Applying Microfluidics for the Production of the Cationic Liposome-Based Vaccine Adjuvant CAF09b
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Liposomes Using Microfluidics
2.3. Preparation of Liposomes with High Shear Mixing
2.4. Physicochemical Characterization of Liposomes
2.5. Quantification of Poly(I:C)
2.6. Immunogenicity Studies
2.7. Statistical Analysis
3. Results
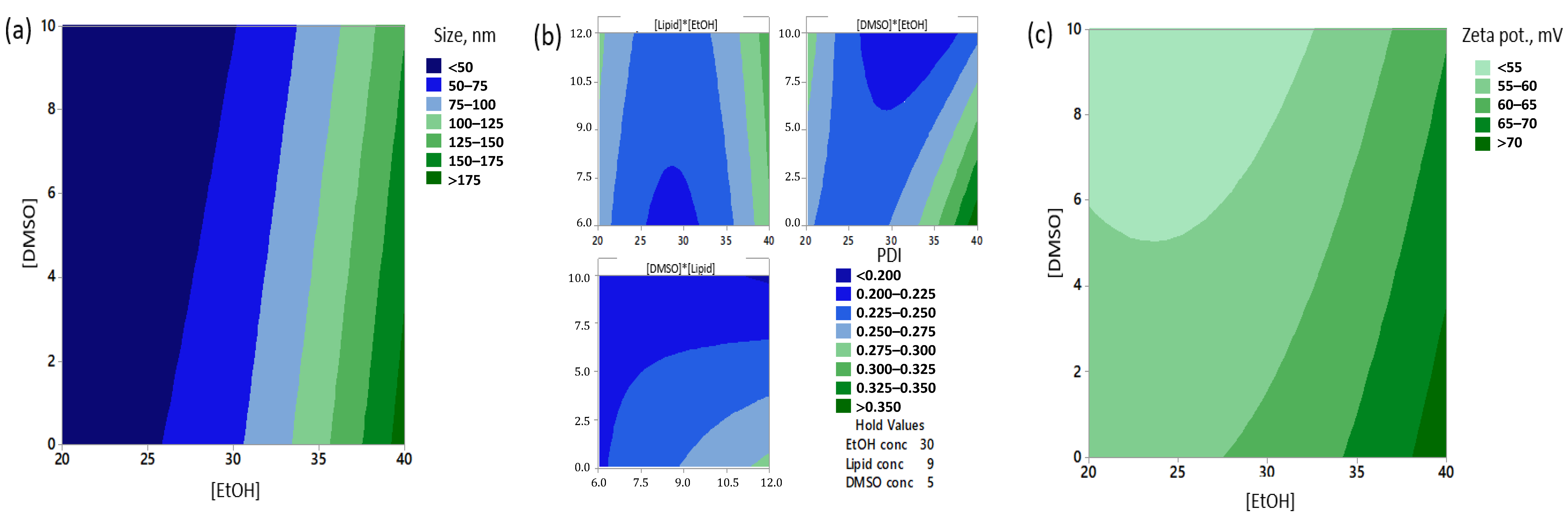
3.1. The Concentration of EtOH Significantly Affected the Particle Sizes, Polydispersity and Zeta Potentials of Freshly Prepared Formulations
3.2. Addition of DMSO Stabilized CAF09b Formulations Stored at 4 °C
3.3. Formulations Prepared with 30% v/v EtOH Have Most Samples at Less Than 100 nm after Storage at 4 °C for 21 Days
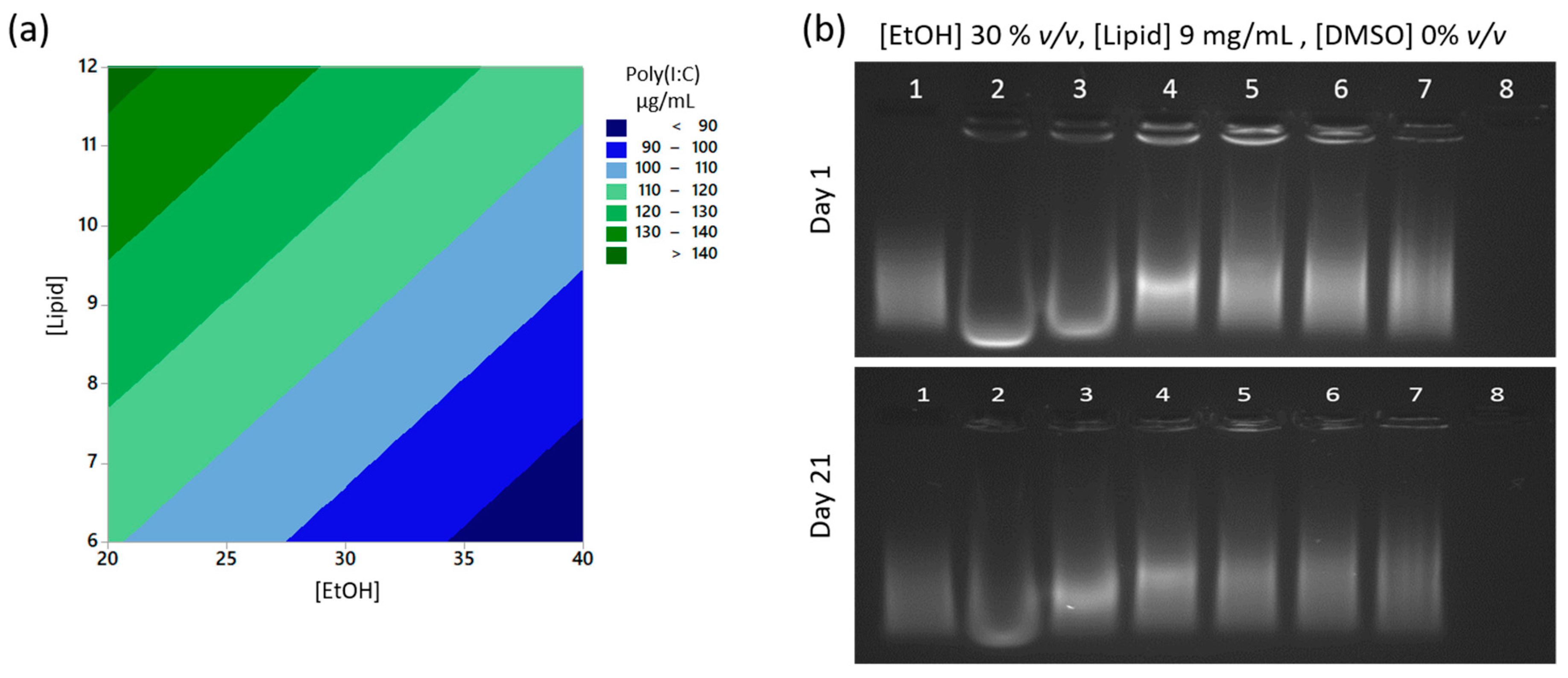
3.4. Poly(I:C) Is Completely Encapsulated Inside the Liposomes after Manufacture by Microfluidics
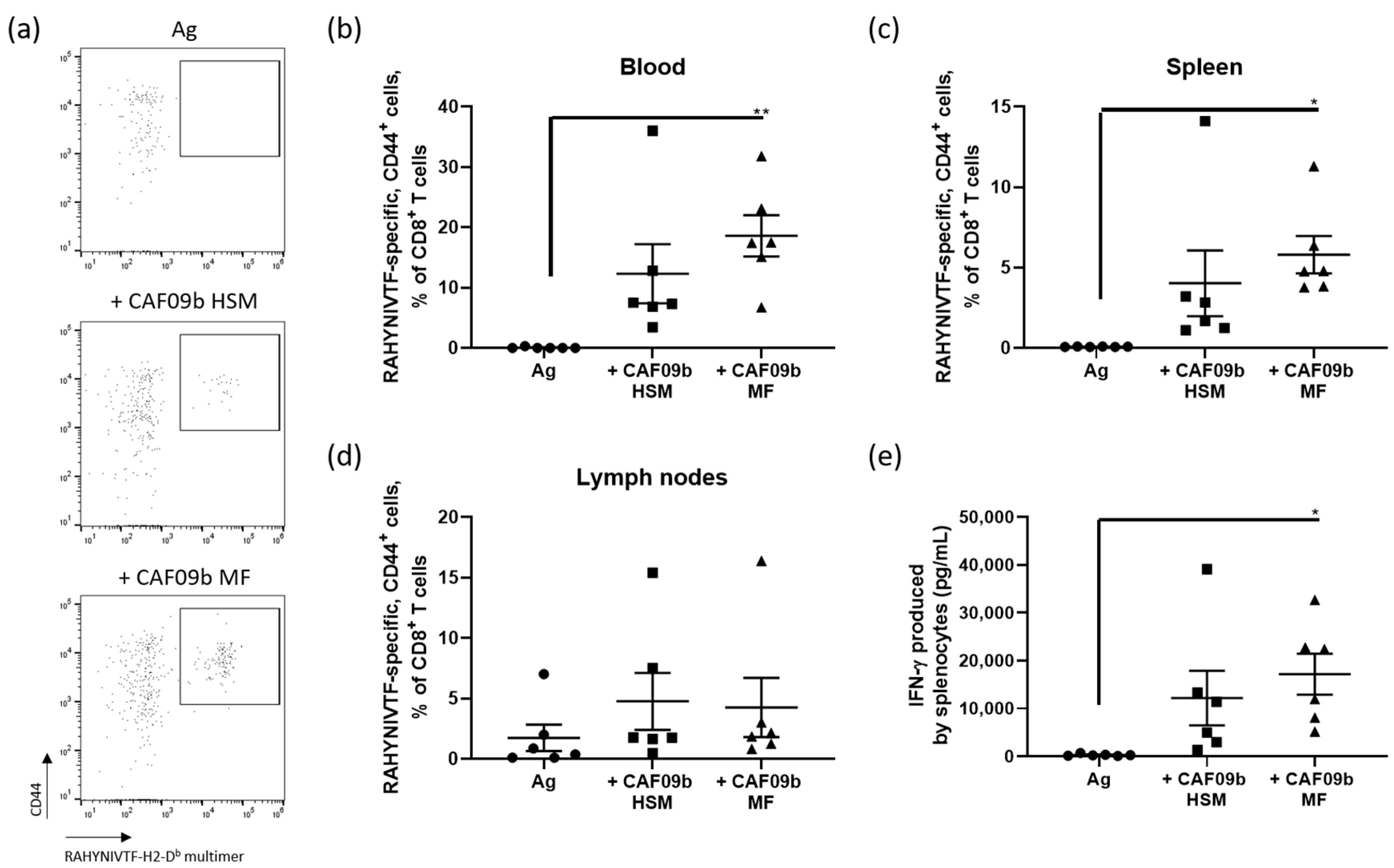
3.5. Antigen-Specific CD8+ T-Cell Immune Responses Induced by CAF09b Prepared by the Microfluidics or High Shear Mixing Methods Are Comparable
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Korsholm, K.S.; Hansen, J.; Karlsen, K.; Filskov, J.; Mikkelsen, M.; Lindenstrøm, T.; Schmidt, S.T.; Andersen, P.; Christensen, D. Induction of CD8+ T-cell responses against subunit antigens by the novel cationic liposomal CAF09 adjuvant. Vaccine 2014, 32, 3927–3935. [Google Scholar] [CrossRef]
- Nordly, P.; Korsholm, K.S.; Pedersen, E.A.; Khilji, T.S.; Franzyk, H.; Jorgensen, L.; Nielsen, H.M.; Agger, E.M.; Foged, C. Incorporation of a synthetic mycobacterial monomycoloyl glycerol analogue stabilizes dimethyldioctadecylammonium liposomes and potentiates their adjuvant effect in vivo. Eur. J. Pharm. Biopharm. 2011, 77, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.T.; Khadke, S.; Korsholm, K.S.; Perrie, Y.; Rades, T.; Andersen, P.; Foged, C.; Christensen, D. The administration route is decisive for the ability of the vaccine adjuvant CAF09 to induce antigen-specific CD8+ T-cell responses: The immunological consequences of the biodistribution profile. J. Control. Release 2016, 239, 107–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahn, A.; Vreeland, W.N.; DeVoe, D.L.; Locascio, L.E.; Gaitan, M. Microfluidic directed formation of liposomes of controlled size. Langmuir 2007, 23, 6289–6293. [Google Scholar] [CrossRef] [PubMed]
