Validation of Liquid Chromatography Coupled with Tandem Mass Spectrometry for the Determination of 12 Tyrosine Kinase Inhibitors (TKIs) and Their Application to Therapeutic Drug Monitoring in Adult and Pediatric Populations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Calibration Standards and Quality Controls
2.3. Sample Preparation
2.4. Instrumental Analysis
2.5. Method Validation
2.5.1. Limits of Detection and Quantification
2.5.2. Linearity
2.5.3. Accuracy and Precision
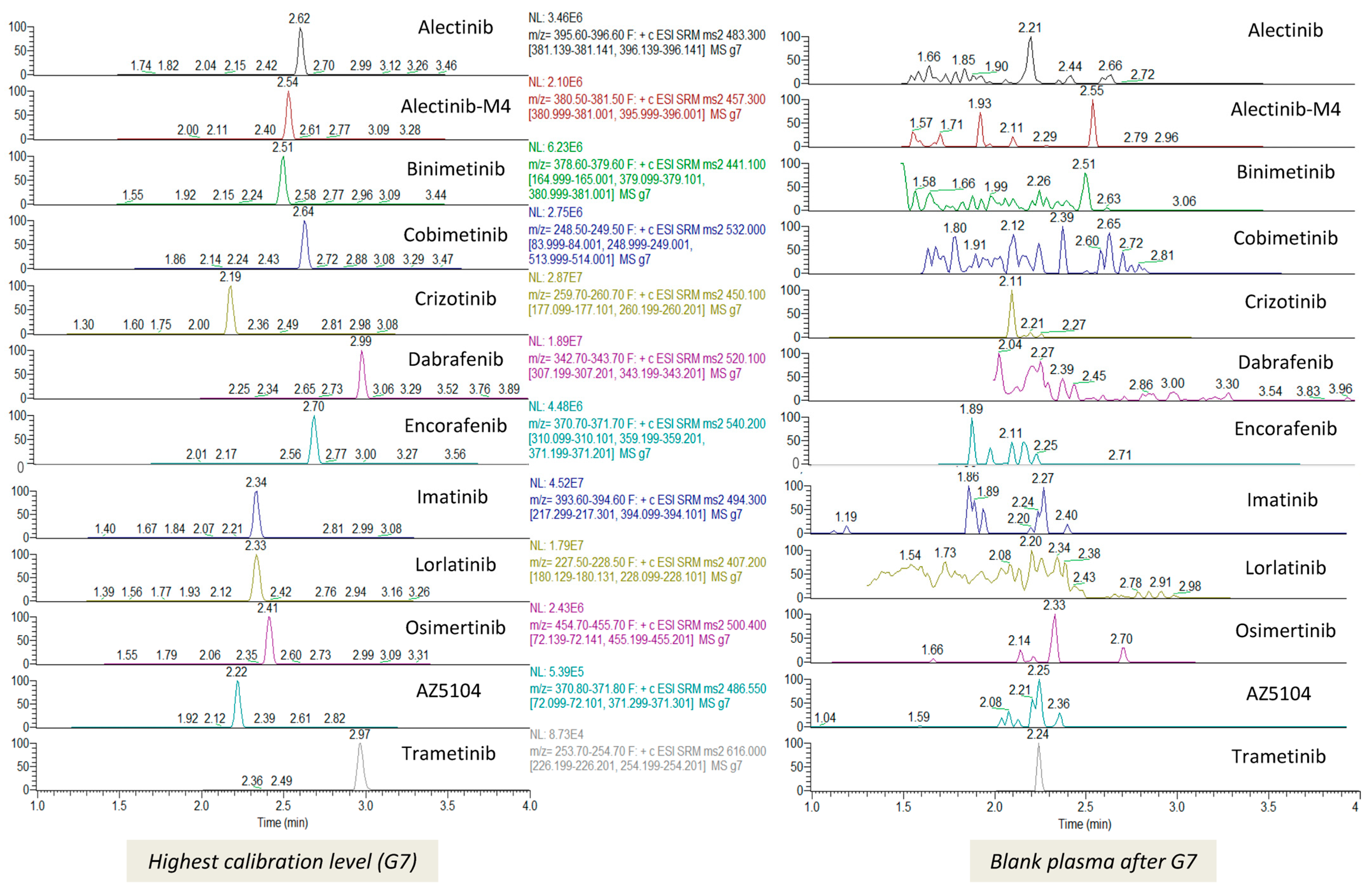
2.5.4. Selectivity and Carryover
2.5.5. Matrix Effect and Recovery
2.5.6. Stability
2.6. Application
2.6.1. Population
2.6.2. Sample Collection
2.6.3. Statistical Analysis
3. Results
3.1. Method Validation
3.2. TKIs Plasma Concentrations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Natoli, C.; Perrucci, B.; Perrotti, F.; Falchi, L.; Iacobelli, S. Tyrosine Kinase Inhibitors. Curr. Cancer Drug Targets 2010, 10, 462–483. [Google Scholar] [CrossRef] [PubMed]
- Cismowski, M.J. Tyrosine Kinase Inhibitors. In xPharm: The Comprehensive Pharmacology Reference; Enna, S.J., Bylund, D.B., Eds.; Elsevier: New York, NY, USA, 2007; pp. 1–4. ISBN 978-0-08-055232-3. [Google Scholar]
- Messinger, Y.H.; Bostrom, B.C.; Olson, D.R.; Gossai, N.P.; Miller, L.H.; Richards, M.K. Langerhans Cell Histiocytosis with BRAF p.N486_P490del or MAP2K1 p.K57_G61del Treated by the MEK Inhibitor Trametinib. Pediatr. Blood Cancer 2020, 67, e28712. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, D.; Cui, L.; Ma, H.-H.; Zhang, L.; Lian, H.-Y.; Zhang, Q.; Zhao, X.-X.; Zhang, L.-P.; Zhao, Y.-Z.; et al. Effectiveness and Safety of Dabrafenib in the Treatment of 20 Chinese Children with BRAFV600E-Mutated Langerhans Cell Histiocytosis. Cancer Res. Treat. 2021, 53, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Donadieu, J.; Larabi, I.A.; Tardieu, M.; Visser, J.; Hutter, C.; Sieni, E.; Kabbara, N.; Barkaoui, M.; Miron, J.; Chalard, F.; et al. Vemurafenib for Refractory Multisystem Langerhans Cell Histiocytosis in Children: An International Observational Study. J. Clin. Oncol. 2019, 37, 2857–2865. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Galindo, C.; Allen, C.E. Langerhans Cell Histiocytosis. Blood 2020, 135, 1319–1331. [Google Scholar] [CrossRef]
- Verougstraete, N.; Stove, V.; Verstraete, A.G.; Stove, C.P. Therapeutic Drug Monitoring of Tyrosine Kinase Inhibitors Using Dried Blood Microsamples. Front. Oncol. 2022, 12, 821807. [Google Scholar] [CrossRef]
- Herviou, P.; Thivat, E.; Richard, D.; Roche, L.; Dohou, J.; Pouget, M.; Eschalier, A.; Durando, X.; Authier, N. Therapeutic Drug Monitoring and Tyrosine Kinase Inhibitors. Oncol. Lett. 2016, 12, 1223–1232. [Google Scholar] [CrossRef]
- Cardoso, E.; Guidi, M.; Blanchet, B.; Schneider, M.P.; Decosterd, L.A.; Buclin, T.; Csajka, C.; Widmer, N. Therapeutic Drug Monitoring of Targeted Anticancer Protein Kinase Inhibitors in Routine Clinical Use: A Critical Review. Ther. Drug Monit. 2020, 42, 33. [Google Scholar] [CrossRef]
