Combined Experimental and Computational Approaches for Ternary Solid Dispersions to Enhance the Oral Bioavailability of Penfluridol
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Carrier Screening and SD Preparation
2.3. Solubility Studies
2.4. In Vitro Drug Release Studies
2.5. Machine Learning
2.6. Structural and Morphological Characterizations
2.7. Pharmacokinetic Studies in Rats
2.8. Analytical Methods
2.9. Pharmacokinetic and Statistical Analysis
3. Results and Discussion
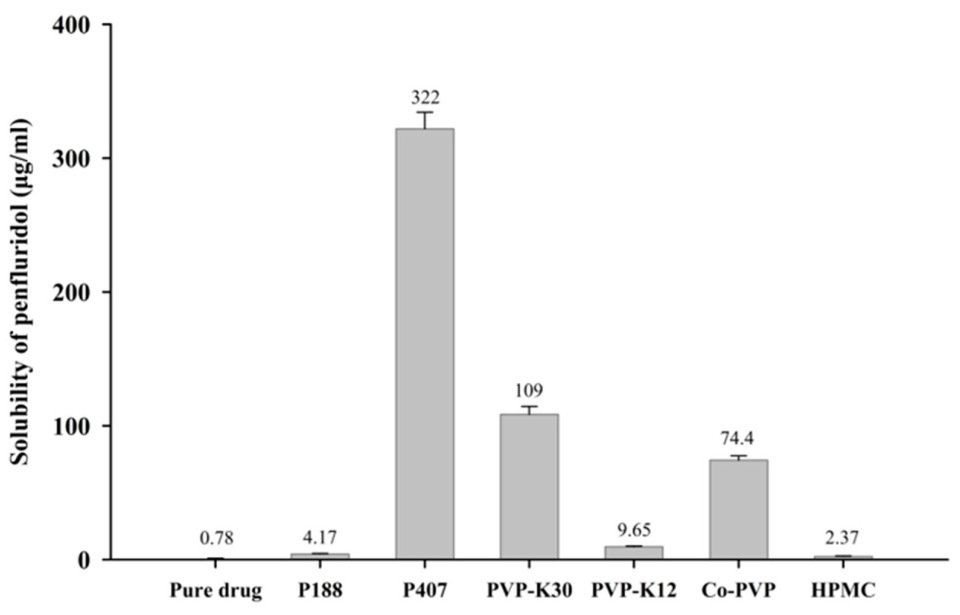
3.1. Carrier Screening and Solubility Enhancement
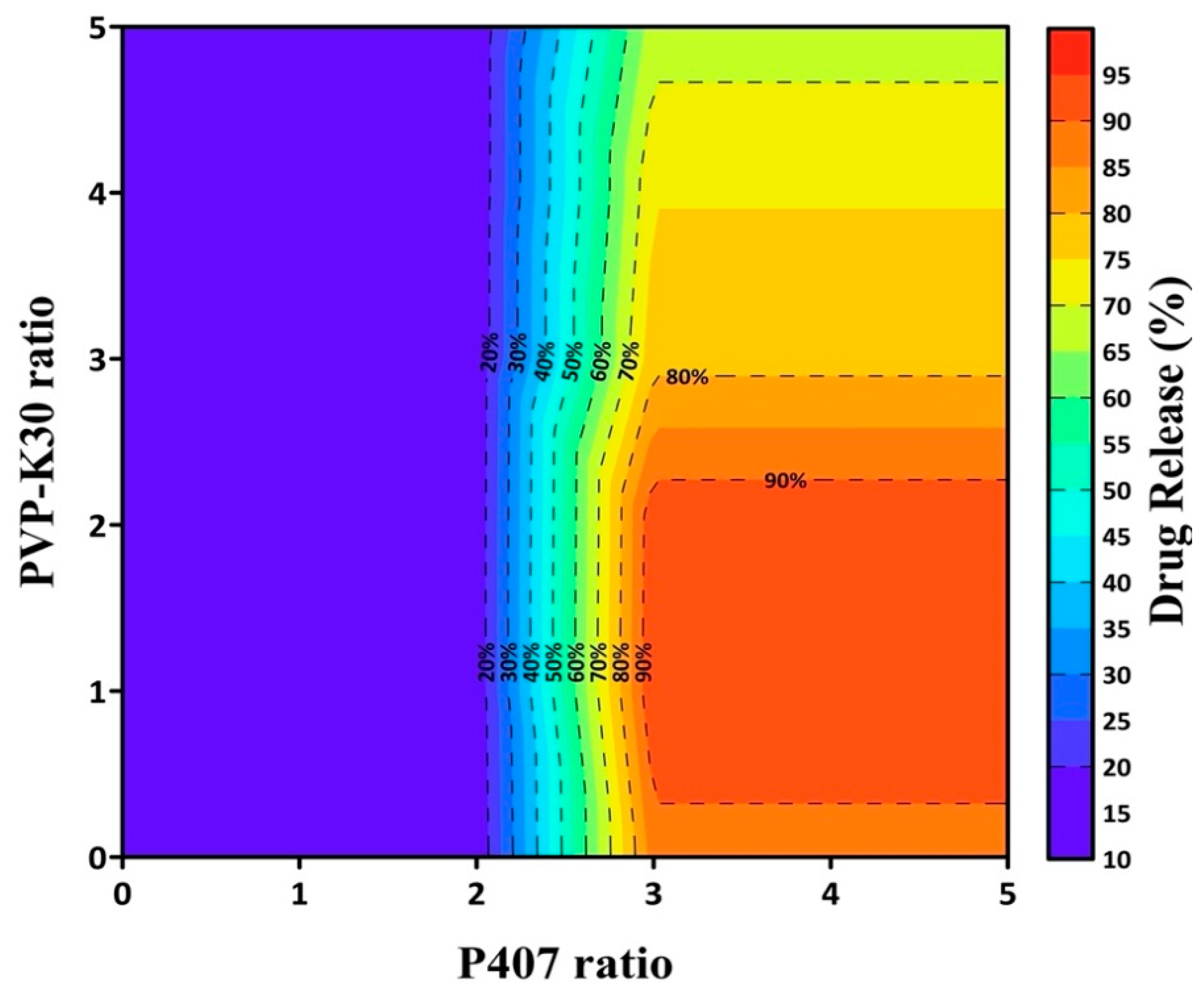
3.2. Formulation Optimization
3.3. In Vitro Characterization of PF-SD5
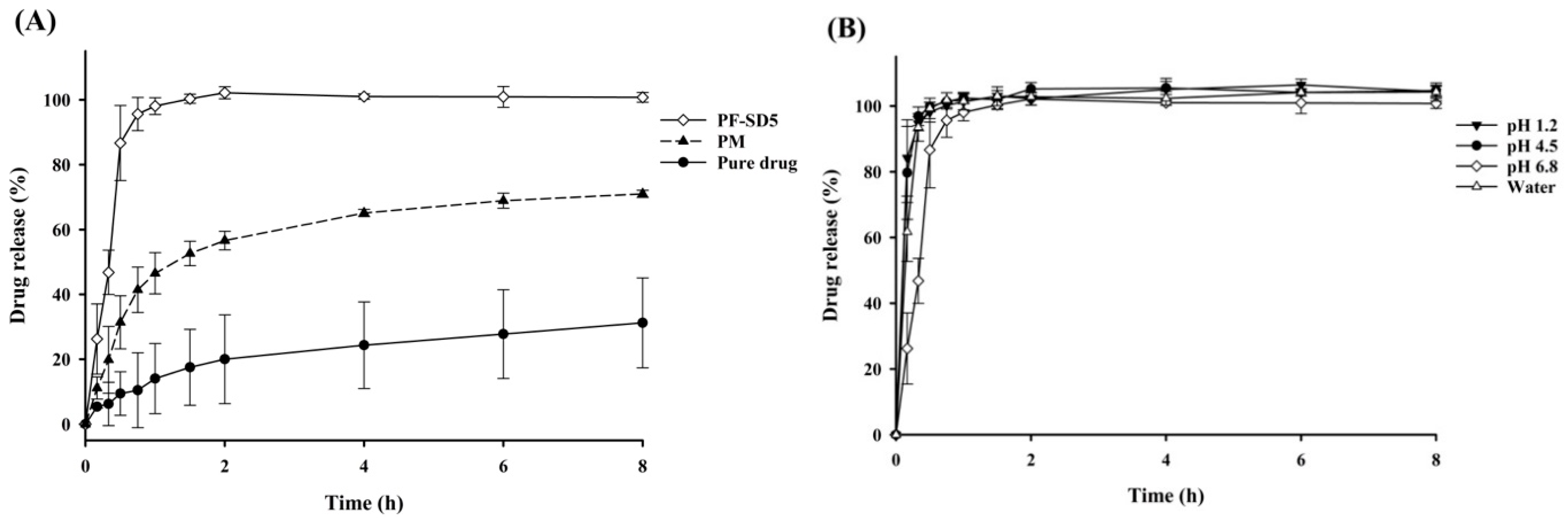
3.3.1. Dissolution Behavior
3.3.2. Structural Characterization of PF-SD5
3.3.3. Morphological Characterization of PF-SD5
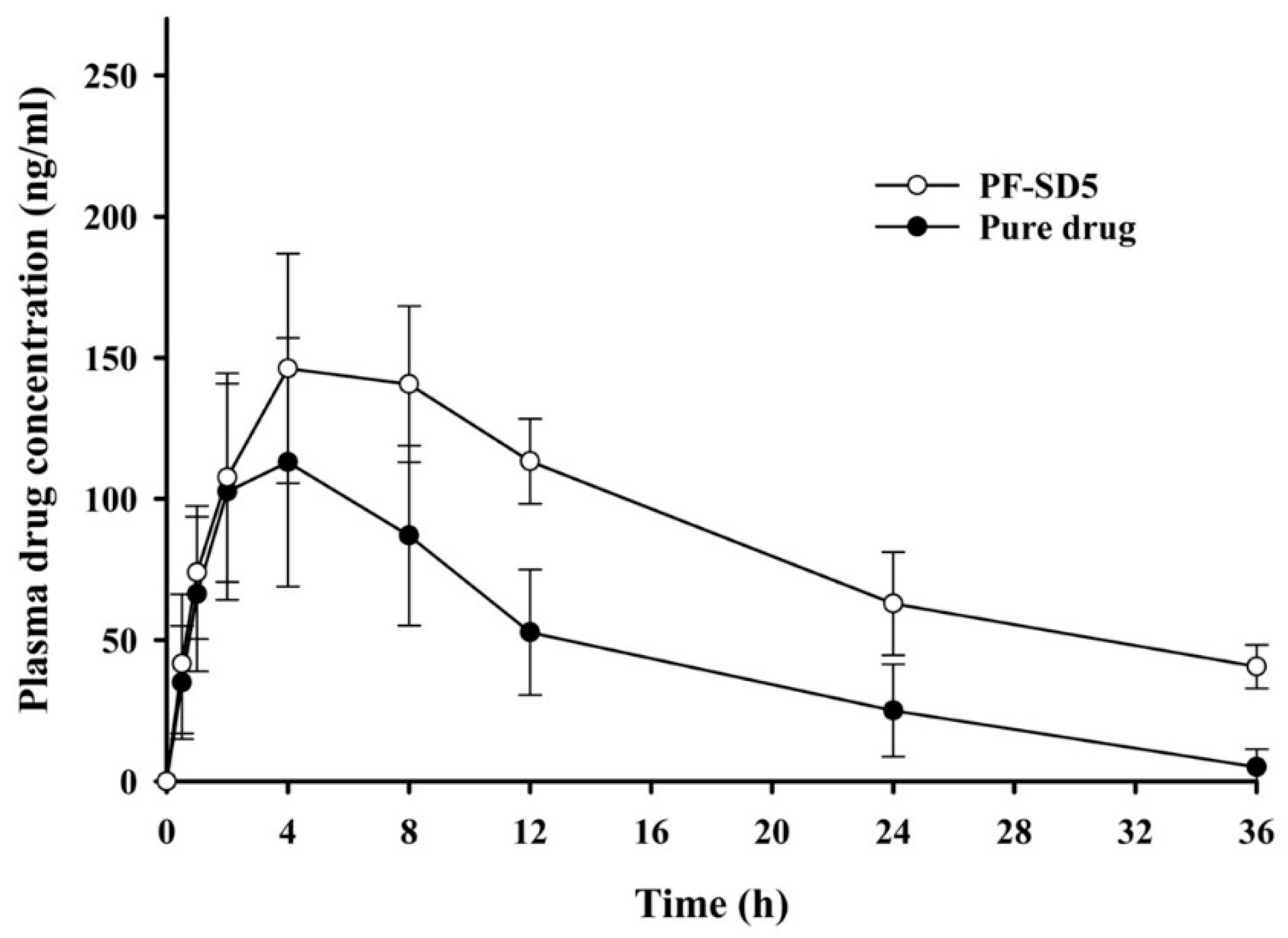
3.4. Pharmacokinetic Study in Rats
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alqahtani, M.S.; Kazi, M.; Alsenaidy, M.A.; Ahmad, M.Z. Advances in oral drug delivery. Front. Pharmacol. 2021, 12, 618411. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Thuy, V.N.; Van, T.V.; Dao, A.H.; Lee, B.-J. Nanostructured lipid carriers and their potential applications for versatile drug delivery via oral administration. OpenNano 2022, 8, 100064. [Google Scholar] [CrossRef]
- Naing, M.D.; Tsume, Y. Dissolution profiles of BCS Class II drugs generated by the gastrointestinal simulator alpha has an edge over the compendial USP II method. Eur. J. Pharm. Biopharm. 2024, 203, 114436. [Google Scholar] [CrossRef] [PubMed]
- Sandrapati, D.K.; Pamujula, N.H.; Katla, V.M. Challenges and solutions in enhancing oral bioavailability of BCS Class II anti-cancer drugs. Int. J. Res. Pharm. Sci. 2025, 16, 101–108. [Google Scholar] [CrossRef]