- Patil, Y.P.; Jadhav, S. Novel methods for liposome preparation. Chem. Phys. Lipids 2014, 177, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Jahn, A.; Stavis, S.M.; Hong, J.S.; Vreeland, W.N.; DeVoe, D.L.; Gaitan, M. Microfluidic mixing and the formation of nanoscale lipid vesicles. ACS Nano 2010, 4, 2077–2087. [Google Scholar] [CrossRef]
- Maeki, M.; Fujishima, Y.; Sato, Y.; Yasui, T.; Kaji, N.; Ishida, A.; Tani, H.; Baba, Y.; Harashima, H.; Tokeshi, M. Understanding the formation mechanism of lipid nanoparticles in microfluidic devices with chaotic micromixers. PLoS ONE 2017, 12, e0187962. [Google Scholar] [CrossRef]
- Zook, J.M.; Vreeland, W.N. Effects of temperature, acyl chain length, and flow-rate ratio on liposome formation and size in a microfluidic hydrodynamic focusing device. Soft Matter 2010, 6, 1352–1360. [Google Scholar] [CrossRef]
- Kastner, E.; Kaur, R.; Lowry, D.; Moghaddam, B.; Wilkinson, A.; Perrie, Y. High-throughput manufacturing of size-tuned liposomes by a new microfluidics method using enhanced statistical tools for characterization. Int. J. Pharm. 2014, 477, 361–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balbino, T.A.; Serafin, J.M.; Malfatti-Gasperini, A.A.; de Oliveira, C.L.P.; Cavalcanti, L.P.; de Jesus, M.B.; de La Torre, L.G. Microfluidic assembly of pDNA/cationic liposome lipoplexes with high pDNA loading for gene delivery. Langmuir 2016, 32, 1799–1807. [Google Scholar] [CrossRef]
- Balbino, T.A.; Serafin, J.M.; Radaic, A.; de Jesus, M.B.; de la Torre, L.G. Integrated microfluidic devices for the synthesis of nanoscale liposomes and lipoplexes. Colloids Surf. B Biointerfaces 2017, 152, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Belliveau, N.M.; Huft, J.; Lin, P.J.C.; Chen, S.; Leung, A.K.K.; Leaver, T.J.; Wild, A.W.; Lee, J.B.; Taylor, R.J.; Tam, Y.K.; et al. Microfluidic synthesis of highly potent limit-size lipid nanoparticles for in vivo delivery of siRNA. Mol. Ther. Nucleic Acids 2012, 1, e37. [Google Scholar] [CrossRef] [PubMed]
- Webb, C.; Khadke, S.; Tandrup Schmidt, S.; Roces, C.B.; Forbes, N.; Berrie, G.; Perrie, Y. The impact of solvent selection: Strategies to guide the manufacturing of liposomes using microfluidics. Pharmaceutics 2019, 11, 653. [Google Scholar] [CrossRef] [Green Version]
