Generation of a Bioengineered Substitute of the Human Sclero-Corneal Limbus Using a Novel Decellularization Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Generation of Human Decellularized Limbal Substitutes
- Protocol 1 (P1) [13]: Double-distilled water (ddH2O) for 24 h; 0.1% SDS (3 incubations of 24 h each) (Sigma-Aldrich, St Louis, MO, USA); PBS (5 incubations of 15 min each).
- Protocol 2 (P2) [13]: ddH2O for 24 h; 0.1% SDS for 24 h; 1.5 M of sodium chloride (NaCl) (Merck, Darmstadt, Germany); PBS (5 incubations of 15 min each).
- Protocol 3 (P3) [13]: ddH2O for 24 h; 0.1% SDS for 24 h; 3 washes in ddH2O (30 min each); 0.6% of Triton X-100 (Sigma-Aldrich, St Louis, MO, USA) for 24 h; 3 washes in ddH2O (30 min each); 1% of SDC for 24 h; 3 washes in ddH2O (30 min each); 100 mg/L of DNAse (Sigma-Aldrich, St Louis, MO, USA) and 20 mg/L of RNAse (Sigma-Aldrich, St Louis, MO, USA) for 45 min; PBS (5 incubations of 15 min each).
- Protocol 4 (P4) [13]: ddH2O for 24 h; 0.1% SDS for 24 h; 3 washes in ddH2O (30 min each); 0.6% of Triton X-100 for 24 h; 3 washes in ddH2O (30 min each); 1% SDC (Sigma-Aldrich, St Louis, MO, USA) for 24 h; 3 washes in ddH2O (30 min each); 0.05% of Trypsin (Sigma-Aldrich, St Louis, MO, USA) for 1 h; 100 mg/L of DNAse and 20 mg/L od RNAse for 45 min; PBS (5 incubations of 15 min each).
- Protocol 5 (P5): 3 washes in ddH2O (15 min each); 0.6 mM of sulfobetaine 16 (SB-16) (Sigma-Aldrich, St Louis, MO, USA) and 125 mM of sulfobetaine 10 (SB-10) (Sigma-Aldrich, St Louis, MO, USA) for 1 h; 3 washes in PBS (30 min each); 1 mg/mL DNAse for 2 h; 4 washes in PBS (30 min each).
- Protocol 6 (P6): 3 washes in ddH2O (15 min each); 0.6 mM of SB-16 and 125 mM of SB-10 for 1 h; 3 washes in PBS (30 min each); 0.3% of SDC for 30 min; 3 washes in PBS (30 min each); 1 mg/mL DNAse for 2 h; 4 washes in PBS (30 min each).
- Protocol 7 (P7) [14]: 3 washes in ddH2O (15 min each); 1% of SDC for 30 min; 3 washes in PBS (30 min each); 1 mg/mL DNAse overnight (O.N.); 4 washes in PBS (30 min each).
2.2. Decellularization Efficiency Analysis
2.3. ECM Preservation Analysis
2.4. Ex Vivo Biocompatibility Evaluation
2.4.1. Assessment of the Potential Cytotoxic Effects of the Tissues Subjected to Decellularization
2.4.2. Evaluation of the Potential Pro-Inflammatory Effects of the Tissues Subjected to Decellularization
2.5. Generation of Cellularized Limbal Substitutes by Tissue Engineering
2.6. Histological, Immunohistochemical and Immunofluorescence Evaluation of the Cellularized Limbal Substitutes Generated by Tissue Engineering and Controls
2.7. Statistical Analyses
3. Results
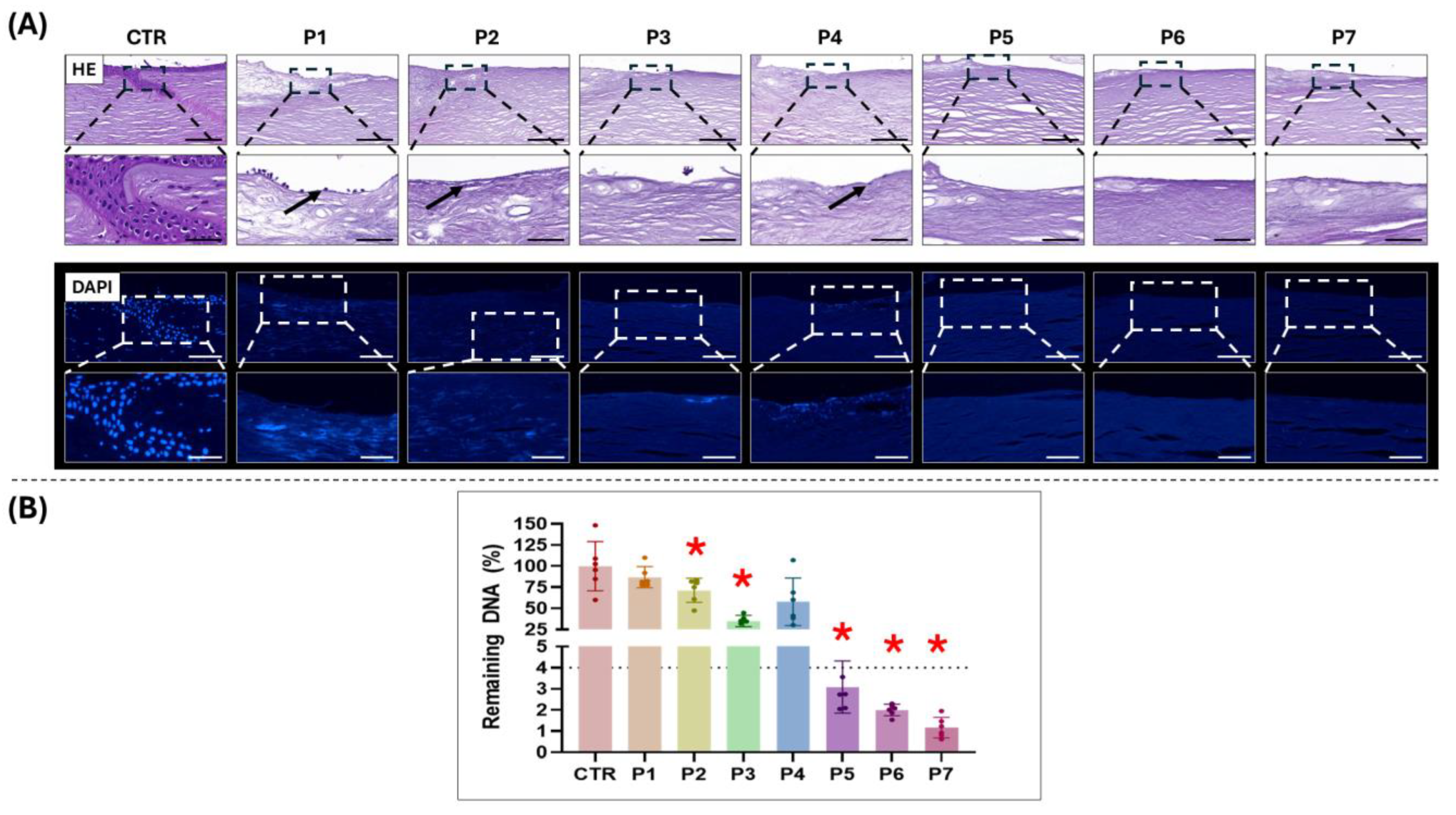
3.1. Evaluation of Decellularization Efficiency
3.2. Analysis of ECM Preservation
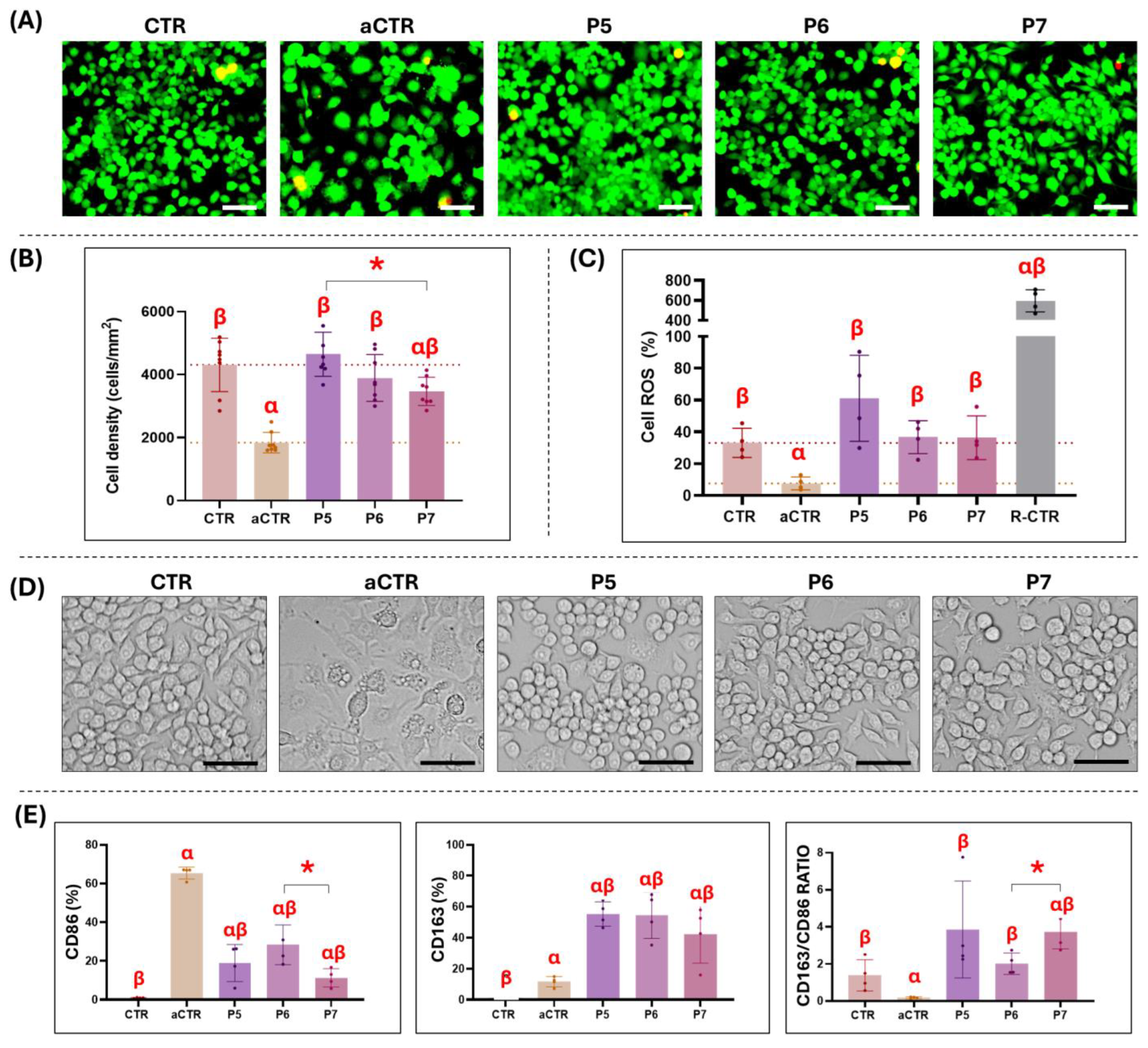
3.3. Ex Vivo Biocompatibility Analysis of Selected Decellularized Tissues
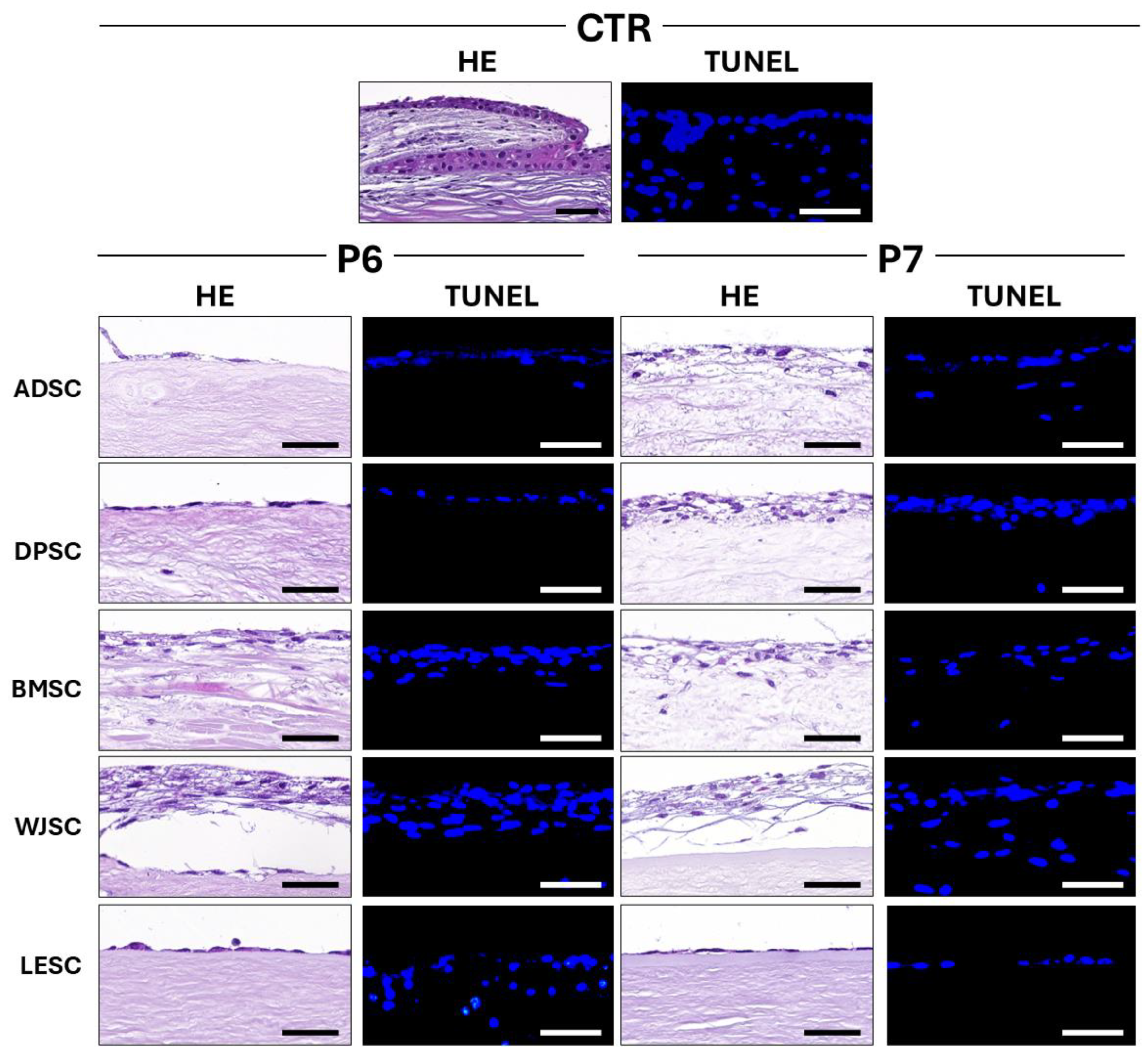
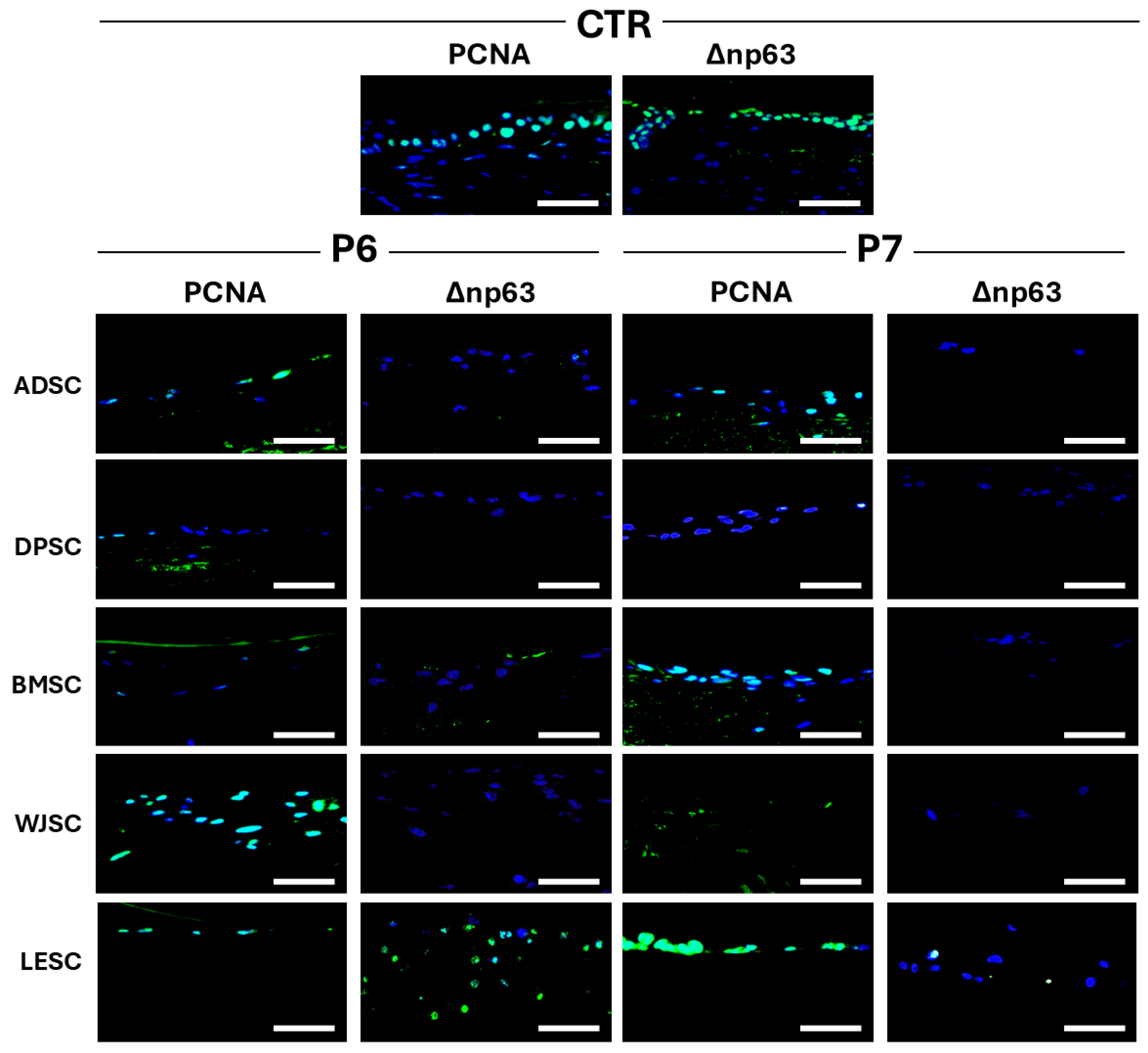
3.4. Characterization of Cellularized Limbal Substitutes Generated by Tissue Engineering
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AB | Alcian Blue |
