The Future of PSMA-Targeted Radionuclide Therapy: An Overview of Recent Preclinical Research
Abstract
:1. Introduction
PSMA-Targeting Small Molecule Inhibitors
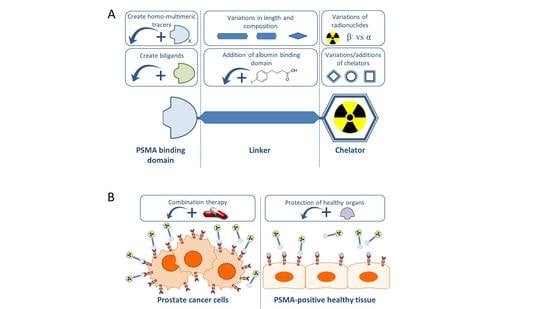
2. Improving PSMA-Targeting Small Molecule Inhibitors
2.1. Addition of an Albumin Binding Domain
2.2. Linker and Chelator Modifications
3. Development of Multi- and Bi-Ligands
3.1. Targeting PSMA and Hepsin/Integrins
3.2. Targeting PSMA and the Gastrin-Releasing Peptide Receptor
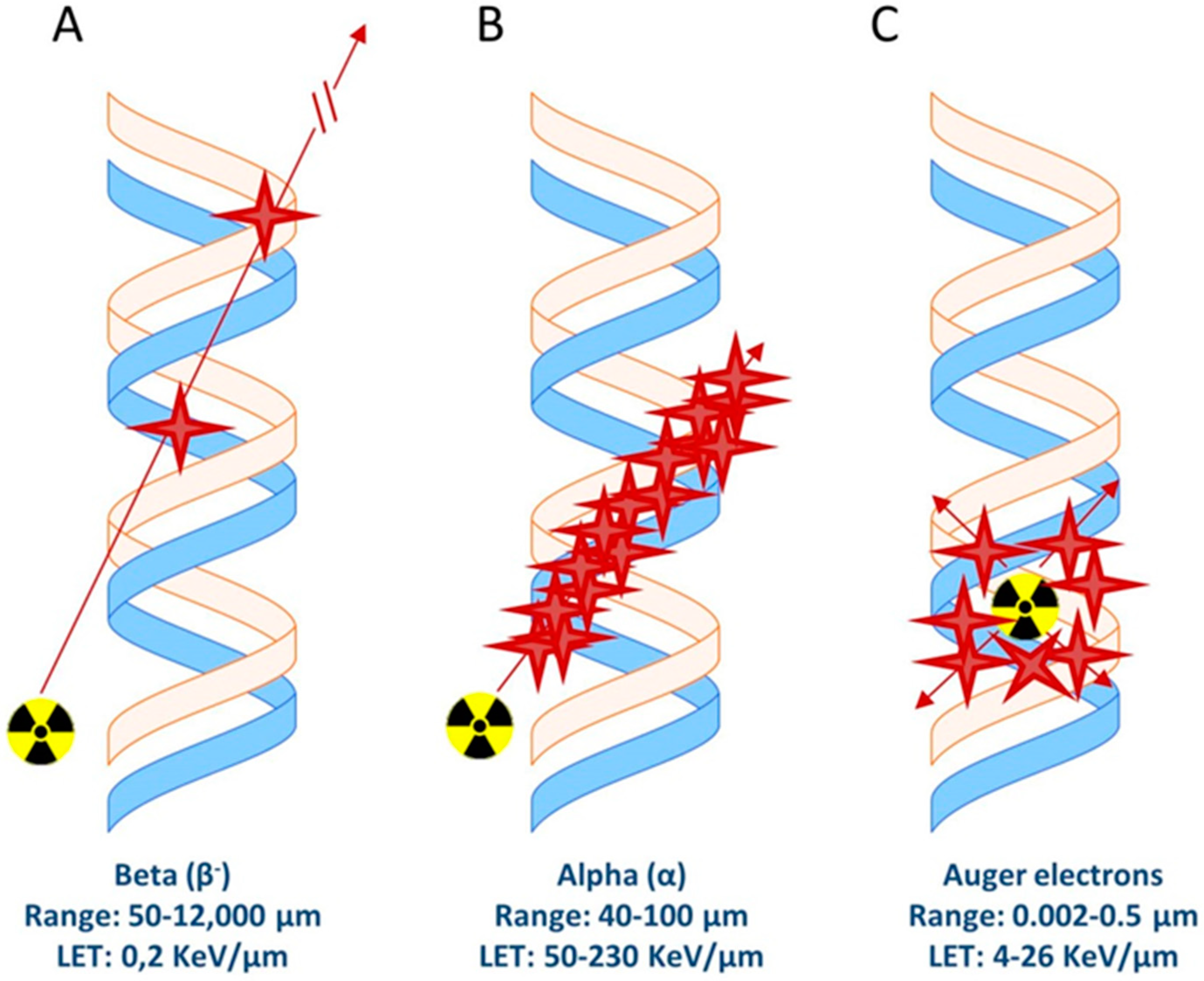
4. Varying Radionuclides for PSMA-TRT
4.1. Beta-Emitters
4.2. Alpha-Emmiters
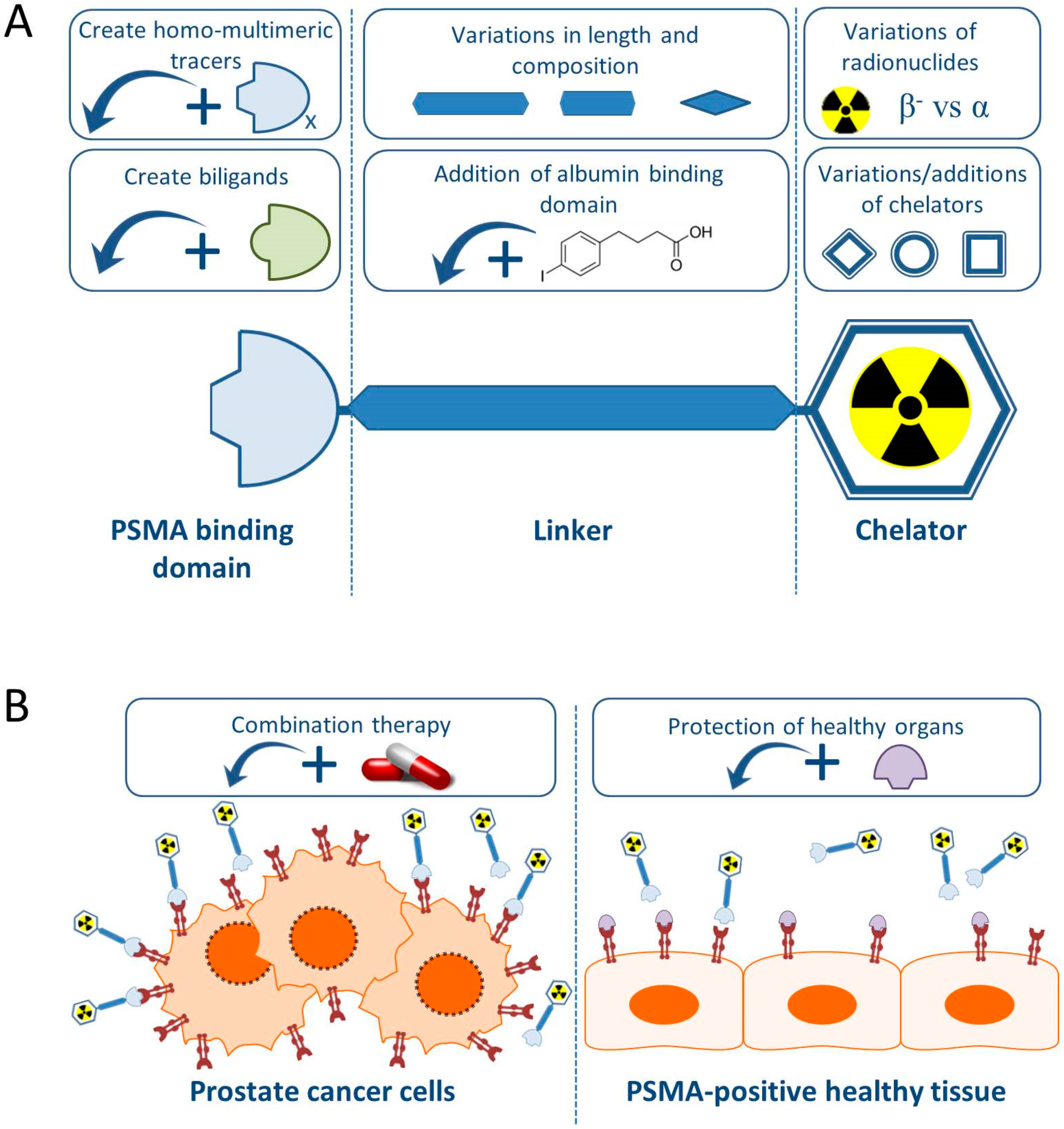
5. Enhancement of Therapy Effect
6. Protection of PSMA Expressing Kidneys and Salivary Glands
7. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.; Gillessen, S.; Heidenreich, A.; Horwich, A.; Committee, E.G. Cancer of the prostate: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26 (Suppl. 5), v69–v77. [Google Scholar] [CrossRef] [PubMed]
- Zaman, M.U.; Fatima, N.; Zaman, A.; Sajid, M.; Zaman, U.; Zaman, S. Diagnostic challenges in prostate cancer and 68Ga-PSMA PET Imaging: A game changer? Asian Pac. J. Cancer Prev. 2017, 18, 2625–2628. [Google Scholar] [PubMed]
- Horoszewicz, J.S.; Kawinski, E.; Murphy, G.P. Monoclonal antibodies to a new antigenic marker in epithelial prostatic cells and serum of prostatic cancer patients. Anticancer Res. 1987, 7, 927–935. [Google Scholar] [PubMed]
- Kawakami, M.; Nakayama, J. Enhanced expression of prostate-specific membrane antigen gene in prostate cancer as revealed by in situ hybridization. Cancer Res. 1997, 57, 2321–2324. [Google Scholar] [PubMed]
- Bostwick, D.G.; Pacelli, A.; Blute, M.; Roche, P.; Murphy, G.P. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: A study of 184 cases. Cancer 1998, 82, 2256–2261. [Google Scholar] [CrossRef]
- Silver, D.A.; Pellicer, I.; Fair, W.R.; Heston, W.D.; Cordon-Cardo, C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin. Cancer Res. 1997, 3, 81–85. [Google Scholar]
- Bouchelouche, K.; Choyke, P.L.; Capala, J. Prostate specific membrane antigen—A target for imaging and therapy with radionuclides. Discov. Med. 2010, 9, 55–61. [Google Scholar]
- Wright, G.L., Jr.; Haley, C.; Beckett, M.L.; Schellhammer, P.F. Expression of prostate-specific membrane antigen in normal, benign, and malignant prostate tissues. Urol. Oncol. 1995, 1, 18–28. [Google Scholar] [CrossRef]
- Backhaus, P.; Noto, B.; Avramovic, N.; Grubert, L.S.; Huss, S.; Bogemann, M.; Stegger, L.; Weckesser, M.; Schafers, M.; Rahbar, K. Targeting PSMA by radioligands in non-prostate disease-current status and future perspectives. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 860–877. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Bruchertseifer, F.; Rathke, H.; Bronzel, M.; Apostolidis, C.; Weichert, W.; Haberkorn, U.; Giesel, F.L.; Morgenstern, A. Targeted α-therapy of metastatic castration-resistant prostate cancer with 225Ac-PSMA-617: Dosimetry Estimate and empiric dose finding. J. Nucl. Med. 2017, 58, 1624–1631. [Google Scholar] [CrossRef] [PubMed]
- Virgolini, I.; Decristoforo, C.; Haug, A.; Fanti, S.; Uprimny, C. Current status of theranostics in prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 471–495. [Google Scholar] [CrossRef] [PubMed]
- Heston, W.D. [Significance of prostate-specific membrane antigen (PSMA). A neurocarboxypeptidase and membrane folate hydrolase]. Bedeutung des prostataspezifischen Membranantigens (PSMA). Eine Neurocarboxypeptidase und Membran-Folat-Hydrolase. Urol. A 1996, 35, 400–407. [Google Scholar] [CrossRef]
- Wustemann, T.; Haberkorn, U.; Babich, J.; Mier, W. Targeting prostate cancer: Prostate-specific membrane antigen based diagnosis and therapy. Med. Res. Rev. 2019, 39, 40–69. [Google Scholar] [CrossRef]
- Teo, M.Y.; Morris, M.J. Prostate-Specific membrane antigen-directed therapy for metastatic castration-resistant prostate cancer. Cancer J. 2016, 22, 347–352. [Google Scholar] [CrossRef]
- Cimadamore, A.; Cheng, M.; Santoni, M.; Lopez-Beltran, A.; Battelli, N.; Massari, F.; Galosi, A.B.; Scarpelli, M.; Montironi, R. New prostate cancer targets for diagnosis, imaging, and therapy: Focus on Prostate-specific membrane antigen. Front. Oncol. 2018, 8, 653. [Google Scholar] [CrossRef]
- Pillai, M.R.A.; Nanabala, R.; Joy, A.; Sasikumar, A.; Russ Knapp, F.F. Radiolabeled enzyme inhibitors and binding agents targeting PSMA: Effective theranostic tools for imaging and therapy of prostate cancer. Nucl. Med. Biol. 2016, 43, 692–720. [Google Scholar] [CrossRef]
- Arsenault, F.; Beauregard, J.M.; Pouliot, F. Prostate-specific membrane antigen for prostate cancer theranostics: From imaging to targeted therapy. Curr. Opin. Support. Palliat. Care 2018, 12, 359–365. [Google Scholar] [CrossRef]