- Tuan, N.M.; Lee, C.H. Penfluridol as a candidate of drug repurposing for anticancer agent. Molecules 2019, 24, 3659. [Google Scholar] [CrossRef]
- Bhattacharyya, D.R.; Bhadra, D.R.; Roy, D.U.; Bhattacharyya, D.S.; Pal, D.J.; Saha, D.S.S. Resurgence of penfluridol: Merits and demerits. East. J. Psychiatry 2021, 18, 23–29. [Google Scholar] [CrossRef]
- Migdaloft, B.H.; Grindel, J.M.; Heykants, J.J.P.; Janssen, P.A.J. Penfluridol: A neuroleptic drug designed for long duration of action. Drug Metab. Rev. 1979, 9, 281–299. [Google Scholar] [CrossRef]
- Claghorn, J.L.; Mathew, R.J.; Mirabi, M. Penfluridol: A long acting oral antipsychotic drug. J. Clin. Psychiatry 1979, 40, 107–109. [Google Scholar]
- Lodhi, D.S.; Panwar, A.S.; Dongre, N. Review study on analysis of the solubility of biopharmaceutical classification system class ii drugs in a self-emulsifying drug delivery system. Asian J. Pharm. Clin. Res. 2021, 15, 36–45. [Google Scholar] [CrossRef]
- Kenguva, G.; Rout, S.R.; Kar, A.; Giri, L.; Mahapatra, S.K.; Shaikh, T.R.; Baidya, D.; Shelke, N.; Dandela, R. Synthesis, characterization and theoretical investigations of the newly developed molecular salts of an anti-psychotic drug (penfluridol). J. Mol. Struct. 2025, 1328, 141392. [Google Scholar] [CrossRef]
- Zhuang, X.; Tian, X.; Zheng, Y.; Lan, N.; Liu, L.; Zhang, R.; Liu, Y. Formulation and physicochemical characterisation of a novel self-microemulsifying delivery system as hydrotropic and solubilising agent for penfluridol. Procedia Eng. 2011, 18, 59–65. [Google Scholar] [CrossRef]
- Kumar, L.; Amin, A.; Bansal, A.K. Salt selection in drug development. Pharm. Technol. 2008, 32, 128–146. [Google Scholar]
- Evans, R. Drawbacks of self-micro emulsifying drug delivery system (SMEDDS) developed by poorly soluble drugs. Res. Rev. Drug Deliv. 2023, 7, 3–4. [Google Scholar]