| aCTR | Control group of activated macrophages |
| ADSC | Adipose-derived Stem Cells |
| ATMP | Advanced Therapy Medicinal Product |
| BMSC | Bone Marrow-Derived Stem Cells |
| CRY αA | Crystallin αA |
| CRY λ | Crystallin λ |
| CTR | Control |
| DAPI | 4’-6-diamidino-2-phenylindole |
| ddH2O | Double-distilled water |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| DPSC | Dental pulp-derived Stem Cells |
| ECM | Extracellular matrix |
| FBS | Fetal Bovine Serum |
| HE | Hematoxylin-eosin |
| KRT5 | Cytokeratin 5 |
| KRT12 | Cytokeratin 12 |
| KRT15 | Cytokeratin 15 |
| LESC | Limbal epithelial stem cell |
| LPS | Lipopolysaccharide |
| LSCD | Limbal Stem Cell Deficiency |
| M1 | Pro-inflammatory phenotype |
| M2 | Pro-regenerative phenotype |
| MSC | Mesenchymal Stem Cell |
| NaCl | Sodium Chloride |
| O.N. | Over night |
| P1 | Protocol 1 |
| P2 | Protocol 2 |
| P3 | Protocol 3 |
| P4 | Protocol 4 |
| P5 | Protocol 5 |
| P6 | Protocol 6 |
| P7 | Protocol 7 |
| PAS | Periodic acid-Schiff |
| PBS | Dulbecco’s Phosphate-Buffered Saline |
| PSR | Picrosirius red |
| ROS | Reactive oxygen species |
| SB-10 | Sulfobetaine 10 |
| SB-16 | Sulfobetaine 16 |
| SDC | Sodium deoxycolate |
| SDS | Sodium dodecyl sulphate |
| TUNEL | Terminal deoxynucleotidyl transferase dUTP nick and labeling |
| WJSC | Wharton’s Jelly-derived Stem Cells |
References
- Vazirani, J.; Nair, D.; Shanbhag, S.; Wurity, S.; Ranjan, A.; Sangwan, V. Limbal Stem Cell Deficiency-Demography and Underlying Causes. Am. J. Ophthalmol. 2018, 188, 99–103. [Google Scholar] [CrossRef]
- Elhusseiny, A.M.; Soleimani, M.; Eleiwa, T.K.; ElSheikh, R.H.; Frank, C.R.; Naderan, M.; Yazdanpanah, G.; Rosenblatt, M.I.; Djalilian, A.R. Current and Emerging Therapies for Limbal Stem Cell Deficiency. Stem Cells Transl. Med. 2022, 11, 259–268. [Google Scholar] [CrossRef]
- Deng, S.X.; Borderie, V.; Chan, C.C.; Dana, R.; Figueiredo, F.C.; Gomes, J.A.P.; Pellegrini, G.; Shimmura, S.; Kruse, F.E.; The International Limbal Stem Cell Deficiency Working Group. Global Consensus on Definition, Classification, Diagnosis, and Staging of Limbal Stem Cell Deficiency. Cornea 2019, 38, 364–375. [Google Scholar] [CrossRef]
- Haagdorens, M.; Van Acker, S.I.; Van Gerwen, V.; Ní Dhubhghaill, S.; Koppen, C.; Tassignon, M.-J.; Zakaria, N. Limbal Stem Cell Deficiency: Current Treatment Options and Emerging Therapies. Stem Cells Int. 2016, 2016, 9798374. [Google Scholar] [CrossRef]
- Sejpal, K.; Bakhtiari, P.; Deng, S.X. Presentation, Diagnosis and Management of Limbal Stem Cell Deficiency. Middle East. Afr. J. Ophthalmol. 2013, 20, 5–10. [Google Scholar] [CrossRef]
- Bonnet, C.; Le, Q.; Cordova, D.; Gonzalez, S.; Tseng, C.-H.; Deng, S.X. Demographics, Prevalence, and Characteristics of Limbal Stem Cell Deficiency in Southern California. Cornea 2025. [Google Scholar] [CrossRef] [PubMed]