- Eiber, M.; Fendler, W.P.; Rowe, S.P.; Calais, J.; Hofman, M.S.; Maurer, T.; Schwarzenboeck, S.M.; Kratowchil, C.; Herrmann, K.; Giesel, F.L. Prostate-specific membrane antigen ligands for imaging and therapy. J. Nucl. Med. 2017, 58 (Suppl. 2), 67s–76s. [Google Scholar] [CrossRef]
- Afshar-Oromieh, A.; Babich, J.W.; Kratochwil, C.; Giesel, F.L.; Eisenhut, M.; Kopka, K.; Haberkorn, U. The rise of PSMA Ligands for diagnosis and therapy of prostate cancer. J. Nucl. Med. 2016, 57 (Suppl. 3), 79S–89S. [Google Scholar] [CrossRef] [PubMed]
- Kopka, K.; Benesova, M.; Barinka, C.; Haberkorn, U.; Babich, J. Glu-ureido-based inhibitors of prostate-specific membrane antigen: Lessons learned during the development of a novel class of low-molecular-weight theranostic radiotracers. J. Nucl. Med. 2017, 58 (Suppl. 2), 17S–26S. [Google Scholar] [CrossRef] [PubMed]
- Diao, W.; Cai, H.; Chen, L.; Jin, X.; Liao, X.; Jia, Z. Recent advances in prostate-specific membrane antigen-based radiopharmaceuticals. Curr. Top. Med. Chem. 2019, 19, 33–56. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.D.; et al. Prostate cancer-specific PET radiotracers: A review on the clinical utility in recurrent disease. Pract. Radiat. Oncol. 2018, 8, 28–39. [Google Scholar] [CrossRef]
- Wester, H.J.; Schottelius, M. PSMA-targeted radiopharmaceuticals for imaging and therapy. Semin. Nucl. Med. 2019, 49, 302–312. [Google Scholar] [CrossRef]
- Benesova, M.; Schafer, M.; Bauder-Wust, U.; Afshar-Oromieh, A.; Kratochwil, C.; Mier, W.; Haberkorn, U.; Kopka, K.; Eder, M. Preclinical Evaluation of a tailor-made DOTA-conjugated PSMA inhibitor with optimized linker moiety for imaging and endoradiotherapy of prostate cancer. J. Nucl. Med. 2015, 56, 914–920. [Google Scholar] [CrossRef]
- Hofman, M.S.; Violet, J.; Hicks, R.J.; Ferdinandus, J.; Thang, S.P.; Akhurst, T.; Iravani, A.; Kong, G.; Ravi Kumar, A.; Murphy, D.G.; et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): A single-centre, single-arm, phase 2 study. Lancet Oncol. 2018, 19, 825–833. [Google Scholar] [CrossRef]
- Sathekge, M.; Bruchertseifer, F.; Knoesen, O.; Reyneke, F.; Lawal, I.; Lengana, T.; Davis, C.; Mahapane, J.; Corbett, C.; Vorster, M.; et al. 225Ac-PSMA-617 in chemotherapy-naive patients with advanced prostate cancer: A pilot study. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 129–138. [Google Scholar] [CrossRef]
- Weineisen, M.; Schottelius, M.; Simecek, J.; Baum, R.P.; Yildiz, A.; Beykan, S.; Kulkarni, H.R.; Lassmann, M.; Klette, I.; Eiber, M.; et al. 68Ga- and 177Lu-labeled PSMA I&T: Optimization of a PSMA-targeted theranostic concept and first proof-of-concept human studies. J. Nucl. Med. 2015, 56, 1169–1176. [Google Scholar]
- Heck, M.M.; Retz, M.; D’Alessandria, C.; Rauscher, I.; Scheidhauer, K.; Maurer, T.; Storz, E.; Janssen, F.; Schottelius, M.; Wester, H.J.; et al. Systemic radioligand therapy with 177Lu labeled prostate specific membrane antigen ligand for imaging and therapy in patients with metastatic castration resistant prostate cancer. J. Urol. 2016, 196, 382–391. [Google Scholar] [CrossRef]
- Hillier, S.M.; Maresca, K.P.; Femia, F.J.; Marquis, J.C.; Foss, C.A.; Nguyen, N.; Zimmerman, C.N.; Barrett, J.A.; Eckelman, W.C.; Pomper, M.G.; et al. Preclinical evaluation of novel glutamate-urea-lysine analogues that target prostate-specific membrane antigen as molecular imaging pharmaceuticals for prostate cancer. Cancer Res. 2009, 69, 6932–6940. [Google Scholar] [CrossRef] [PubMed]
- Afshar-Oromieh, A.; Haberkorn, U.; Zechmann, C.; Armor, T.; Mier, W.; Spohn, F.; Debus, N.; Holland-Letz, T.; Babich, J.; Kratochwil, C. Repeated PSMA-targeting radioligand therapy of metastatic prostate cancer with 131I-MIP-1095. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 950–959. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.A.; Coleman, R.E.; Goldsmith, S.J.; Vallabhajosula, S.; Petry, N.A.; Cho, S.; Armor, T.; Stubbs, J.B.; Maresca, K.P.; Stabin, M.G.; et al. First-in-man evaluation of 2 high-affinity PSMA-avid small molecules for imaging prostate cancer. J. Nucl. Med. 2013, 54, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Hillier, S.M.; Maresca, K.P.; Lu, G.; Merkin, R.D.; Marquis, J.C.; Zimmerman, C.N.; Eckelman, W.C.; Joyal, J.L.; Babich, J.W. 99mTc-labeled small-molecule inhibitors of prostate-specific membrane antigen for molecular imaging of prostate cancer. J. Nucl. Med. 2013, 54, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Schmidkonz, C.; Hollweg, C.; Beck, M.; Reinfelder, J.; Goetz, T.I.; Sanders, J.C.; Schmidt, D.; Prante, O.; Bauerle, T.; Cavallaro, A.; et al. 99mTc-MIP-1404-SPECT/CT for the detection of PSMA-positive lesions in 225 patients with biochemical recurrence of prostate cancer. Prostate 2018, 78, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Robu, S.; Schottelius, M.; Eiber, M.; Maurer, T.; Gschwend, J.; Schwaiger, M.; Wester, H.J. Preclinical evaluation and first patient application of 99mTc-PSMA-I&S for SPECT imaging and radioguided surgery in prostate cancer. J. Nucl. Med. 2017, 58, 235–242. [Google Scholar] [PubMed]