- Hjorth-Hansen, H. TKI Safety. HemaSphere 2019, 3, 51–53. [Google Scholar] [CrossRef]
- Hehlmann, R.; Cortes, J.E.; Zyczynski, T.; Gambacorti-Passerini, C.; Goldberg, S.L.; Mauro, M.J.; Michallet, M.; Simonsson, B.; Williams, L.A.; Gajavelli, S.; et al. Tyrosine Kinase Inhibitor Interruptions, Discontinuations and Switching in Patients with Chronic-phase Chronic Myeloid Leukemia in Routine Clinical Practice: SIMPLICITY. Am. J. Hematol. 2019, 94, 46–54. [Google Scholar] [CrossRef]
- Verheijen, R.B.; Yu, H.; Schellens, J.H.M.; Beijnen, J.H.; Steeghs, N.; Huitema, A.D.R. Practical Recommendations for Therapeutic Drug Monitoring of Kinase Inhibitors in Oncology. Clin. Pharmacol. Ther. 2017, 102, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Widmer, N.; Bardin, C.; Chatelut, E.; Paci, A.; Beijnen, J.; Levêque, D.; Veal, G.; Astier, A. Review of Therapeutic Drug Monitoring of Anticancer Drugs Part Two–Targeted Therapies. Eur. J. Cancer 2014, 50, 2020–2036. [Google Scholar] [CrossRef] [PubMed]
- Van Veelen, A.; van Geel, R.; de Beer, Y.; Dingemans, A.-M.; Stolk, L.; Ter Heine, R.; de Vries, F.; Croes, S. Validation of an Analytical Method Using HPLC-MS/MS to Quantify Osimertinib in Human Plasma and Supplementary Stability Results. Biomed. Chromatogr. 2020, 34, e4771. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, X.; Chen, Z.; Jiang, S.; Wang, L.; Tao, M.; Miao, L. Method Development and Validation for Simultaneous Determination of Six Tyrosine Kinase Inhibitors and Two Active Metabolites in Human Plasma/Serum Using UPLC–MS/MS for Therapeutic Drug Monitoring. J. Pharm. Biomed. Anal. 2022, 211, 114562. [Google Scholar] [CrossRef] [PubMed]
- Sparidans, R.W.; Li, W.; Schinkel, A.H.; Schellens, J.H.M.; Beijnen, J.H. Bioanalytical Liquid Chromatography-Tandem Mass Spectrometric Assay for the Quantification of the ALK Inhibitors Alectinib, Brigatinib and Lorlatinib in Plasma and Mouse Tissue Homogenates. J. Pharm. Biomed. Anal. 2018, 161, 136–143. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, S.; Chen, M.; Huang, S.; Zhang, M.; Bao, W.; Bao, A.; Zhang, P.; Guo, H.; Liu, Z.; et al. Simultaneous and Rapid Determination of 12 Tyrosine Kinase Inhibitors by LC-MS/MS in Human Plasma: Application to Therapeutic Drug Monitoring in Patients with Non-Small Cell Lung Cancer. J. Chromatogr. B 2021, 1175, 122752. [Google Scholar] [CrossRef]
- Huynh, H.H.; Pressiat, C.; Sauvageon, H.; Madelaine, I.; Maslanka, P.; Lebbé, C.; Thieblemont, C.; Goldwirt, L.; Mourah, S. Development and Validation of a Simultaneous Quantification Method of 14 Tyrosine Kinase Inhibitors in Human Plasma Using LC-MS/MS. Ther. Drug Monit. 2017, 39, 43–54. [Google Scholar] [CrossRef]
- Merienne, C.; Rousset, M.; Ducint, D.; Castaing, N.; Titier, K.; Molimard, M.; Bouchet, S. High Throughput Routine Determination of 17 Tyrosine Kinase Inhibitors by LC–MS/MS. J. Pharm. Biomed. Anal. 2018, 150, 112–120. [Google Scholar] [CrossRef]
