Pressure-Induced Geopolymerization in Alkali-Activated Fly Ash
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Materials
2.2. Sample Preparation
2.3. Experimental Methods
- -
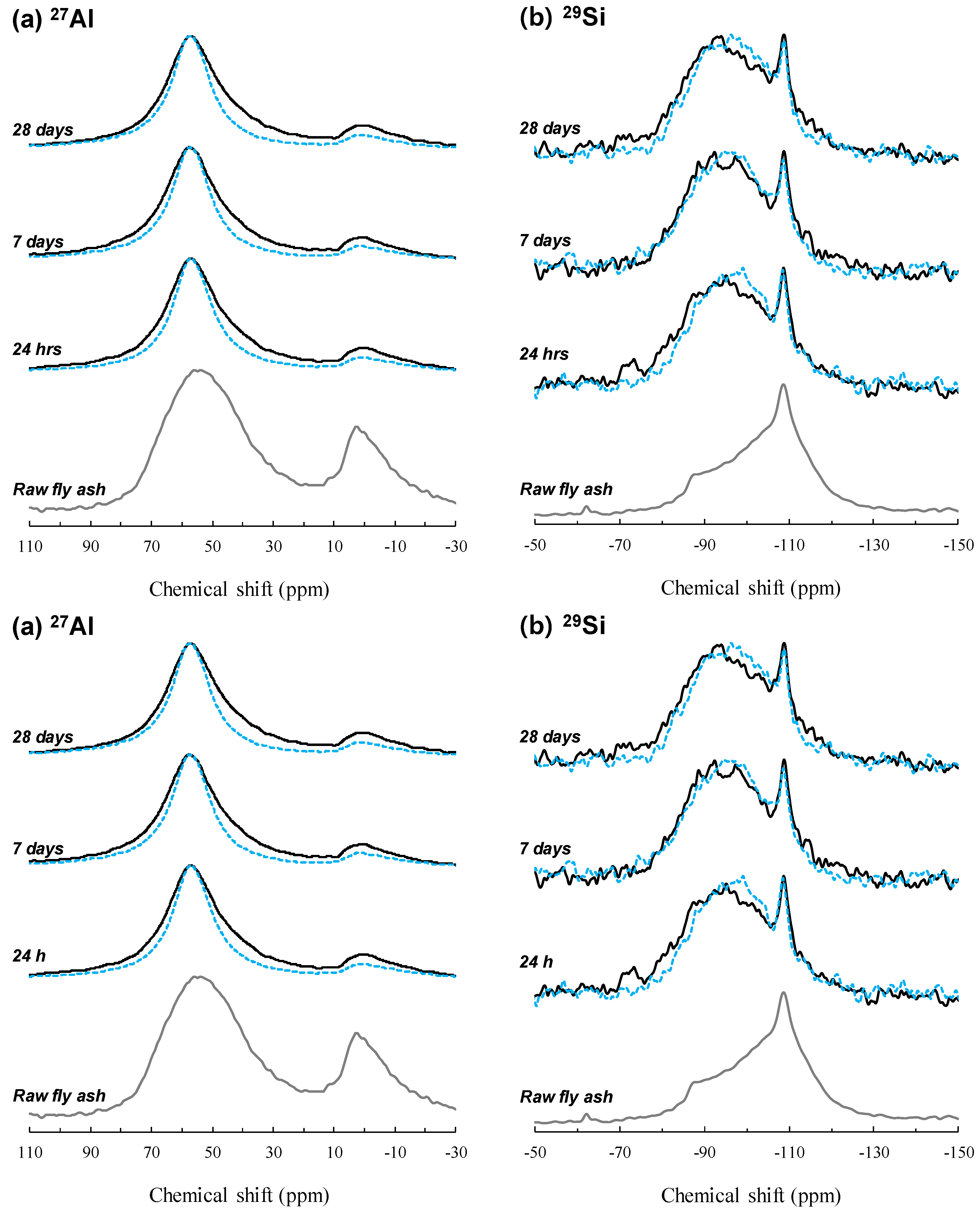
- 29Si MAS NMR spectra: data acquisition at a transmitter frequency of 119.14 MHz, a pulse length of 30° (2.2 µs), a spinning rate of 10 kHz, and a relaxation delay of 22 s, using a 5 mm HX-CPMAS probe and a 5 mm zirconia rotor. The chemical shifts were referenced to TMS (0 ppm).