- Choy, C.J.; Ling, X.; Geruntho, J.J.; Beyer, S.K.; Latoche, J.D.; Langton-Webster, B.; Anderson, C.J.; Berkman, C.E. 177Lu-Labeled phosphoramidate-based PSMA inhibitors: The effect of an albumin binder on biodistribution and therapeutic efficacy in prostate tumor-bearing mice. Theranostics 2017, 7, 1928–1939. [Google Scholar] [CrossRef]
- Kelly, J.M.; Amor-Coarasa, A.; Nikolopoulou, A.; Wustemann, T.; Barelli, P.; Kim, D.; Williams, C., Jr.; Zheng, X.; Bi, C.; Hu, B.; et al. Dual-target binding ligands with modulated pharmacokinetics for endoradiotherapy of prostate cancer. J. Nucl. Med. 2017, 58, 1442–1449. [Google Scholar] [CrossRef]
- Kiess, A.P.; Minn, I.; Chen, Y.; Hobbs, R.; Sgouros, G.; Mease, R.C.; Pullambhatla, M.; Shen, C.J.; Foss, C.A.; Pomper, M.G. Auger radiopharmaceutical therapy targeting prostate-specific membrane antigen. J. Nucl. Med. 2015, 56, 1401–1407. [Google Scholar] [CrossRef]
- Chakravarty, R.; Siamof, C.M.; Dash, A.; Cai, W. Targeted α-therapy of prostate cancer using radiolabeled PSMA inhibitors: A game changer in nuclear medicine. Am. J. Nucl. Med. Mol. Imaging 2018, 8, 247–267. [Google Scholar]
- Kulkarni, H.R.; Singh, A.; Langbein, T.; Schuchardt, C.; Mueller, D.; Zhang, J.; Lehmann, C.; Baum, R.P. Theranostics of prostate cancer: From molecular imaging to precision molecular radiotherapy targeting the prostate specific membrane antigen. Br. J. Radiol. 2018, 91, 20180308. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.S.; Heston, W.D.W. should low molecular weight PSMA targeted ligands get bigger and use albumin ligands for PSMA targeting? Theranostics 2017, 7, 1940–1941. [Google Scholar] [CrossRef] [PubMed]
- Dennis, M.S.; Zhang, M.; Meng, Y.G.; Kadkhodayan, M.; Kirchhofer, D.; Combs, D.; Damico, L.A. Albumin binding as a general strategy for improving the pharmacokinetics of proteins. J. Biol. Chem. 2002, 277, 35035–35043. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, S.; Huang, M. Structure and enzymatic activities of human serum albumin. Curr. Pharm. Des. 2015, 21, 1831–1836. [Google Scholar] [CrossRef]
- Infusino, I.; Panteghini, M. Serum albumin: Accuracy and clinical use. Clin. Chim. Acta 2013, 419, 15–18. [Google Scholar] [CrossRef]
- Wang, Z.; Jacobson, O.; Tian, R.; Mease, R.C.; Kiesewetter, D.O.; Niu, G.; Pomper, M.G.; Chen, X. Radioligand therapy of prostate cancer with a long-lasting prostate-specific membrane antigen targeting agent 90Y-DOTA-EB-MCG. Bioconjug. Chem. 2018, 29, 2309–2315. [Google Scholar] [CrossRef]
- Merlot, A.M.; Kalinowski, D.S.; Richardson, D.R. Unraveling the mysteries of serum albumin-more than just a serum protein. Front. Physiol. 2014, 5, 299. [Google Scholar] [CrossRef]
- Muller, C.; Struthers, H.; Winiger, C.; Zhernosekov, K.; Schibli, R. DOTA conjugate with an albumin-binding entity enables the first folic acid-targeted 177Lu-radionuclide tumor therapy in mice. J. Nucl. Med. 2013, 54, 124–131. [Google Scholar] [CrossRef]
- Kelly, J.; Amor-Coarasa, A.; Ponnala, S.; Nikolopoulou, A.; Williams, C., Jr.; Schlyer, D.; Zhao, Y.; Kim, D.; Babich, J.W. Trifunctional PSMA-targeting constructs for prostate cancer with unprecedented localization to LNCaP tumors. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1841–1851. [Google Scholar] [CrossRef]
- Benesova, M.; Umbricht, C.A.; Schibli, R.; Muller, C. Albumin-binding PSMA ligands: Optimization of the tissue distribution profile. Mol. Pharm. 2018, 15, 934–946. [Google Scholar] [CrossRef]
- Kuo, H.T.; Merkens, H.; Zhang, Z.; Uribe, C.F.; Lau, J.; Zhang, C.; Colpo, N.; Lin, K.S.; Benard, F. Enhancing treatment efficacy of 177Lu-PSMA-617 with the conjugation of an albumin-binding motif: Preclinical dosimetry and endoradiotherapy studies. Mol. Pharm. 2018, 15, 5183–5191. [Google Scholar] [CrossRef] [PubMed]
