Understanding the Impact of Sustainable Pharmaceutical Packaging on the Chemical Stability of Silodosin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Excipient Compatibility Studies
2.3. Long-Term ICH Stability Study
2.4. HPLC Quantification of SLD and Related Substances
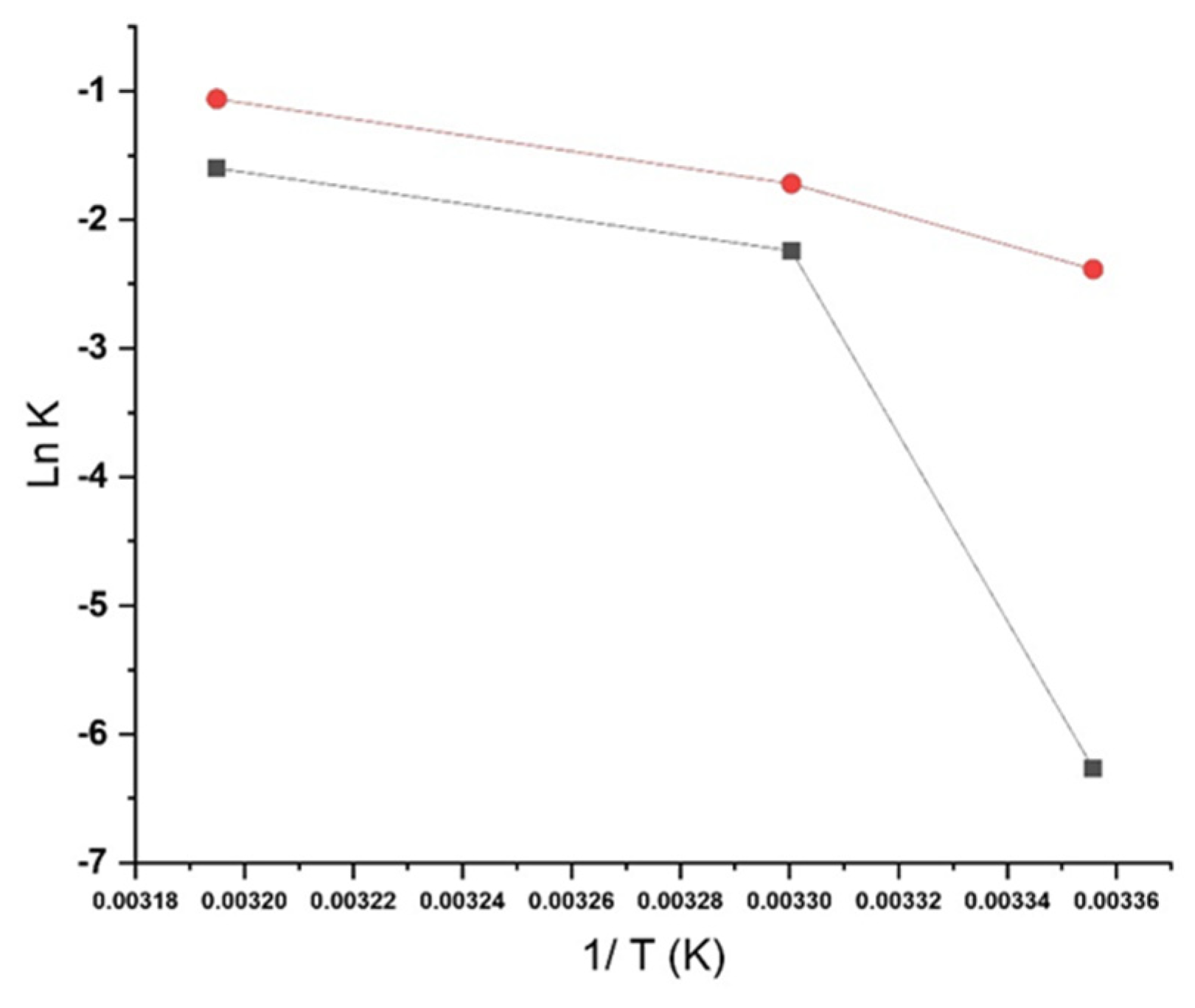
2.5. Stability Modeling
2.6. Permeability of Packing Materials
2.7. Statistics
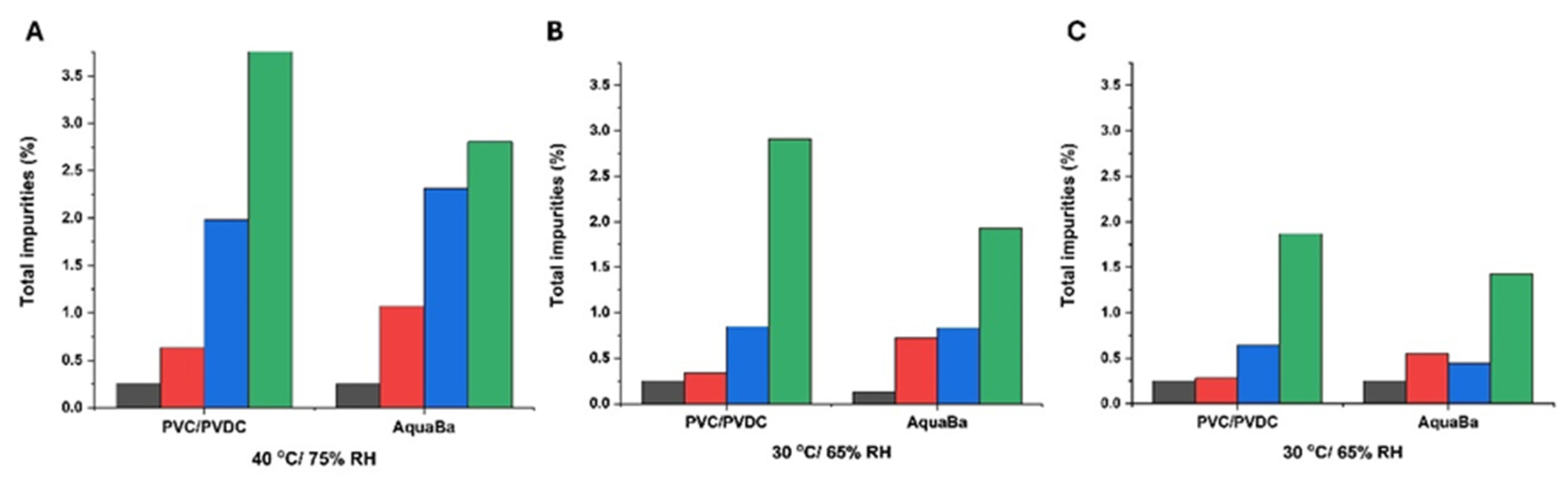
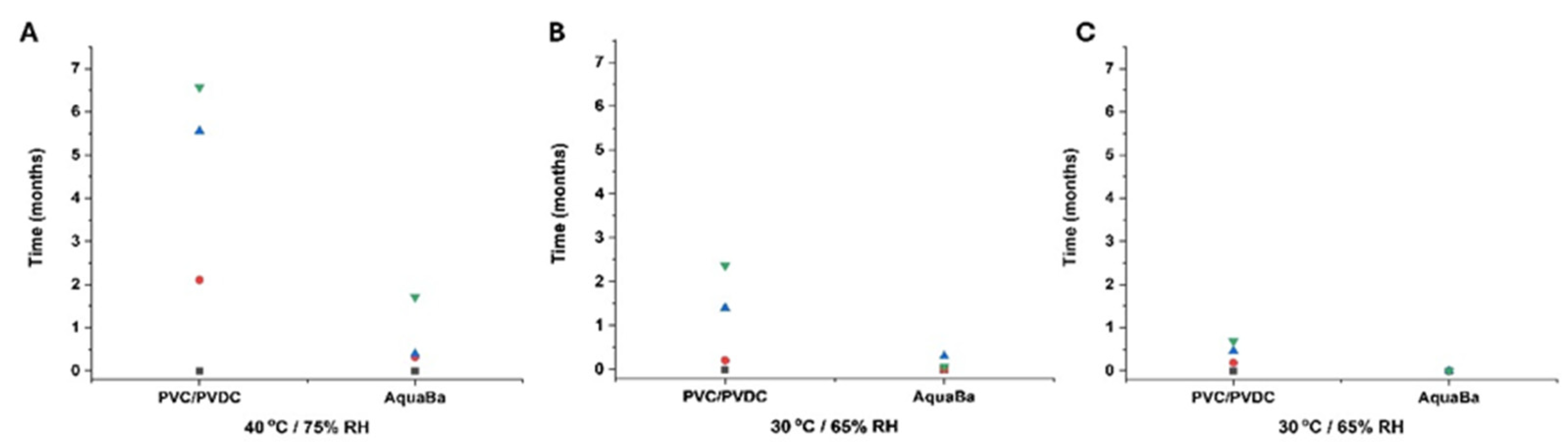
3. Results and Discussion
3.1. HPLC Validation
3.2. Excipient Compatibility Studies
3.3. Long-Term ICH Stability Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jindan, L.; Xiao, W.; Liping, X. Evolving Role of Silodosin for the Treatment of Urological Disorders—A Narrative Review. Drug Des. Devel Ther. 2022, 16, 2861–2884. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Alvarez, I.; Sanchez-Dengra, B.; Rodriguez-Galvez, R.; Ruiz-Picazo, A.; Gonzalez-Alvarez, M.; Garcia-Arieta, A.; Bermejo, M. Exploring a Bioequivalence Failure for Silodosin Products Due to Disintegrant Excipients. Pharmaceutics 2022, 14, 2565. [Google Scholar] [CrossRef] [PubMed]