- Michelon, M.; Oliveira, D.R.B.; de Figueiredo Furtado, G.; de la Torre, L.G.; Cunha, R.L. High-throughput continuous production of liposomes using hydrodynamic flow-focusing microfluidic devices. Colloids Surf. B Biointerfaces 2017, 156, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Shrimal, P.; Jadeja, G.; Patel, S. A review on novel methodologies for drug nanoparticle preparation: Microfluidic approach. Chem. Eng. Res. Des. 2020, 153, 728–756. [Google Scholar] [CrossRef]
- Carugo, D.; Bottaro, E.; Owen, J.; Stride, E.; Nastruzzi, C. Liposome production by microfluidics: Potential and limiting factors. Sci. Rep. 2016, 6, 25876. [Google Scholar] [CrossRef] [Green Version]
- Bramosanti, M.; Chronopoulou, L.; Grillo, F.; Valletta, A.; Palocci, C. Microfluidic-assisted nanoprecipitation of antiviral-loaded polymeric nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2017, 532, 369–376. [Google Scholar] [CrossRef]
- Maeki, M.; Kimura, N.; Sato, Y.; Harashima, H.; Tokeshi, M. Advances in microfluidics for lipid nanoparticles and extracellular vesicles and applications in drug delivery systems. Adv. Drug Deliv. Rev. 2018, 128, 84–100. [Google Scholar] [CrossRef]
- Forbes, N.; Hussain, M.T.; Briuglia, M.L.; Edwards, D.P.; ter Horst, J.H.; Szita, N.; Perrie, Y. Rapid and scale-independent microfluidic manufacture of liposomes entrapping protein incorporating in-line purification and at-line size monitoring. Int. J. Pharm. 2019, 556, 68–81. [Google Scholar] [CrossRef] [Green Version]
- Feitosa, E.; Jansson, J.; Lindman, B. The effect of chain length on the melting temperature and size of dialkyldimethylammonium bromide vesicles. Chem. Phys. Lipids 2006, 142, 128–132. [Google Scholar] [CrossRef]
- Sedighi, M.; Sieber, S.; Rahimi, F.; Shahbazi, M.-A.; Rezayan, A.H.; Huwyler, J.; Witzigmann, D. Rapid optimization of liposome characteristics using a combined microfluidics and design-of-experiment approach. Drug Deliv. Trans. Res. 2019, 9, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Perrie, Y.; Gregoriadis, G. Liposome-entrapped plasmid DNA: Characterisation studies. Biochim. Biophys. Acta 2000, 1475, 125–132. [Google Scholar] [CrossRef]