- Umbricht, C.A.; Benesova, M.; Schibli, R.; Muller, C. Preclinical development of novel PSMA-targeting radioligands: Modulation of albumin-binding properties to improve prostate cancer therapy. Mol. Pharm. 2018, 15, 2297–2306. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.M.; Amor-Coarasa, A.; Ponnala, S.; Nikolopoulou, A.; Williams, C., Jr.; DiMagno, S.G.; Babich, J. Albumin-binding PSMA ligands: Implications for expanding the therapeutic window. J. Nucl. Med. 2018, 60, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.M.; Amor-Coarasa, A.; Ponnala, S.; Nikolopoulou, A.; Williams, C., Jr.; Thiele, N.A.; Schlyer, D.; Wilson, J.J.; DiMagno, S.G.; Babich, J.W. A single dose of 225Ac-RPS-074 induces a complete tumor response in a LNCaP xenograft model. J. Nucl. Med. 2018, 60, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Benesova, M.; Bauder-Wust, U.; Schafer, M.; Klika, K.D.; Mier, W.; Haberkorn, U.; Kopka, K.; Eder, M. Linker Modification strategies to control. the prostate-specific membrane antigen (PSMA)-Targeting and pharmacokinetic properties of DOTA-conjugated PSMA inhibitors. J. Med. Chem. 2016, 59, 1761–1775. [Google Scholar] [CrossRef]
- Huang, S.S.; Wang, X.; Zhang, Y.; Doke, A.; DiFilippo, F.P.; Heston, W.D. Improving the biodistribution of PSMA-targeting tracers with a highly negatively charged linker. Prostate 2014, 74, 702–713. [Google Scholar] [CrossRef]
- Wustemann, T.; Bauder-Wust, U.; Schafer, M.; Eder, M.; Benesova, M.; Leotta, K.; Kratochwil, C.; Haberkorn, U.; Kopka, K.; Mier, W. Design of internalizing PSMA-specific glu-ureido-based radiotherapeuticals. Theranostics 2016, 6, 1085–1095. [Google Scholar] [CrossRef]
- Schmidt, A.; Wirtz, M.; Farber, S.F.; Osl, T.; Beck, R.; Schottelius, M.; Schwaiger, M.; Wester, H.J. Effect of carbohydration on the theranostic tracer PSMA I&T. ACS Omega 2018, 3, 8278–8287. [Google Scholar]
- Kuo, H.T.; Pan, J.; Zhang, Z.; Lau, J.; Merkens, H.; Zhang, C.; Colpo, N.; Lin, K.S.; Benard, F. Effects of Linker modification on tumor-to-kidney contrast of 68Ga-labeled PSMA-targeted imaging probes. Mol. Pharm. 2018, 15, 3502–3511. [Google Scholar] [CrossRef]
- Ray Banerjee, S.; Chen, Z.; Pullambhatla, M.; Lisok, A.; Chen, J.; Mease, R.C.; Pomper, M.G. Preclinical comparative study of 68Ga-labeled DOTA, NOTA, and HBED-CC chelated Radiotracers for targeting PSMA. Bioconjug. Chem. 2016, 27, 1447–1455. [Google Scholar] [CrossRef]
- Ray Banerjee, S.; Pullambhatla, M.; Foss, C.A.; Falk, A.; Byun, Y.; Nimmagadda, S.; Mease, R.C.; Pomper, M.G. Effect of chelators on the pharmacokinetics of 99mTc-labeled imaging agents for the prostate-specific membrane antigen (PSMA). J. Med. Chem. 2013, 56, 6108–6121. [Google Scholar] [CrossRef] [PubMed]
- Wurzer, A.; Seidl, C.; Morgenstern, A.; Bruchertseifer, F.; Schwaiger, M.; Wester, H.J.; Notni, J. Dual-nuclide Radiopharmaceuticals for positron emission tomography based dosimetry in radiotherapy. Chemistry 2018, 24, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Guan, B.; Nham, K.; Hao, G.; Sun, X.; Simanek, E.E. Tumor uptake of triazine dendrimers decorated with four, sixteen, and sixty-four PSMA-targeted ligands: Passive versus active tumor targeting. Biomolecules 2019, 9, 421. [Google Scholar] [CrossRef] [PubMed]
- Schafer, M.; Bauder-Wust, U.; Leotta, K.; Zoller, F.; Mier, W.; Haberkorn, U.; Eisenhut, M.; Eder, M. A dimerized urea-based inhibitor of the prostate-specific membrane antigen for 68Ga-PET imaging of prostate cancer. EJNMMI Res. 2012, 2, 23. [Google Scholar] [CrossRef]
- Liolios, C.; Schafer, M.; Haberkorn, U.; Eder, M.; Kopka, K. Novel bispecific PSMA/GRPr targeting radioligands with optimized pharmacokinetics for improved PET imaging of prostate cancer. Bioconjug. Chem. 2016, 27, 737–751. [Google Scholar] [CrossRef] [PubMed]
- Eder, M.; Schafer, M.; Bauder-Wust, U.; Haberkorn, U.; Eisenhut, M.; Kopka, K. Preclinical evaluation of a bispecific low-molecular heterodimer targeting both PSMA and GRPR for improved PET imaging and therapy of prostate cancer. Prostate 2014, 74, 659–668. [Google Scholar] [CrossRef]
- Wu, Q.; Parry, G. Hepsin and prostate cancer. Front. Biosci. 2007, 12, 5052–5059. [Google Scholar] [CrossRef]
- Subedi, M.; Minn, I.; Chen, J.; Kim, Y.; Ok, K.; Jung, Y.W.; Pomper, M.G.; Byun, Y. Design, synthesis and biological evaluation of PSMA/hepsin-targeted heterobivalent ligands. Eur. J. Med. Chem. 2016, 118, 208–218. [Google Scholar] [CrossRef] [Green Version]
- Shallal, H.M.; Minn, I.; Banerjee, S.R.; Lisok, A.; Mease, R.C.; Pomper, M.G. Heterobivalent agents targeting PSMA and integrin-αvβ3. Bioconjug. Chem. 2014, 25, 393–405. [Google Scholar] [CrossRef]
- Escudero-Castellanos, A.; Ocampo-Garcia, B.; Ferro-Flores, G.; Santos-Cuevas, C.; Morales-Avila, E.; Luna-Gutierrez, M.; Isaac-Olive, K. Synthesis and preclinical evaluation of the 177Lu-DOTA-PSMA(inhibitor)-Lys3-bombesin heterodimer designed as a radiotheranostic probe for prostate cancer. Nucl. Med. Commun. 2019, 40, 278–286. [Google Scholar] [CrossRef]
- Iagaru, A. Will GRPR compete with PSMA as a target in prostate cancer? J. Nucl. Med. 2017, 58, 1883–1884. [Google Scholar] [CrossRef] [PubMed]