- Naharros-Molinero, A.; Caballo-Gonzalez, M.A.; de la Mata, F.J.; Garcia-Gallego, S. Shell Formulation in Soft Gelatin Capsules: Design and Characterization. Adv. Healthc. Mater. 2024, 13, e2302250. [Google Scholar] [CrossRef] [PubMed]
- ICH Q8 (R2) Pharmaceutical Development. Reference Code: EMA/CHMP/ICH/167068/2004. 2014. Available online: https://www.ema.europa.eu/en/ich-q8-r2-pharmaceutical-development-scientific-guideline (accessed on 15 March 2025).
- ICH: Q 1 A (R2): Stability Testing of New Drug Substances and Products. Reference Code CPMP/ICH/2736/99. 2003. Available online: https://www.ema.europa.eu/en/ich-q1a-r2-stability-testing-new-drug-substances-drug-products-scientific-guideline (accessed on 15 March 2025).
- ICH: Q 3 A (R2): Impurities in New Drug Substances. Reference Code: CPMP/ICH/2737/99. 2006. Available online: https://www.ema.europa.eu/en/ich-q3a-r2-impurities-new-drug-substances-scientific-guideline (accessed on 15 March 2025).
- Zadbuke, N.; Shahi, S.; Gulecha, B.; Padalkar, A.; Thube, M. Recent trends and future of pharmaceutical packaging technology. J. Pharm. Bioallied Sci. 2013, 5, 98–110. [Google Scholar] [CrossRef] [PubMed]
- AquaBa Pahrmaceutical Packing Material. Available online: https://kaatimex.bg/wp-content/uploads/2015/12/Bilcare_Aquaba.pdf (accessed on 15 March 2025).
