Effect of Activating Solution Modulus on the Synthesis of Sustainable Geopolymer Binders Using Spent Oil Bleaching Earths as Precursor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Geopolymers Synthesis
2.3. Characterisation of Geopolymers
3. Results and Discussion
3.1. Reaction Degree
3.2. Fourier Transform Infrared Spectroscopy (FTIR)
3.3. Mineralogy of Geopolymer Binders
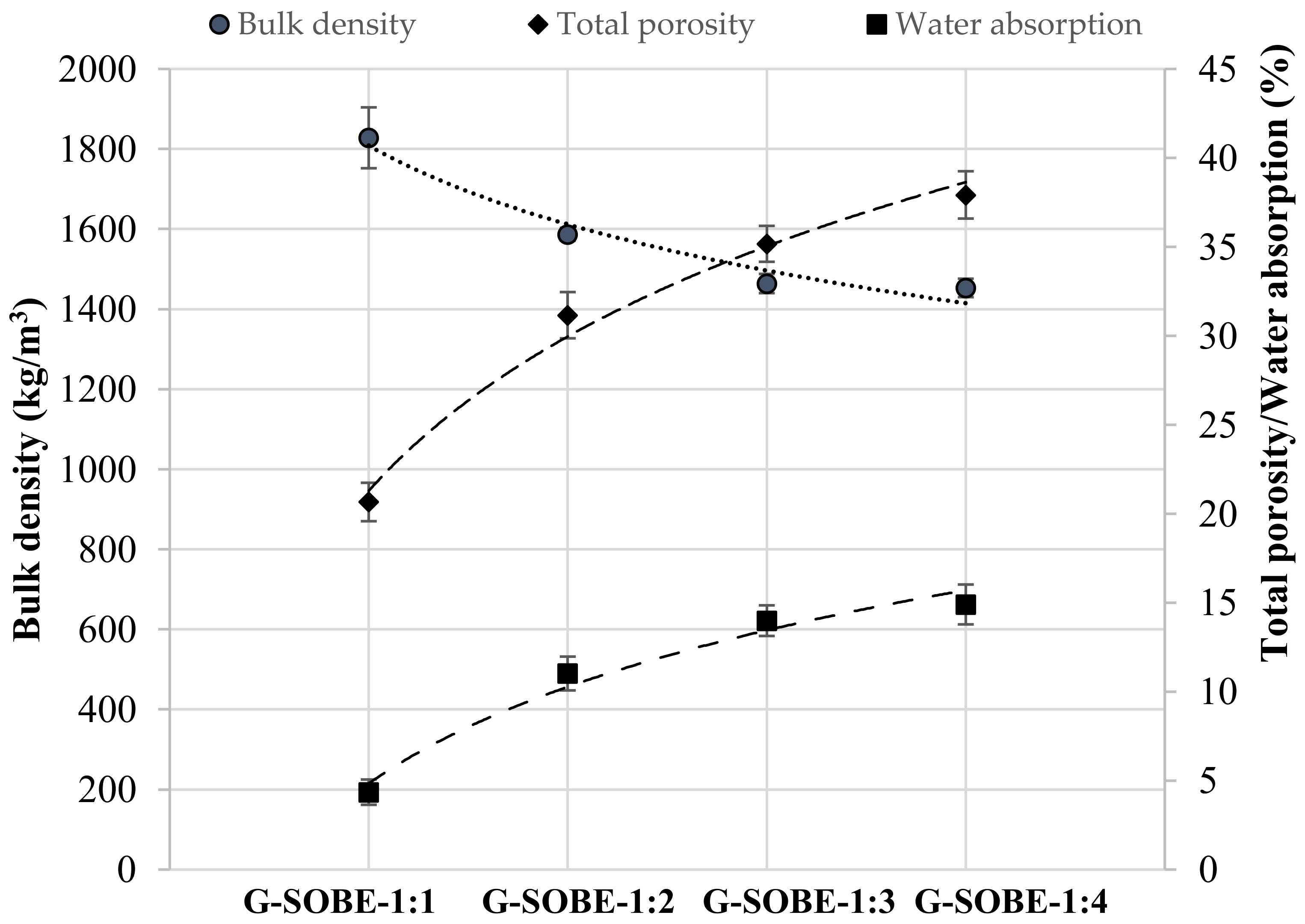
3.4. Bulk Density, Total Porosity and Water Absorption of Geopolymer Binders
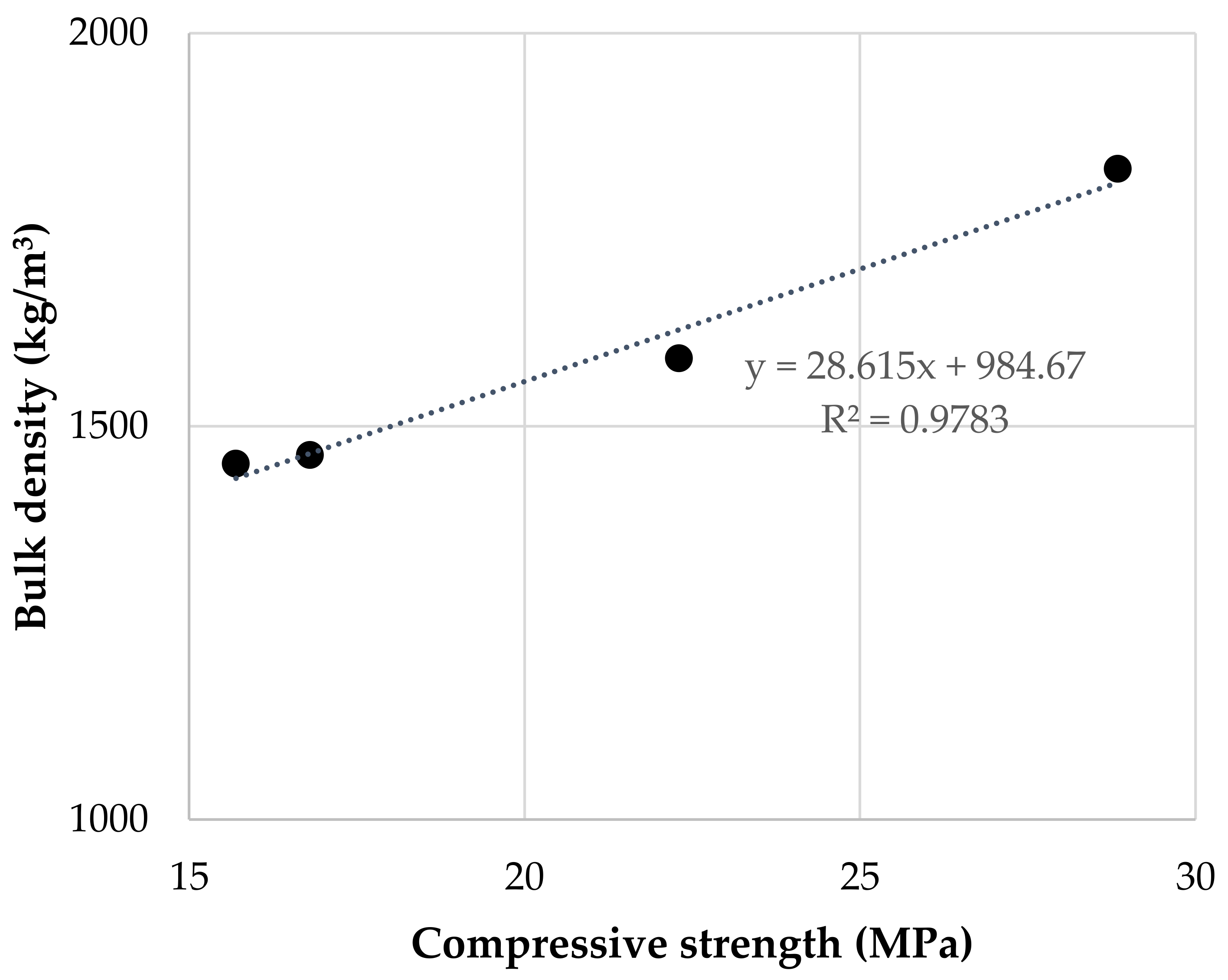
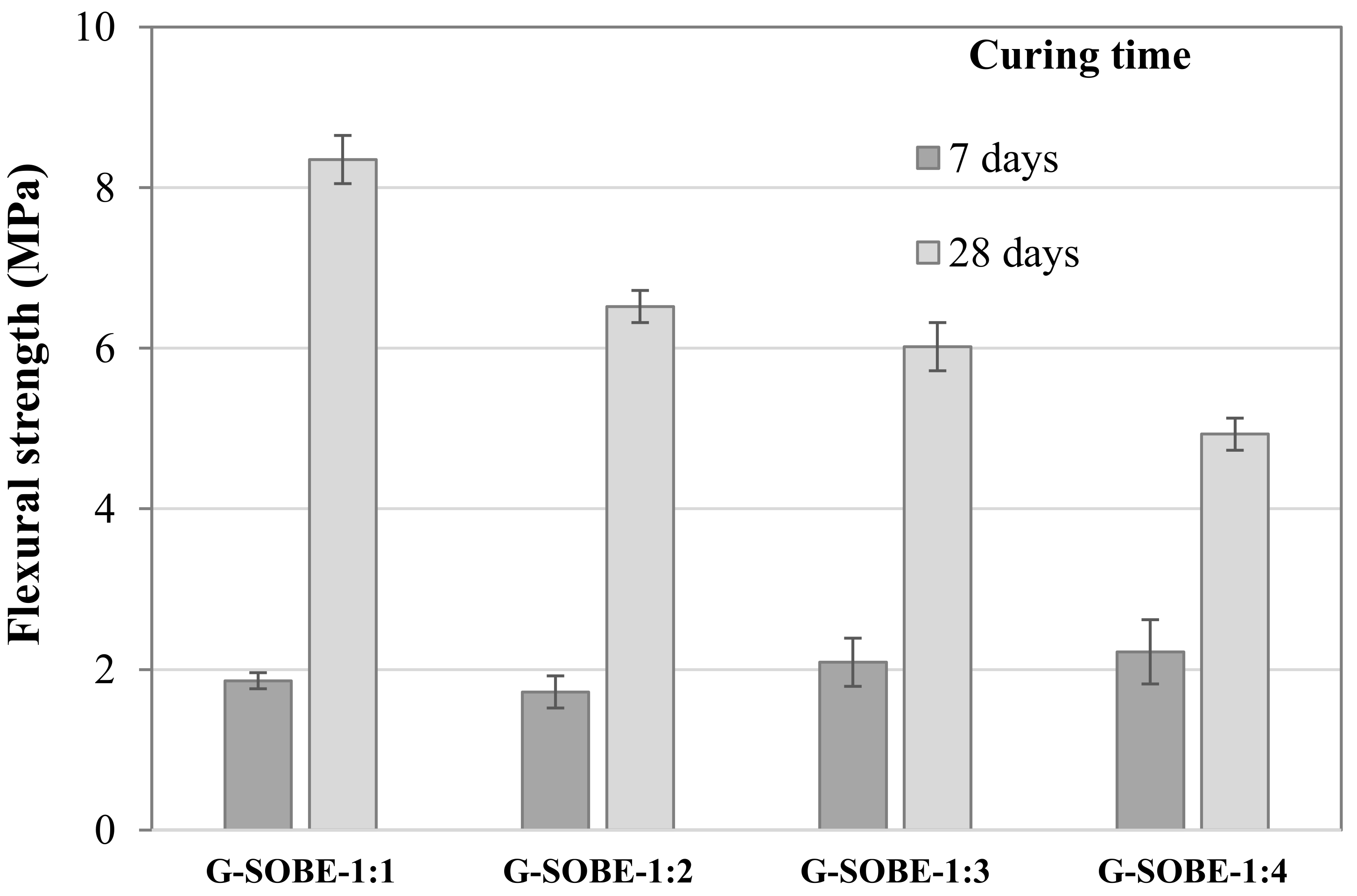
3.5. Compressive and Flexural Strength of Geopolymer Binders
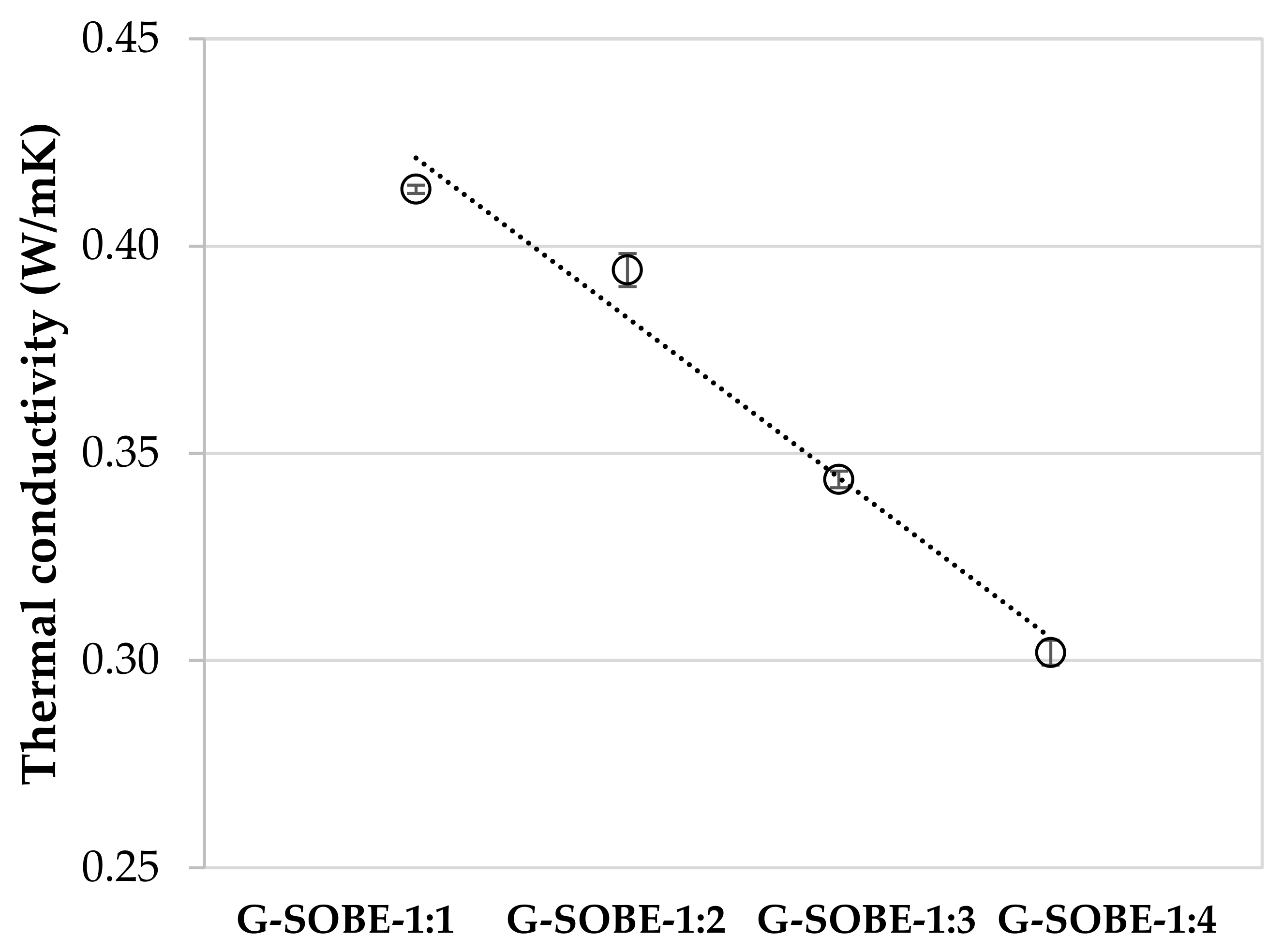
3.6. Thermal Conductivity
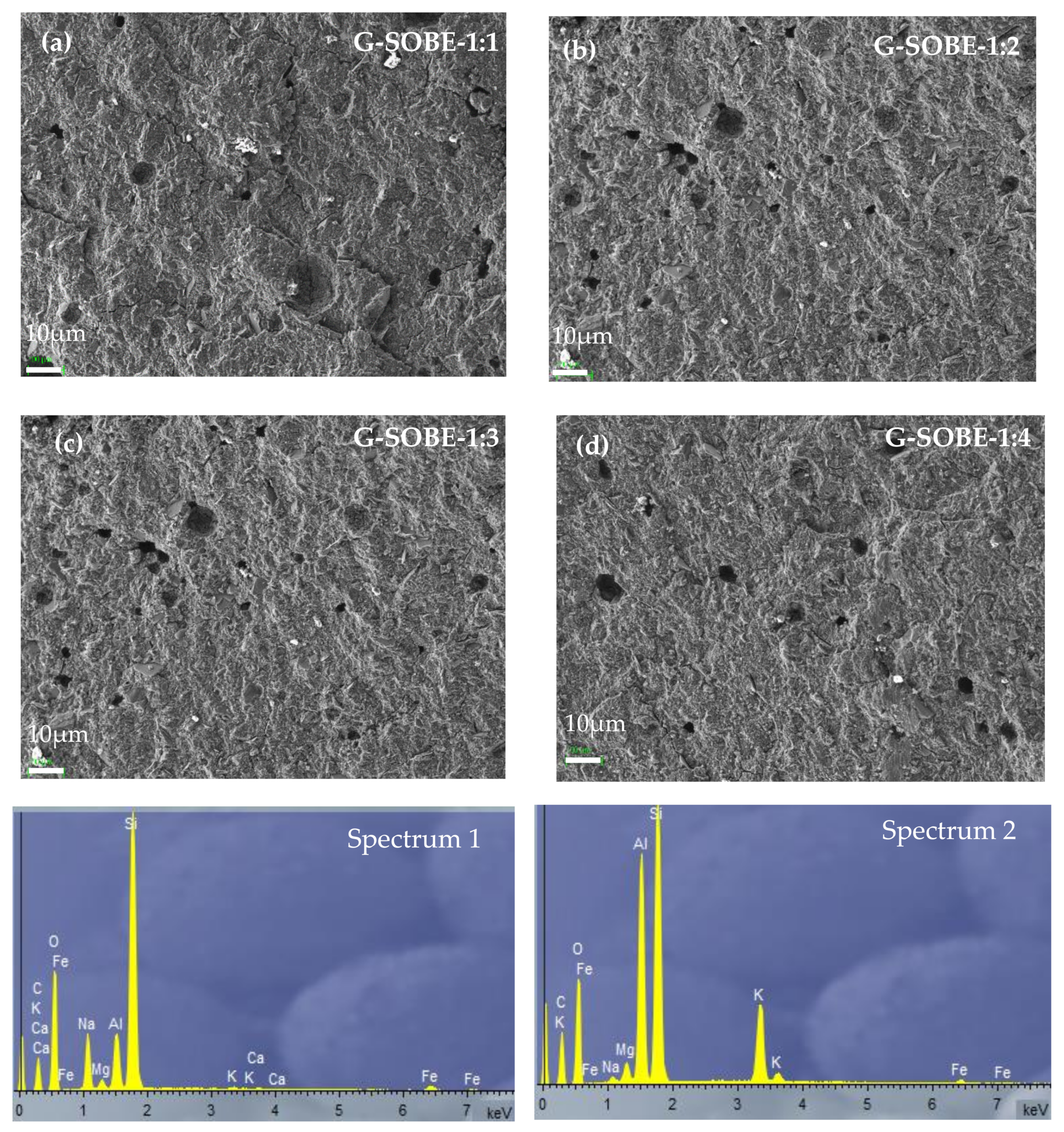