- Yazdanpanah, G.; Haq, Z.; Kang, K.; Jabbehdari, S.; Rosenblatt, M.L.; Djalilian, A.R. Strategies for Reconstructing the Limbal Stem Cell Niche. Ocul. Surf. 2019, 17, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Schlötzer-Schrehardt, U.; Dietrich, T.; Saito, K.; Sorokin, L.; Sasaki, T.; Paulsson, M.; Kruse, F.E. Characterization of Extracellular Matrix Components in the Limbal Epithelial Stem Cell Compartment. Exp. Eye Res. 2007, 85, 845–860. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiari, P.; Djalilian, A. Update on Limbal Stem Cell Transplantation. Middle East. Afr. J. Ophthalmol. 2010, 17, 9–14. [Google Scholar] [CrossRef]
- Pellegrini, G.; Ardigò, D.; Milazzo, G.; Iotti, G.; Guatelli, P.; Pelosi, D.; De Luca, M. Navigating Market Authorization: The Path Holoclar Took to Become the First Stem Cell Product Approved in the European Union. Stem Cells Transl. Med. 2018, 7, 146–154. [Google Scholar] [CrossRef]
- Pellegrini, G.; Traverso, C.E.; Franzi, A.T.; Zingirian, M.; Cancedda, R.; De Luca, M. Long-Term Restoration of Damaged Corneal Surfaces with Autologous Cultivated Corneal Epithelium. Lancet 1997, 349, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Posarelli, M.; Romano, D.; Tucci, D.; Giannaccare, G.; Scorcia, V.; Taloni, A.; Pagano, L.; Borgia, A. Ocular-Surface Regeneration Therapies for Eye Disorders: The State of the Art. BioTech 2023, 12, 48. [Google Scholar] [CrossRef]
- Sánchez-Porras, D.; Caro-Magdaleno, M.; González-Gallardo, C.; García-García, Ó.D.; Garzón, I.; Carriel, V.; Campos, F.; Alaminos, M. Generation of a Biomimetic Substitute of the Corneal Limbus Using Decellularized Scaffolds. Pharmaceutics 2021, 13, 1718. [Google Scholar] [CrossRef]
- Polisetti, N.; Roschinski, B.; Schlötzer-Schrehardt, U.; Maier, P.; Schlunck, G.; Reinhard, T. A Decellularized Human Limbal Scaffold for Limbal Stem Cell Niche Reconstruction. Int. J. Mol. Sci. 2021, 22, 10067. [Google Scholar] [CrossRef]
- Isidan, A.; Liu, S.; Chen, A.M.; Zhang, W.; Li, P.; Smith, L.J.; Hara, H.; Cooper, D.K.C.; Ekser, B. Comparison of Porcine Corneal Decellularization Methods and Importance of Preserving Corneal Limbus through Decellularization. PLoS ONE 2021, 16, e0243682. [Google Scholar] [CrossRef]
- Spaniol, K.; Witt, J.; Mertsch, S.; Borrelli, M.; Geerling, G.; Schrader, S. Generation and Characterisation of Decellularised Human Corneal Limbus. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 547–557. [Google Scholar] [CrossRef]
- Polisetti, N.; Schmid, A.; Schlötzer-Schrehardt, U.; Maier, P.; Lang, S.J.; Steinberg, T.; Schlunck, G.; Reinhard, T. A Decellularized Human Corneal Scaffold for Anterior Corneal Surface Reconstruction. Sci. Rep. 2021, 11, 2992. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Fernández, P.; Etayo-Escanilla, M.; Sánchez-Porras, D.; Blanco-Elices, C.; Campos, F.; Carriel, V.; García-García, Ó.D.; Chato-Astrain, J. A Novel In Vitro Pathological Model for Studying Neural Invasion in Non-Melanoma Skin Cancer. Gels 2024, 10, 252. [Google Scholar] [CrossRef] [PubMed]
- Vela-Romera, A.; Carriel, V.; Martín-Piedra, M.A.; Aneiros-Fernández, J.; Campos, F.; Chato-Astrain, J.; Prados-Olleta, N.; Campos, A.; Alaminos, M.; Garzón, I. Characterization of the Human Ridged and Non-Ridged Skin: A Comprehensive Histological, Histochemical and Immunohistochemical Analysis. Histochem. Cell Biol. 2019, 151, 57–73. [Google Scholar] [CrossRef]