- Pharmaceutical Pakcing Material PVC. Available online: https://rijerplastic.com/news-detail/Pharmaceutical-packaging-material-PVC. (accessed on 15 March 2025).
- Van Dooren, A.A. PVC as pharmaceutical packaging material. A literature survey with special emphasis on plasticized PVC bags. Pharm. Weekbl. Sci. 1991, 13, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Jaime, S.; Alves, R.; Bocoli, P. Moisture and oxygen barrier properties of glass, PET and HDPE bottles for pharmaceutical products. J. Drug Deliv. Sci. Technol. 2022, 71, 103330. [Google Scholar] [CrossRef]
- Pan, Z.; Bora, M.; Gee, R.; Dauskardt, R.H. Water vapor transmission rate measurement for moisture barriers using infrared imaging. Mater. Chem. Phys. 2023, 308, 128289. [Google Scholar] [CrossRef]
- WVTR of Different Packing Materials. Available online: https://liveoresearch.com/en/product/aquaba/?utm_source=chatgpt.com (accessed on 12 November 2025).
- Moir, P. Study of a Range of Blister Packaging Materials Using the New Technique of Moisture Profiling to Give Early Indications of Moisture Barrier Propertie. Available online: https://f1000research-files.f1000.com/posters/docs/f1000research-113128.pdf?_ga=undefined (accessed on 12 November 2025).
