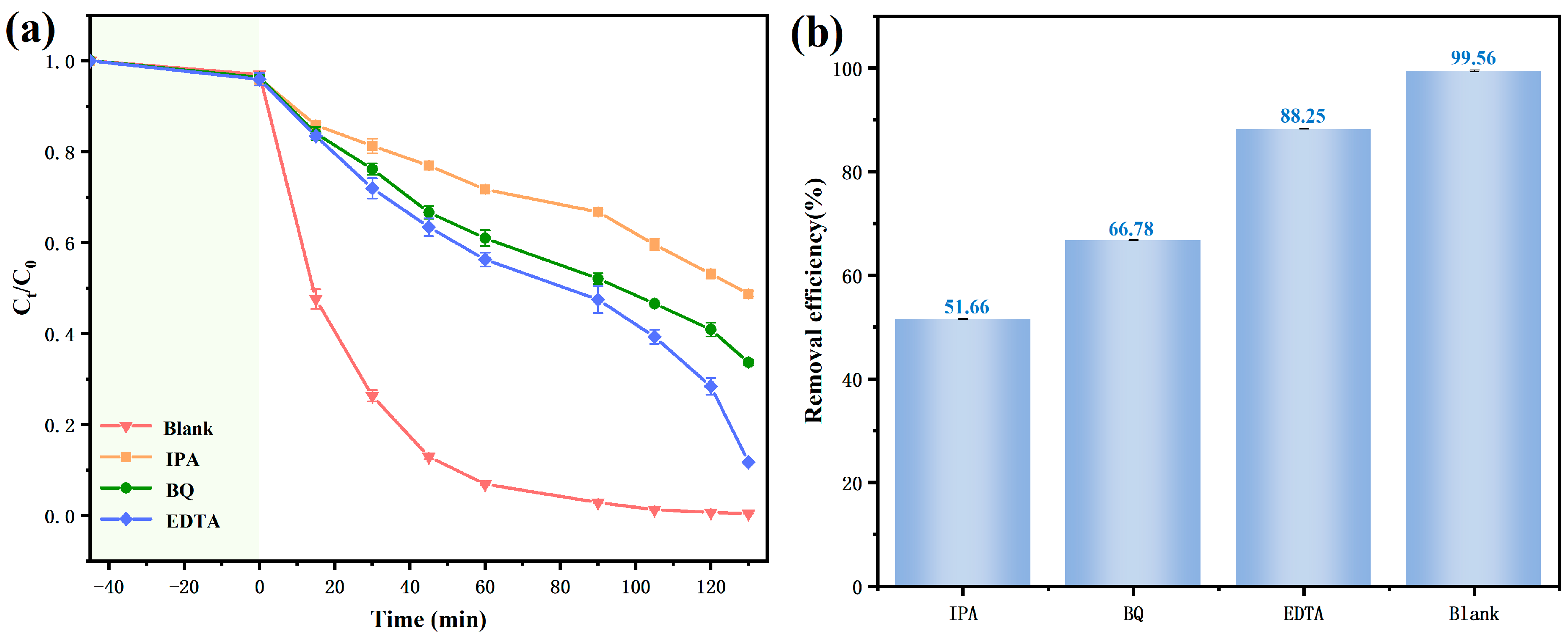
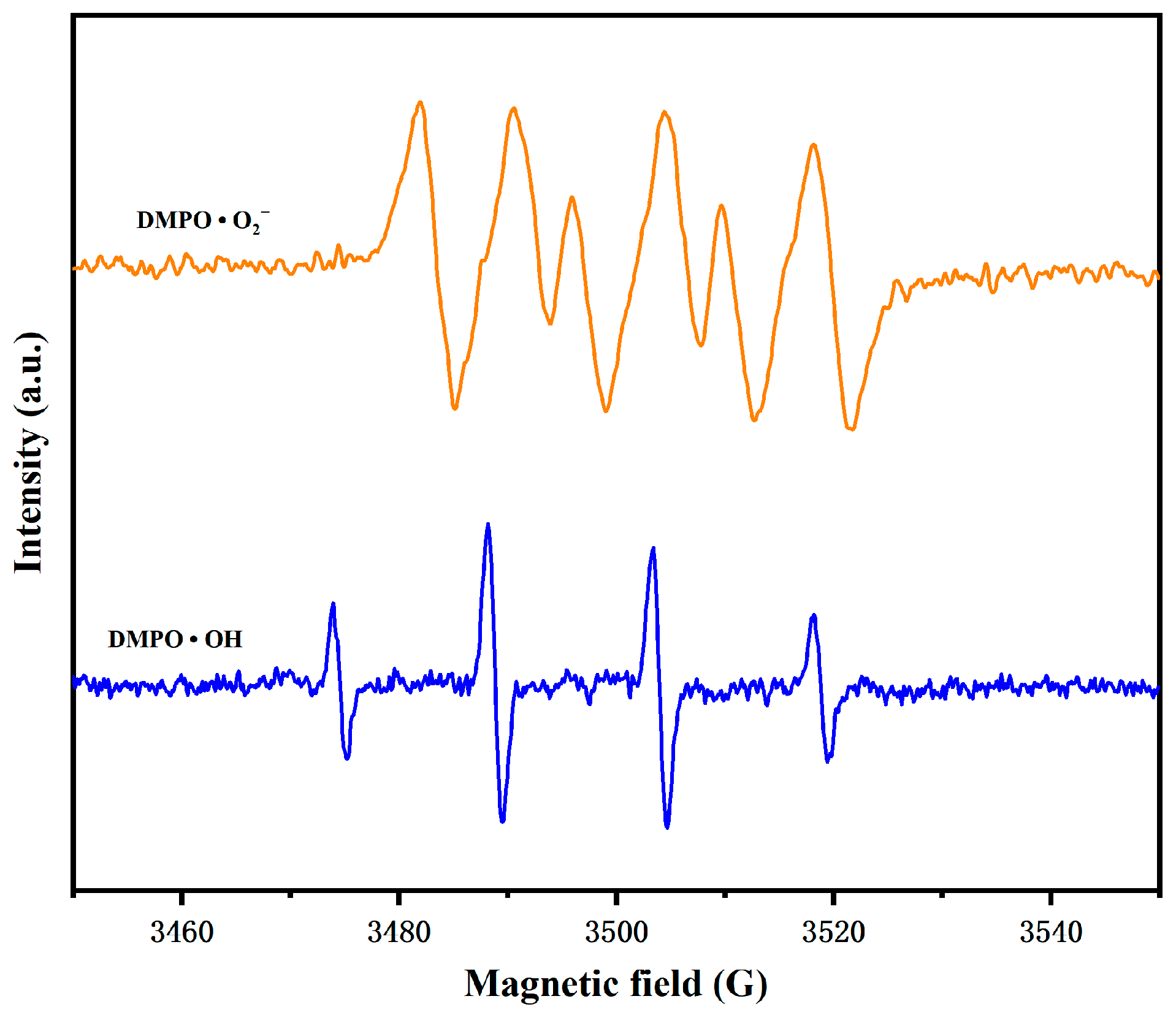
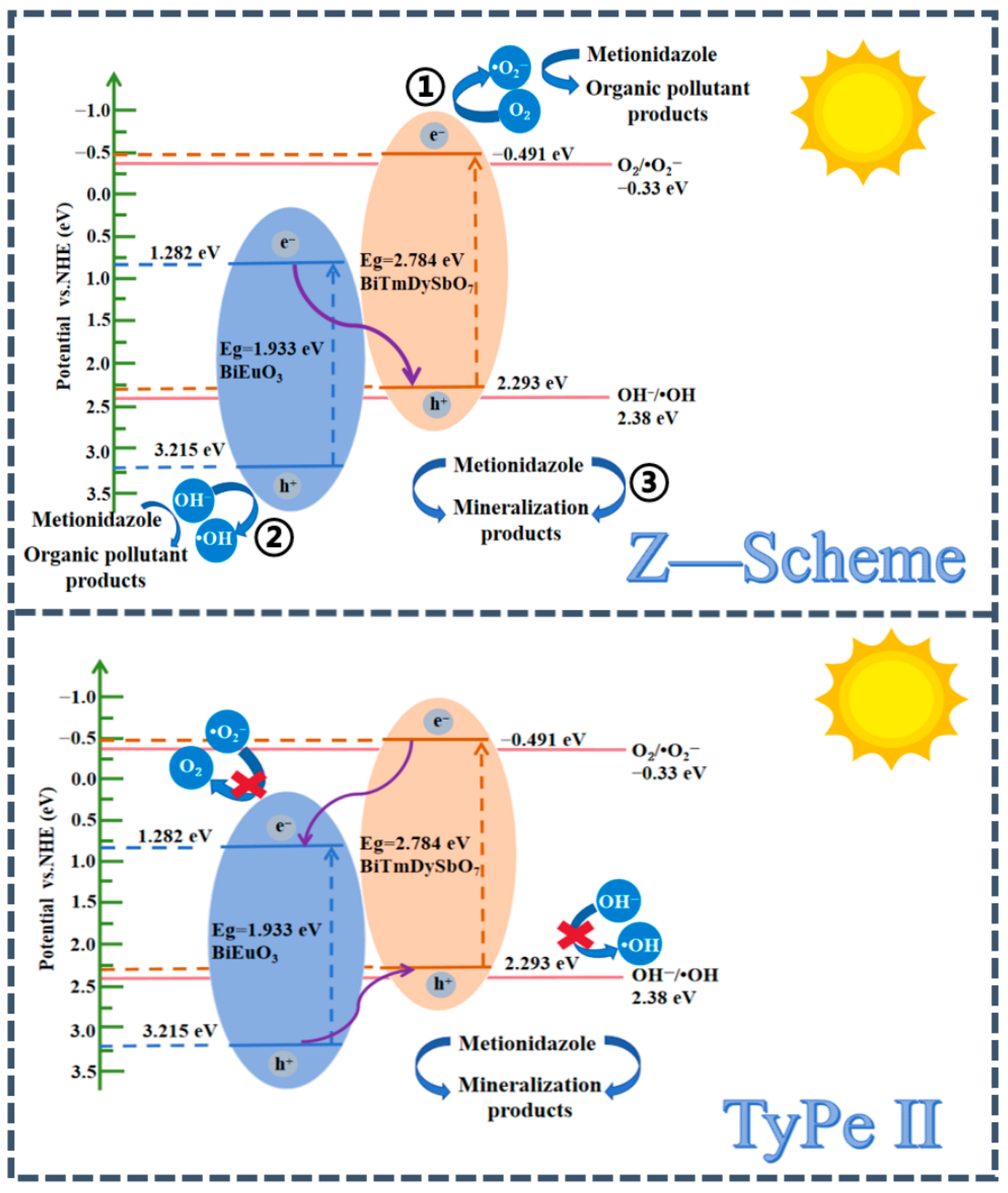
3.3. Research on the Photocatalytic Mechanism of BBHP
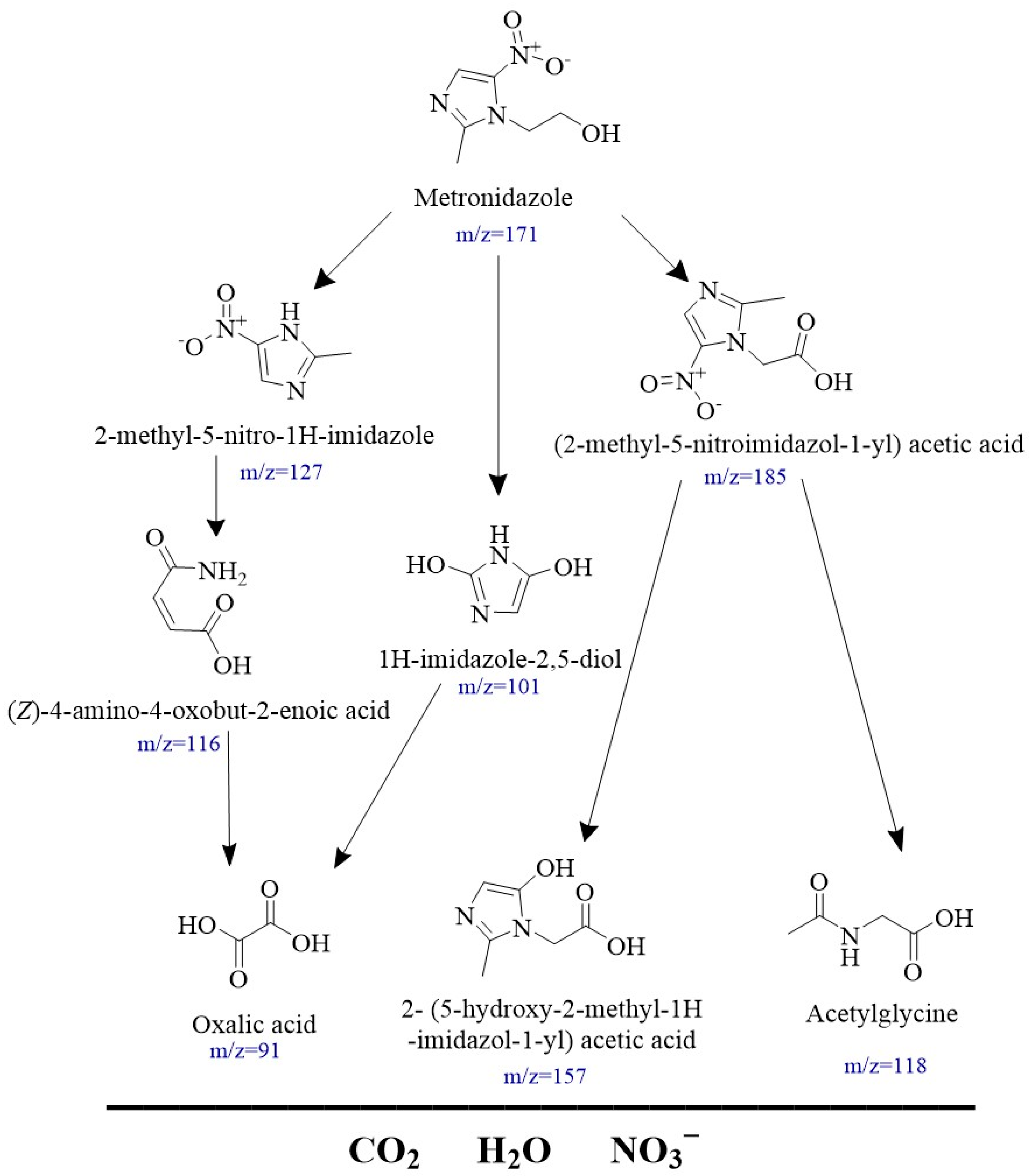
3.4. Degradation Pathway of the MNZ
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, Y.H.; Peng, B.; Liao, J.G.; Cao, W.C.; Liu, Y.J.; Nie, X.D.; Li, Z.W.; Rui, O.Y. Recent advances in the role of dissolved organic matter during antibiotics photodegradation in the aquatic environment. Sci. Total Environ. 2024, 916, 170101. [Google Scholar] [CrossRef] [PubMed]
- Chamkal, N.; Lhlou, I.; Bandadi, L.; Ounine, K. Hospital Antibiotics Usage: Environmental Hazard and Promotion of Antibiotic Resistant Bacteria. Ann. Ig. Med. Prev. Comunita 2022, 34, 266–278. [Google Scholar]
- Chen, J.; Xiang, Q.; Wu, J.Y.; Huang, X.B.; Wang, C.; Wei, D.Q.; Lv, Y. Different Effects of Antibiotics on Klebsiella pneumoniae and Escherichia coli Resistance Induced by Antibiotics: A Retrospective Study from China. Microb. Drug Resist. 2022, 28, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Le, T.H.; Truong, T.; Tran, L.-T.; Nguyen, D.-H.; Pham, T.P.T.; Ng, C. Antibiotic resistance in the aquatic environments: The need for an interdisciplinary approach. Int. J. Environ. Sci. Technol. 2023, 20, 3395–3408. [Google Scholar] [CrossRef]
- Sampaio, C.P.P.; Biondo-Simoes, M.D.P.; Trindade, C.T.; Olandowski, M.; Matias, J.E.F. Metronidazole concentration in the bloodstream following its topical application, at different concentration levels, on experimental skin wounds during healing by secondary intention. Acta Cir. Bras. 2019, 34, e20190010000004. [Google Scholar] [CrossRef]
- Acikan, I.; Sayeste, E.; Bozoglan, A.; Artas, G.; Isayev, A.; Kirtay, M.; Ozercan, I.H.; Yaman, F.; Dundar, S.; Icen, V. Evaluation of the Effects of Topical Application of Chlorhexidine, Ozone, and Metronidazole on Palatal Wound Healing: A Histopathological Study. J. Craniofac. Surg. 2022, 33, 1929–1933. [Google Scholar] [CrossRef]
- Rafiq, M.; Ullah, R.; Ahmed, A.; Usman, M.; Yu, B.; Shen, Y.Q.; Cong, H.L. Highly efficient adsorption assisted photocatalytic decontamination of metronidazole by Co3O4 doped graphitic carbon nitrides. Ferroelectrics 2024, 618, 1715–1730. [Google Scholar] [CrossRef]
- Vasseghian, Y.; Dragoi, E.N.; Almomani, F.; Le, V.T. Graphene-based materials for metronidazole degradation: A comprehensive review. Chemosphere 2022, 286, 131727. [Google Scholar] [CrossRef]
- Banu, T.; Rashid, M.H.; Tofail, S.A.M.; Ul Haq, E.; Gulshan, F. Photocatalytic degradation of metronidazole (MNZ) antibiotic by CuO nanoparticles for environmental protection from pharmaceutical pollution. Surf. Interface Anal. 2023, 55, 430–436. [Google Scholar] [CrossRef]
- Saha, M.; Sarkar, A. Review on Multiple Facets of Drug Resistance: A Rising Challenge in the 21st Century. J. Xenobiot. 2021, 11, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Thai, V.; Dang, V.D.; Thuy, N.T.; Pandit, B.; Vo, T.K.Q.; Khedulkar, A.P. Fluoroquinolones: Fate, effects on the environment and selected removal methods. J. Clean. Prod. 2023, 418, 137762. [Google Scholar] [CrossRef]
- Bueno, I.; He, H.; Kinsley, A.C.; Ziemann, S.J.; Degn, L.R.; Nault, A.J.; Beaudoin, A.L.; Singer, R.S.; Wammer, K.H.; Arnold, W.A. Biodegradation, photolysis, and sorption of antibiotics in aquatic environments: A scoping review. Sci. Total Environ. 2023, 897, 165301. [Google Scholar] [CrossRef]
- Zhu, T.T.; Su, Z.X.; Lai, W.X.; Zhang, Y.B.; Liu, Y.W. Insights into the fate and removal of antibiotics and antibiotic resistance genes using biological wastewater treatment technology. Sci. Total Environ. 2021, 776, 145906. [Google Scholar] [CrossRef]