- Ávila-Fernández, P.; Etayo-Escanilla, M.; Sánchez-Porras, D.; Fernández-Valadés, R.; Campos, F.; Garzón, I.; Carriel, V.; Alaminos, M.; García-García, Ó.D. Spatiotemporal Characterization of Extracellular Matrix Maturation in Human Artificial Stromal-Epithelial Tissue Substitutes. BMC Biol. 2024, 22, 263. [Google Scholar] [CrossRef]
- Berasain, J.; Ávila-Fernández, P.; Cárdenas-Pérez, R.; Cànaves-Llabrés, A.I.; Etayo-Escanilla, M.; Alaminos, M.; Carriel, V.; García-García, Ó.D.; Chato-Astrain, J.; Campos, F. Genipin Crosslinking Promotes Biomechanical Reinforcement and Pro-Regenerative Macrophage Polarization in Bioartificial Tubular Substitutes. Biomed. Pharmacother. 2024, 174, 116449. [Google Scholar] [CrossRef]
- Martin-Piedra, M.A.; Alfonso-Rodriguez, C.A.; Zapater, A.; Durand-Herrera, D.; Chato-Astrain, J.; Campos, F.; Sanchez-Quevedo, M.C.; Alaminos, M.; Garzon, I. Effective Use of Mesenchymal Stem Cells in Human Skin Substitutes Generated by Tissue Engineering. Eur. Cell Mater. 2019, 37, 233–249. [Google Scholar] [CrossRef]
- Garzón, I.; Chato-Astrain, J.; González-Gallardo, C.; Ionescu, A.; Cardona, J.d.l.C.; Mateu, M.; Carda, C.; Pérez, M.D.M.; Martín-Piedra, M.Á.; Alaminos, M. Long-Term in Vivo Evaluation of Orthotypical and Heterotypical Bioengineered Human Corneas. Front. Bioeng. Biotechnol. 2020, 8, 681. [Google Scholar] [CrossRef] [PubMed]
- Thokala, P.; Singh, A.; Singh, V.K.; Rathi, V.M.; Basu, S.; Singh, V.; MacNeil, S.; Sangwan, V.S. Economic, Clinical and Social Impact of Simple Limbal Epithelial Transplantation for Limbal Stem Cell Deficiency. Br. J. Ophthalmol. 2022, 106, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Schlötzer-Schrehardt, U.; Latta, L.; Gießl, A.; Zenkel, M.; Fries, F.N.; Käsmann-Kellner, B.; Kruse, F.E.; Seitz, B. Dysfunction of the Limbal Epithelial Stem Cell Niche in Aniridia-Associated Keratopathy. Ocul. Surf. 2021, 21, 160–173. [Google Scholar] [CrossRef]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An Overview of Tissue and Whole Organ Decellularization Processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef]
- Moffat, D.; Ye, K.; Jin, S. Decellularization for the Retention of Tissue Niches. J. Tissue Eng. 2022, 13, 20417314221101151. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Lou, D.; Ma, L.; Gao, C. Optimizing Detergent Concentration and Processing Time to Balance the Decellularization Efficiency and Properties of Bioprosthetic Heart Valves. J. Biomed. Mater. Res. A 2019, 107, 2235–2243. [Google Scholar] [CrossRef]
- Saeid Nia, M.; Floder, L.M.; Seiler, J.A.; Puehler, T.; Pommert, N.S.; Berndt, R.; Meier, D.; Sellers, S.L.; Sathananthan, J.; Zhang, X.; et al. Optimization of Enzymatic and Chemical Decellularization of Native Porcine Heart Valves for the Generation of Decellularized Xenografts. Int. J. Mol. Sci. 2024, 25, 4026. [Google Scholar] [CrossRef]
- Lovati, A.B.; D’Arrigo, D.; Odella, S.; Tos, P.; Geuna, S.; Raimondo, S. Nerve Repair Using Decellularized Nerve Grafts in Rat Models. A Review of the Literature. Front. Cell. Neurosci. 2018, 12, 427. [Google Scholar] [CrossRef]