- Serrano, D.R.; Fernandez-Garcia, R.; Mele, M.; Healy, A.M.; Lalatsa, A. Designing Fast-Dissolving Orodispersible Films of Amphotericin B for Oropharyngeal Candidiasis. Pharmaceutics 2019, 11, 369. [Google Scholar] [CrossRef] [PubMed]
- Serrano, D.R.; Walsh, D.; O’Connell, P.; Mugheirbi, N.A.; Worku, Z.A.; Bolas-Fernandez, F.; Galiana, C.; Dea-Ayuela, M.A.; Healy, A.M. Optimising the in vitro and in vivo performance of oral cocrystal formulations via spray coating. Eur. J. Pharm. Biopharm. 2018, 124, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gonzalez, O.; Ballesteros, M.P.; Torrado, J.J.; Serrano, D.R. Application of Accelerated Predictive Stability Studies in Extemporaneously Compounded Formulations of Chlorhexidine to Assess the Shelf Life. Molecules 2023, 28, 7925. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gonzalez, O.; Ramirez, I.O.; Ramirez, B.I.; O’Connell, P.; Ballesteros, M.P.; Torrado, J.J.; Serrano, D.R. Drug Stability: ICH versus Accelerated Predictive Stability Studies. Pharmaceutics 2022, 14, 2324. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Garcia, R.; Walsh, D.; O’Connell, P.; Passero, L.F.D.; de Jesus, J.A.; Laurenti, M.D.; Dea-Ayuela, M.A.; Ballesteros, M.P.; Lalatsa, A.; Bolas-Fernandez, F.; et al. Targeted Oral Fixed-Dose Combination of Amphotericin B-Miltefosine for Visceral Leishmaniasis. Mol. Pharm. 2025, 22, 1437–1448. [Google Scholar] [CrossRef] [PubMed]
- Directorate for the Quality of Medicines & HealthCare. European Pharmacopoeia, 11th ed.; Directorate for the Quality of Medicines & HealthCare: Strasbourg, France, 2022. [Google Scholar]
- Vishnuvardhan, C.; Saibaba, B.; Allakonda, L.; Swain, D.; Gananadhamu, S.; Srinivas, R.; Satheeshkumar, N. LC-ESI-MS/MS evaluation of forced degradation behaviour of silodosin: In vitro anti cancer activity evaluation of silodosin and major degradation products. J. Pharm. Biomed. Anal. 2017, 134, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pandeti, S.; Narender, T.; Prabhakar, S.; Reddy, T.J. Characterization of degradation products of silodosin under stress conditions by liquid chromatography/Fourier transform mass spectrometry. Rapid Commun. Mass Spectrom. 