- Shah, V.M.; Nguyen, D.X.; Patel, P.; Cote, B.; Al-Fatease, A.; Pham, Y.; Huynh, M.G.; Woo, Y.; Alani, A.W.G. Liposomes produced by microfluidics and extrusion: A comparison for scale-up purposes. Nanomedicine 2019, 18, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Hussain, M.T.; Roces, C.B.; Anderluzzi, G.; Kastner, E.; Salmaso, S.; Kirby, D.J.; Perrie, Y. Microfluidics based manufacture of liposomes simultaneously entrapping hydrophilic and lipophilic drugs. Int. J. Pharm. 2016, 514, 160–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kastner, E.; Verma, V.; Lowry, D.; Perrie, Y. Microfluidic-controlled manufacture of liposomes for the solubilisation of a poorly water soluble drug. Int. J. Pharm. 2015, 485, 122–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stetter, F.W.S.; Hugel, T. The nanomechanical properties of lipid membranes are significantly influenced by the presence of ethanol. Biophys. J. 2013, 104, 1049–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forbes, N.; Shin, J.E.; Ogunyankin, M.; Zasadzinski, J.A. Inside-outside self-assembly of light-activated fast-release liposomes. Phys. Chem. Chem. Phys. 2015, 17, 15569–15578. [Google Scholar] [CrossRef]
- Adachi, T.; Takahashi, H.; Ohki, K.; Hatta, I. Interdigitated structure of phospholipid-alcohol systems studied by X-ray diffraction. Biophys. J. 1995, 68, 1850–1855. [Google Scholar] [CrossRef] [Green Version]
- Patra, M.; Salonen, E.; Terama, E.; Vattulainen, I.; Faller, R.; Lee, B.W.; Holopainen, J.; Karttunen, M. Under the influence of alcohol: The effect of ethanol and methanol on lipid bilayers. Biophys. J. 2006, 90, 1121–1135. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.H.; Dea, P.K. Insights into the dynamics of DMSO in phosphatidylcholine bilayers. Biophys. Chem. 2001, 94, 33–40. [Google Scholar] [CrossRef]
- Yamashita, Y.; Kinoshita, K.; Yamazaki, M. Low concentration of DMSO stabilizes the bilayer gel phase rather than the interdigitated gel phase in dihexadecylphosphatidylcholine membrane. Biochim. Biophys. Acta 2000, 1467, 395–405. [Google Scholar] [CrossRef] [Green Version]