- Ananias, H.J.; van den Heuvel, M.C.; Helfrich, W.; de Jong, I.J. Expression of the gastrin-releasing peptide receptor, the prostate stem cell antigen and the prostate-specific membrane antigen in lymph node and bone metastases of prostate cancer. Prostate 2009, 69, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Bandari, R.P.; Jiang, Z.; Reynolds, T.S.; Bernskoetter, N.E.; Szczodroski, A.F.; Bassuner, K.J.; Kirkpatrick, D.L.; Rold, T.L.; Sieckman, G.L.; Hoffman, T.J.; et al. Synthesis and biological evaluation of copper-64 radiolabeled [DUPA-6-Ahx-(NODAGA)-5-Ava-BBN(7–14)NH2], a novel bivalent targeting vector having affinity for two distinct biomarkers (GRPr/PSMA) of prostate cancer. Nucl. Med. Biol. 2014, 41, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Abouzayed, A.; Yim, C.B.; Mitran, B.; Rinne, S.S.; Tolmachev, V.; Larhed, M.; Rosenstrom, U.; Orlova, A. Synthesis and preclinical evaluation of radio-iodinated GRPR/PSMA bispecific heterodimers for the theranostics application in prostate cancer. Pharmaceutics 2019, 11, 358. [Google Scholar] [CrossRef]
- Kassis, A.I. Therapeutic radionuclides: Biophysical and radiobiologic principles. Semin. Nucl. Med. 2008, 38, 358–366. [Google Scholar] [CrossRef]
- Rahbar, K.; Ahmadzadehfar, H.; Kratochwil, C.; Haberkorn, U.; Schafers, M.; Essler, M.; Baum, R.P.; Kulkarni, H.R.; Schmidt, M.; Drzezga, A.; et al. German Multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J. Nucl. Med. 2017, 58, 85–90. [Google Scholar] [CrossRef]
- Kratochwil, C.; Giesel, F.L.; Stefanova, M.; Benesova, M.; Bronzel, M.; Afshar-Oromieh, A.; Mier, W.; Eder, M.; Kopka, K.; Haberkorn, U. PSMA-Targeted radionuclide therapy of metastatic castration-resistant prostate cancer with 177Lu-labeled PSMA-617. J. Nucl. Med. 2016, 57, 1170–1176. [Google Scholar] [CrossRef]
- Champion, C.; Quinto, M.A.; Morgat, C.; Zanotti-Fregonara, P.; Hindie, E. Comparison between 117117, 67Cu, 47Sc and 161Tb, with 117117. Theranostics 2016, 6, 1611–1618. [Google Scholar] [CrossRef]
- Muller, C.; Umbricht, C.A.; Gracheva, N.; Tschan, V.J.; Pellegrini, G.; Bernhardt, P.; Zeevaart, J.R.; Koster, U.; Schibli, R.; van der Meulen, N.P. Terbium-161 for PSMA-targeted radionuclide therapy of prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1919–1930. [Google Scholar] [CrossRef] [Green Version]
- Nonnekens, J.; Chatalic, K.L.; Molkenboer-Kuenen, J.D.; Beerens, C.E.; Bruchertseifer, F.; Morgenstern, A.; Veldhoven-Zweistra, J.; Schottelius, M.; Wester, H.J.; van Gent, D.C.; et al. 213Bi-labeled prostate-specific membrane antigen-targeting agents induce DNA double-strand breaks in prostate cancer xenografts. Cancer Biother. Radiopharm. 2017, 32, 67–73. [Google Scholar] [CrossRef]
- Graf, F.; Fahrer, J.; Maus, S.; Morgenstern, A.; Bruchertseifer, F.; Venkatachalam, S.; Fottner, C.; Weber, M.M.; Huelsenbeck, J.; Schreckenberger, M.; et al. DNA double strand breaks as predictor of efficacy of the alpha-particle emitter Ac-225 and the electron emitter Lu-177 for somatostatin receptor targeted radiotherapy. PLoS ONE 2014, 9, e88239. [Google Scholar] [CrossRef] [PubMed]
- Zustovich, F.; Barsanti, R. Targeted α therapies for the treatment of bone metastases. Int. J. Mol. Sci. 2017, 19, 74. [Google Scholar] [CrossRef] [PubMed]
- Kiess, A.P.; Minn, I.; Vaidyanathan, G.; Hobbs, R.F.; Josefsson, A.; Shen, C.; Brummet, M.; Chen, Y.; Choi, J.; Koumarianou, E.; et al. (2S)-2-(3-(1-Carboxy-5-(4–211At-Astatobenzamido)Pentyl)Ureido)-pentanedioic acid for PSMA-targeted α-particle radiopharmaceutical therapy. J. Nucl. Med. 2016, 57, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Bruchertseifer, F.; Rathke, H.; Hohenfellner, M.; Giesel, F.L.; Haberkorn, U.; Morgenstern, A. Targeted α-therapy of metastatic castration-resistant prostate cancer with 225Ac-PSMA-617: Swimmer-plot analysis suggests efficacy regarding duration of tumor control. J. Nucl. Med. 2018, 59, 795–802. [Google Scholar] [CrossRef]
- Dos Santos, J.C.; Schafer, M.; Bauder-Wust, U.; Lehnert, W.; Leotta, K.; Morgenstern, A.; Kopka, K.; Haberkorn, U.; Mier, W.; Kratochwil, C. Development and dosimetry of 203Pb/212Pb-labelled PSMA ligands: Bringing “the lead” into PSMA-targeted alpha therapy? Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Ray Banerjee, S.; Minn, I.L.; Kumar, V.; Josefsson, A.; Lisok, A.; Brummet, M.; Chen, J.; Kiess, A.; Baidoo, K.; Brayton, C. Preclinical evaluation of 203/212Pb-labeled low-molecular-weight compounds for targeted radiopharmaceutical therapy of prostate cancer. J. Nucl. Med. 2019. [Google Scholar] [CrossRef]
- Malcolm, J.; Falzone, N.; Lee, B.Q.; Vallis, K.A. Targeted radionuclide therapy: New advances for improvement of patient management and response. Cancers 2019, 11, 268. [Google Scholar] [CrossRef]