- Jin, Q.; Liu, W.; Dong, Y.B.; Lu, Y.R.; Yang, C.; Lin, H. Single atom catalysts for degradation of antibiotics from aqueous environments by advanced oxidation processes: A review. J. Clean. Prod. 2023, 423, 138688. [Google Scholar] [CrossRef]
- Zheng, N.; Shi, J.T.; Nie, L.J.; Xue, K.K.; Gao, Y.H.; Shi, J.H. Photo-Fenton degradation of oxytetracycline by g-C3N4/CQDs/FeOCl with in-situ hydrogen peroxide production: Degradation pathway and toxicity analysis of intermediate products. J. Water Process. Eng. 2025, 69, 106615. [Google Scholar] [CrossRef]
- Nasiri, A.; Golestani, N.; Rajabi, S.; Hashemi, M. Facile and green synthesis of recyclable, environmentally friendly, chemically stable, and cost-effective magnetic nanohybrid adsorbent for tetracycline adsorption. Heliyon 2024, 10, e24179. [Google Scholar] [CrossRef]
- Li, J.H.; Zhao, W.; Guo, Y.; Wei, Z.B.; Han, M.S.; He, H.; Yang, S.G.; Sun, C. Facile synthesis and high activity of novel BiVO4/FeVO4 heteroj unction photocatalyst for degradation of metronidazole. Appl. Surf. Sci. 2015, 351, 270–279. [Google Scholar] [CrossRef]
- Derikvandi, H.; Nezamzadeh-Ejhieh, A. Designing of experiments for evaluating the interactions of influencing factors on the photocatalytic activity of NiS and SnS2: Focus on coupling, supporting and nanoparticles. J. Colloid Interface Sci. 2017, 490, 628–641. [Google Scholar] [CrossRef]
- Derikvandi, H.; Nezamzadeh-Ejhieh, A. Synergistic effect of p-n heterojunction, supporting and zeolite nanoparticles in enhanced photocatalytic activity of NiO and SnO2. J. Colloid Interface Sci. 2017, 490, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Boxi, S.S.; Paria, S. Visible light induced enhanced photocatalytic degradation of organic pollutants in aqueous media using Ag doped hollow TiO2 nanospheres. RSC Adv. 2015, 5, 37657–37668. [Google Scholar] [CrossRef]
- Hu, X.; Hu, X.J.; Peng, Q.Q.; Zhou, L.; Tan, X.F.; Jiang, L.H.; Tang, C.F.; Wang, H.; Liu, S.H.; Wang, Y.Q.; et al. Mechanisms underlying the photocatalytic degradation pathway of ciprofloxacin with heterogeneous TiO2. Chem. Eng. J. 2020, 380, 122366. [Google Scholar] [CrossRef]
- Guo, J.F.; Li, S.M.; Duan, L.; Guo, P.; Li, X.J.; Cui, Q.Q.; Wang, H.; Jiang, Q. Preparation of Si doped molecularly imprinted TiO2 photocatalyst and its degradation to antibiotic wastewater. Integr. Ferroelectr. 2016, 168, 170–182. [Google Scholar] [CrossRef]
- Zhou, Y.W.; Cai, F.; Li, W.; Qiu, J.; Xu, L.; Liu, Q.Y.; Zhang, Y.L. Photocatalytic degradation of a typical macrolide antibiotic roxithromycin using polypropylene fibre sheet supported N-TiO2/graphene oxide composite materials. Environ. Technol. 2023, 44, 3354–3366. [Google Scholar]
- Rameel, M.I.; Wali, M.; Al-Humaidi, J.Y.; Liaqat, F.; Khan, M.A. Enhanced photocatalytic degradation of levofloxacin over heterostructured C3N4/Nb2O5 system under visible light. Heliyon 2023, 9, e20479. [Google Scholar] [CrossRef]
- Wen, X.J.; Niu, C.G.; Zhang, L.; Liang, C.; Zeng, G.M. A novel Ag2O/CeO2 heterojunction photocatalysts for photocatalytic degradation of enrofloxacin: Possible degradation pathways, mineralization activity and an in depth mechanism insight. Appl. Catal. B-Environ. Energy 2018, 221, 701–714. [Google Scholar] [CrossRef]
- Ahmed, Y.; Dutta, K.R.; Akhtar, P.; Hossen, M.A.; Alam, M.J.; Alharbi, O.A.; Almohamadi, H.; Mohammad, A.W. Emerging strategies in the sustainable removal of antibiotics using semiconductor-based photocatalysts. Beilstein J. Nanotechnol. 2025, 16, 264–285. [Google Scholar] [CrossRef]
- Khan, J.A.; Ahamad, S.; Ansari, M.A.H.; Tauqeer, M.; Park, C.H.; Park, J.P.; Choi, C.H.; Mohammad, A. State-of-the-art in ZnS-based nanoarchitects for visible-light photocatalytic degradation of antibiotics and organic dyes. J. Water Process. Eng. 2024, 67, 106151. [Google Scholar] [CrossRef]