- Ricci, M.; Oliva, R.; Del Vecchio, P.; Paolantoni, M.; Morresi, A.; Sassi, P. DMSO-induced perturbation of thermotropic properties of cholesterol-containing DPPC liposomes. Biochim. Biophys. Acta. 2016, 1858, 3024–3031. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, M.F.; Jennings, G.T. Vaccine delivery: A matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 2010, 10, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Badiee, A.; Khamesipour, A.; Samiei, A.; Soroush, D.; Shargh, V.H.; Kheiri, M.T.; Barkhordari, F.; Robert Mc Master, W.; Mahboudi, F.; Jaafari, M.R. The role of liposome size on the type of immune response induced in BALB/c mice against leishmaniasis: Rgp63 as a model antigen. Exp. Parasitol. 2012, 132, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Joshi, V.; Geary, S.; Salem, A. Biodegradable particles as vaccine delivery systems: Size matters. AAPS J. 2013, 15, 85–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fifis, T.; Gamvrellis, A.; Crimeen-Irwin, B.; Pietersz, G.A.; Li, J.; Mottram, P.L.; McKenzie, I.F.C.; Plebanski, M. Size-dependent immunogenicity: Therapeutic and protective properties of nano-vaccines against tumors. J. Immunol. 2004, 173, 3148–3154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, S.D.; Scholzen, A.; Minigo, G.; David, C.; Apostolopoulos, V.; Mottram, P.L.; Plebanski, M. Pathogen recognition and development of particulate vaccines: Does size matter? Methods 2006, 40, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Manolova, V.; Flace, A.; Bauer, M.; Schwarz, K.; Saudan, P.; Bachmann, M.F. Nanoparticles target distinct dendritic cell populations according to their size. Eur. J. Immunol. 2008, 38, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- Swartz, M.A.; Hubbell, J.A.; Reddy, S.T. Lymphatic drainage function and its immunological implications: From dendritic cell homing to vaccine design. Semin. Immunol. 2008, 20, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Malcolm, D.W.; Varghese, J.J.; Sorrells, J.E.; Ovitt, C.E.; Benoit, D.S.W. The effects of biological fluids on colloidal stability and siRNA delivery of a pH-responsive micellar nanoparticle delivery system. ACS Nano 2018, 12, 187–197. [Google Scholar] [CrossRef]
- Khadke, S.; Roces, C.B.; Cameron, A.; Devitt, A.; Perrie, Y. Formulation and manufacturing of lymphatic targeting liposomes using microfluidics. J. Control. Release 2019, 307, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Henriksen-Lacey, M.; Christensen, D.; Bramwell, V.W.; Lindenstrøm, T.; Agger, E.M.; Andersen, P.; Perrie, Y. Liposomal cationic charge and antigen adsorption are important properties for the efficient