- Bueno, J.M.; Gualda, E.J.; Artal, P. Analysis of Corneal Stroma Organization with Wavefront Optimized Nonlinear Microscopy. Cornea 2011, 30, 692–701. [Google Scholar] [CrossRef]
- Subasinghe, S.K.; Ogbuehi, K.C.; Mitchell, L.; Dias, G.J. Animal Model with Structural Similarity to Human Corneal Collagen Fibrillar Arrangement. Anat. Sci. Int. 2021, 96, 286–293. [Google Scholar] [CrossRef]
- Sanchez, I.; Martin, R.; Ussa, F.; Fernandez-Bueno, I. The Parameters of the Porcine Eyeball. Graefes Arch. Clin. Exp. Ophthalmol. 2011, 249, 475–482. [Google Scholar] [CrossRef]
- Nicoli, S.; Ferrari, G.; Quarta, M.; Macaluso, C.; Govoni, P.; Dallatana, D.; Santi, P. Porcine Sclera as a Model of Human Sclera for in Vitro Transport Experiments: Histology, SEM, and Comparative Permeability. Mol. Vis. 2009, 15, 259–266. [Google Scholar]
- Beckmann, I.; Meisel-Mikołajczyk, F.; Wallenburg, H.C. The Effects of Deoxycholate and Sodium Dodecyl Sulphate on the Serological Reactivity of Antigens Isolated from Six Bacteroides Reference Strains. Antonie Van. Leeuwenhoek 1990, 57, 71–76. [Google Scholar] [CrossRef]
- Qu, K.; Yuan, Z.; Wang, Y.; Song, Z.; Gong, X.; Zhao, Y.; Mu, Q.; Zhan, Q.; Xu, W.; Wang, L. Structures, Properties, and Applications of Zwitterionic Polymers. ChemPhysMater 2022, 1, 294–309. [Google Scholar] [CrossRef]
- Song, Y.H.; Maynes, M.A.; Hlavac, N.; Visosevic, D.; Daramola, K.O.; Porvasnik, S.L.; Schmidt, C.E. Development of Novel Apoptosis-Assisted Lung Tissue Decellularization Methods. Biomater. Sci. 2021, 9, 3485–3498. [Google Scholar] [CrossRef] [PubMed]
- Keane, T.J.; Swinehart, I.T.; Badylak, S.F. Methods of Tissue Decellularization Used for Preparation of Biologic Scaffolds and in Vivo Relevance. Methods 2015, 84, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Hudson, T.W.; Zawko, S.; Deister, C.; Lundy, S.; Hu, C.Y.; Lee, K.; Schmidt, C.E. Optimized Acellular Nerve Graft Is Immunologically Tolerated and Supports Regeneration. Tissue Eng. 2004, 10, 1641–1651. [Google Scholar] [CrossRef] [PubMed]
- Rajab, T.K.; O’Malley, T.J.; Tchantchaleishvili, V. Decellularized Scaffolds for Tissue Engineering: Current Status and Future Perspective. Artif. Organs 2020, 44, 1031–1043. [Google Scholar] [CrossRef]
- Whitehead, K.M.; Hendricks, H.K.L.; Cakir, S.N.; de Castro Brás, L.E. ECM Roles and Biomechanics in Cardiac Tissue Decellularization. Am. J. Physiol. Heart Circ. Physiol. 2022, 323, H585–H596. [Google Scholar] [CrossRef]
- Marin-Tapia, H.A.; Romero-Salazar, L.; Arteaga-Arcos, J.C.; Rosales-Ibáñez, R.; Mayorga-Rojas, M. Micro-Mechanical Properties of Corneal Scaffolds from Two Different Bio-Models Obtained by an Efficient Chemical Decellularization. J. Mech. Behav. Biomed. Mater. 2021, 119, 104510. [Google Scholar] [CrossRef] [PubMed]
- Rico-Sánchez, L.; Garzón, I.; González-Andrades, M.; Ruíz-García, A.; Punzano, M.; Lizana-Moreno, A.; Muñoz-Ávila, J.I.; Sánchez-Quevedo, M.D.C.; Martínez-Atienza, J.; Lopez-Navas, L.; et al. Successful Development and Clinical Translation of a Novel Anterior Lamellar Artificial Cornea. J. Tissue Eng. Regen. Med. 2019, 13, 2142–2154. [Google Scholar] [CrossRef]