- Gong, Y.Y.; Wu, Y.J.; Xu, Y.; Li, L.; Li, C.; Liu, X.J.; Niu, L.Y. All-solid-state Z-scheme CdTe/TiO2 heterostructure photocatalysts with enhanced visible-light photocatalytic degradation of antibiotic waste water. Chem. Eng. J. 2018, 350, 257–267. [Google Scholar] [CrossRef]
- Fan, G.D.; Zhou, J.F.; Ruan, F.Y.; Li, Y.; Tian, H.Y.; Fan, D.; Chen, Q.Q.; Li, N. The Z-scheme photocatalyst S-BiOBr/Bi2Sn2O7 with 3D/0D interfacial structure for the efficient degradation of organic pollutants. Sep. Purif. Technol. 2023, 309, 123099. [Google Scholar] [CrossRef]
- Lv, D.D.; Zhang, D.F.; Sun, Q.Z.; Wu, J.D.; Zhang, L.; Pu, X.P.; Ma, H.Y.; Dou, J.M. A novel method for the synthesis of BiOCl/Bi2Sn2O7 heterojunction photocatalysts with enhanced visible light photocatalytic properties. Nanotechnology 2016, 27, 385602. [Google Scholar] [CrossRef] [PubMed]
- Khodabakhsh, M.; Yilmaz, B.; Firoozi, S.; Haghshenas, D.F.; Unal, U. Enhanced Photocatalytic Properties of Restacked Unilamellar [SrTa2O7]2− Nanosheets of Aurivillius Phase Layered Perovskites. ACS Omega 2023, 8, 10607–10617. [Google Scholar] [CrossRef]
- Zhang, W.J.; Ma, Z.; Du, L.; Li, H. Role of PEG4000 in sol-gel synthesis of Sm2Ti2O7 photocatalyst for enhanced activity. J. Alloys Compd. 2017, 704, 26–31. [Google Scholar] [CrossRef]
- Naceur, B.; Abdelkader, E.; Nadjia, L.; Sellami, M.; Noureddine, B. Synthesis and characterization of Bi1.56Sb1.48Co0.96O7 pyrochlore sun-light-responsive photocatalyst. Mater. Res. Bull. 2016, 74, 491–501. [Google Scholar] [CrossRef]
- Murcia-López, S.; Hidalgo, M.C.; Navío, J.A. Synthesis characterization and photocatalytic activity of Bi-doped TiO2 photocatalysts under simulated solar irradiation. Appl. Catal. A-Gen. 2011, 404, 59–67. [Google Scholar] [CrossRef]
- Mezyen, M.; El Fidha, G.; Bitri, N.; Harrathi, F.; Ly, I.; Llobet, E. Visible light activated SnO2: Dy thin films for the photocatalytic degradation of methylene blue. RSC Adv. 2023, 13, 31151–31166. [Google Scholar] [CrossRef] [PubMed]
- Rao, Z.P.; Shi, G.S.; Wang, Z.; Mahmood, A.; Xie, X.F.; Sun, J. Photocatalytic degradation of gaseous VOCs over Tm3+-TiO2: Revealing the activity enhancement mechanism and different reaction paths. Chem. Eng. J. 2020, 395, 125078. [Google Scholar] [CrossRef]
- Gandelman, H.; da Silva, A.L.; Ramos, B.; Gouvêa, D. Interface excess on Sb-doped TiO2 photocatalysts and its influence on photocatalytic activity. Ceram. Int. 2021, 47, 619–625. [Google Scholar] [CrossRef]
- Ran, Y.; Zhong, J.; Li, J.; Li, M.; Tian, C. Substantially boosted photocatalytic detoxification activity of TiO2 benefited from Eu doping. Environ. Technol. 2023, 44, 1313–1321. [Google Scholar] [CrossRef]
- Alzard, R.H.; Siddig, L.A.; Abdelhamid, A.S.; Ramachandran, T.; Alzamly, A. Structural analysis and photocatalytic activities of bismuth-lanthanide oxide perovskites. J. Solid State Chem. 2024, 329, 124359. [Google Scholar] [CrossRef]
- Zhi, G.W.; Hao, L.Y.; Chen, W.J.; Sheng, Q.Y.; Liu, L.; Wang, W.; Yao, H.Y. Z-scheme CuSbS2/ZnO Heterojunction for Enhanced Photocatalytic Degradation of RhB. Chem. Sel. 2022, 7, e202202457. [Google Scholar] [CrossRef]
- Tang, Z.L.; Deng, X.; Chen, X.T.; Jiang, C.M.; Cai, L.; Guo, T.L. Ag2S/TiO2 Z-scheme heterojunction under magnetic field: Enhanced photocatalytic degradation of tetracycline. J. Alloys Compd. 2025, 1010, 177752. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, Y.K.; Zhao, G.Q.; Li, C.F.; Liu, L.K.; Jiao, F.P. Enhanced visible light photocatalytic degradation of rhodamine B by Z-scheme CuWO4/g-C3N4 heterojunction. J. Mater. Sci.-Mater. Electron. 2021, 32, 2731–2743. [Google Scholar] [CrossRef]
- Liu, J.H.; Zhang, B.B.; Huang, Z.Y.; Wang, W.Y.; Xi, X.G.; Dong, P.Y. MOF-Derived In2O3 Microrod-Decorated MgIn2S4 Nanosheets: Z-Scheme Heterojunction for Efficient Photocatalytic Degradation of Tetracycline. Langmuir 2023, 39, 17458–17470. [Google Scholar] [CrossRef]