deposition of antigen at the injection site and ability of the vaccine to induce a CMI response. J. Control. Release 2010, 145, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.T.; Olsen, C.L.; Franzyk, H.; Wørzner, K.; Korsholm, K.S.; Rades, T.; Andersen, P.; Foged, C.; Christensen, D. Comparison of two different PEGylation strategies for the liposomal adjuvant CAF09: Towards induction of CTL responses upon subcutaneous vaccine administration. Eur. J. Pharm. Biopharm. 2019, 140, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Hamborg, M.; Jorgensen, L.; Bojsen, A.; Christensen, D.; Foged, C. Protein antigen adsorption to the DDA/TDB liposomal adjuvant: Effect on protein structure, stability, and liposome physicochemical characteristics. Pharm. Res. 2013, 30, 140–155. [Google Scholar] [CrossRef] [PubMed]
- Roces, C.B.; Khadke, S.; Christensen, D.; Perrie, Y. Scale-Independent Microfluidic Production of Cationic Liposomal Adjuvants and Development of Enhanced Lymphatic Targeting Strategies. Mol. Pharm. 2019, 16, 4372–4386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ICH Expert Working Group. ICH Harmonised Guideline. Impurities: Guideline for Residual Solvents Q3C(R6); Currrent Step 4 Version; International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use: Geneva, Switzerland, 2016; pp. 1–38. [Google Scholar]
- Dimov, N.; Kastner, E.; Hussain, M.; Perrie, Y.; Szita, N. Formation and purification of tailored liposomes for drug delivery using a module-based micro continuous-flow system. Sci. Rep. 2017, 7, 12045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammed, A.R.; Bramwell, V.W.; Coombes, A.G.A.; Perrie, Y. Lyophilisation and sterilisation of liposomal vaccines to produce stable and sterile products. Methods 2006, 40, 30–38. [Google Scholar] [CrossRef]

| Parameter | Abbreviation | Low Level | Middle Level | High Level |
|---|---|---|---|---|
| EtOH conc. % v/v (corresponding FRR) | [EtOH] | 20 (4:1) | 30 (2.35:1) | 40 (1.5:1) |
| Total lipid conc. after microfluidics *, mg/mL | [Lipid] | 6 | 9 | 12 |
| DMSO conc. % v/v | [DMSO] | 0 | 5 | 10 |
| Sample No. | DoE Parameters | Day 0 | Day 1 4 °C | Day 21 4 °C | Day 1 RT | Day 21 RT | Day 7 4 °C | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [Lipid] mg/mL | [DMSO] % v/v | [EtOH] % v/v | Size, nm | PDI | Zp, mV | Size, nm | PDI | Size Ratio | Size, nm | PDI | Size Ratio | Size, nm | PDI | Size Ratio | Size, nm | PDI | Size Ratio | Poly(I:C), mg/mL | |