- Daly, K.A.; Liu, S.; Agrawal, V.; Brown, B.N.; Johnson, S.A.; Medberry, C.J.; Badylak, S.F. Damage Associated Molecular Patterns within Xenogeneic Biologic Scaffolds and Their Effects on Host Remodeling. Biomaterials 2012, 33, 91–101. [Google Scholar] [CrossRef]
- Piatnitskaia, S.; Rafikova, G.; Bilyalov, A.; Chugunov, S.; Akhatov, I.; Pavlov, V.; Kzhyshkowska, J. Modelling of Macrophage Responses to Biomaterials in Vitro: State-of-the-Art and the Need for the Improvement. Front. Immunol. 2024, 15, 1349461. [Google Scholar] [CrossRef] [PubMed]
- Pacienza, N.; Lee, R.H.; Bae, E.-H.; Kim, D.-K.; Liu, Q.; Prockop, D.J.; Yannarelli, G. In Vitro Macrophage Assay Predicts the In Vivo Anti-Inflammatory Potential of Exosomes from Human Mesenchymal Stromal Cells. Mol. Ther. Methods Clin. Dev. 2019, 13, 67–76. [Google Scholar] [CrossRef]
- Fuchs, A.-K.; Syrovets, T.; Haas, K.A.; Loos, C.; Musyanovych, A.; Mailänder, V.; Landfester, K.; Simmet, T. Carboxyl- and Amino-Functionalized Polystyrene Nanoparticles Differentially Affect the Polarization Profile of M1 and M2 Macrophage Subsets. Biomaterials 2016, 85, 78–87. [Google Scholar] [CrossRef]
- Li, W.; Ye, Q.; Jiang, Z.; Xia, D.; Yan, Z.; Wang, D.; Chen, Y.; Cao, T.; Wang, J.; Lin, C.; et al. A Cross-Linked Macropore Hydrogel Based on M1 Macrophage Lysate and Alginate Regulates Tumor-Associated Macrophages for the Treatment of Melanoma. Int. J. Biol. Macromol. 2024, 269, 132089. [Google Scholar] [CrossRef]
- Brown, B.N.; Londono, R.; Tottey, S.; Zhang, L.; Kukla, K.A.; Wolf, M.T.; Daly, K.A.; Reing, J.E.; Badylak, S.F. Macrophage Phenotype as a Predictor of Constructive Remodeling Following the Implantation of Biologically Derived Surgical Mesh Materials. Acta Biomater. 2012, 8, 978–987. [Google Scholar] [CrossRef]
- Zhou, J.; Xi, Y.; Wu, T.; Zeng, X.; Yuan, J.; Peng, L.; Fu, H.; Zhou, C. A Potential Therapeutic Approach for Ulcerative Colitis: Targeted Regulation of Mitochondrial Dynamics and Mitophagy through Phytochemicals. Front. Immunol. 2024, 15, 1506292. [Google Scholar] [CrossRef] [PubMed]
- Mezhenskyi, O.; Andriienkova, K.; Goldanova, T.; Antmen, E.; Soubrie, T.; Vrana, N.E. Macrophage Phenotype Detection Methodology on Textured Surfaces via Nuclear Morphology Using Machine Learning. Adv. Intell. Discov. 2025, 0, e202500055. [Google Scholar] [CrossRef]
- Mia, S.; Warnecke, A.; Zhang, X.-M.; Malmström, V.; Harris, R.A. An Optimized Protocol for Human M2 Macrophages Using M-CSF and IL-4/IL-10/TGF-β Yields a Dominant Immunosuppressive Phenotype. Scand. J. Immunol. 2014, 79, 305–314. [Google Scholar] [CrossRef]
- Liu, L.; Guo, H.; Song, A.; Huang, J.; Zhang, Y.; Jin, S.; Li, S.; Zhang, L.; Yang, C.; Yang, P. Progranulin Inhibits LPS-Induced Macrophage M1 Polarization via NF-кB and MAPK Pathways. BMC Immunol. 2020, 21, 32. [Google Scholar] [CrossRef]
- Pelegrin, P.; Surprenant, A. Dynamics of Macrophage Polarization Reveal New Mechanism to Inhibit IL-1beta Release through Pyrophosphates. EMBO J. 2009, 28, 2114–2127. [Google Scholar] [CrossRef]