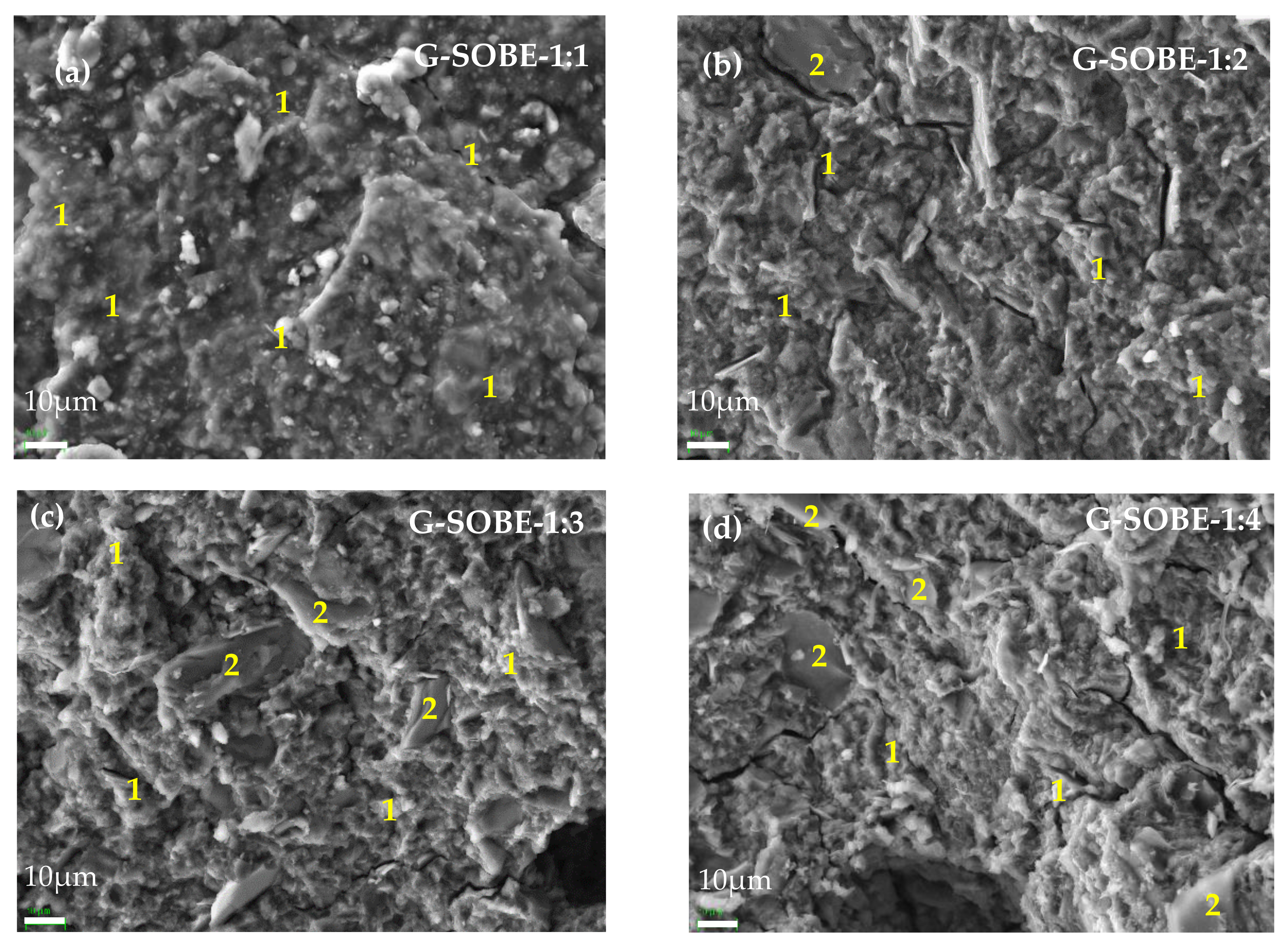
3.7. Microstructure of Geopolymer Binders
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Asociación de Productores de Cemento (ASOCEM). World Overview of the Cement Industry. 2018. Available online: http://www.asocem.org.pe/archivo/files/Vision%20General%20de%20la%20Industria%20del%20Cemento%20y%20sus%20Principales%20Actores.pdf (accessed on 22 May 2021).