- Herbrink, M.; de Vries, N.; Rosing, H.; Huitema, A.D.R.; Nuijen, B.; Schellens, J.H.M.; Beijnen, J.H. Quantification of 11 Therapeutic Kinase Inhibitors in Human Plasma for Therapeutic Drug Monitoring Using Liquid Chromatography Coupled with Tandem Mass Spectrometry. Ther. Drug Monit. 2016, 38, 649–656. [Google Scholar] [CrossRef]
- Rousset, M.; Dutriaux, C.; Bosco-Lévy, P.; Prey, S.; Pham-Ledard, A.; Dousset, L.; Gérard, E.; Bouchet, S.; Canal-Raffin, M.; Titier, K.; et al. Trough Dabrafenib Plasma Concentrations Can Predict Occurrence of Adverse Events Requiring Dose Reduction in Metastatic Melanoma. Clin. Chim. Acta 2017, 472, 26–29. [Google Scholar] [CrossRef]
- Food and Drug Administration. Center for Drug Evaluation and Research Trametinib Clinical Pharmacology and Biopharmaceutics Review; Food and Drug Administration: Roma, Italy, 2013.
- European Medicines Agency. Mektovi. In Summary of Product Characteristics; European Medicines Agency: Amsterdam, The Netherlands, 2018. [Google Scholar]
- European Medicines Agency. Cotellic. In Summary of Product Characteristics; European Medicines Agency: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Heinzerling, L.; Eigentler, T.K.; Fluck, M.; Hassel, J.C.; Heller-Schenck, D.; Leipe, J.; Pauschinger, M.; Vogel, A.; Zimmer, L.; Gutzmer, R. Tolerability of BRAF/MEK Inhibitor Combinations: Adverse Event Evaluation and Management. ESMO Open 2019, 4, e000491. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Cao, J.; Chang, J.; Zhang, Z.; Yang, L.; Wang, J.; Cantarini, M.; Zhang, L. Pharmacokinetics of Osimertinib in Chinese Patients with Advanced NSCLC: A Phase 1 Study. J. Clin. Pharmacol. 2018, 58, 504–513. [Google Scholar] [CrossRef]
- Food and Drug Administration. Center for Drug Evaluation and Research Crizotinib Clinical Pharmacology and Biopharmaceutics Review; Food and Drug Administration: Roma, Italy, 2011.
- European Medicines Agency. Alecensa. In Summary of Product Charateristics; European Medicines Agency: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Demetri, G.D.; Wang, Y.; Wehrle, E.; Racine, A.; Nikolova, Z.; Blanke, C.D.; Joensuu, H.; von Mehren, M. Imatinib Plasma Levels Are Correlated with Clinical Benefit in Patients with Unresectable/Metastatic Gastrointestinal Stromal Tumors. J. Clin. Oncol. 2009, 27, 3141–3147. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; O’Gorman, M.T.; James, L.P.; Klamerus, K.J.; Mugundu, G.; Pithavala, Y.K. Pharmacokinetics of Lorlatinib After Single and Multiple Dosing in Patients with Anaplastic Lymphoma Kinase (ALK)-Positive Non-Small Cell Lung Cancer: Results from a Global Phase I/II Study. Clin. Pharmacokinet. 2021, 60, 1313–1324. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Guideline on Bioanalytical Method Validation; European Medicines Agency: London, UK, 2011; p. 23. [Google Scholar]
- International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use ICH Harmonised Tripartite Guideline—Validation of Analytical Procedures: Text and Methodology Q2(R1). Available online: https://www.scirp.org/reference/ReferencesPapers?ReferenceID=1584467 (accessed on 12 October 2023).