- Chatterjee, B.; Hamed Almurisi, S.; Ahmed Mahdi Dukhan, A.; Mandal, U.K.; Sengupta, P. Controversies with self-emulsifying drug delivery system from pharmacokinetic point of view. Drug Deliv. 2016, 23, 3639–3652. [Google Scholar] [CrossRef]
- Bikiaris, D.N. Solid dispersions, part I: Recent evolutions and future opportunities in manufacturing methods for dissolution rate enhancement of poorly water-soluble drugs. Expert Opin. Drug Deliv. 2011, 8, 1501–1519. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Walia, M.; Harikumar, S.L. Solubility enhancement by solid dispersion method: A review. J. Drug Deliv. Ther. 2013, 3, 148–155. [Google Scholar] [CrossRef]
- Brouwers, J.; Brewster, M.E.; Augustijns, P. Supersaturating drug delivery systems: The answer to solubility-limited oral bioavailability? J. Pharm. Sci. 2009, 98, 2549–2572. [Google Scholar] [CrossRef]
- Vasconcelos, T.; Sarmento, B.; Costa, P. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov. Today 2007, 12, 1068–1075. [Google Scholar] [CrossRef]
- Nair, A.R.; Lakshman, Y.D.; Anand, V.S.K.; Sree, K.S.N.; Bhat, K.; Dengale, S.J. Overview of extensively employed polymeric carriers in solid dispersion technology. AAPS PharmSciTech 2020, 21, 309. [Google Scholar] [CrossRef]
- Bannigan, P.; Aldeghi, M.; Bao, Z.; Häse, F.; Aspuru-Guzik, A.; Allen, C. Machine learning directed drug formulation development. Adv. Drug Deliv. Rev. 2021, 175, 113806. [Google Scholar] [CrossRef]
- Protopapa, C.; Siamidi, A.; Eneli, A.A.; Elbadawi, M.; Vlachou, M. Machine learning predicts drug release profiles and kinetic parameters based on tablets’ formulations. AAPS J. 2025, 27, 124. [Google Scholar] [CrossRef] [PubMed]