- Gill, M.R.; Falzone, N.; Du, Y.; Vallis, K.A. Targeted radionuclide therapy in combined-modality regimens. Lancet Oncol. 2017, 18, e414–e423. [Google Scholar] [CrossRef]
- Wardman, P. Chemical radiosensitizers for use in radiotherapy. Clin. Oncol. 2007, 19, 397–417. [Google Scholar] [CrossRef]
- Palacios, D.A.; Miyake, M.; Rosser, C.J. Radiosensitization in prostate cancer: Mechanisms and targets. BMC Urol. 2013, 13, 4. [Google Scholar] [CrossRef]
- Tesson, M.; Rae, C.; Nixon, C.; Babich, J.W.; Mairs, R.J. Preliminary evaluation of prostate-targeted radiotherapy using 131I-MIP-1095 in combination with radiosensitising chemotherapeutic drugs. J. Pharm. Pharmacol. 2016, 68, 912–921. [Google Scholar] [CrossRef] [PubMed]
- Perlmutter, M.A.; Lepor, H. Androgen deprivation therapy in the treatment of advanced prostate cancer. Rev. Urol. 2007, 9 (Suppl. 1), S3–S8. [Google Scholar] [PubMed]
- Mottet, N.; Bellmunt, J.; Bolla, M.; Briers, E.; Cumberbatch, M.G.; De Santis, M.; Fossati, N.; Gross, T.; Henry, A.M.; Joniau, S.; et al. EAU-ESTRO-SIOG Guidelines on prostate cancer. Part. 1: Screening, diagnosis, and local treatment with curative intent. Eur. Urol. 2017, 71, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Mengdi, Q.; Almasan, A.; Gurkan-Cavusoglu, E. Computational analysis of androgen receptor dependent radiosensitivity in prostate cancer. In Proceedings of the 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Lake Buena Vista, FL, USA, 17–20 August 2016; pp. 1426–1429. [Google Scholar]
- Schmidt-Hansen, M.; Hoskin, P.; Kirkbride, P.; Hasler, E.; Bromham, N. Hormone and radiotherapy versus hormone or radiotherapy alone for non-metastatic prostate cancer: A systematic review with meta-analyses. Clin. Oncol. 2014, 26, e21–e46. [Google Scholar] [CrossRef]
- Jones, C.U.; et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N. Engl. J. Med. 2011, 365, 107–118. [Google Scholar] [CrossRef]
- D’Amico, A.V.; Manola, J.; Loffredo, M.; Renshaw, A.A.; DellaCroce, A.; Kantoff, P.W. 6-month androgen suppression plus radiation therapy vs. radiation therapy alone for patients with clinically localized prostate cancer: A randomized controlled trial. JAMA 2004, 292, 821–827. [Google Scholar] [CrossRef]
- Goodwin, J.F.; Schiewer, M.J.; Dean, J.L.; Schrecengost, R.S.; de Leeuw, R.; Han, S.; Ma, T.; Den, R.B.; Dicker, A.P.; Feng, F.Y.; et al. A hormone-DNA repair circuit governs the response to genotoxic insult. Cancer Discov. 2013, 3, 1254–1271. [Google Scholar] [CrossRef]
- Sekhar, K.R.; Wang, J.; Freeman, M.L.; Kirschner, A.N. Radiosensitization by enzalutamide for human prostate cancer is mediated through the DNA damage repair pathway. PLoS ONE 2019, 14, e0214670. [Google Scholar] [CrossRef]
- Chou, F.J.; Chen, Y.; Chen, D.; Niu, Y.; Li, G.; Keng, P.; Yeh, S.; Chang, C. Preclinical study using androgen receptor (AR) degradation enhancer to increase radiotherapy efficacy via targeting radiation-increased AR to better suppress prostate cancer progression. EBioMedicine 2019, 40, 504–516. [Google Scholar] [CrossRef] [Green Version]
- Meller, B.; Bremmer, F.; Sahlmann, C.O.; Hijazi, S.; Bouter, C.; Trojan, L.; Meller, J.; Thelen, P. Alterations in androgen deprivation enhanced prostate-specific membrane antigen (PSMA) expression in prostate cancer cells as a target for diagnostics and therapy. EJNMMI Res. 2015, 5, 66. [Google Scholar] [CrossRef]
- Leitsmann, C.; Thelen, P.; Schmid, M.; Meller, J.; Sahlmann, C.O.; Meller, B.; Trojan, L.; Strauss, A. Enhancing PSMA-uptake with androgen deprivation therapy—A new way to detect prostate cancer metastases? Int. Braz. J. Urol. 2019, 45, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.L., Jr.; Grob, B.M.; Haley, C.; Grossman, K.; Newhall, K.; Petrylak, D.; Troyer, J.; Konchuba, A.; Schellhammer, P.F.; Moriarty, R. Upregulation of prostate-specific membrane antigen after androgen-deprivation therapy. Urology 1996, 48, 326–334. [Google Scholar] [CrossRef]
- Liu, T.; Wu, L.Y.; Fulton, M.D.; Johnson, J.M.; Berkman, C.E. Prolonged androgen deprivation leads to downregulation of androgen receptor and prostate-specific membrane antigen in prostate cancer cells. Int. J. Oncol. 2012, 41, 2087–2092. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.S.; Reuter, V.E.; Heston, W.D.; Hutchinson, B.; Grauer, L.S.; Gaudin, P.B. Short term neoadjuvant androgen deprivation therapy does not affect prostate specific membrane antigen expression in prostate tissues. Cancer 2000, 88, 407–415. [Google Scholar] [CrossRef]
- Luckerath, K.; Wei, L.; Fendler, W.P.; Evans-Axelsson, S.; Stuparu, A.D.; Slavik, R.; Mona, C.E.; Calais, J.; Rettig, M.; Reiter, R.E.; et al. Preclinical evaluation of PSMA expression in response to androgen receptor blockade for theranostics in prostate cancer. EJNMMI Res. 2018, 8, 96. [Google Scholar] [CrossRef] [PubMed]