- Bouchet, S.; Chauzit, E.; Ducint, D.; Castaing, N.; Canal-Raffin, M.; Moore, N.; Titier, K.; Molimard, M. Simultaneous Determination of Nine Tyrosine Kinase Inhibitors by 96-Well Solid-Phase Extraction and Ultra Performance LC/MS-MS. Clin. Chim. Acta 2011, 412, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Miura, M.; Takahashi, N. Routine Therapeutic Drug Monitoring of Tyrosine Kinase Inhibitors by HPLC–UV or LC–MS/MS Methods. Drug Metab. Pharmacokinet. 2016, 31, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Rousset, M.; Titier, K.; Bouchet, S.; Dutriaux, C.; Pham-Ledard, A.; Prey, S.; Canal-Raffin, M.; Molimard, M. An UPLC-MS/MS Method for the Quantification of BRAF Inhibitors (Vemurafenib, Dabrafenib) and MEK Inhibitors (Cobimetinib, Trametinib, Binimetinib) in Human Plasma. Application to Treated Melanoma Patients. Clin. Chim. Acta 2017, 470, 8–13. [Google Scholar] [CrossRef]
- Veerman, G.D.M.; Lam, M.H.; Mathijssen, R.H.J.; Koolen, S.L.W.; de Bruijn, P. Quantification of Afatinib, Alectinib, Crizotinib and Osimertinib in Human Plasma by Liquid Chromatography/Triple-Quadrupole Mass Spectrometry; Focusing on the Stability of Osimertinib. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1113, 37–44. [Google Scholar] [CrossRef]
- Panuwet, P.; Hunter, R.E.; D’Souza, P.E.; Chen, X.; Radford, S.A.; Cohen, J.R.; Marder, M.E.; Kartavenka, K.; Ryan, P.B.; Barr, D.B. Biological Matrix Effects in Quantitative Tandem Mass Spectrometry-Based Analytical Methods: Advancing Biomonitoring. Crit. Rev. Anal. Chem. 2016, 46, 93–105. [Google Scholar] [CrossRef]
- De Nicolò, A.; Cantù, M.; D’Avolio, A. Matrix Effect Management in Liquid Chromatography Mass Spectrometry: The Internal Standard Normalized Matrix Effect. Bioanalysis 2017, 9, 1093–1105. [Google Scholar] [CrossRef]
- Baselt, R.C. Imatinib. In Disposition of Toxic Drugs and Chemicals in Man; Biomedical Publications: Foster City, CA, USA, 2017. [Google Scholar]
- Baselt, R.C. Trametinib. In Disposition of Toxic Drugs and Chemicals in Man; Biomedical Publications: Foster City, CA, USA, 2017. [Google Scholar]
- Van Veelen, A.; van Geel, R.; Schoufs, R.; de Beer, Y.; Stolk, L.M.; Hendriks, L.E.L.; Croes, S. Development and Validation of an HPLC-MS/MS Method to Simultaneously Quantify Alectinib, Crizotinib, Erlotinib, Gefitinib and Osimertinib in Human Plasma Samples, Using One Assay Run. Biomed. Chromatogr. 2021, 35, e5224. [Google Scholar] [CrossRef] [PubMed]
- Krens, S.D.; van der Meulen, E.; Jansman, F.G.A.; Burger, D.M.; van Erp, N.P. Quantification of Cobimetinib, Cabozantinib, Dabrafenib, Niraparib, Olaparib, Vemurafenib, Regorafenib and Its Metabolite Regorafenib M2 in Human Plasma by UPLC–MS/MS. Biomed. Chromatogr. 2020, 34, e4758. [Google Scholar] [CrossRef] [PubMed]
- Hefnawy, M.M.; Alanazi, M.M.; Al-Hossaini, A.M.; Alnasser, A.I.; El-Azab, A.S.; Jardan, Y.A.B.; Attwa, M.W.; El-Gendy, M.A. A Rapid and Sensitive Liquid Chromatography-Tandem Mass Spectrometry Bioanalytical Method for the Quantification of Encorafenib and Binimetinib as a First-Line Treatment for Advanced (Unresectable or Metastatic) Melanoma—Application to a Pharmacokinetic Study. Molecules 2022, 28, 79. [Google Scholar] [CrossRef] [PubMed]