- Andrew, R.M. Global CO2 emissions from cement production. Earth Syst. Sci. Data 2018, 10, 195–217. [Google Scholar] [CrossRef] [Green Version]
- Deja, J.; Uliasz-Bocheńczyk, A.; Mokrzycki, E. CO2 emissions from Polish cement industry. Int. J. Greenh. Gas Control. 2010, 4, 583–588. [Google Scholar] [CrossRef]
- Zhou, W.; Yan, C.; Duan, P.; Liu, Y.; Zhang, Z.; Qiu, X.; Li, D. A comparative study of high- and low-Al2O3 fly ash based-geopolymers: The role of mix proportion factors and curing temperature. Mater. Des. 2016, 95, 63–74. [Google Scholar] [CrossRef]
- Ouellet-Plamondon, C.; Habert, G. Life cycle assessment (LCA) of alkali-activated cements and concretes. In Handbook of Alkali-Activated Cements, Mortars and Concretes; Elsevier: Amsterdam, The Netherlands, 2015; Volume 1, pp. 663–686. [Google Scholar]
- Maddalena, R.; Roberts, J.; Hamilton, A. Can Portland cement be replaced by low-carbon alternative materials? A study on the thermal properties and carbon emissions of innovative cements. J. Clean. Prod. 2018, 186, 933–942. [Google Scholar] [CrossRef]
- Robayo-Salazar, R.; Arcila, J.M.M.; de Gutiérrez, R.M.; Martínez, E. Life cycle assessment (LCA) of an alkali-activated binary concrete based on natural volcanic pozzolan: A comparative analysis to OPC concrete. Constr. Build. Mater. 2018, 176, 103–111. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer. Chemistry and Applications, 3rd ed.; Institut Geopolymer: Saint-Quentin, France, 2008; pp. 1–585. [Google Scholar]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Van Deventer, J.S. The role of inorganic polymer technology in the development of ‘green concrete’. Cem. Concr. Res. 2007, 37, 1590–1597. [Google Scholar] [CrossRef]
- Grutzeck, M.W.; Siemer, D.D. Zeolites Synthesized from Class F Fly Ash and Sodium Aluminate Slurry. J. Am. Ceram. Soc. 2005, 80, 2449–2453. [Google Scholar] [CrossRef]
- Hung, T.; Pernica, D.; Kroisová, D.; Bortnovsky, O.; Rylichova, V.; Louda, P. Composites Base on Geopolymer Matrices: Preliminary Fabrication, Mechanical Properties and Future Applications. In Advanced Materials Research; Trans Tech Publications Ltd.: Bäch, Switzerland, 2008; Volume 55, pp. 477–480. [Google Scholar]
- Cheng, T.; Chiu, J. Fire-resistant geopolymer produced by granulated blast furnace slag. Miner. Eng. 2003, 16, 205–210. [Google Scholar] [CrossRef]
- Bakharev, T. Resistance of Geopolymer Materials to Acid Attack. Cem. Concr. Res. 2005, 35, 658–670. [Google Scholar] [CrossRef]
- Bell, J.; Gordon, M.; Kriven, W. Use of Geopolymeric Cements as a Refractory Adhesive for Metal and Ceramic Joins. Ceram. Eng. Sci. Proc. 2008, 24, 407–413. [Google Scholar] [CrossRef]
- Khale, D.; Chaudhary, R. Mechanism of geopolymerization and factors influencing its development: A review. J. Mater. Sci. 2007, 42, 729–746. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L. Durability of Alkali-Activated Materials: Progress and Perspectives. J. Am. Ceram. Soc. 2014, 97, 997–1008. [Google Scholar] [CrossRef]
- Karakoç, M.B.; Türkmen, I.; Maraş, M.M.; Kantarci, F.; Demirboğa, R. Sulfate resistance of ferrochrome slag based geopolymer concrete. Ceram. Int. 2016, 42, 1254–1260. [Google Scholar] [CrossRef]
- Davidovits, J. Mineral Polymers and Methods of Making Them. U.S. Patent No. 4.349.386, 1982. Available online: https://patentimages.storage.googleapis.com/56/1b/2e/9e4535535ead87/US4349386.pdf (accessed on 12 May 2021).
- Barbosa, V.F.; MacKenzie, K.; Thaumaturgo, C. Synthesis and characterisation of materials based on inorganic polymers of alumina and silica: Sodium polysialate polymers. Int. J. Inorg. Mater. 2000, 2, 309–317. [Google Scholar] [CrossRef]
- Samuel, R.; Puppala, A.; Radovic, M. Sustainability Benefits Assessment of Metakaolin-Based Geopolymer Treatment of High Plasticity Clay. Sustainability 2020, 12, 10495. [Google Scholar] [CrossRef]
- Bonet-Martínez, E.; García-Cobo, P.; Pérez-Villarejo, L.; Castro, E.; Eliche-Quesada, D. Effect of Olive-Pine Bottom Ash on Properties of Geopolymers Based on Metakaolin. Materials 2020, 13, 901. [Google Scholar] [CrossRef] [Green Version]
- Liew, Y.-M.; Heah, C.-Y.; Mustafa, A.B.M.; Kamarudin, H. Structure and properties of clay-based geopolymer cements: A review. Prog. Mater. Sci. 2016, 83, 595–629. [Google Scholar] [CrossRef]
- Gómez-Casero, M.; Moral-Moral, F.; Pérez-Villarejo, L.; Sánchez-Soto, P.; Eliche-Quesada, D. Synthesis of clay geopolymers using olive pomace fly ash as an alternative activator. Influence of the additional commercial alkaline activator used. J. Mater. Res. Technol. 2021, 12, 1762–1776. [Google Scholar] [CrossRef]
- Novais, R.M.; Buruberri, L.H.; Ascensão, G.; Seabra, M.P.; Labrincha, J. Porous biomass fly ash-based geopolymers with tailored thermal conductivity. J. Clean. Prod. 2016, 119, 99–107. [Google Scholar] [CrossRef]
- Tennakoon, C.; Sagoe-Crentsil, K.; Nicolas, R.S.; Sanjayan, J.G. Characteristics of Australian brown coal fly ash blended geopolymers. Constr. Build. Mater. 2015, 101, 396–409. [Google Scholar] [CrossRef]
- Azad, N.; Samarakoon, S. Utilization of Industrial By-Products/Waste to Manufacture Geopolymer Cement/Concrete. Sustainability 2021, 13, 873. [Google Scholar] [CrossRef]
- Torres, M.; Puertas, F. Waste glass in the geopolymer preparation. Mechanical and microstructural characterisation. J. Clean. Prod. 2015, 90, 397–408. [Google Scholar] [CrossRef]
- Novais, R.M.; Ascensão, G.; Seabra, M.P.; Labrincha, J. Waste glass from end-of-life fluorescent lamps as raw material in geopolymers. Waste Manag. 2016, 52, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Nikolov, A.; Nugteren, H.; Rostovsky, I. Optimization of geopolymers based on natural zeolite clinoptilolite by calcination and use of aluminate activators. Constr. Build. Mater. 2020, 243, 118257. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer cement. Geopolymer Sci. Tech. 2013, 21, 1–11. [Google Scholar]
- Cui, Y.; Wang, D.; Wang, Y.; Sun, R.; Rui, Y. Effects of the n(H2O: Na2Oeq) ratio on the geopolymerization process and 609 microstructures of fly ash-based geopolymers. J. Non Cryst. Solids 2019, 511, 19–28. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, S.; Zuo, Y.; Chen, W.; Ye, G. Chemical deformation of metakaolin based geopolymer. Cem. Concr. Res. 2019, 120, 108–118. [Google Scholar] [CrossRef]
- Koleżyński, A.; Król, M.; Żychowicz, M. The structure of geopolymers-Theoretical studies. J. Mol. Struct. 2018, 1163, 465–471. [Google Scholar] [CrossRef]
- Yusuf, M.O.; Palomo, A.M.W.; Grutzeck, M.W.; Blanco, M.T. Alkali-activated fly ashes: A cement for the future. Cem. Concr. Res. 1999, 29, 1323–1329. [Google Scholar] [CrossRef]
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. One-part alkali-activated materials: A review. Cement Concr. Res. 2018, 103, 21–34. [Google Scholar] [CrossRef]
- Komljenović, M.; Baščarević, Z.; Bradić, V. Mechanical and microstructural properties of alkali-activated fly ash geopol-620 ymers. J. Hazard. Mater. 2010, 181, 35–42. [Google Scholar] [CrossRef]
- Weng, L.; Sagoe-Crentsil, K. Dissolution processes, hydrolysis and condensation reactions during geopolymer synthesis: 622 Part I—low Si/Al ratio systems. J. Mater. Sci. 2007, 42, 2997–3006. [Google Scholar] [CrossRef]
- Lizcano, M.; Kim, H.S.; Basu, S.; Radovic, M. Mechanical properties of sodium and potassium activated metakaolin-624 based geopolymers. J. Mater. Sci. 2012, 47, 2607–2616. [Google Scholar] [CrossRef]
- Van Jaarsveld, J.G.S.; van Deventer, J.S.J.; Lukey, G.C. The effect of composition and temperature on the properties of fly 626 ash- and kaolinite-based geopolymers. Chem. Eng. J. 2002, 89, 63–73. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D. Geopolymerisation: A review and prospects for the minerals industry. Miner. Eng. 2007, 20, 1261–1277. [Google Scholar] [CrossRef]
- Aventum. Available online: https://www.aventum.net/2017/05/16/tierras-de-blanqueo-de-aceites-hay-alternativas-al-630 vertedero/ (accessed on 28 May 2021).