- -
- 27Al MAS NMR spectra: data acquisition at a transmitter frequency of 156.32 MHz, a pulse length of 30° (1.8 µs), a spinning rate of 22 kHz, and a relaxation delay of 2 s, using a 2.5 mm HX-CPMAS probe and a 2.5 mm low Al zirconia rotor. The chemical shifts were referenced to aqueous AlCl3 (0 ppm).
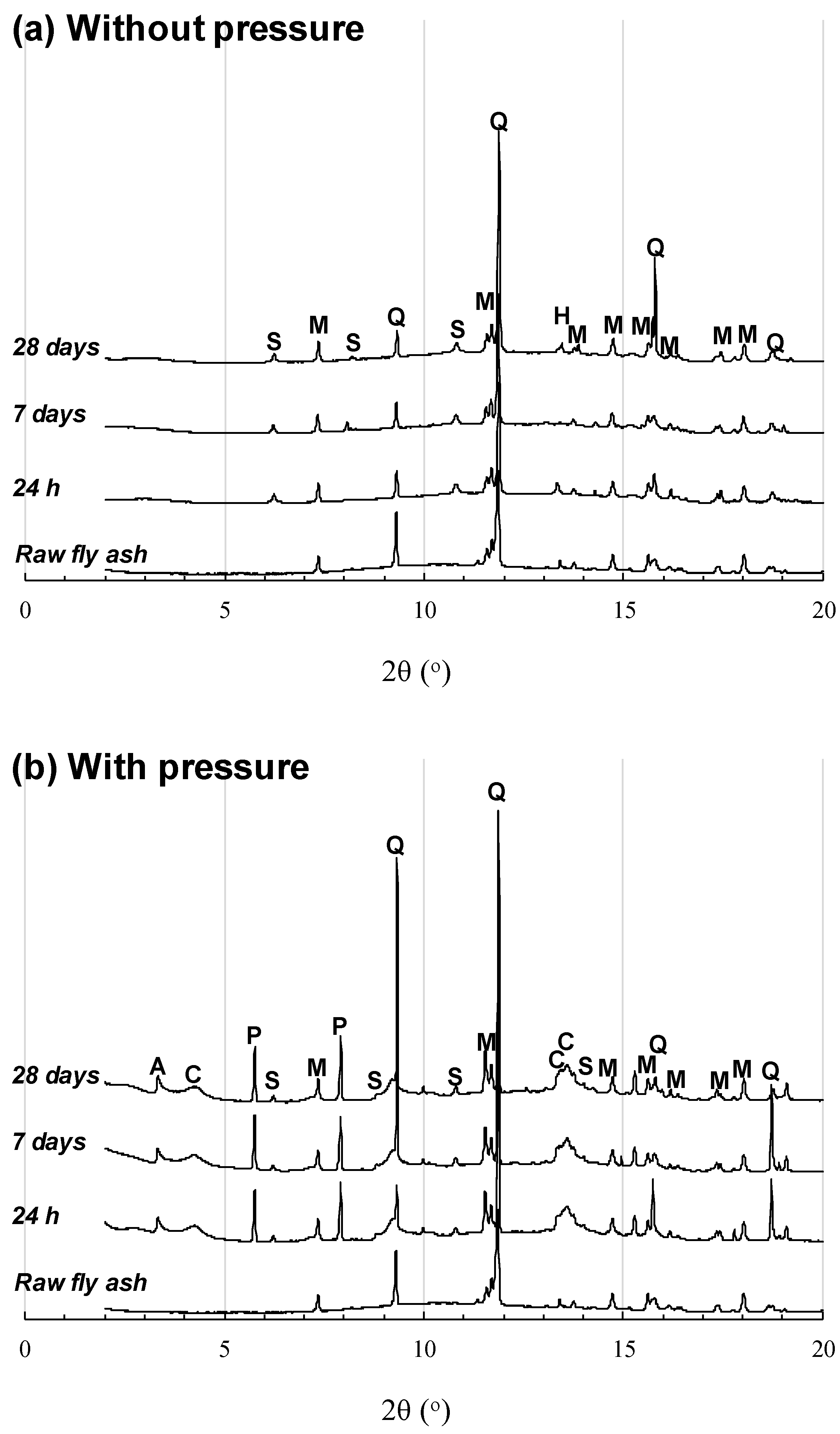
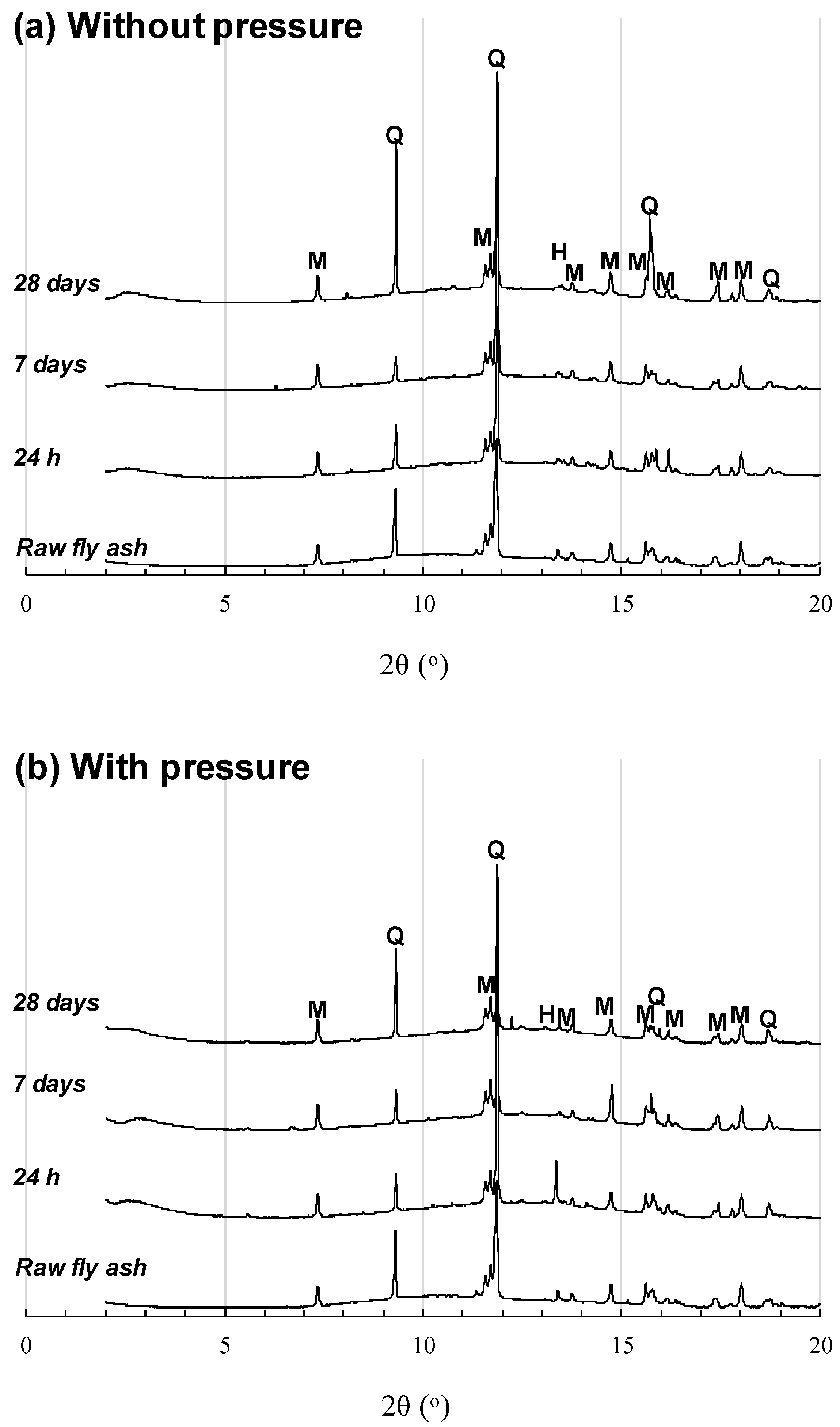
3. Results
3.1. NaOH-Activated Samples
3.2. Sodium Silicate-Series Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Provis, J.L. Alkali-activated materials. Cem. Concr. Res. 2017, in press. [Google Scholar] [CrossRef]
- Provis, J.L.; Palomo, A.; Shi, C. Advances in understanding alkali-activated materials. Cem. Concr. Res. 2015, 78, 110–125. [Google Scholar] [CrossRef] [Green Version]
- McLellan, B.C.; Williams, R.P.; Lay, J.; van Riessen, A.; Corder, G.D. Costs and carbon emissions for geopolymer pastes in comparison to ordinary portland cement. J. Clean. Prod. 2011, 19, 1080–1090. [Google Scholar] [CrossRef] [Green Version]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; van Deventer, J.S. The role of inorganic polymer technology in the development of ‘green concrete’. Cem. Concr. Res. 2007, 37, 1590–1597. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L. Durability of Alkali-Activated Materials: Progress and Perspectives. J. Am. Ceram. Soc. 2014, 97, 997–1008. [Google Scholar] [CrossRef]
- Provis, J.L. Geopolymers and other alkali activated materials: Why, how, and what? Mater. Struct. 2014, 47, 11–25. [Google Scholar] [CrossRef]
- Jang, J.; Park, S.; Lee, H. Physical barrier effect of geopolymeric waste form on diffusivity of cesium and strontium. J. Hazard. Mater. 2016, 318, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Kriven, W.M. Geopolymer-Based Composites. In Comprehensive Composite Materials II; Beaumont, P.W.R., Zweben, C.H., Eds.; Elsevier: Oxford, UK, 2018; pp. 269–280. [Google Scholar]
- Moon, J.; Bae, S.; Celik, K.; Yoon, S.; Kim, K.-H.; Kim, K.S.; Monteiro, P.J. Characterization of natural pozzolan-based geopolymeric binders. Cem. Concr. Compos. 2014, 53, 97–104. [Google Scholar] [CrossRef]