- Bharathi, M.; Kamaraj, R.; Murugaanandam, S.; Navyaja, K.; Kumar, T.S. A data-driven approach to predict the in vitro dissolution time of sustained-release tablets using raw material databases and machine learning algorithms. Pharmacia 2024, 71, 1–7. [Google Scholar] [CrossRef]
- Yang, Y.; Ye, Z.; Su, Y.; Zhao, Q.; Li, X.; Ouyang, D. Deep learning for in vitro prediction of pharmaceutical formulations. Acta Pharm. Sin. B 2019, 9, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Grigoryan, A.; Helfrich, S.; Lequeux, V.; Lapras, B.; Marchand, C.; Merienne, C.; Bruno, F.; Mazet, R.; Pirot, F. Smart formulation: AI-driven web platform for optimization and stability prediction of compounded pharmaceuticals using KNIME. Pharmaceuticals 2025, 18, 1240. [Google Scholar] [CrossRef]
- Momeni, M.; Afkanpour, M.; Rakhshani, S.; Mehrabian, A.; Tabesh, H. A prediction model based on artificial intelligence techniques for disintegration time and hardness of fast disintegrating tablets in pre-formulation tests. BMC Med. Inform. Decis. Mak. 2024, 24, 88. [Google Scholar] [CrossRef]
- Kolašinac, N.; Kachrimanis, K.; Homšek, I.; Grujić, B.; Đurić, Z.; Ibrić, S. Solubility enhancement of desloratadine by solid dispersion in poloxamers. Int. J. Pharm. 2012, 436, 161–170. [Google Scholar] [CrossRef]
- Rusdin, A.; Mohd Gazzali, A.; Ain Thomas, N.; Megantara, S.; Aulifa, D.L.; Budiman, A.; Muchtaridi, M. Advancing drug delivery paradigms: Polyvinyl pyrolidone (PVP)-based amorphous solid dispersion for enhanced physicochemical properties and therapeutic efficacy. Polymers 2024, 16, 286. [Google Scholar] [CrossRef]
- Kyaw Oo, M.; Mandal, U.K.; Chatterjee, B. Polymeric behavior evaluation of PVP K30-poloxamer binary carrier for solid dispersed nisoldipine by experimental design. Pharm. Dev. Technol. 2017, 22, 2–12. [Google Scholar] [CrossRef]