- Blanco-Elices, C.; Morales-Álvarez, C.; Chato-Astrain, J.; González-Gallardo, C.; Ávila-Fernández, P.; Campos, F.; Carmona, R.; Martín-Piedra, M.Á.; Garzón, I.; Alaminos, M. Development of Stromal Differentiation Patterns in Heterotypical Models of Artificial Corneas Generated by Tissue Engineering. Front. Bioeng. Biotechnol. 2023, 11, 1124995. [Google Scholar] [CrossRef]
- Lee, A.-Y.; Lee, J.; Kim, C.-L.; Lee, K.S.; Lee, S.-H.; Gu, N.-Y.; Kim, J.-M.; Lee, B.C.; Koo, O.J.; Song, J.-Y.; et al. Comparative Studies on Proliferation, Molecular Markers and Differentiation Potential of Mesenchymal Stem Cells from Various Tissues (Adipose, Bone Marrow, Ear Skin, Abdominal Skin, and Lung) and Maintenance of Multipotency during Serial Passages in Miniature Pig. Res. Vet. Sci. 2015, 100, 115–124. [Google Scholar] [CrossRef]
- Bains, K.K.; Young, R.D.; Koudouna, E.; Lewis, P.N.; Quantock, A.J. Cell-Cell and Cell-Matrix Interactions at the Presumptive Stem Cell Niche of the Chick Corneal Limbus. Cells 2023, 12, 2334. [Google Scholar] [CrossRef]
- Martin-Piedra, M.A.; Carmona, G.; Campos, F.; Carriel, V.; Fernández-González, A.; Campos, A.; Cuende, N.; Garzón, I.; Gacto, P.; Alaminos, M. Histological Assessment of Nanostructured Fibrin-Agarose Skin Substitutes Grafted in Burnt Patients. A Time-Course Study. Bioeng. Transl. Med. 2023, 8, e10572. [Google Scholar] [CrossRef]
- Koppula, P.R.; Chelluri, L.K.; Polisetti, N.; Vemuganti, G.K. Histocompatibility Testing of Cultivated Human Bone Marrow Stromal Cells—A Promising Step towards Pre-Clinical Screening for Allogeneic Stem Cell Therapy. Cell Immunol. 2009, 259, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, R.M.; Vajda, F.; Wibowo, J.A.; Figueiredo, F.; Connon, C.J. YAP, ΔNp63, and β-Catenin Signaling Pathways Are Involved in the Modulation of Corneal Epithelial Stem Cell Phenotype Induced by Substrate Stiffness. Cells 2019, 8, 347. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Arrabal, O.; Blanco-Elices, C.; González-Gallardo, C.; Sánchez-Porras, D.; Etayo-Escanilla, M.; Fernández, P.Á.; Chato-Astrain, J. Histological, Histochemical, and Immunohistochemical Characterization of NANOULCOR Nanostructured Fibrin-Agarose Human Cornea Substitutes Generated by Tissue Engineering. BMC Med. 2024, 22, 531. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.-H.; Wang, Y.-C.; Liu, S.-P.; Shih, T.-R.; Lin, H.-L.; Chen, Y.-M.; Sung, J.-H.; Lu, C.-H.; Wei, J.-R.; Wang, Z.-W.; et al. Decellularization and Recellularization Technologies in Tissue Engineering. Cell Transplant. 2014, 23, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Milazzo, G.; De Luca, M.; Pellegrini, G. Holoclar: First of Its Kind in More Ways than One. Cell Gene Therapy Insights 2016, 2, 183–197. [Google Scholar] [CrossRef]


| Reagents | Protocols | ||||||
|---|---|---|---|---|---|---|---|
| P1 [13] | P2 [13] | P3 [13] | P4 [13] | P5 | P6 | P7 [14] | |
| ddH2O | 24 h | 24 h | 24 h | 24 h | 3 × 15 min | 3 × 15 min | 3 × 15 min |
| SDS (0.1%) | 3 × 24 h | 24 h | 24 h | 24 h | - | - | - |