- Loh, S.K.; Cheong, K.Y.; Salimon, J. Surface-active physicochemical characteristics of spent bleaching earth on soil-plant interaction and water-nutrient uptake: A review. Appl. Clay Sci. 2017, 140, 59–65. [Google Scholar] [CrossRef]
- Tang, J.; Mu, B.; Zheng, M.; Wang, A. One-Step Calcination of the Spent Bleaching Earth for the Efficient Removal of Heavy Metal Ions. ACS Sustain. Chem. Eng. 2015, 3, 1125–1135. [Google Scholar] [CrossRef]
- Tang, J.; Mu, B.; Wang, W.; Zheng, M.; Wang, A. Fabrication of manganese dioxide/carbon/attapulgite composites derived from spent bleaching earth for adsorption of Pb(ii) and Brilliant green. RSC Adv. 2016, 6, 36534–36543. [Google Scholar] [CrossRef]
- Tang, J.; Mu, B.; Zong, L.; Zheng, M.; Wang, A. Facile and green fabrication of magnetically recyclable carboxyl-functionalized attapulgite/carbon nanocomposites derived from spent bleaching earth for wastewater treatment. Chem. Eng. J. 2017, 322, 102–114. [Google Scholar] [CrossRef]
- Mana, M.; Ouali, M.S.; Lindheimer, M.; de Menorval, L.C. Removal of lead from aqueous solutions with a treated spent bleaching earth. J. Hazard. Mater. 2008, 159, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Kheang Loh, S.; James, S.; Ngatiman, M.; Yein Cheong, K.; May Choo, Y.; Soon Lim, W. Enhancement of palm oil refinery waste—Spent bleaching earth (SBE) into bio organic fertilizer and their effects on crop biomass growth. Ind. Crops Prod. 2013, 49, 775–781. [Google Scholar] [CrossRef] [Green Version]
- Dijkstra, A.J. What to Do with Spent Bleaching Earth? A Review. J. Am. Oil Chem. Soc. 2020, 97, 565–575. [Google Scholar] [CrossRef]
- Srisang, S.; Srisang, N. Recycling spent bleaching earth and oil palm ash to tile production: Impact on properties, utilization, and microstructure. J. Clean. Prod. 2021, 294, 126336. [Google Scholar] [CrossRef]
- Eliche-Quesada, D.; Iglesias, F.A.C. Utilisation of spent filtration earth or spent bleaching earth from the oil refinery industry in clay products. Ceram. Int. 2014, 40, 16677–16687. [Google Scholar] [CrossRef]
- Boey, P.-L.; Ganesan, S.; Maniam, G.P.; Ali, D.M.H. Ultrasound aided in situ transesterification of crude palm oil adsorbed on spent bleaching clay. Energy Convers. Manag. 2011, 52, 2081–2084. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Jiménez, A.; Palomo, A. Mid-infrared spectroscopic studies of alkali-activated fly ash structure. Microporous Mesoporous Mater. 2005, 86, 207–214. [Google Scholar] [CrossRef]
- UNE-EN 1015-11:2020. Methods of Test for Mortar for Masonary—Part 11: Determination of Flexural and Compressive Strength of Hardened Mortar; Asociación Española de Normalización: Madrid, Spain, 2020. [Google Scholar]
- UNE-EN 1015-10:2020. Methods of Test for Mortar for Masonry—Part 10: Determination of Dry Bulk Density of Hardened Mortar; Asociación Española de Normalización: Madrid, Spain, 2020. [Google Scholar]
- ISO 8302:1991. Thermal Insulation-Determination of Steady-State Thermal Resistance and Related Properties-Guarded Hot Plate Apparatus; International Standards Organization: Geneva, Switzerland, 1991. [Google Scholar]
- Larson, A.C.; Von Dreele, R.B. General Structure Analysis System (GSAS). Los Alamos Natl. Lab. Rep. LAUR 2004, 96, 748. [Google Scholar]
- Toby, B.H. EXPGUI, a graphical user interface forGSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef] [Green Version]