- Higano, C.S. Side effects of androgen deprivation therapy: Monitoring and minimizing toxicity. Urology 2003, 61 (Suppl. 2), 32–38. [Google Scholar] [CrossRef]
- Sarnelli, A.; Belli, M.L.; Di Iorio, V.; Mezzenga, E.; Celli, M.; Severi, S.; Tardelli, E.; Nicolini, S.; Oboldi, D.; Uccelli, L.; et al. Dosimetry of 177Lu-PSMA-617 after mannitol infusion and glutamate tablet administration: Preliminary results of EUDRACT/RSO 2016–002732–32 IRST protocol. Molecules 2019, 24, 621. [Google Scholar] [CrossRef]
- Von Eyben, F.E.; Baumann, G.S.; Baum, R.P. PSMA diagnostics and treatments of prostate cancer become mature. Clin. Transl. Imaging 2018, 6, 145–148. [Google Scholar] [CrossRef] [Green Version]
- Langbein, T.; Chausse, G.; Baum, R.P. Salivary gland toxicity of PSMA radioligand therapy: Relevance and preventive strategies. J. Nucl. Med. 2018, 59, 1172–1173. [Google Scholar] [CrossRef]
- Rathke, H.; Kratochwil, C.; Hohenberger, R.; Giesel, F.L.; Bruchertseifer, F.; Flechsig, P.; Morgenstern, A.; Hein, M.; Plinkert, P.; Haberkorn, U.; et al. Initial clinical experience performing sialendoscopy for salivary gland protection in patients undergoing 225Ac-PSMA-617 RLT. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 139–147. [Google Scholar] [CrossRef]
- Baum, R.P.; Langbein, T.; Singh, A.; Shahinfar, M.; Schuchardt, C.; Volk, G.F.; Kulkarni, H. Injection of botulinum toxin for preventing salivary gland toxicity after PSMA radioligand therapy: An empirical proof of a promising concept. Nucl Med. Mol. Imaging 2018, 52, 80–81. [Google Scholar] [CrossRef] [PubMed]
- Chatalic, K.L.; Heskamp, S.; Konijnenberg, M.; Molkenboer-Kuenen, J.D.; Franssen, G.M.; Clahsen-van Groningen, M.C.; Schottelius, M.; Wester, H.J.; van Weerden, W.M.; Boerman, O.C.; et al. Towards personalized treatment of prostate cancer: PSMA I&T, a promising prostate-specific membrane antigen-targeted theranostic agent. Theranostics 2016, 6, 849–861. [Google Scholar] [PubMed]
- Liu, T.; Liu, C.; Xu, X.; Liu, F.; Guo, X.; Li, N.; Wang, X.; Yang, J.; Yang, X.; Zhu, H.; et al. Preclinical evaluation and pilot clinical study of Al18F-PSMA-BCH for prostate cancer imaging. J. Nucl. Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Umbricht, C.A.; Benesova, M.; Schmid, R.M.; Turler, A.; Schibli, R.; van der Meulen, N.P.; Muller, C. 44Sc-PSMA-617 for radiotheragnostics in tandem with 177Lu-PSMA-617-preclinical investigations in comparison with 68Ga-PSMA-11 and 68Ga-PSMA-617. EJNMMI Res. 2017, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Giesel, F.L.; Leotta, K.; Eder, M.; Hoppe-Tich, T.; Youssoufian, H.; Kopka, K.; Babich, J.W.; Haberkorn, U. PMPA for nephroprotection in PSMA-targeted radionuclide therapy of prostate cancer. J. Nucl. Med. 2015, 56, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Bacich, D.J.; Pinto, J.T.; Tong, W.P.; Heston, W.D. Cloning, expression, genomic localization, and enzymatic activities of the mouse homolog of prostate-specific membrane antigen/NAALADase/folate hydrolase. Mamm. Genome 2001, 12, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Simons, B.W.; Turtle, N.F.; Ulmert, D.H.; Abou, D.S.; Thorek, D.L.J. PSMA expression in the Hi-Myc model; extended utility of a representative model of prostate adenocarcinoma for biological insight and as a drug discovery tool. Prostate 2019, 79, 678–685. [Google Scholar] [CrossRef]
- Tonnesmann, R.; Meyer, P.T.; Eder, M.; Baranski, A.C. [177Lu]Lu-PSMA-617 Salivary gland uptake characterized by quantitative in vitro autoradiography. Pharmaceuticals 2019, 12, 18. [Google Scholar] [CrossRef]
- Rupp, N.J.; Umbricht, C.A.; Pizzuto, D.A.; Lenggenhager, D.; Topfer, A.; Muller, J.; Muhlematter, U.J.; Ferraro, D.A.; Messerli, M.; Morand, G.B.; et al. First clinico-pathological evidence of a non PSMA-related uptake mechanism for 68Ga-PSMA-11 in salivary glands. J. Nucl. Med. 2019. [Google Scholar] [CrossRef]
- Roy, J.; Warner, B.; Basuli, F.; Williams, M.; Wong, K.; Ton, A.; Chiorini, J.; Choyke, P.; Lin, F.; Jagoda, E. Identifying an appropriate animal model to examine preservation of salivary function with PSMA targeted radiotherapies. J. Nucl. Med. 2018, 59 (Suppl. 1), 1255. [Google Scholar]
- Rousseau, E.; Lau, J.; Kuo, H.T.; Zhang, Z.; Merkens, H.; Hundal-Jabal, N.; Colpo, N.; Lin, K.S.; Benard, F. Monosodium glutamate reduces 68Ga-PSMA-11 uptake in salivary glands and kidneys in a preclinical prostate cancer model. J. Nucl. Med. 2018, 59, 1865–1868. [Google Scholar] [CrossRef] [PubMed]