- Singh, G.B.; Subramaniam, K.V. Evaluation of sodium content and sodium hydroxide molarity on compressive strength of alkali activated low-calcium fly ash. Cem. Concr. Compos. 2017, 81, 122–132. [Google Scholar] [CrossRef]
- Walkley, B.; Rees, G.J.; Nicolas, R.S.; van Deventer, J.S.; Hanna, J.V.; Provis, J.L. New Structural Model of Hydrous Sodium Aluminosilicate Gels and the Role of Charge-Balancing Extra-Framework Al. J. Phys. Chem. C 2018, 122, 5673–5685. [Google Scholar] [CrossRef]
- Jang, J.; Park, S.; Kim, G.; Lee, H.-K. Stability of MgO-modified geopolymeric gel structure exposed to a CO2-rich environment. Constr. Build. Mater. 2017, 151, 178–185. [Google Scholar] [CrossRef]
- Park, S.-M.; Jang, J.-G.; Chae, S.; Lee, H.-K. An NMR Spectroscopic Investigation of Aluminosilicate Gel in Alkali-Activated Fly Ash in a CO2-Rich Environment. Materials 2016, 9, 308. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Lee, H. Influence of the slag content on the chloride and sulfuric acid resistances of alkali-activated fly ash/slag paste. Cem. Concr. Compos. 2016, 72, 168–179. [Google Scholar] [CrossRef]
- Khalid, H.R.; Lee, N.; Park, S.; Abbas, N.; Lee, H. Synthesis of geopolymer-supported zeolites via robust one-step method and their adsorption potential. J. Hazard. Mater. 2018, 353, 522–533. [Google Scholar] [CrossRef] [PubMed]
- Al-Zboon, K.; Al-Harahsheh, M.S.; Hani, F.B. Fly ash-based geopolymer for Pb removal from aqueous solution. J. Hazard. Mater. 2011, 188, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.G.; Park, S.M.; Lee, H.K. Cesium and Strontium Retentions Governed by Aluminosilicate Gel in Alkali-Activated Cements. Materials 2017, 10, 447. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Sun, Z.; Tao, D.; Xu, Y.; Li, P.; Cui, H.; Zhai, J. Immobilization of simulated radionuclide 133Cs+ by fly ash-based geopolymer. J. Hazard. Mater. 2013, 262, 325–331. [Google Scholar] [CrossRef] [PubMed]
- De Silva, P.; Sagoe-Crenstil, K. Medium-term phase stability of Na2O–Al2O3–SiO2–H2O geopolymer systems. Cem. Concr. Res. 2008, 38, 870–876. [Google Scholar] [CrossRef]