- Spatari, C.; Li, W.; Schinkel, A.H.; Ragno, G.; Schellens, J.H.M.; Beijnen, J.H.; Sparidans, R.W. Bioanalytical Assay for the Quantification of the ALK Inhibitor Lorlatinib in Mouse Plasma Using Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1083, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Steeghs, N.; Nijenhuis, C.M.; Schellens, J.H.M.; Beijnen, J.H.; Huitema, A.D.R. Practical Guidelines for Therapeutic Drug Monitoring of Anticancer Tyrosine Kinase Inhibitors: Focus on the Pharmacokinetic Targets. Clin. Pharmacokinet. 2014, 53, 305–325. [Google Scholar] [CrossRef]
- Anforth, R.; Liu, M.; Nguyen, B.; Uribe, P.; Kefford, R.; Clements, A.; Long, G.V.; Fernandez-Peñas, P. Acneiform Eruptions: A Common Cutaneous Toxicity of the MEK Inhibitor Trametinib. Australas. J. Dermatol. 2014, 55, 250–254. [Google Scholar] [CrossRef]
- Crombag, M.B.S.; van Doremalen, J.G.C.; Janssen, J.M.; Rosing, H.; Schellens, J.H.M.; Beijnen, J.H.; Steeghs, N.; Huitema, A.D.R. Therapeutic Drug Monitoring of Small Molecule Kinase Inhibitors in Oncology in a Real-world Cohort Study: Does Age Matter? Br. J. Clin. Pharmacol. 2018, 84, 2770–2778. [Google Scholar] [CrossRef]
- Escudero-Ortiz, V.; Domínguez-Leñero, V.; Catalán-Latorre, A.; Rebollo-Liceaga, J.; Sureda, M. Relevance of Therapeutic Drug Monitoring of Tyrosine Kinase Inhibitors in Routine Clinical Practice: A Pilot Study. Pharmaceutics 2022, 14, 1216. [Google Scholar] [CrossRef]
- Alvarez, J.-C.; Funck-Brentano, E.; Abe, E.; Etting, I.; Saiag, P.; Knapp, A. A LC/MS/MS Micro-Method for Human Plasma Quantification of Vemurafenib. Application to Treated Melanoma Patients. J. Pharm. Biomed. Anal. 2014, 97, 29–32. [Google Scholar] [CrossRef]
- Kondyli, M.; Larouche, V.; Saint-Martin, C.; Ellezam, B.; Pouliot, L.; Sinnett, D.; Legault, G.; Crevier, L.; Weil, A.; Farmer, J.-P.; et al. Trametinib for Progressive Pediatric Low-Grade Gliomas. J. Neurooncol. 2018, 140, 435–444. [Google Scholar] [CrossRef]
- Selt, F.; van Tilburg, C.M.; Bison, B.; Sievers, P.; Harting, I.; Ecker, J.; Pajtler, K.W.; Sahm, F.; Bahr, A.; Simon, M.; et al. Response to Trametinib Treatment in Progressive Pediatric Low-Grade Glioma Patients. J. Neurooncol. 2020, 149, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Ronsley, R.; Hounjet, C.D.; Cheng, S.; Rassekh, S.R.; Duncan, W.J.; Dunham, C.; Gardiner, J.; Ghag, A.; Ludemann, J.P.; Wensley, D.; et al. Trametinib Therapy for Children with Neurofibromatosis Type 1 and Life-Threatening Plexiform Neurofibroma or Treatment-Refractory Low-Grade Glioma. Cancer Med. 2021, 10, 3556–3564. [Google Scholar] [CrossRef] [PubMed]
- Tardieu, M.; Néron, A.; Duvert-Lehembre, S.; Amine Larabi, I.; Barkaoui, M.; Emile, J.-F.; Seigneurin, A.; Boralevi, F.; Donadieu, J. Cutaneous Adverse Events in Children Treated with Vemurafenib for Refractory BRAFV600E Mutated Langerhans Cell Histiocytosis. Pediatr. Blood Cancer 2021, 68, e29140. [Google Scholar] [CrossRef] [PubMed]


| Molecule | Target (TK) | Indication (Adults) | Target Concentrations (ng/mL) | Reference |
|---|---|---|---|---|
| Dabrafenib | BRAF | melanoma BRAF+ NSCLC BRAF+ | Cmin ≥ 15 | [21] |