2017, 31, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Waterman, K.C.; Adami, R.C. Accelerated aging: Prediction of chemical stability of pharmaceuticals. Int. J. Pharm. 2005, 293, 101–125. [Google Scholar] [CrossRef] [PubMed]

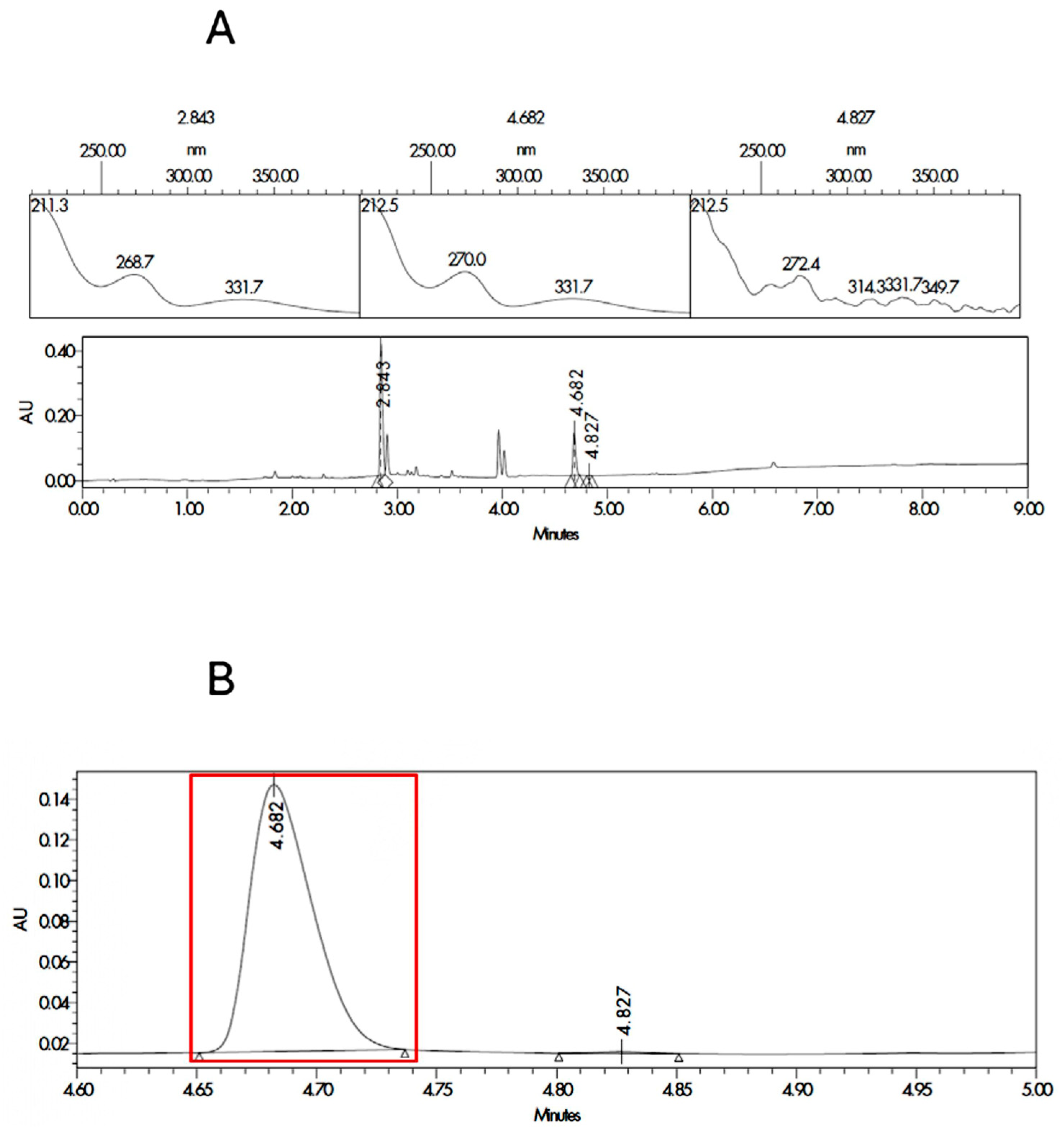
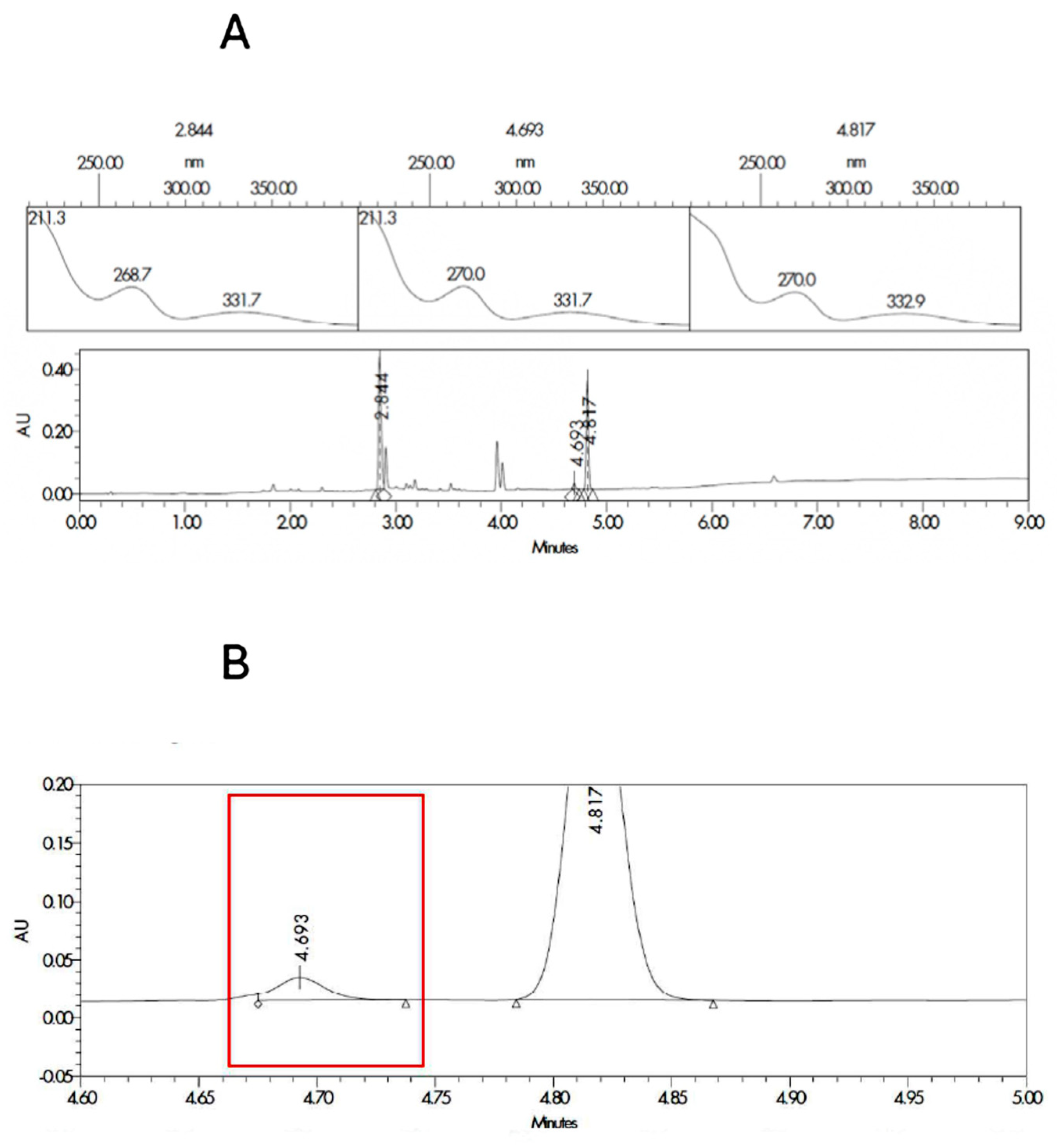
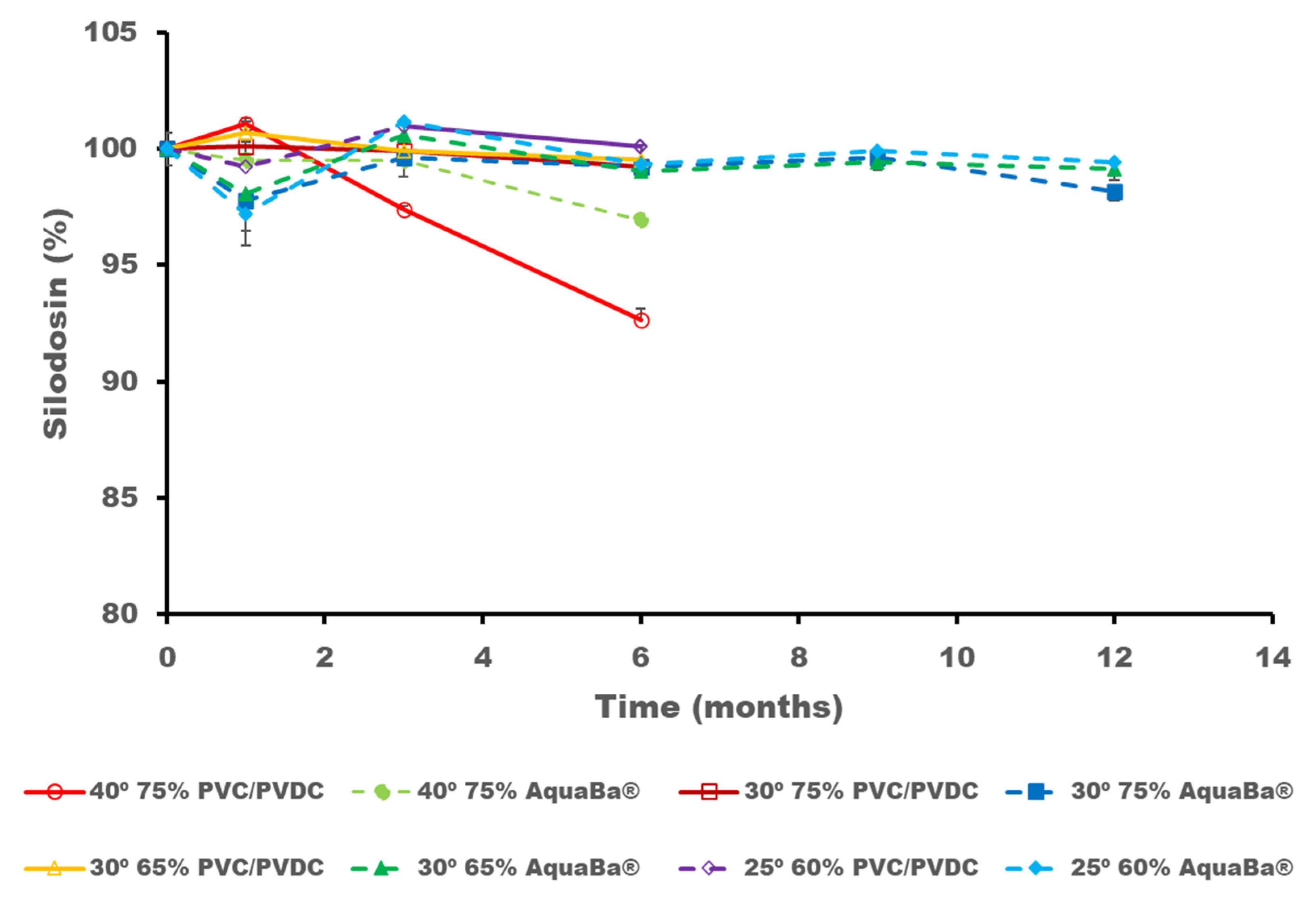
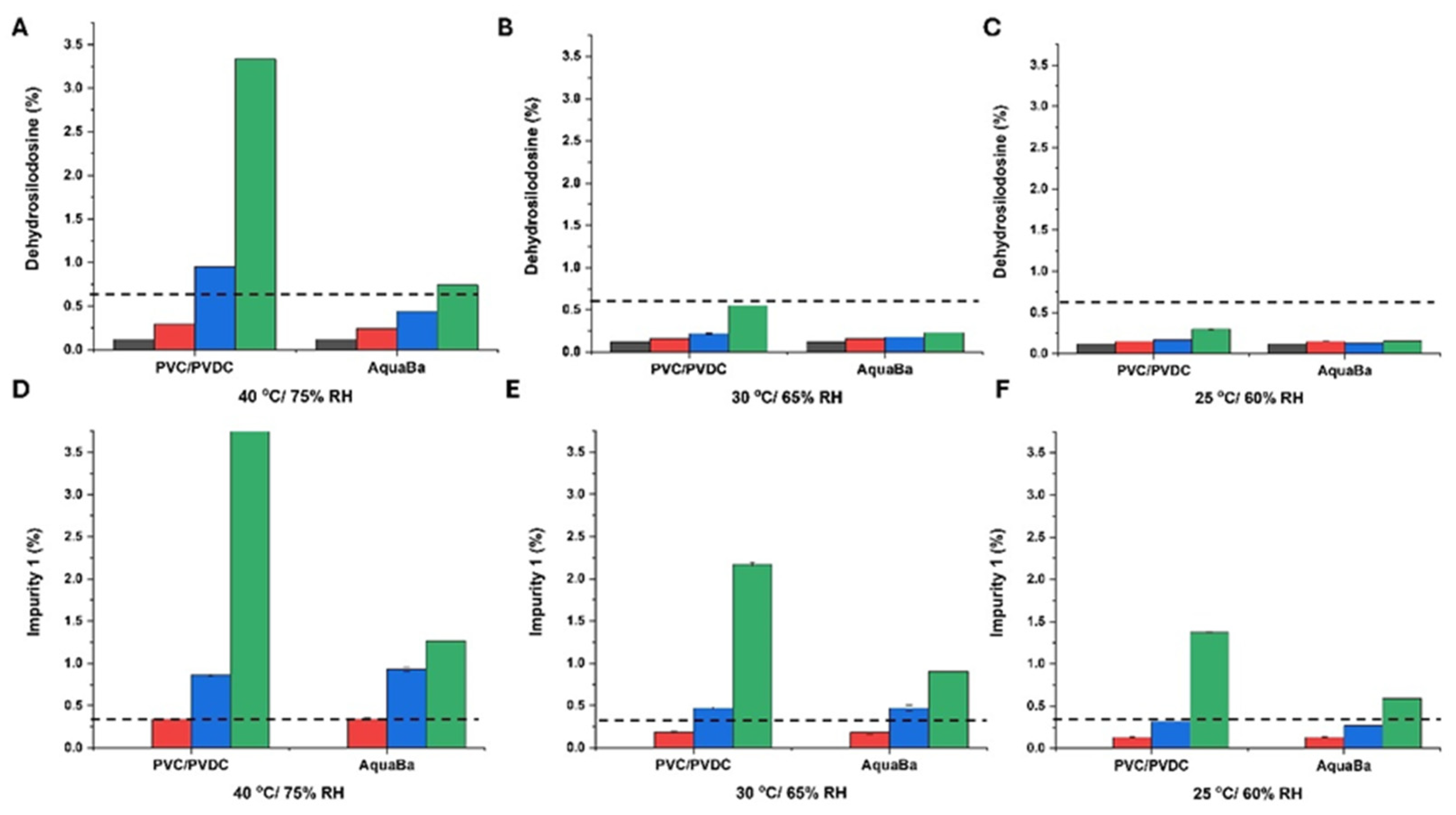
| Sample Code | Component |
|---|---|
| 1 | SLD |
| 2 | Capryol® 90 |
| 3 | Lauroyl macrogol-32 glycerides |
| 4 | BHT |
| 5 | SLD + Capryol® 90 |
| 6 | SLD + Lauroyl macrogol-32 glycerides |
| 7 | SLD + BHT |
| 8 | SLD + Capryol® 90 + Lauroyl macrogol-32 glycerides |
| 9 | SLD + Capryol® 90 + Lauroyl macrogol-32 glycerides + BHT |
| 10 | Capryol® 90 + Lauroyl macrogol-32 glycerides |
| 11 | Capryol® 90 + Lauroyl macrogol-32 glycerides + BHT |
| Time (min) | % Mobile Phase A | % Mobile Phase B |
|---|---|---|
| 0.01 | 78 | 22 |
| 13.00 | 78 | 22 |
| 28.00 | 15 | 85 |
| 37.00 | 78 | 22 |
| 45.00 | 78 | 22 |
| Kinetic Profile | 40 °C/75% RH | 30 °C/65% RH | 25 °C/60% RH | Average |