| 1 | 6 | 0 | 40 | 186 | 0.34 | 66 | 185 | 0.38 | 1.0 | 7082 | 0.87 | 38.1 | 187 | 0.38 | 1.0 | 6350 | 0.93 | 34.2 | 79 |
| 2 | 6 | 5 | 40 | 322 | 0.48 | 82 | 521 | 0.55 | 1.6 | 3319 | 1.00 | 10.3 | 313 | 0.87 | 1.0 | 1121 | 0.94 | 3.5 | 87 |
| 3 | 6 | 10 | 40 | 158 | 0.26 | 57 | 155 | 0.26 | 1.0 | 474 | 0.51 | 3.0 | 159 | 0.28 | 1.0 | 742 | 0.78 | 4.7 | 69 |
| 4 | 9 | 0 | 40 | 181 | 0.36 | 61 | 171 | 0.44 | 0.9 | 175 | 0.42 | 1.0 | 176 | 0.41 | 1.0 | 173 | 0.41 | 1.0 | 131 |
| 5 | 9 | 5 | 40 | 174 | 0.33 | 72 | 174 | 0.31 | 1.0 | 200 | 0.38 | 1.2 | 181 | 0.39 | 1.0 | 276 | 0.61 | 1.6 | 148 |
| 6 | 9 | 10 | 40 | 198 | 0.35 | 59 | 183 | 0.36 | 0.9 | 961 | 0.78 | 4.9 | 191 | 0.40 | 1.0 | 505 | 0.52 | 2.6 | 126 |
| 7 | 12 | 0 | 40 | 183 | 0.40 | 68 | 186 | 0.33 | 1.0 | 204 | 0.37 | 1.1 | 184 | 0.42 | 1.0 | 220 | 0.41 | 1.2 | 119 |
| 8 | 12 | 5 | 40 | 194 | 0.36 | 58 | 202 | 0.32 | 1.0 | 209 | 0.36 | 1.1 | 206 | 0.33 | 1.1 | 234 | 0.39 | 1.2 | 105 |
| 9 | 12 | 10 | 40 | 138 | 0.20 | 58 | 150 | 0.21 | 1.1 | 144 | 0.22 | 1.0 | 154 | 0.24 | 1.1 | 185 | 0.31 | 1.3 | 103 |
| 10 | 6 | 0 | 30 | 59 | 0.25 | 64 | 55 | 0.22 | 0.9 | 61 | 0.22 | 1.0 | 58 | 0.23 | 1.0 | 71 | 0.21 | 1.2 | 124 |
| 11 | 6 | 5 | 30 | 52 | 0.17 | 45 | 53 | 0.18 | 1.0 | 55 | 0.16 | 1.1 | 56 | 0.19 | 1.1 | 77 | 0.34 | 1.5 | 101 |
| 12 | 6 | 10 | 30 | 83 | 0.13 | 54 | 84 | 0.15 | 1.0 | 90 | 0.17 | 1.1 | 85 | 0.15 | 1.0 | 94 | 0.21 | 1.1 | 67 |
| 13 | 9 | 0 | 30 | 82 | 0.24 | 62 | 103 | 0.26 | 1.3 | 150 | 0.23 | 1.8 | 59 | 0.24 | 0.7 | 68 | 0.24 | 0.8 | 107 |
| 14 | 9 | 5 | 30 | 58 | 0.24 | 65 | 67 | 0.22 | 1.2 | 100 | 0.22 | 1.7 | 59 | 0.23 | 1.0 | 68 | 0.25 | 1.2 | 110 |
| 15 | 9 | 5 | 30 | 56 | 0.23 | 45 | 76 | 0.23 | 1.4 | 112 | 0.22 | 2.0 | 64 | 0.27 | 1.2 | 71 | 0.27 | 1.3 | 75 |
| 16 | 9 | 5 | 30 | 65 | 0.22 | 56 | 69 | 0.21 | 1.1 | 98 | 0.20 | 1.5 | 66 | 0.23 | 1.0 | 74 | 0.25 | 1.1 | 108 |
| 17 | 9 | 5 | 30 | 49 | 0.19 | 45 | 75 | 0.22 | 1.5 | 116 | 0.22 | 2.3 | 58 | 0.23 | 1.2 | 68 | 0.25 | 1.4 | 81 |
| 18 | 9 | 5 | 30 | 62 | 0.23 | 55 | 64 | 0.19 | 1.0 | 96 | 0.21 | 1.5 | 61 | 0.20 | 1.0 | 71 | 0.23 | 1.1 | 86 |
| 19 | 9 | 5 | 30 | 52 | 0.22 | 60 | 67 | 0.22 | 1.3 | 102 | 0.20 | 1.9 | 55 | 0.22 | 1.0 | 65 | 0.23 | 1.2 | 110 |