| Trametinib | MEK | melanoma BRAF+ NSCLC BRAF+ | Cmin ≥ 10.6 | [22] |
| Binimetinib | MEK | melanoma BRAF+ | Cmax = 654 | [23] |
| Cobimetinib | MEK 1/2 | melanoma BRAF+ | Cmax = 270 | [24] |
| Encorafenib | BRAF | melanoma BRAF+ metastatic colorectal cancer | Cmax = 3100 | [25] |
| Osimertinib | EGFFR | NSCLC EGFR+ | Cmin ≥ 90 (40 mg dosage) Cmin ≥ 160 (80 mg dosage) | [26] |
| AZ5104 (metabolite) | EGFR | - | ||
| Crizotinib | ALK | NSCLC ALK+ ou ROS1+ | Cmin ≥ 235 | [27] |
| Alectinib | ALK, RET | NSCLC ALK+ | Cmin ≥ 570 | [28] |
| Alectinib M4 (metabolite) | ALK | Cmin ≥ 220 | [28] | |
| Imatinib | Bcr-Abl, c-Kit, PDGFR, SCFR, DDR | Chronic myeloid leukemia Acute lymphocytic leukemia Gastrointestinal stromal tumor Myelodysplastic/myeloproliferative syndromes | Cmin ≥ 1100 | [29] |
| Lorlatinib | ALK ROS1 | NSCLC ALK+ | Cmax = 575 | [30] |
| Molecules | G1 | G2 | G3 | G4 | G5 | G6 | G7 | QC1 | QC2 | QC3 | QC4 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AZ5104 (Osimertinib metabolite) | 0.2 | 1 | 2 | 10 | 20 | 100 | 200 | 0.3 | 3 | 15 | 150 |
| Trametinib | |||||||||||
| Alectinib | 2 | 10 | 20 | 100 | 200 | 1000 | 2000 | 3 | 30 | 150 | 1500 |
| Alectinib M4 (metabolite) | |||||||||||
| Binimetinib | |||||||||||
| Cobimetinib | |||||||||||
| Crizotinib | |||||||||||
| Lorlatinib | |||||||||||
| Osimertinib | |||||||||||
| Dabrafenib | 5 | 25 | 50 | 250 | 500 | 2500 | 5000 | 7.5 | 75 | 375 | 3750 |
| Imatinib | |||||||||||
| Encorafenib |
| Compound | Rt (min) | Parent Ion m/z | Fragment Ions m/z | Collision Energy (eV) |
|---|---|---|---|---|
| Alectinib | 2.7 | 483.3 | 381.1 | 36 |
| 396.1 | 23 | |||
| Alectinib-D8 | 2.7 | 491.7 | 396.2 | 24 |
| 381.1 | 36 | |||
| Alectinib M4 | 2.6 | 457.3 | 381 | 33 |
| 396 | 30 | |||
| AZ5104 | 2.1 | 486.5 | 72.1 | 28 |
| 371.3 | 32 | |||
| Binimetinib | 2.6 | 441.1 | 379.1 | 19 |
| 165 | 33 | |||
| Bimetinib-13CD4 | 2.6 | 449.1 | 381 | 19 |
| 165 | 31 | |||
| Cobimetinib | 2.8 | 532 | 84 | 29 |
| 249 | 30 | |||
| Cobimetinib-13C6 | 2.8 | 538.1 | 84 | 29 |
| 255 | 32 | |||
| Crizotinib | 2.2 | 450.1 | 177.1 | 38 |
| 260.2 | 23 | |||
| Crizotinib-13C2D5 | 2.2 | 457.2 | 177.1 | 42 |
| 267.2 | 26 | |||
| Dabrafenib | 3.2 | 520.1 | 307.2 | 33 |
| 343.2 | 26 | |||
| Dabrafenib-D9 | 3.2 | 529.1 | 316.3 | 34 |
| 352.3 | 28 | |||
| Encorafenib | 2.8 | 540.2 | 310.1 | 38 |
| 371.2 | 36 | |||
| Encorafenib-13C2D3 | 2.8 | 544.2 | 310 | 34 |
| 371 | 37 | |||
| Imatinib | 2.3 | 494.3 | 217.3 | 25 |
| 394.1 | 26 | |||
| Imatinib-D8 | 2.3 | 502.4 | 225.1 | 26 |
| 394.1 | 27 | |||
| Lorlatinib | 2.3 | 407.2 | 228.1 | 2 |
| 180.1 | 23 | |||
| Osimertinib | 2.4 | 500.4 | 72.14 | 25 |
| 455.2 | 21 | |||
| Osimertinib-13CD3 | 2.4 | 504.7 | 389.2 | 34 |
| 459.2 | 21 | |||
| Trametinib | 3.1 | 616 | 226.2 | 46 |
| 254.2 | 37 | |||
| Trametinib-13C6 | 3.1 | 622 | 232.2 | 48 |
| 260.2 | 39 |
| Dabrafenib | Trametinib | Cobimetinib | Binimetinib | Encorafenib | Alectinib | Imatinib | Osimertinib | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adults | Children | Adults | Children | Adults | Children | Adults | Children | Adults | Adults | Adults | Adults | |