- Pan, B.; Chen, W.; Zhou, L.X.; Lai, X.J.; Qin, J.N.; Wang, C.Y. CoFe2O4/carbon nitride Z-scheme heterojunction photocatalytic PMS activation for efficient tetracycline degradation: Accelerated electron transfer. Process Saf. Environ. Protect. 2024, 191, 2522–2532. [Google Scholar] [CrossRef]
- Wang, C.; Liu, H.Y.; Wang, G.F.; Huang, W.Y.; Wei, Z.W.; Fang, H.Y.; Shen, F. Visible light driven S-scheme heterojunction Zn3In2S6/Bi2MoO6 for efficient degradation of metronidazole. J. Alloys Compd. 2022, 917, 165507. [Google Scholar] [CrossRef]
- Wang, X.Y.; Zhang, R.Y.; Luo, J.; Zhang, H.F.; Yan, Q.Z.; Zhao, J.W.; Liang, Z.; Shi, W.L.; Lu, C.Y. Constructing an S-scheme ZnSnO3-CuBi2O4 heterojunction modulation to boost visible-light-induced photothermal-photocatalytic degradation of tetracycline. J. Environ. Chem. Eng. 2025, 13, 115612. [Google Scholar] [CrossRef]
- Saha, S.; Chanda, S.; Dutta, A.; Kumar, U.; Ranjan, R.; Sinha, T.P. Dielectric relaxation and anti-ferromagnetic coupling of BiEu03 and BiGdO3. J. Magn. Magn. Mater. 2014, 360, 80–86. [Google Scholar] [CrossRef]
- Li, S.G.; Chen, F.; An, Q.; Tang, R.F.; Huang, H.W. Triggering Overall Crystal Polarization by Octahedron Elongation Distortion for Highly Boosted and Selective Aerobic Photo-Oxidation. Adv. Funct. Mater. 2024, 34, 2409035. [Google Scholar] [CrossRef]
- Peng, Z.Y.; Jiang, Y.H.; Wang, X.Q.; Zhang, R.X.; Xu, H.Q.; Xiao, Y.; Jing, X.; Zhang, J.M.; Liu, Y.; Ni, L. Novel CdIn2S4 nano-octahedra/TiO2 hollow hybrid heterostructure: In-situ synthesis, synergistic effect and enhanced dual-functional photocatalytic activities. Ceram. Int. 2019, 45, 15942–15953. [Google Scholar] [CrossRef]
- Guo, B.; Zhao, C.C.; Liu, X.X.; Yu, Z.; Zhou, L.J.; Yuan, H.M.; Zhao, Z. Effects of Surface Hydrothermal Carbon Layer on the Photocatalytic Activity of Magnetic NiFe2O4 Octahedron. Chem. J. Chin. Univ.-Chin. 2022, 43, 20220472. [Google Scholar]
- Nguyen, V.Q.; Mady, A.H.; Mahadadalkar, M.A.; Baynosa, M.L.; Kumar, D.R.; Rabie, A.M.; Lee, J.; Kim, W.K.; Shim, J.J. Highly active Z-scheme heterojunction photocatalyst of anatase TiO2 octahedra covered with C-MoS2 nanosheets for efficient degradation of organic pollutants under solar light. J. Colloid Interface Sci. 2022, 606, 337–352. [Google Scholar] [CrossRef]
- Ma, D.C.; Tang, J.W.; He, G.W.; Xue, Y.; Pan, S.; Liu, F.J.; Zhao, J.Z. Enhancing photocatalytic degradation of rhodamine B with visible-light-driven HCl-assisted Bi2O3 photocatalysts: Activity, mechanism, and pathways. Mater. Sci. Semicond. Process 2024, 181, 108672. [Google Scholar] [CrossRef]
- Bouziani, A.; Yahya, M.; Bianchi, C.L.; Falletta, E.; Celik, G. Ternary Polyaniline@Bi2O3-BiOCl Nanocomposites as Innovative Highly Active Photocatalysts for the Removal of the Dye under Solar Light Irradiation. Nanomaterials 2023, 13, 713. [Google Scholar] [CrossRef] [PubMed]
- Punitha, T.; Balamurugan, P.; Kumar, G.S.; Sasikruba, S. Ferromagnetic nanostructures of chromium-doped dysprosium iron oxide (DyCrxFe1−xO3) with visible light photocatalysis capabilities. Results Surf. Interfaces 2025, 18, 100452. [Google Scholar] [CrossRef]
- Zargar, R.A.; Imran, M.; Arora, M.; Nagal, V.; Mearaj, T.; Manthrammel, M.A.; Shkir, M.; Hafiz, A.K. Low-cost synthesis of lanthanides (Eu3+ and Sm3+)-intercalated TiO2 nanostructures: A detailed study on structural, optical and photocatalytic applications. J. Mater. Sci.-Mater. Electron. 2022, 33, 26931–26942. [Google Scholar] [CrossRef]
- Karnchana, N.; Phuruangrat, A.; Thongtem, S.; Thongtem, T. Combustion synthesis and characterization of visible-light-driven Tm-doped ZnO nanoparticles used for photodegradation of methylene blue. Dig. J. Nanomater. Biostruct. 2021, 16, 1243–1252. [Google Scholar] [CrossRef]