- Palomo, A.; Alonso, S.; Fernandez-Jiménez, A.; Sobrados, I.; Sanz, J. Alkaline activation of fly ashes: NMR study of the reaction products. J. Am. Ceram. Soc. 2004, 87, 1141–1145. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A. Characterisation of fly ashes. Potential reactivity as alkaline cements. Fuel 2003, 82, 2259–2265. [Google Scholar] [CrossRef]
- Jang, J.G.; Lee, H.-K. Effect of fly ash characteristics on delayed high-strength development of geopolymers. Constr. Build. Mater. 2016, 102, 260–269. [Google Scholar] [CrossRef]
- Lee, N.; Khalid, H.R.; Lee, H. Synthesis of mesoporous geopolymers containing zeolite phases by a hydrothermal treatment. Microporous Mesoporous Mater. 2016, 229, 22–30. [Google Scholar] [CrossRef]
- Xu, R.; Pang, W.; Yu, J.; Huo, Q.; Chen, J. Chemistry of Zeolites and Related Porous Materials: Synthesis and Structure; John Wiley & Sons: New York, NY, USA, 2009. [Google Scholar]
- Lee, N.; Khalid, H.R.; Lee, H. Adsorption characteristics of cesium onto mesoporous geopolymers containing nano-crystalline zeolites. Microporous Mesoporous Mater. 2017, 242, 238–244. [Google Scholar] [CrossRef]
- Oh, J.E.; Moon, J.; Mancio, M.; Clark, S.M.; Monteiro, P.J. Bulk modulus of basic sodalite, Na8[AlSiO4]6(OH)2·2H2O, a possible zeolitic precursor in coal-fly-ash-based geopolymers. Cem. Concr. Res. 2011, 41, 107–112. [Google Scholar] [CrossRef]
- De Lacaillerie, J.-B.d.E.; Fretigny, C.; Massiot, D. MAS NMR spectra of quadrupolar nuclei in disordered solids: The Czjzek model. J. Magn. Reson. 2008, 192, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Merwin, L.; Sebald, A.; Rager, H.; Schneider, H. 29Si and 27Al MAS NMR spectroscopy of mullite. Phys. Chem. Miner. 1991, 18, 47–52. [Google Scholar] [CrossRef]
- Madani, A.; Aznar, A.; Sanz, J.; Serratosa, J. Silicon-29 and aluminum-27 NMR study of zeolite formation from alkali-leached kaolinites: Influence of thermal preactivation. J. Phys. Chem. 1990, 94, 760–765. [Google Scholar] [CrossRef]