- Thompson, P.; Cox, D.E.; Hastings, J.B. Rietveld refinement of Debye-Scherrer synchrotron X-ray data from Al2O3. J. Appl. Crystallogr. 1987, 20, 79–83. [Google Scholar] [CrossRef] [Green Version]
- Viani, A.; Gualtieri, A.; Artioli, G. The nature of disorder in montmorillonite by simulation of X-ray powder patterns. Am. Miner. 2002, 87, 966–975. [Google Scholar] [CrossRef]
- Gualtieri, A. Accuracy of XRPD QPA using the combined Rietveld—RIR method. J. Appl. Crystallogr. 2000, 33, 267–278. [Google Scholar] [CrossRef]
- García-Lodeiro, I.; Fernández-Jiménez, A.; Blanco, M.T.; Palomo, A. FTIR study of the sol-gel synthesis of cementitious gels: C-S-H and N-A-S-H. J. Sol-Gel Sci. Technol. 2008, 45, 63–72. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, J.; Hou, L.; Lu, Z.; Li, J.; Wang, J. Pore structure and properties of porous geopolymer based on pre-swelled bentonite. Constr. Build. Mater. 2020, 254, 119226. [Google Scholar] [CrossRef]
- Gionis, V.; Kacandes, G.H.; Kastritis, I.D.; Chryssikos, G.D. On the structure of palygorskite by mid- and near-infrared spectroscopy. Am. Miner. 2006, 91, 1125–1133. [Google Scholar] [CrossRef]
- Bergamonti, L.; Taurino, R.; Cattani, L.; Ferretti, D.; Bondioli, F. Lightweight hybrid organic-inorganic geopolymers obtained using polyurethane waste. Constr. Build. Mater. 2018, 185, 285–292. [Google Scholar] [CrossRef]
- Rattanasak, U.; Chindaprasirt, P. Influence of NaOH solution on the synthesis of fly ash geopolymer. Miner. Eng. 2009, 22, 1073–1078. [Google Scholar] [CrossRef]
- Jiang, J.; Yang, Y.; Hou, L.; Lu, Z.; Li, J.; Niu, Y. Facile preparation and hardened properties of porous geopolymer-supported zeolite based on swelled bentonite. Constr. Build. Mater. 2019, 228, 117040. [Google Scholar] [CrossRef]
- Longhi, M.A.; Rodríguez, E.D.; Bernal, S.A.; Provis, J.L.; Kirchheim, A.P. Valorisation of a kaolin mining waste for the production of geopolymers. J. Clean. Prod. 2016, 115, 265–272. [Google Scholar] [CrossRef]
- Yaseri, S.; Verki, V.M.; Mahdikhani, M. Utilization of high volume cement kiln dust and rice husk ash in the production of sustainable geopolymer. J. Clean. Prod. 2019, 230, 592–602. [Google Scholar] [CrossRef]
- Rożek, P.; Król, M.; Mozgawa, W. Spectroscopic studies of fly ash-based geopolymers. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 198, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Król, M.; Rożek, P.; Mozgawa, W. Synthesis of the Sodalite by Geopolymerization Process Using Coal Fly Ash. Pol. J. Environ. Stud. 2017, 26, 2611–2617. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; Provis, J.L.; van Deventer, J.S. The effect of silica availability on the mechanism of geopolymerisation. Cem. Concr. Res. 2011, 41, 210–216. [Google Scholar] [CrossRef]
- Criado, M.; Fernández-Jiménez, A.; Palomo, A. Alkali activation of fly ash: Effect of the SiO2/Na2O ratio: Part I: FTIR study. Microporous Mesoporous Mater. 2007, 106, 180–191. [Google Scholar] [CrossRef]
- Barbosa, V.F.; MacKenzie, K. Thermal behaviour of inorganic geopolymers and composites derived from sodium polysialate. Mater. Res. Bull. 2003, 38, 319–331. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D.; Vlachou, A.; Bartzas, G.; Galetakis, M. Effect of synthesis parameters on the quality of con-struction and demolition wastes (CDW) geopolymers. Adv. Powder Technol. 2015, 26, 368–376. [Google Scholar] [CrossRef] [Green Version]