- Makhloufi, R.; Hachani, S.E.; Fettah, A.; Messai, B. Wet Chemical Synthesis of Sb4O5Cl2 Used as an Effective Photocatalyst for Methylene Blue and Crystal Violet Degradation under Visible Light Irradiation. Ann. Chim.-Sci. Mater. 2022, 46, 69–74. [Google Scholar] [CrossRef]
- Thirumal, V.; Yuvakkumar, R.; Kumar, P.S.; Keerthana, S.P.; Ravi, G.; Thambidurai, M.; Dang, C.; Velauthapillai, D. Facile hydrothermal synthesis of MXene@antimony nanoneedle composites for toxic pollutants removal. Environ. Res. 2022, 210, 112904. [Google Scholar] [CrossRef]
- Rauf, N.; Ilyas, S.; Heryanto, H.; Rahmat, R.; Fahri, A.N.; Rahmi, M.H.; Tahir, D. The Correlation between Structural and Optical Properties of Zinc Hydroxide Nanoparticle in Supports Photocatalytic Performance. Opt. Mater. 2021, 112, 110780. [Google Scholar] [CrossRef]
- Jalali, S.; Ardjmand, M.; Ramavandi, B.; Nosratinia, F. Elimination of amoxicillin using zeolite Y-sea salt as a good catalyst for activation of hydrogen peroxide: Investigating degradation pathway and the effect of wastewater chemistry. J. Environ. Manag. 2022, 302, 114045. [Google Scholar] [CrossRef]
- Asaithambi, S.; Murugan, R.; Sakthivel, P.; Karuppaiah, M.; Rajendran, S.; Ravi, G. Influence of Ni Doping in SnO2 Nanoparticles with Enhanced Visible Light Photocatalytic Activity for Degradation of Methylene Blue Dye. J. Nanosci. Nanotechnol. 2019, 19, 4438–4446. [Google Scholar] [CrossRef]
- Cadis, A.I.; Rus, F.S.; Goncalves, J.N.; Ivanovici, M. Preparing a Ca-Bi-O System by the Precipitation Method and Studying Its Intermediate Structural Properties for Applications in Water Treatment. Inorganics 2023, 11, 79. [Google Scholar] [CrossRef]
- Regmi, C.; Kshetri, Y.K.; Ray, S.K.; Pandey, R.P.; Lee, S.W. Utilization of visible to NIR light energy by Yb+3, Er+3 and Tm+3 doped BiVO4 for the photocatalytic degradation of methylene blue. Appl. Surf. Sci. 2017, 392, 61–70. [Google Scholar] [CrossRef]
- Slimani, Y.; Khan, A.; Nawaz, M.; Hossain, M.K.; Thakur, A. Efficient rhodamine B dye photocatalytic degradation in aqueous media using novel ZnO nanomaterials co-doped with Ce and Dy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 321, 124725. [Google Scholar] [CrossRef] [PubMed]
- Nozari, M.; Dehaghi, S.M. Structural properties of TiO2/Sb nanoparticles with different molar ratios and investigation the photocatalytic degradation of AZO dyes under UV irradiation in different condition. Fresenius Environ. Bull. 2016, 25, 3962–3970. [Google Scholar]
- Singh, A.; Dutta, D.P.; Roy, M.; Tyagi, A.K.; Fulekar, M.H. Sonochemical synthesis, characterization, and photocatalytic properties of Bi2−xSbxWO6 nanorods. J. Mater. Sci. 2014, 49, 2085–2097. [Google Scholar] [CrossRef]
- Patil, M.S.; Kitchamsetti, N.; Mulani, S.R.; Rondiya, S.R.; Deshpande, N.G.; Patil, R.A.; Cross, R.W.; Dzade, N.Y.; Sharma, K.K.; Patil, P.S.; et al. Photocatalytic behavior of Ba(Sb/Ta)2O6 perovskite for reduction of organic pollutants: Experimental and DFT correlation. J. Taiwan Inst. Chem. Eng. 2021, 122, 201–209. [Google Scholar] [CrossRef]
- Shandilya, P.; Mittal, D.; Soni, M.; Raizada, P.; Lim, J.H.; Jeong, D.Y.; Dewedi, R.P.; Saini, A.K.; Singh, P. Islanding of EuVO4 on high-dispersed fluorine doped few layered graphene sheets for efficient photocatalytic mineralization of phenolic compounds and bacterial disinfection. J. Taiwan Inst. Chem. Eng. 2018, 93, 528–542. [Google Scholar] [CrossRef]
- Cao, S.B.; Wu, L.; Li, J.; Li, Y.; Da, K.; Chen, W.T.; Xue, R.T.; Yang, J.; Fan, X.M. Unraveling the generation mechanism of singlet oxygen in Bi2WxMo1−xO6 solid solution and roles in photocatalytic degradation of gaseous toluene under visible-light irradiation. J. Environ. Chem. Eng. 2024, 12, 112321. [Google Scholar] [CrossRef]