| Number of patients | 95 | 8 | 106 | 10 | 4 | 14 | 24 | 1 | 20 | 1 | 7 | 9 |
| Average age (min–max) (years) | 58 [25–90] | 11 [3–16] | 62 [43–90] | 9 [1–17] | 55 [23–86] | 3.2 [0.1–13] | 62 [33–84] | 2 | 60 [33–83] | 66 | 60 [40–81] | 75.5 [45–86] |
| Sex ratio | 1.2 | 1 | 2.3 | 2.3 | 3 | 1 | 1.6 | - | 0.6 | - | 1.3 | 0.3 |
| Average dose (min–max) (mg/kg) | 4 [1.5–7.3] | 2 [0.4–4.7] | 0.028 [0.01–0.05] | 0.023 [0.01–0.05] | 0.5 [0.3–0.6] | 1.2 [0.9–2.7] | 1.2 [0.8–2.4] | 6.9 | 5.8 [3.8–12.2] | 21 | 4.5 [1.4–7.5] | 1.2 [0.6–2] |
| Number of analyzed samples | 288 | 22 | 355 | 67 | 4 | 82 | 77 | 8 | 77 | 3 | 13 | 10 |
| Molecules | LOD (ng/mL) (n = 6) | LOQ (ng/mL) (n = 6) | Linearity Range (ng/mL) (n = 6) | R2 | Accuracy (Bias, %) | Precision CV (%) | Recovery (n = 6) | Normalized Matrix Effect (NME) (n = 6) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intra-Day (n = 6) | Inter-Day (n = 18) | Intra-Day (n = 6) | Inter-Day (n = 18) | QC2 | QC4 | QC2 | QC4 | |||||||||
| Mean Value | CV (%) | Mean Value | CV (%) | Mean Value | CV (%) | Mean Value | CV (%) | |||||||||
| Dabrafenib | 0.5 | 5 | 5–5000 | 0.9984 | 88.3–103.7 | 89–102.9 | 3.5–5.5 | 2.1–8.4 | 103.5 | 14.2 | 70.1 | 9.1 | 100 | 9.4 | 100 | 5.1 |
| Trametinib | 0.5 | 1 | 1–200 | 0.9980 | 91.8–111.3 | 92.4–106.3 | 4.7–10.8 | 2–14.9 | 94.8 | 10.4 | 85.1 | 6.4 | 120 | 7.9 | 100 | 6.3 |
| Cobimetinib | 0.4 | 2 | 2–2000 | 0.9986 | 88.3–103.1 | 89.6–102 | 4.7–7.3 | 2.5–4.9 | 97.8 | 5.8 | 113.8 | 7.1 | 120 | 3.2 | 100 | 5.5 |
| Encorafenib | 1 | 5 | 5–5000 | 0.9990 | 90.8–106.3 | 92.8–103.2 | 3.9–7.4 | 5.2–14.8 | 88.3 | 15.1 | 114.9 | 8.8 | 110 | 10.8 | 100 | 9.9 |
| Binimetinib | 0.2 | 2 | 2–2000 | 0.9989 | 89.5–103.1 | 92.7–99.5 | 3.4–6.7 | 2.5–8.4 | 110 | 11.1 | 107.7 | 10 | 100 | 8.6 | 100 | 6.5 |
| Osimertinib | 2 | 2 | 2–2000 | 0.9959 | 96.9–113.7 | 100.3–109.8 | 4.3–8.1 | 5.6–7.9 | 106.1 | 10 | 98.3 | 12.5 | 100 | 7.5 | 120 | 12.8 |
| AZ5104 | 0.1 | 0.2 | 0.2–200 | 0.9987 | 88.7–113.6 | 93.8–107.4 | 5.8–9.8 | 9.9–14.6 | 109.8 | 9 | 112.5 | 11.8 | 220 | 8.9 | 210 | 8.8 |
| Crizotinib | 2 | 10 | 10–2000 | 0.9974 | 85.1–109 | 87.5–108.5 | 2.8–6.3 | 1–12.3 | 119.7 | 17.6 | 118.0 | 10.5 | 130 | 8.6 | 100 | 5.5 |
| Alectinib | 0.2 | 2 | 2–2000 | 0.9984 | 85.4–103.9 | 89.8–102.4 | 5.3–8.8 | 1.9–14.4 | 106.4 | 12.1 | 113.1 | 13.8 | 70 | 6.7 | 70 | 10.9 |
| Alectinib M4 | 0.2 | 2 | 2–2000 | 1 | 86.8–106.3 | 91.3–103.1 | 6.4–10.2 | 11.2–11.8 | 107.8 | 4 | 113.1 | 9.8 | 110 | 4.8 | 110 | 9.1 |
| Imatinib | 2.5 | 5 | 5–5000 | 0.9990 | 88.7–97.5 | 92–94.9 | 3.9–5.9 | 6.1–11 | 109.3 | 6 | 111.6 | 9.2 | 120 | 3.3 | 110 | 6.7 |
| Lorlatinib | 0.2 | 2 | 2–2000 | 0.9980 | 89.5–105.3 | 95.7–99 | 3.7–6.9 | 8–14.6 | 104.6 | 8.1 | 114.2 | 14.5 | 80 | 5.3 | 110 | 15.1 |
| Compound | Measured Value (µg/L) | Mean (µg/L) | Z-Score |
|---|---|---|---|
| Alectinib | 296 | 316 | −0.27 |
| Binimetinib | 150 | 137 | 0.64 |
| Cobimetinib | 74 | 64.6 | 1.12 |
| Crizotinib | 78 | 72.8 | 1.3 |
| Dabrafenib | 58 | 53.1 | 1.1 |
| Encorafenib | 50 | 44.1 | 0.94 |
| Imatinib | 528 | 446 | 1.38 |
| Trametinib | 5.5 | 4.74 | 0.98 |