- Kazea, C.R.; Yankw, J.N.; Nanae, D.A.; Tchakoutea, H.K.; Kamseu, E.; Melo, U.C.; Leonelli, C.; Rahierd, H. Effect of silicate modulus on the setting, mechanical strength and microstructure of iron-rich aluminosilicate (laterite) based-geopolymer cured at room temperature. Ceram. Int. 2018, 44, 21442–21450. [Google Scholar] [CrossRef]
- Lecomte, I.; Henrist, C.; Liégeois, M.; Maseri, F.; Rulmont, A.; Cloots, R. (Micro)-structural comparison between geopolymers, alkali-activated slag cement and Portland cement. J. Eur. Ceram. Soc. 2006, 26, 3789–3797. [Google Scholar] [CrossRef]
- Zhang, G.; Ke, Y.; He, J.; Qin, M.; Shen, H.; Lu, S.; Xu, J. Effects of organo-modified montmorillonite on the tribology performance of bismaleimide-based nanocomposites. Mater. Des. 2015, 86, 138–145. [Google Scholar] [CrossRef]
- Anh, H.N.; Ahn, H.; Jo, H.Y.; Kim, G.-Y. Effect of alkaline solutions on bentonite properties. Environ. Earth Sci. 2017, 76, 374. [Google Scholar] [CrossRef]
- Provis, J.L.; Lukey, A.G.C.; Van Deventer, J.S.J. Do Geopolymers Actually Contain Nanocrystalline Zeolites? A Reexamination of Existing Results. Chem. Mater. 2005, 17, 3075–3085. [Google Scholar] [CrossRef]
- Leong, H.Y.; Ong, D.E.L.; Sanjayan, J.G.; Nazari, A. The effect of different Na2O and K2O ratios of alkali activator on compressive strength of fly ash based-geopolymer. Constr. Build. Mater. 2016, 106, 500–511. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M.; Johari, M.A.M.; Rahman, M.K.; Maslehuddin, M. Effect of alkaline activators and binder content on the properties of natural pozzolan-based alkali activated concrete. Constr. Build. Mater. 2017, 147, 648–660. [Google Scholar] [CrossRef]
- Toniolo, N.; Rincon, A.; Roether, J.; Ercole, P.; Bernardo, E.; Boccaccini, A. Extensive reuse of soda-lime waste glass in fly ash-based geopolymers. Constr. Build. Mater. 2018, 188, 1077–1084. [Google Scholar] [CrossRef]
- Hanjitsuwan, S.; Hunpratub, S.; Thongbai, P.; Maensiri, S.; Sata, V.; Chindaprasirt, P. Effects of NaOH concentrations on physical and electrical properties of high calcium fly ash geopolymer paste. Cem. Concr. Compos. 2014, 45, 9–14. [Google Scholar] [CrossRef]
- Glid, M.; Sobrados, I.; Ben Rhaiem, H.; Sanz, J.; Amara, A.B.H. Alkaline activation of metakaolinite-silica mixtures: Role of dissolved silica concentration on the formation of geopolymers. Ceram. Int. 2017, 43, 12641–12650. [Google Scholar] [CrossRef]
- Hameed, A.M.; Rawdhan, R.R.; Al-Mishhadani, S.A. Effect of various factors on the manufacturing of geopolymer mortar. Arch. Sci. 2017, 1, 111. [Google Scholar]
- Xu, H.; Van Deventer, J.S.J. The geopolymerisation of alumino-silicate minerals. Int. J. Miner. Process. 2000, 59, 247–266. [Google Scholar] [CrossRef] [Green Version]
- Hardjito, D.; Wallah, S.E.; Sumajouw, D.M.J.; Rangan, B.V. Fly Ash-Based Geopolymer Concrete. Aust. J. Struct. Eng. 2005, 6, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Huiskes, D.; Keulen, A.; Yu, Q.; Brouwers, H. Design and performance evaluation of ultra-lightweight geopolymer concrete. Mater. Des. 2016, 89, 516–526. [Google Scholar] [CrossRef]