- Liang, M.J.; Deng, N.; Xiang, X.Y.; Mei, Y.; Yang, Z.Y.; Yang, Y.; Yang, S.J. Bi/BiVO4&Bi4V2O11 Composite Catalysts: Preparation and Photocatalytic Performance. Chin. J. Inorg. Chem. 2019, 35, 263–270. [Google Scholar]
- Wang, Q.; Wu, H.; Gao, Q.Y.; Lin, D.G.; Fan, Y.J.; Duan, R.; Cong, Y.Q.; Zhang, Y. Fabrication of visible-light-active Bi/BiOI-Bi2O3 composite with enhanced photocatalytic activity. J. Colloid Interface Sci. 2019, 548, 255–264. [Google Scholar] [CrossRef]
- Heryanto, H.; Mutmainna, I.; Rahmi, M.H.; Ola, A.T.T.; Tang, N.F.R.; Mohamed, M.A.; Tahir, D. Favourable peak diffraction shift moments as a function of Mg doping on ZnO matrix as a promising catalyst for methylene blue waste. Mater. Chem. Phys. 2024, 313, 128772. [Google Scholar] [CrossRef]
- Wang, C.; Liu, H.Y.; Wang, G.F.; Ye, F.; Tao, S.K.; Li, X.; Qiu, P.; Huang, Z.H. Ni (II) doping induced lattice distortion in Zn3In2S6/BiOBr-OVs for boosting photocatalytic removal of antibiotics and Cr (VI) performance. Sep. Purif. Technol. 2023, 324, 124457. [Google Scholar] [CrossRef]
- Li, X.Y.; Li, W.J.; Gu, S.N.; Liu, X.T.; Li, H.D.; Ren, C.J.; Ma, X.H.; Zhou, H.L. Efficient ytterbium-doped Bi2WO6 photocatalysts: Synthesis, the formation of oxygen vacancies and boosted superoxide yield for enhanced visible-light photocatalytic activity. J. Alloys Compd. 2021, 851, 156935. [Google Scholar] [CrossRef]
- Makula, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.G.; Ye, J.H.; Arakawa, H. Substitution effects of In3+ by Fe3+ on photocatalytic and structural properties of Bi2InNbO7 photocatalysts. J. Mol. Catal. A-Chem. 2001, 168, 289–297. [Google Scholar] [CrossRef]
- Wu, S.Q.; Hu, H.Y.; Lin, Y.; Zhang, J.L.; Hu, Y.H. Visible light photocatalytic degradation of tetracycline over TiO2. Chem. Eng. J. 2020, 382, 122842. [Google Scholar] [CrossRef]
- Nain, P.; Pawar, M.; Rani, S.; Kumar, S.; Khan, M.A.M. (Ho, Yb) co-doping effects on structural, optical, photocatalytic and thermal properties of TiO2 NPs prepared by hydrothermal approach. Phys. B 2025, 706, 417132. [Google Scholar] [CrossRef]
- Sukhadeve, G.K.; Janbandhu, S.Y.; Kumar, R.; Lataye, D.H.; Ramteke, D.D.; Gedam, R.S. Visible light assisted photocatalytic degradation of Indigo Carmine dye and NO2 removal by Fe doped TiO2 nanoparticles. Ceram. Int. 2022, 48, 29121–29135. [Google Scholar] [CrossRef]
- Bansal, N.; Kumar, A. Photocatalytic degradation of organophosphorus pesticide Chlorpyrifos using CdS/NiS nanocomposite. Ionics 2024, 30, 4901–4916. [Google Scholar] [CrossRef]
- Geldasa, F.T.; Kebede, M.A.; Shura, M.W.; Andoshe, D.M.; Tegegne, N.A.; Hone, F.G. Facile synthesis of different metals doped α-PbO nanoparticles for photocatalytic degradation of Methylene Blue dye. Phys. Scr. 2023, 98, 065701. [Google Scholar] [CrossRef]
- Beura, R.; Pachaiappan, R.; Thangadurai, P. A detailed study on Sn4+ doped ZnO for enhanced photocatalytic degradation. Appl. Surf. Sci. 2018, 433, 887–898. [Google Scholar] [CrossRef]
- Agulo, I.J.; Rosario, P.; Yague, K.; Balod, M.J.; Empizo, M.J.F.; Agulto, V.C.; Shimizu, T.; Madarang, M.A.; Ngaloy, R.; Sarukura, N. Photoluminescence and enhanced photocatalytic activity of mechanically activated graphite-zinc oxide composites. Mater. Res. Express 2023, 10, 065601. [Google Scholar] [CrossRef]
- Dang, H.F.; Li, B.Q.; Li, C.P.; Zang, Y.H.; Xu, P.R.; Zhao, X.C.; Fan, H.B.; Qiu, Y.F. One-dimensional Au/SiC heterojunction nanocomposites with enhanced photocatalytic and photoelectrochemical performances: Kinetics and mechanism insights. Electrochim. Acta 2018, 267, 24–33. [Google Scholar] [CrossRef]