| 20 | 9 | 10 | 30 | 52 | 0.27 | 52 | 53 | 0.28 | 1.0 | 60 | 0.34 | 1.2 | 54 | 0.25 | 1.0 | 64 | 0.21 | 1.2 | 109 |
| 21 | 12 | 0 | 30 | 57 | 0.25 | 50 | 130 | 0.28 | 2.3 | 235 | 0.37 | 4.1 | 55 | 0.28 | 1.0 | 64 | 0.28 | 1.1 | 121 |
| 22 | 12 | 5 | 30 | 71 | 0.28 | 57 | 94 | 0.22 | 1.3 | 144 | 0.23 | 2.0 | 72 | 0.24 | 1.0 | 79 | 0.26 | 1.1 | 140 |
| 23 | 12 | 10 | 30 | 56 | 0.17 | 52 | 69 | 0.19 | 1.2 | 99 | 0.17 | 1.8 | 61 | 0.17 | 1.1 | 67 | 0.16 | 1.2 | 142 |
| 24 | 6 | 0 | 20 | 42 | 0.25 | 59 | 121 | 0.24 | 2.8 | 3400 | 1.00 | 80.3 | 48 | 0.26 | 1.1 | 75 | 0.33 | 1.8 | 118 |
| 25 | 6 | 5 | 20 | 47 | 0.43 | 55 | 79 | 0.28 | 1.7 | 114 | 0.24 | 2.4 | 61 | 0.35 | 1.3 | 66 | 0.50 | 1.4 | 133 |
| 26 | 6 | 10 | 20 | 40 | 0.29 | 49 | 45 | 0.25 | 1.1 | 62 | 0.25 | 1.5 | 45 | 0.25 | 1.1 | 64 | 0.38 | 1.6 | 129 |
| 27 | 9 | 0 | 20 | 60 | 0.36 | 58 | 355 | 0.61 | 5.9 | 3157 | 1.00 | 52.3 | 45 | 0.32 | 0.8 | 87 | 0.35 | 1.4 | 124 |
| 28 | 9 | 5 | 20 | 55 | 0.28 | 53 | 130 | 0.27 | 2.4 | 244 | 0.34 | 4.4 | 47 | 0.36 | 0.9 | 72 | 0.33 | 1.3 | 122 |
| 29 | 9 | 10 | 20 | 44 | 0.36 | 45 | 108 | 0.27 | 2.5 | 929 | 0.81 | 21.1 | 52 | 0.31 | 1.2 | 81 | 0.49 | 1.8 | 124 |
| 30 | 12 | 0 | 20 | 49 | 0.27 | 53 | 1123 | 0.84 | 23.0 | 2887 | 1.00 | 59.3 | 58 | 0.32 | 1.2 | 117 | 0.33 | 2.4 | 143 |
| 31 | 12 | 5 | 20 | 43 | 0.30 | 52 | 124 | 0.27 | 2.9 | 586 | 0.65 | 13.7 | 44 | 0.27 | 1.0 | 52 | 0.27 | 1.2 | 134 |
| 32 | 12 | 10 | 20 | 48 | 0.27 | 53 | 97 | 0.28 | 2.0 | 175 | 0.28 | 3.7 | 48 | 0.27 | 1.0 | 56 | 0.30 | 1.2 | 153 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, S.T.; Christensen, D.; Perrie, Y. Applying Microfluidics for the Production of the Cationic Liposome-Based Vaccine Adjuvant CAF09b. Pharmaceutics 2020, 12, 1237. https://doi.org/10.3390/pharmaceutics12121237
Schmidt ST, Christensen D, Perrie Y. Applying Microfluidics for the Production of the Cationic Liposome-Based Vaccine Adjuvant CAF09b. Pharmaceutics. 2020; 12(12):1237. https://doi.org/10.3390/pharmaceutics12121237
Chicago/Turabian StyleSchmidt, Signe Tandrup, Dennis Christensen, and Yvonne Perrie. 2020. "Applying Microfluidics for the Production of the Cationic Liposome-Based Vaccine Adjuvant CAF09b" Pharmaceutics 12, no. 12: 1237. https://doi.org/10.3390/pharmaceutics12121237