- Park, S.; Jang, J.; Lee, N.; Lee, H. Physicochemical properties of binder gel in alkali-activated fly ash/slag exposed to high temperatures. Cem. Concr. Res. 2016, 89, 72–79. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L.; Walkley, B.; Nicolas, R.S.; Gehman, J.D.; Brice, D.G.; Kilcullen, A.R.; Duxson, P.; van Deventer, J.S. Gel nanostructure in alkali-activated binders based on slag and fly ash, and effects of accelerated carbonation. Cem. Concr. Res. 2013, 53, 127–144. [Google Scholar] [CrossRef] [Green Version]
- Kowalczyk, G.; Roberts, J.E. Solid state 29Si NMR determination of crystalline silica in natural iron oxide pigments. Anal. Chim. Acta 1994, 286, 25–35. [Google Scholar] [CrossRef]
- White, C.E.; Provis, J.L.; Bloomer, B.; Henson, N.J.; Page, K. In situ X-ray pair distribution function analysis of geopolymer gel nanostructure formation kinetics. Phys. Chem. Chem. Phys. 2013, 15, 8573–8582. [Google Scholar] [CrossRef] [PubMed]
- White, C.E.; Provis, J.L.; Llobet, A.; Proffen, T.; van Deventer, J.S. Evolution of local structure in geopolymer gels: An in situ neutron pair distribution function analysis. J. Am. Ceram. Soc. 2011, 94, 3532–3539. [Google Scholar] [CrossRef]
- Lloyd, R. Accelerated ageing of geopolymers. In Geopolymers: Structure, Processing, Properties and Industrial Applications; Woodhead Publishing: Sawston, UK, 2009; pp. 139–166. [Google Scholar]
- Rashid, S.; Barnes, P.; Bensted, J.; Turrillas, X. Conversion of calcium aluminate cement hydrates re-examined with synchrotron energy-dispersive diffraction. J. Mater. Sci. Lett. 1994, 13, 1232–1234. [Google Scholar] [CrossRef]
- Rees, C.A.; Provis, J.L.; Lukey, G.C.; van Deventer, J.S. Attenuated total reflectance fourier transform infrared analysis of fly ash geopolymer gel aging. Langmuir 2007, 23, 8170–8179. [Google Scholar] [CrossRef] [PubMed]
- Rees, L.V.; Chandrasekhar, S. Formation of zeolite from the system Na2O-Al2O3-SiO2-H2O in alkaline medium (pH > 10). Zeolites 1993, 13, 524–533. [Google Scholar] [CrossRef]