| NaCl (1.5 M) | - | 2 × 24 h | - | - | - | - | - |
| ddH2O | - | - | 3 × 30 min | 3 × 30 min | - | - | - |
| Triton X-100 (0.6%) | - | - | 24 h | 24 h | - | - | - |
| ddH2O | - | - | 3 × 30 min | 3 × 30 min | - | - | - |
| SDC (1%) | - | - | 24 h | 24 h | - | - | 30 min |
| SB mix solution (SB-16 0.6 mM; SB-10 125 mM) | - | - | - | - | 1 h | 1 h | - |
| PBS | - | - | - | - | 3 × 30 min | 3 × 30 min | 3 × 30 min |
| SDC (0.3%) | - | - | - | - | - | 30 min | - |
| PBS | - | - | - | - | - | 3 × 30 min | - |
| ddH2O | - | - | 3 × 30 min | 3 × 30 min | - | - | - |
| Trypsin (0.05%) | - | - | - | 1 h | - | - | - |
| Enzymatic solution (DNAse 100 mg/L; RNAse 20 mg/L) | - | - | 45 min | 45 min | - | - | - |
| DNAse 1 mg/mL | - | - | - | - | 2 h | 2 h | O.N. (12 h) |
| PBS | 5 × 15 min | 5 × 15 min | 5 × 15 min | 5 × 15 min | 4 × 30 min | 4 × 30 min | 4 × 30 min |
| TOTAL PROCESSING TIME (h) | 97.25 | 97.25 | 102.5 | 103.5 | 7.25 | 9.25 | 16.75 |
| PCNA | Δnp63 | AE1/AE3 | KRT5 | KRT12 | KRT15 | CRYαA | CRYλ | |
|---|---|---|---|---|---|---|---|---|
| CTR | +++ | +++ | +++ | +++ | ++ | +++ | +++ | +++ |
| P6-ADSC | ++ | - | ++ | - | - | - | - | ++ |
| P6-DPSC | + | - | ++ | - | - | - | - | ++ |
| P6-BMSC | ± | ± | ++ | - | - | - | +++ | ++ |
| P6-WJSC | +++ | - | ++ | - | - | - | ++ | ++ |
| P6-LESC | ++ | ++ | ++ | - | - | - | - | ++ |
| P7-ADSC | ++ | - | ++ | - | - | - | ± | ++ |
| P7-DPSC | ± | - | ++ | - | - | - | - | ++ |
| P7-BMSC | ++ | - | ++ | - | - | - | + | ++ |
| P7-WJSC | + | - | ++ | - | - | - | ++ | ++ |
| P7-LESC | ++ | + | ++ | - | - | - | - | ++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ávila-Fernández, P.; Sánchez-Porras, D.; Etayo-Escanilla, M.; González-Gallardo, C.; Alaminos, M.; Chato-Astrain, J.; Campos, F.; García-García, Ó.D. Generation of a Bioengineered Substitute of the Human Sclero-Corneal Limbus Using a Novel Decellularization Method. Pharmaceutics 2025, 17, 1540. https://doi.org/10.3390/pharmaceutics17121540
Ávila-Fernández P, Sánchez-Porras D, Etayo-Escanilla M, González-Gallardo C, Alaminos M, Chato-Astrain J, Campos F, García-García ÓD. Generation of a Bioengineered Substitute of the Human Sclero-Corneal Limbus Using a Novel Decellularization Method. Pharmaceutics. 2025; 17(12):1540. https://doi.org/10.3390/pharmaceutics17121540
Chicago/Turabian StyleÁvila-Fernández, Paula, David Sánchez-Porras, Miguel Etayo-Escanilla, Carmen González-Gallardo, Miguel Alaminos, Jesús Chato-Astrain, Fernando Campos, and Óscar Darío García-García. 2025. "Generation of a Bioengineered Substitute of the Human Sclero-Corneal Limbus Using a Novel Decellularization Method" Pharmaceutics 17, no. 12: 1540. https://doi.org/10.3390/pharmaceutics17121540
APA StyleÁvila-Fernández, P., Sánchez-Porras, D., Etayo-Escanilla, M., González-Gallardo, C., Alaminos, M., Chato-Astrain, J., Campos, F., & García-García, Ó. D. (2025). Generation of a Bioengineered Substitute of the Human Sclero-Corneal Limbus Using a Novel Decellularization Method. Pharmaceutics, 17(12), 1540. https://doi.org/10.3390/pharmaceutics17121540