- Ali, I.S.M.; Sajad, U.A.; Abdul Rasool, B.K. Solid dispersion systems for enhanced dissolution of poorly water-soluble candesartan cilexetil: In vitro evaluation and simulated pharmacokinetics studies. PLoS ONE 2024, 19, e0303900. [Google Scholar] [CrossRef]
- Altaani, B.; Obaidat, R.; Malkawi, W. Enhancement of dissolution of atorvastatin through preparation of polymeric solid dispersions using supercritical fluid technology. Res. Pharm. Sci. 2020, 15, 123–136. [Google Scholar] [CrossRef]
- Ei-Badry, M.; Hassan, M.A.; Elsaghir, H. Performance of poloxamer 407 as hydrophilic carrier on the binary mixtures with nimesulide. Farmacia 2013, 61, 1137. [Google Scholar]
- Zhou, R.; Wang, F.; Chang, M.; Yue, H.; Shi, L.; Zhao, Y. Preparation and evaluation of solid dispersion of asiatic acid with PVPK30. Dig. J. Nanomater. Biostruct. 2012, 7, 1015–1020. [Google Scholar]
- Sharma, A.; Jain, C.P.; Tanwar, Y.S. Preparation and characterization of solid dispersions of carvedilol with poloxamer 188. J. Chil. Chem. Soc. 2013, 58, 1553–1557. [Google Scholar] [CrossRef]
- Febriyenti, F.; Indra, P.; Zaini, E.; Ismed, F.; Lucida, H. Preparation and characterization of quercetin-polyvinylpyrrolidone K-30 spray dried solid dispersion. J. Pharm. Pharmacogn. Res. 2020, 8, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Hancock, B.C.; Parks, M. What is the true solubility advantage for amorphous pharmaceuticals? Pharm. Res. 2000, 17, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Takeuchi, H. Dose-dependent effects of antipsychotics on efficacy and adverse effects in schizophrenia. Behav. Brain Res. 2021, 402, 113098. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, J.J.; Reilly, K.R.; Fu, D.-J.; Alphs, L. Comparison of the peak-to-trough fluctuation in plasma concentration of long-acting injectable antipsychotics and their oral equivalents. Innov. Clin. Neurosci. 2012, 9, 17–23. [Google Scholar]