- Zhu, X.D.; Song, H.J.; Feng, W.; Wen, G.L.; Li, H.Y.; Zhou, J. Photocurrent response and photocatalytic activity of Nd-doped TiO2 thin films prepared by sol-gel method. J. Adv. Oxid. Technol. 2017, 20, 20160190. [Google Scholar] [CrossRef]
- Al Bacha, S.; Saitzek, S.; Kabbour, H.; McCabe, E.E. Iron Oxychalcogenides and Their Photocurrent Responses. Inorg. Chem. 2024, 63, 3292–3302. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Li, X.; Liu, S.N.; Liu, Y.; Kong, T.; Zhang, H.Y.; Duan, X.G.; Chen, C.M.; Wang, S.B. Roles of Catalyst Structure and Gas Surface Reaction in the Generation of Hydroxyl Radicals for Photocatalytic Oxidation. ACS Catal. 2022, 12, 2770–2780. [Google Scholar] [CrossRef]
- Zhuang, Y.; Luan, J.F. Improved photocatalytic property of peony-like InOOH for degrading norfloxacin. Chem. Eng. J. 2020, 382, 122770. [Google Scholar] [CrossRef]
- Wei, F.; Xing, H.D.; Xiu, Z.Y.; Li, J.D.; Xing, D.F.; Han, X.J. Z-scheme TiO2-Au@CN heterojunction for simultaneous water purification of disinfection and organic pollutant removal by simulated solar light. Mater. Res. Bull. 2023, 168, 100829. [Google Scholar] [CrossRef]
- Yuan, Y.; Guo, R.T.; Hong, L.F.; Ji, X.Y.; Lin, Z.D.; Li, Z.S.; Pan, W.G. A review of metal oxide-based Z-scheme heterojunction photocatalysts: Actualities and developments. Mater. Today Energy 2021, 21, 100829. [Google Scholar] [CrossRef]
- Xu, S.; Gong, S.Q.; Jiang, H.; Shi, P.H.; Fan, J.C.; Xu, Q.J.; Min, Y.L. Z-scheme heterojunction through interface engineering for broad spectrum photocatalytic water splitting. Appl. Catal. B-Environ. 2020, 267, 118661. [Google Scholar] [CrossRef]
- Shabbir, A.; Sardar, S.; Mumtaz, A. Mechanistic investigations of emerging type-II, Z-scheme and S-scheme heterojunctions for photocatalytic applications—A review. J. Alloys Compd. 2024, 1003, 175683. [Google Scholar] [CrossRef]
- Yin, N.; Chen, H.Y.; Yuan, X.Z.; Zhang, Y.; Zhang, M.J.; Guo, J.Y.; Zhang, Y.Y.; Qiao, L.; Liu, M.S.; Song, K.X. Highly efficient photocatalytic degradation of norfloxacin via Bi2Sn2O7/PDIH Z-scheme heterojunction: Influence and mechanism. J. Hazard. Mater. 2022, 436, 129317. [Google Scholar] [CrossRef]
- Jia, T.; Wu, J.; Xiao, Y.X.; Liu, Q.Z.; Wu, Q.; Qi, Y.F.; Qi, X.M. Self-grown oxygen vacancies-rich CeO2/BiOBr Z-scheme heterojunction decorated with rGO as charge transfer channel for enhanced photocatalytic oxidation of elemental mercury. J. Colloid Interf. Sci. 2021, 587, 402–416. [Google Scholar] [CrossRef]
- Lu, C.Y.; Guo, F.; Yan, Q.Z.; Zhang, Z.J.; Li, D.; Wang, L.P.; Zhou, Y.H. Hydrothermal synthesis of type II ZnIn2S4/BiPO4 heterojunction photocatalyst with dandelion-like microflower structure for enhanced photocatalytic degradation of tetracycline under simulated solar light. J. Alloys Compd. 2019, 811, 151976. [Google Scholar] [CrossRef]
- Nkudede, E.; Xing, Q.Z.; Pang, Z.Y.; Liu, N.H.; Yan, X.W.; Sulemana, H.; Yusuf, B.A.; Di, J.; Yin, S.; Xia, J.X. A novel Type-II Bi-based oxyhalide: Bi4O5I2/BiOBr microspheres for improved photocatalytic performance. Mater. Sci. Semicond. Process. 2024, 182, 108660. [Google Scholar] [CrossRef]
- Xue, M.; Zhao, J.Z.; Chen, J.J.; Ouyang, W.; Wang, Y.Q.; Yin, S.; Li, H.M.; Xia, J.X. Improving charge separation Bi19S27Cl3/BiOCl Z-scheme heterojunction for efficient visible-light photocatalytic degradation of 2-mercaptobenzothiazole. Colloid Surf. A-Physicochem. Eng. Asp. 2025, 710, 136136. [Google Scholar] [CrossRef]
- Feng, C.; Hu, M.; Alharbi, J.; Rueping, M.; Zhang, H. Bridging Scales in Solar-Driven Water Splitting: Pathways to System Integration. Adv. Mater. 2025, e06690. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.C.; Tuo, B.Y.; Song, X. Photocatalytic degradation of methyl orange by Bi20TiO32–montmorillonite composite. Micro Nano Lett. 2020, 15, 561–565. [Google Scholar] [CrossRef]