- Katović, A.; Subotić, B.; Šmit, I.; Despotović, L.A. Crystallization of tetragonal (B8) and cubic (B1) modifications of zeolite NaP from freshly prepared gel. Part 1. Mechanism of the crystallization. Zeolites 1989, 9, 45–53. [Google Scholar] [CrossRef]
- Provis, J.L.; Lukey, G.C.; van Deventer, J.S. Do geopolymers actually contain nanocrystalline zeolites? A reexamination of existing results. Chem. Mater. 2005, 17, 3075–3085. [Google Scholar] [CrossRef]
- Kotova, O.; Shabalin, I.; Shushkov, D.; Kocheva, L. Hydrothermal synthesis of zeolites from coal fly ash. Adv. Appl. Ceram. 2016, 115, 152–157. [Google Scholar] [CrossRef]
- Król, M.; Morawska, J.; Mozgawa, W.; Pichór, W. Low-temperature synthesis of zeolite from perlite waste—Part I: Review of methods and phase compositions of resulting products. Mater. Sci.-Pol. 2014, 32, 503–513. [Google Scholar] [CrossRef]
- Duxson, P.; Mallicoat, S.; Lukey, G.; Kriven, W.; van Deventer, J. The effect of alkali and Si/Al ratio on the development of mechanical properties of metakaolin-based geopolymers. Colloids Surf. A Physicochem. Eng. Asp. 2007, 292, 8–20. [Google Scholar] [CrossRef]

| (wt%) | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | P2O5 | TiO2 | K2O | SO3 | LOI |
|---|---|---|---|---|---|---|---|---|---|---|
| 57.0 | 21.0 | 10.0 | 4.8 | 1.3 | 1.5 | 1.5 | 1.4 | 1.0 | 2.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.M.; Khalid, H.R.; Seo, J.H.; Yoon, H.N.; Son, H.M.; Kim, S.H.; Lee, N.K.; Lee, H.K.; Jang, J.G. Pressure-Induced Geopolymerization in Alkali-Activated Fly Ash. Sustainability 2018, 10, 3538. https://doi.org/10.3390/su10103538
Park SM, Khalid HR, Seo JH, Yoon HN, Son HM, Kim SH, Lee NK, Lee HK, Jang JG. Pressure-Induced Geopolymerization in Alkali-Activated Fly Ash. Sustainability. 2018; 10(10):3538. https://doi.org/10.3390/su10103538
Chicago/Turabian StylePark, Sol Moi, Hammad Raza Khalid, Joon Ho Seo, Hyun No Yoon, Hyeong Min Son, Seon Hyeok Kim, Nam Kon Lee, Haeng Ki Lee, and Jeong Gook Jang. 2018. "Pressure-Induced Geopolymerization in Alkali-Activated Fly Ash" Sustainability 10, no. 10: 3538. https://doi.org/10.3390/su10103538