- Jackson, P.F.; Cole, D.C.; Slusher, B.S.; Stetz, S.L.; Ross, L.E.; Donzanti, B.A.; Trainor, D.A. Design, synthesis, and biological activity of a potent inhibitor of the neuropeptidase N-acetylated α-linked acidic dipeptidase. J. Med. Chem. 1996, 39, 619–622. [Google Scholar] [CrossRef] [PubMed]
- Soeda, F.; Watabe, T.; Naka, S.; Liu, Y.; Horitsugi, G.; Neels, O.C.; Kopka, K.; Tatsumi, M.; Shimosegawa, E.; Giesel, F.L.; et al. Impact of 18F-PSMA-1007 uptake in prostate cancer using different peptide concentrations: Preclinical PET/CT study in mice. J. Nucl. Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Wurzer, A.; Pollmann, J.; Schmidt, A.; Reich, D.; Wester, H.J.; Notni, J. Molar activity of Ga-68 labeled PSMA inhibitor conjugates determines PET imaging results. Mol. Pharm. 2018, 15, 4296–4302. [Google Scholar] [CrossRef] [PubMed]
- Majer, P.; Jancarik, A.; Krecmerova, M.; Tichy, T.; Tenora, L.; Wozniak, K.; Wu, Y.; Pommier, E.; Ferraris, D.; Rais, R.; et al. Discovery of orally available prodrugs of the glutamate carboxypeptidase II (GCPII) inhibitor 2-phosphonomethylpentanedioic acid (2-PMPA). J. Med. Chem. 2016, 59, 2810–2819. [Google Scholar] [CrossRef] [PubMed]
- Matteucci, F.; Mezzenga, E.; Caroli, P.; Di Iorio, V.; Sarnelli, A.; Celli, M.; Fantini, L.; Moretti, A.; Galassi, R.; De Giorgi, U.; et al. Reduction of 68Ga-PSMA renal uptake with mannitol infusion: Preliminary results. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 2189–2194. [Google Scholar] [CrossRef] [PubMed]
| Compound | Radionuclides | PSMA Binding Motif | First Reference | Clinical Trials |
|---|---|---|---|---|
| PSMA-617 | Lutetium-177 Actinium-225 Indium-111 Gallium-68 Yttrium-90 | Urea based (Glu-urea-lys) | [26] | [27,28] |
| PSMA-I&T | Lutetium-177 Actinium-225 Indium-111 Gallium-68 | Urea based (Glu-urea-lys) | [29] | [30] |
| MIP-1095 | Iodine-123 Iodine-131 | Urea based (Glu-urea-lys) | [31] | [32] |
| MIP-1072 | Iodine-123 | Urea based (Glu-urea-lys) | [31] | [33] |
| MIP-1404/-1405 | Technetium-99m | Urea based (Glu-urea-lys) | [34] | [35] |
| PSMA I&S | Technetium-99m | Urea based (Glu-urea-lys) | [36] | - |
| CTT1400/CTT1402 | Lutetium-177 | phosphoramidate-based | [37] | - |
| RPS-027 | Iodine-131 Astatine-211 | Urea based (Glu-urea-lys) | [38] | - |
| DCIBzL | Iodine-125 Iodine-131 | Urea based (Glu-urea-lys) | [39] | - |
| Compound | Radionuclide | Albumin Binding Domain | In Vivo Model | Injected | Tumor-to-kidney-Ratio 24 h p.i. | Ref. |
|---|---|---|---|---|---|---|
| CTT1402 | Lutetium-177 | 4-(p-iodophenyl) butyric acid | NCr nude mice + PC3-PIP tumors | 1.85 ± 0.07 MBq 4 ± 1 MBq/nmol | 0.14 | [37] |
| RPS-027 | Iodine-131 | 4-(4-iodophenyl) butanoic acid | NCr-nu/nu mice + LNCaP cell xenografts | ∼370 kBq/10 μCi | ±2.5 * | [38] |
| DOTA-PSMA-ALB-02 | Lutetium-177 | 4-(p-iodophenyl) butyric acid | PC-3 PIP/flu | No data | 7.16 | [50] |
| HTK01169 | Lutetium-177 | N-[4-(p-iodophenyl)butanoyl]-Glu | LNCaP | No data | 0.45 | [51] |
| DOTA-EB-MCG | Yttrium-90 | truncated Evans blue | Athymic Nude-Foxn1nu, Envigo + PC3-PIP tumors | 3.7–5.1 MBq | ±4 * | [46] |
| RPS-072 | Lutetium-177 | 4-(4-iodophenyl) butanoic acid | Male BALB/C athymic nu/nu mice + LNCaP tumors | 0.36–1.3 MBq 13–23 pmol | ±4.5 * | [53] |
| RPS-074 | Actinium-225 | 4-(4-iodophenyl) butanoic acid | Male BALB/C athymic nu/nu mice + LNCaP tumors | 105 kBq 142 pmol | 4.3 | [54] |
| PSMA-ALB-53 | Lutetium-177 | 4-(p-iodophenyl)-moiety | PC3-PIP tumors | 5MBq 100 pmol | 2.17 | [52] |
| PSMA-ALB-56 | Lutetium-177 | p-(tolyl)-moiety | PC3-PIP tumors | 5MBq 100 pmol | 10.8 | [52] |
| Radionuclide | Particle Emission | T1/2 | Eavg KeV (β− or α) |
|---|---|---|---|
| Scandium-47 | β− | 3.3 days | 162 |
| Copper-67 | β− | 2.6 days | 141 |
| Iodine-131 | β− | 8.0 days | 181 |
| Terbium-161 | β− | 6.9 days | 154 |
| Lutetium-177 | β− | 6.7 days | 140 |
| Astatine-211 | α | 7.2 h | 5868 |
| Lead-212 | β− | 10.6 h | 130 |
| Bismuth-213 | α | 46 min | 1390 (max) |
| Actinium-225 | α | 9.9 days | 5915 |
| Thorium-227 | α | 18.7 days | 6145 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruigrok, E.A.M.; van Weerden, W.M.; Nonnekens, J.; de Jong, M. The Future of PSMA-Targeted Radionuclide Therapy: An Overview of Recent Preclinical Research. Pharmaceutics 2019, 11, 560. https://doi.org/10.3390/pharmaceutics11110560
Ruigrok EAM, van Weerden WM, Nonnekens J, de Jong M. The Future of PSMA-Targeted Radionuclide Therapy: An Overview of Recent Preclinical Research. Pharmaceutics. 2019; 11(11):560. https://doi.org/10.3390/pharmaceutics11110560
Chicago/Turabian StyleRuigrok, Eline A.M., Wytske M. van Weerden, Julie Nonnekens, and Marion de Jong. 2019. "The Future of PSMA-Targeted Radionuclide Therapy: An Overview of Recent Preclinical Research" Pharmaceutics 11, no. 11: 560. https://doi.org/10.3390/pharmaceutics11110560