| Dabrafenib | Trametinib | Cobimetinib | Binimetinib | Encorafenib | Alectinib | Alectinib M4 | Imatinib | Osimertinib | AZ5104 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adults (n = 288) | Children (n = 22) | Adults (n = 355) | Children (n = 67) | Adults (n = 4) | Children (n = 82) | Adults (n = 92) | Children (n = 8) | Adults (n = 77) | Adults (n = 3) | Adults (n = 3) | Adults (n = 13) | Adults (n = 10) | Adults (n = 10) | |
| Concentration range CI 95% (ng/mL) | [88–122] | [26–178] | [11–13] | [11–14] | [24–94] | [74–118] | [51–64] | [37–204] | [16–21] | [292–424] | [125–170] | [1132–1702] | [122–320] | [16–56] |
| IQR (ng/mL) | [29–114] | [15–131] | [8–14] | [6.5–17] | [46.5–83] | [34–114] | [38–69] | [62–143] | [10–22] | [302–408] | [132–168] | [1254–1742] | [95–321] | [11–47] |
| Median concentration (ng/mL) | 53.5 | 18.4 | 11 | 9.7 | 70 | 64.3 | 52 | 100 | 15 | 346 | 156 | 1618 | 207 | 25 |
| Mean concentration (ng/mL) | 105.3 | 102.3 | 12.4 | 12.6 | 59.3 | 96.3 | 57.4 | 120 | 18.6 | 358 | 147 | 1417 | 221 | 32 |
| Dose–weight-adjusted median concentration (ng/mL/mg/kg) | 14 | 19.1 | 386.3 | 538.5 | 138.1 | 62.6 | 41.6 | 14.6 | 2.6 | 16.7 | 7.5 | 321.7 | 211.4 | 21.7 |
| Dose–weight-adjusted mean concentration (ng/mL/mg/kg) | 28.3 | 62.3 | 443.8 | 578.4 | 145.7 | 85.4 | 48.8 | 17.5 | 3.4 | 17.3 | 7.1 | 363.6 | 196.7 | 25.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellouard, M.; Donadieu, J.; Thiebot, P.; Giroux Leprieur, E.; Saiag, P.; Etting, I.; Dugues, P.; Abe, E.; Alvarez, J.-C.; Larabi, I.-A. Validation of Liquid Chromatography Coupled with Tandem Mass Spectrometry for the Determination of 12 Tyrosine Kinase Inhibitors (TKIs) and Their Application to Therapeutic Drug Monitoring in Adult and Pediatric Populations. Pharmaceutics 2024, 16, 5. https://doi.org/10.3390/pharmaceutics16010005
Bellouard M, Donadieu J, Thiebot P, Giroux Leprieur E, Saiag P, Etting I, Dugues P, Abe E, Alvarez J-C, Larabi I-A. Validation of Liquid Chromatography Coupled with Tandem Mass Spectrometry for the Determination of 12 Tyrosine Kinase Inhibitors (TKIs) and Their Application to Therapeutic Drug Monitoring in Adult and Pediatric Populations. Pharmaceutics. 2024; 16(1):5. https://doi.org/10.3390/pharmaceutics16010005
Chicago/Turabian StyleBellouard, Marie, Jean Donadieu, Pauline Thiebot, Etienne Giroux Leprieur, Philippe Saiag, Isabelle Etting, Pamela Dugues, Emuri Abe, Jean-Claude Alvarez, and Islam-Amine Larabi. 2024. "Validation of Liquid Chromatography Coupled with Tandem Mass Spectrometry for the Determination of 12 Tyrosine Kinase Inhibitors (TKIs) and Their Application to Therapeutic Drug Monitoring in Adult and Pediatric Populations" Pharmaceutics 16, no. 1: 5. https://doi.org/10.3390/pharmaceutics16010005
APA StyleBellouard, M., Donadieu, J., Thiebot, P., Giroux Leprieur, E., Saiag, P., Etting, I., Dugues, P., Abe, E., Alvarez, J.-C., & Larabi, I.-A. (2024). Validation of Liquid Chromatography Coupled with Tandem Mass Spectrometry for the Determination of 12 Tyrosine Kinase Inhibitors (TKIs) and Their Application to Therapeutic Drug Monitoring in Adult and Pediatric Populations. Pharmaceutics, 16(1), 5. https://doi.org/10.3390/pharmaceutics16010005