|---|---|---|---|---|
| Dehydrosilodosin | ||||
| Order 0 | 0.85 | 0.87 | 0.87 | 0.86 |
| Order 1 | 1.00 | 0.88 | 0.88 | 0.92 |
| order 2 | 0.99 | 0.87 | 0.87 | 0.91 |
| Avrami | 0.96 | 0.75 | 0.75 | 0.82 |
| Difussion | 0.90 | 0.85 | 0.80 | 0.85 |
| Impurity 1 | ||||
| Order 0 | 0.85 | 0.87 | 0.87 | 0.86 |
| Order 1 | 0.99 | 0.99 | 0.99 | 0.99 |
| order 2 | 0.92 | 0.92 | 0.93 | 0.92 |
| Avrami | 0.91 | 0.92 | 0.92 | 0.92 |
| Difussion | 0.85 | 0.85 | 0.85 | 0.85 |
| Kinetic Profile | 40 °C/75% RH | 30 °C/65% RH | 25 °C/60% RH | Average |
|---|---|---|---|---|
| Dehydrosilodosin | ||||
| Order 0 | 0.98 | 0.84 | * | 0.91 |
| Order 1 | 0.98 | 0.96 | * | 0.97 |
| order 2 | 0.99 | 0.92 | * | 0.96 |
| Avrami | 0.99 | 0.86 | * | 0.93 |
| Difussion | 0.64 | 0.72 | * | 0.77 |
| Impurity 1 | ||||
| Order 0 | 0.91 | 0.9 | 0.91 | 0.91 |
| Order 1 | 0.99 | 0.99 | 0.99 | 0.99 |
| order 2 | 0.92 | 0.93 | 0.93 | 0.93 |
| Avrami | 0.91 | 0.92 | 0.92 | 0.92 |