- Yun, T.S.; Jeong, Y.J.; Han, T.-S.; Youm, K.-S. Evaluation of thermal conductivity for thermally insulated concretes. Energy Build. 2013, 61, 125–132. [Google Scholar] [CrossRef]
- Pan, Z.; Feng, K.N.; Gong, K.; Zou, B.; Korayem, A.H.; Sanjayan, J.; Duan, W.H.; Collins, F. Damping and microstructure of fly ash-based geopolymers. J. Mater. Sci. 2012, 48, 3128–3137. [Google Scholar] [CrossRef]
- Albitar, M.; Ali, M.M.; Visintin, P.; Drechsler, M. Durability evaluation of geopolymer and conventional concretes. Constr. Build. Mater. 2017, 136, 374–385. [Google Scholar] [CrossRef]
- Zhang, Z.; Provis, J.L.; Reid, A.; Wang, H. Mechanical, thermal insulation, thermal resistance and acoustic absorption properties of geopolymer foam concrete. Cem. Concr. Compos. 2015, 62, 97–105. [Google Scholar] [CrossRef]
- Rashad, A.M.; Sadek, D.M.; Hassan, H.A. An investigation on blast-furnace stag as fine aggregate in alkali-activated slag mortars subjected to elevated temperatures. J. Clean. Prod. 2016, 112, 1086–1096. [Google Scholar] [CrossRef]
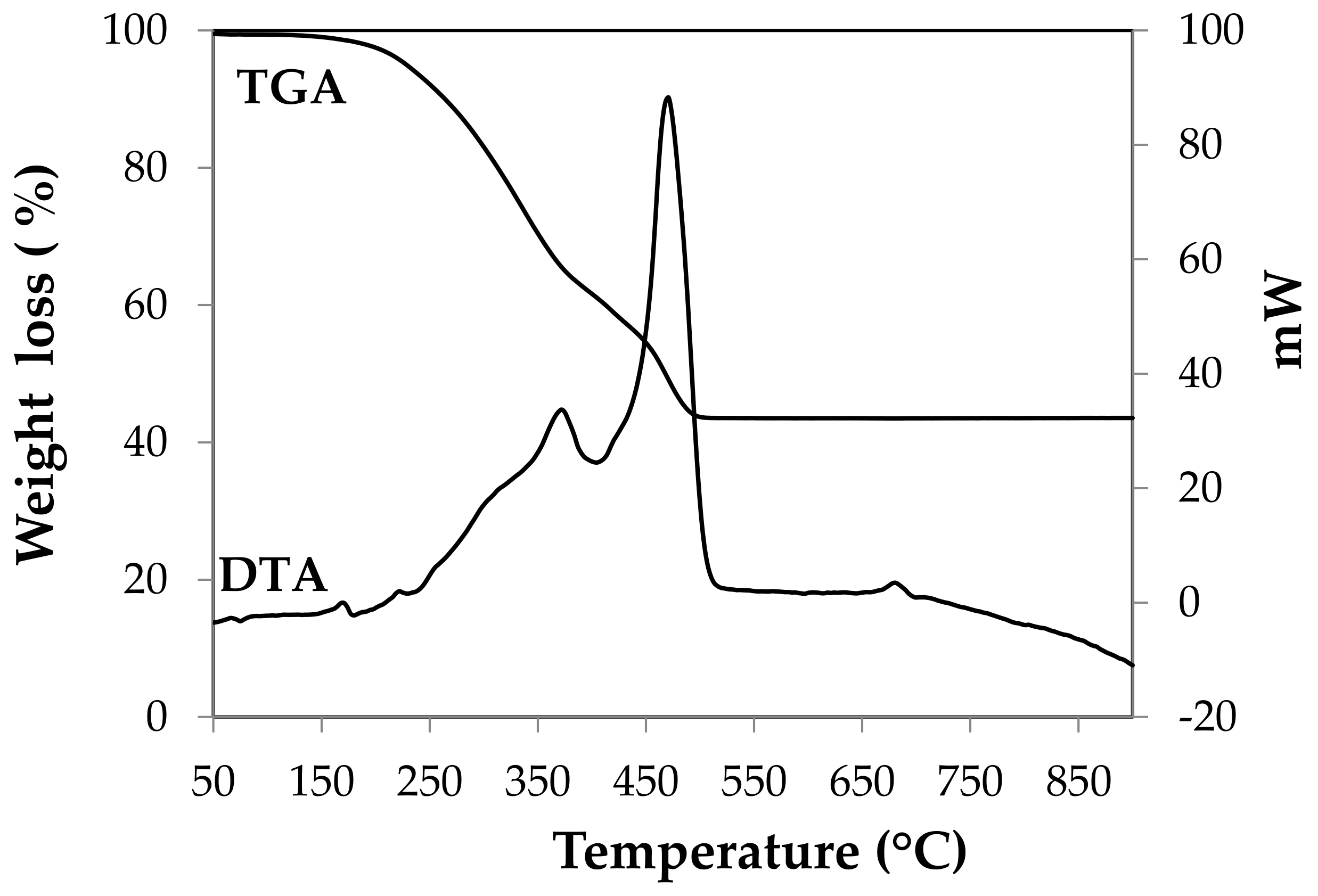
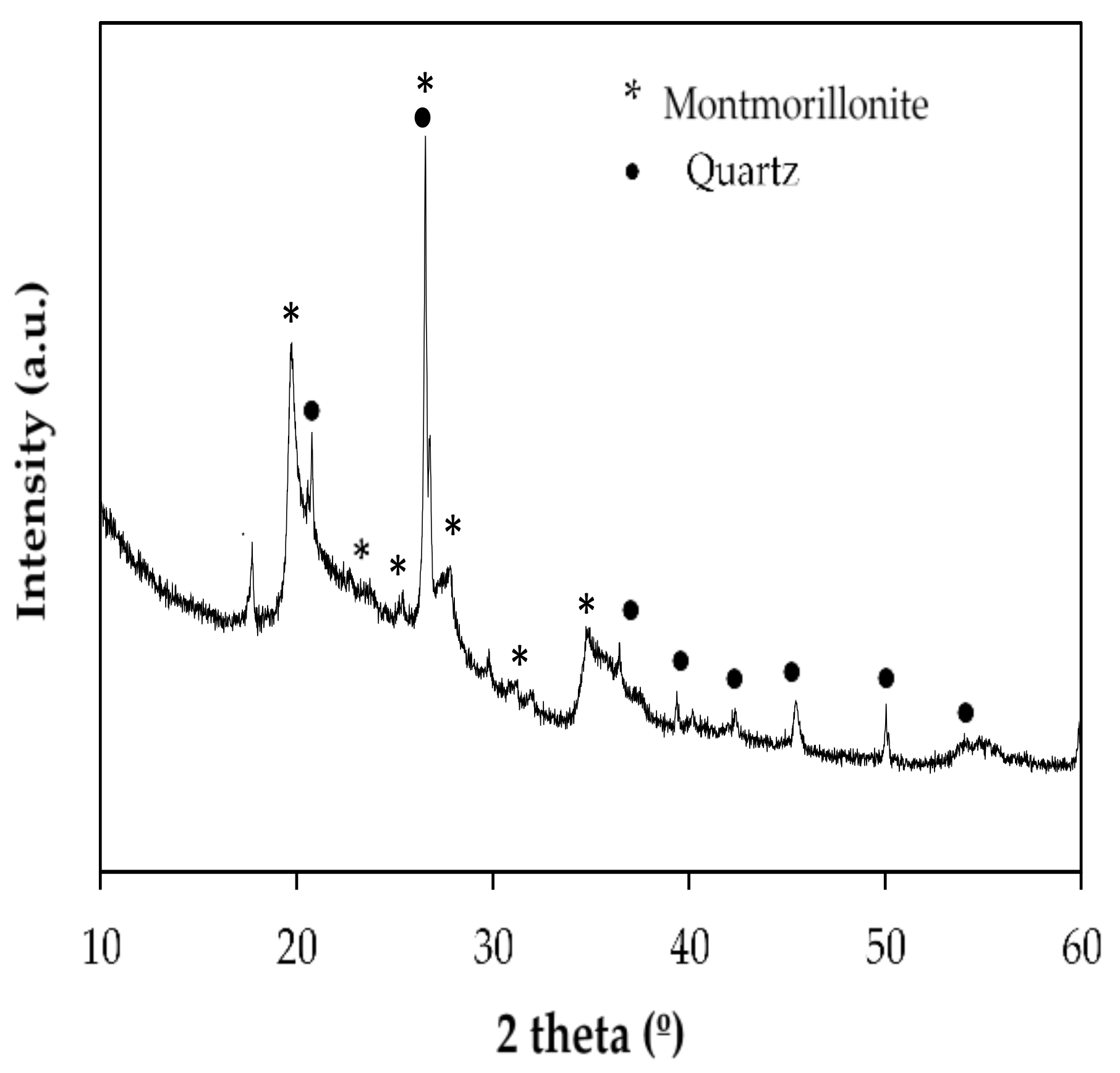
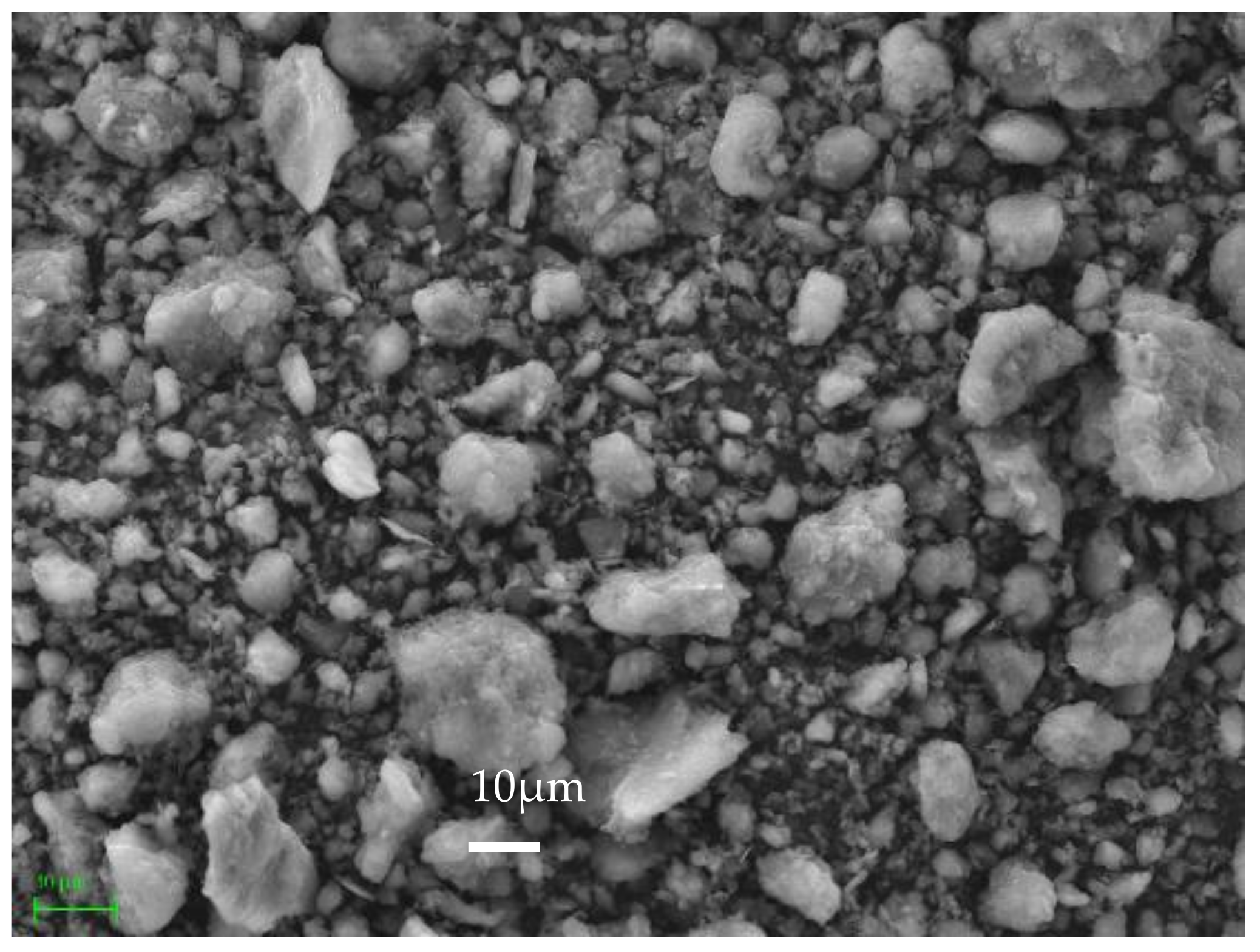

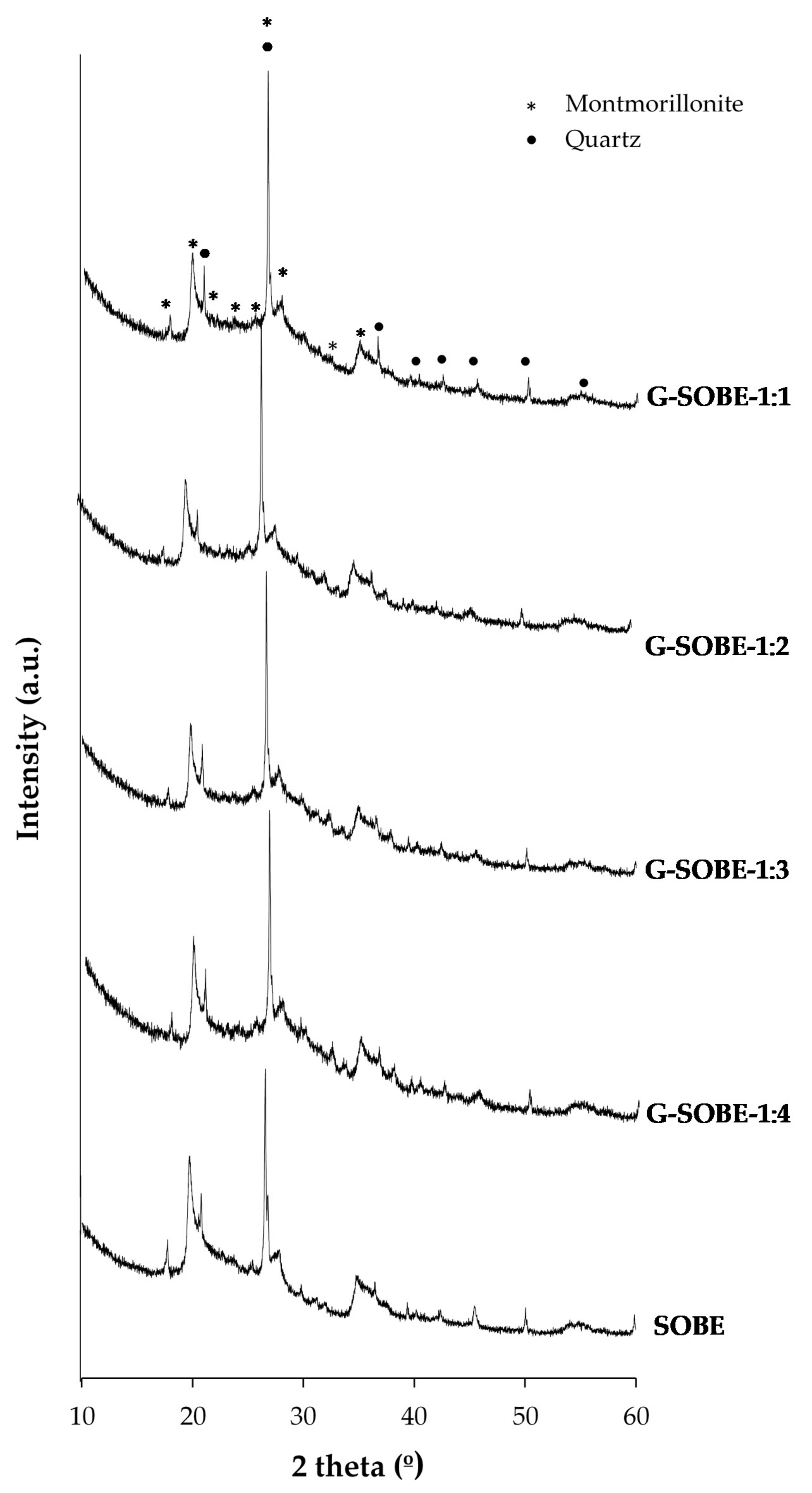

| Raw Material | Chemical Composition | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | MgO | K2O | CaO | TiO2 | Na2O | P2O5 | SO3 | MnO | LOI | |
| SOBE | 68.35 | 14.43 | 3.21 | 2.05 | 0.90 | 0.35 | 0.33 | 0.18 | 0.09 | 0.04 | 0.01 | 9.96 |
| Geopolymer | Water/Binder (Mass Ratio) | NaOH (g) | Na2SiO3 | Water (g) | Ms | Na+ (mol kg−1) | Si/Al (Molar Ratio) | Si/Na (Molar Ratio) |
|---|---|---|---|---|---|---|---|---|
| G-SOBE-1:1 | 0.71 | 27.54 | 140 | 112.5 | 1.27 | 6 | 5.22 | 2.75 |
| G-SOBE-1:2 | 0.76 | 36.72 | 93.32 | 149.93 | 0.78 | 6 | 4.82 | 2.34 |
| G-SOBE-1:3 | 0.79 | 41.31 | 70.00 | 168.69 | 0.56 | 6 | 4.62 | 2.16 |
| G-SOBE-1:4 | 0.81 | 44.07 | 56.00 | 179.93 | 0.44 | 6 | 4.50 | 2.06 |
| Sample | G-SOBE-1:1 | G-SOBE-1:2 | G-SOBE-1:3 | G-SOBE-1:4 |
|---|---|---|---|---|
| Reaction degree (%) | 52.5 | 47.3 | 45.7 | 43.5 |
| Phase Composition (wt%) | ||
|---|---|---|
| Sample | Montmorillonite | α-Quartz |
| Raw material | 82.4 ± 0.2 | 17.6 ± 0.3 |
| G-SOBE-1:1 | 81.8 ± 0.2 | 18.2 ± 0.2 |
| G-SOBE-1:2 | 81.3 ± 0.2 | 18.7 ± 0.3 |
| G-SOBE-1:3 | 81.7 ± 0.2 | 18.3 ± 0.5 |
| G-SOBE-1:4 | 80.5 ± 0.2 | 19.5 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado-Plana, P.; Rodríguez-Expósito, A.; Bueno-Rodríguez, S.; Pérez-Villarejo, L.; Tobaldi, D.M.; Labrincha, J.A.; Eliche-Quesada, D. Effect of Activating Solution Modulus on the Synthesis of Sustainable Geopolymer Binders Using Spent Oil Bleaching Earths as Precursor. Sustainability 2021, 13, 7501. https://doi.org/10.3390/su13137501
Delgado-Plana P, Rodríguez-Expósito A, Bueno-Rodríguez S, Pérez-Villarejo L, Tobaldi DM, Labrincha JA, Eliche-Quesada D. Effect of Activating Solution Modulus on the Synthesis of Sustainable Geopolymer Binders Using Spent Oil Bleaching Earths as Precursor. Sustainability. 2021; 13(13):7501. https://doi.org/10.3390/su13137501
Chicago/Turabian StyleDelgado-Plana, P., A. Rodríguez-Expósito, S. Bueno-Rodríguez, L. Pérez-Villarejo, D. M. Tobaldi, J. A. Labrincha, and D. Eliche-Quesada. 2021. "Effect of Activating Solution Modulus on the Synthesis of Sustainable Geopolymer Binders Using Spent Oil Bleaching Earths as Precursor" Sustainability 13, no. 13: 7501. https://doi.org/10.3390/su13137501