- Khan, S.S.; Subhiksha, V.; Harini, G.; Syed, A.; Janani, B.; Steffy, J.P.; Elgorban, A.M.; Abid, I.; Wong, L.S. Integrating N doped KNbO3 tailored with Sr2V2O7 for escalated performance towards photocatalytic degradation of metronidazole: Insights into mechanism, degradation pathway and its toxicity evaluation. Surf. Interfaces 2024, 51, 104610. [Google Scholar] [CrossRef]
- Sre, V.V.; Janani, B.; Syed, A.; Elgorban, A.M.; Abid, I.; Wong, L.S.; Khan, S.S. Unveiling the enhanced photocatalytic degradation of metronidazole over green-synthesized Ag@ZnCdS QDs Schottky heterojunction: Pathway, toxicity evaluation and mechanistic insights. J. Water Process. Eng. 2024, 62, 105325. [Google Scholar] [CrossRef]
- Li, Z.; Meng, A.; Li, W.; Xiong, G.; Ye, M.; Meng, Y.; Li, Z. Co-Catalyst-Free Al6Si2O13/Cd8.05Zn1.95S10 Nanocomposites for Visible-Light-Driven Stable H2 Evolution and DDVP Degradation. Catalysts 2025, 15, 564. [Google Scholar] [CrossRef]
- Kaiba, A.; Alansi, A.M.; Oubelkacem, A.; Chabri, I.; Hameed, S.T.; Afzal, N.; Rafique, M.; Qahtan, T.F. Sunlight-Driven Synthesis of TiO2/(MA)2SnCl4 Nanocomposite Films for Enhanced Photocatalytic Degradation of Organic Pollutants. Catalysts 2025, 15, 214. [Google Scholar] [CrossRef]
- Saira; Abd El-Fattah, W.; Shahid, M.; Ashraf, S.; Sandhu, Z.A.; Guesmi, A.; Ben Hamadi, N.; Farhan, M.; Raza, M.A. Solar-Activated Titanium-Based Cu4O3/ZrO2/TiO2 Ternary Nano-Heterojunction for Rapid Photocatalytic Degradation of the Textile Dye Everzol Yellow 3RS. Catalysts 2025, 15, 751. [Google Scholar] [CrossRef]
- Si, P.F.; Feng, Y.J.; Wang, X.; Yue, X.P.; Li, D.F.; Zhang, S.; Liang, B.; Gao, Y.J.; Zhou, A.J. Enhanced degradation of Metronidazole by the coupling of photocatalytic and microbial fuel cell: Mechanism and electrochemistry characteristic. J. Environ. Chem. Eng. 2023, 11, 109707. [Google Scholar] [CrossRef]
- Zhao, J.; He, Q.; Yao, B.H.; Zhang, Q.; Zhang, T.; Yang, M.K.; Liu, Y. Preparation and Photocatalytic Property of Er3NbO7/LSC-100 Chelating Resin Composites. Integr. Ferroelectr. 2015, 159, 73–86. [Google Scholar] [CrossRef]
- Wu, Y.; Xia, F.F.; Li, C.Z.; Jayswal, M.R.; Prakash, O.; Muddassir, M. Two new Ni(II) and Co(II)-based coordination polymers based on mixed linkers as photocatalysts for antibiotics decomposition. Inorg. Chim. Acta 2023, 558, 121723. [Google Scholar] [CrossRef]
- Tran, M.L.; Fu, C.C.; Juang, R.S. Effects of water matrix components on degradation efficiency and pathways of antibiotic metronidazole by UV/TiO2 photocatalysis. J. Mol. Liq. 2019, 276, 32–38. [Google Scholar] [CrossRef]
- Abbasi-Asl, H.; Sabzehmeidani, M.M.; Ghaedi, M. Efficient degradation of metronidazole antibiotic by TiO2/Ag3PO4/g–C3N4 ternary composite photocatalyst in a continuous flow-loop photoreactor. J. Environ. Chem. Eng. 2021, 9, 105963. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luan, J.; Li, Z.; Yao, Y.; Wang, J.; Hao, L. Metronidazole Degradation via Visible Light-Driven Z-Scheme BiTmDySbO7/BiEuO3 Heterojunction Photocatalyst. Sustainability 2025, 17, 10024. https://doi.org/10.3390/su172210024
Luan J, Li Z, Yao Y, Wang J, Hao L. Metronidazole Degradation via Visible Light-Driven Z-Scheme BiTmDySbO7/BiEuO3 Heterojunction Photocatalyst. Sustainability. 2025; 17(22):10024. https://doi.org/10.3390/su172210024
Chicago/Turabian StyleLuan, Jingfei, Zhe Li, Ye Yao, Jian Wang, and Liang Hao. 2025. "Metronidazole Degradation via Visible Light-Driven Z-Scheme BiTmDySbO7/BiEuO3 Heterojunction Photocatalyst" Sustainability 17, no. 22: 10024. https://doi.org/10.3390/su172210024
APA StyleLuan, J., Li, Z., Yao, Y., Wang, J., & Hao, L. (2025). Metronidazole Degradation via Visible Light-Driven Z-Scheme BiTmDySbO7/BiEuO3 Heterojunction Photocatalyst. Sustainability, 17(22), 10024. https://doi.org/10.3390/su172210024