| Difussion | 0.85 | 0.85 | 0.85 | 0.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Visa, C.; Rodriguez, R.; Rincón, Á.; Peña, S.; Serrano, D.R.; Torrado, J.J. Understanding the Impact of Sustainable Pharmaceutical Packaging on the Chemical Stability of Silodosin. Pharmaceutics 2025, 17, 1548. https://doi.org/10.3390/pharmaceutics17121548
Visa C, Rodriguez R, Rincón Á, Peña S, Serrano DR, Torrado JJ. Understanding the Impact of Sustainable Pharmaceutical Packaging on the Chemical Stability of Silodosin. Pharmaceutics. 2025; 17(12):1548. https://doi.org/10.3390/pharmaceutics17121548
Chicago/Turabian StyleVisa, Celia, Roi Rodriguez, Ángela Rincón, Soledad Peña, Dolores Remedios Serrano, and Juan José Torrado. 2025. "Understanding the Impact of Sustainable Pharmaceutical Packaging on the Chemical Stability of Silodosin" Pharmaceutics 17, no. 12: 1548. https://doi.org/10.3390/pharmaceutics17121548
APA StyleVisa, C., Rodriguez, R., Rincón, Á., Peña, S., Serrano, D. R., & Torrado, J. J. (2025). Understanding the Impact of Sustainable Pharmaceutical Packaging on the Chemical Stability of Silodosin. Pharmaceutics, 17(12), 1548. https://doi.org/10.3390/pharmaceutics17121548