| Formulation | Ratio (w/w/w) | ||
|---|---|---|---|
| Penfluridol | P407 | PVP-K30 | |
| PF-SD1 | 1 | 5 | 0 |
| PF-SD2 | 1 | 5 | 1 |
| PF-SD3 | 1 | 5 | 3 |
| PF-SD4 | 1 | 5 | 5 |
| PF-SD5 | 1 | 3 | 1 |
| PF-SD6 | 1 | 2 | 1 |
| PF-SD7 | 1 | 1 | 1 |
| Formulation | Solubility (μg/mL) |
|---|---|
| Pure drug | 0.78 0.02 |
| PM | 54.6 8.81 * |
| PF-SD5 | 91.2 6.29 * |
| Parameters | Pure Drug | PF-SD5 |
|---|---|---|
| Cmax (ng/mL) | 115 ± 45.4 | 154 ± 38.2 |
| Tmax (h) | 4.3 ± 2.0 | 6.0 ± 2.2 |
| AUC (ng × h/mL) | 1661 ± 610 | 3144 ± 429 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamani, M.; Kim, G.L.; Kil, S.M.; Han, H.-K. Combined Experimental and Computational Approaches for Ternary Solid Dispersions to Enhance the Oral Bioavailability of Penfluridol. Pharmaceutics 2025, 17, 1546. https://doi.org/10.3390/pharmaceutics17121546
Mamani M, Kim GL, Kil SM, Han H-K. Combined Experimental and Computational Approaches for Ternary Solid Dispersions to Enhance the Oral Bioavailability of Penfluridol. Pharmaceutics. 2025; 17(12):1546. https://doi.org/10.3390/pharmaceutics17121546
Chicago/Turabian StyleMamani, Masoud, Gyu Lin Kim, Su Min Kil, and Hyo-Kyung Han. 2025. "Combined Experimental and Computational Approaches for Ternary Solid Dispersions to Enhance the Oral Bioavailability of Penfluridol" Pharmaceutics 17, no. 12: 1546. https://doi.org/10.3390/pharmaceutics17121546
APA StyleMamani, M., Kim, G. L., Kil, S. M., & Han, H.-K. (2025). Combined Experimental and Computational Approaches for Ternary Solid Dispersions to Enhance the Oral Bioavailability of Penfluridol. Pharmaceutics, 17(12), 1546. https://doi.org/10.3390/pharmaceutics17121546







