Diagnosis of Dengue Infection Using Conventional and Biosensor Based Techniques
Abstract
:1. Introduction
2. Dengue as a Global Health Concern
3. Causes for Emergence of Dengue
4. A Brief History of Dengue
5. Laboratory Diagnosis and Its Significance
| Clinical symptoms | Differential diagnoses |
|---|---|
| Flu-like | Influenza, Measles, Chikungunya, Adenovirus infection, Infectious mononucleosis, Acute HIV seroconversion illness |
| Rash | Rubella, Zika fever, West Nile fever, Measles, Scarlet fever, Meningococcal infection, Chikungunya, Drug |
| Diarrhoea | Rotavirus infection, Norovirus, Cytomegalovirus, Viral hepatitis, Food poisoning |
| Muscle/Joint pain | West Nile fever, Zika fever, Chikungunya |
| Neurological manifestation | Meningoencephalitis, Tick-borne encephalitis, West Nile fever, Japanese encephalitis, Febrile seizures |
| Bleeding tendency | Yellow fever, Ebola, Marburg |
| Thrombocytopenia | Rubella, Epstein-Barr virus, Parvovirus |
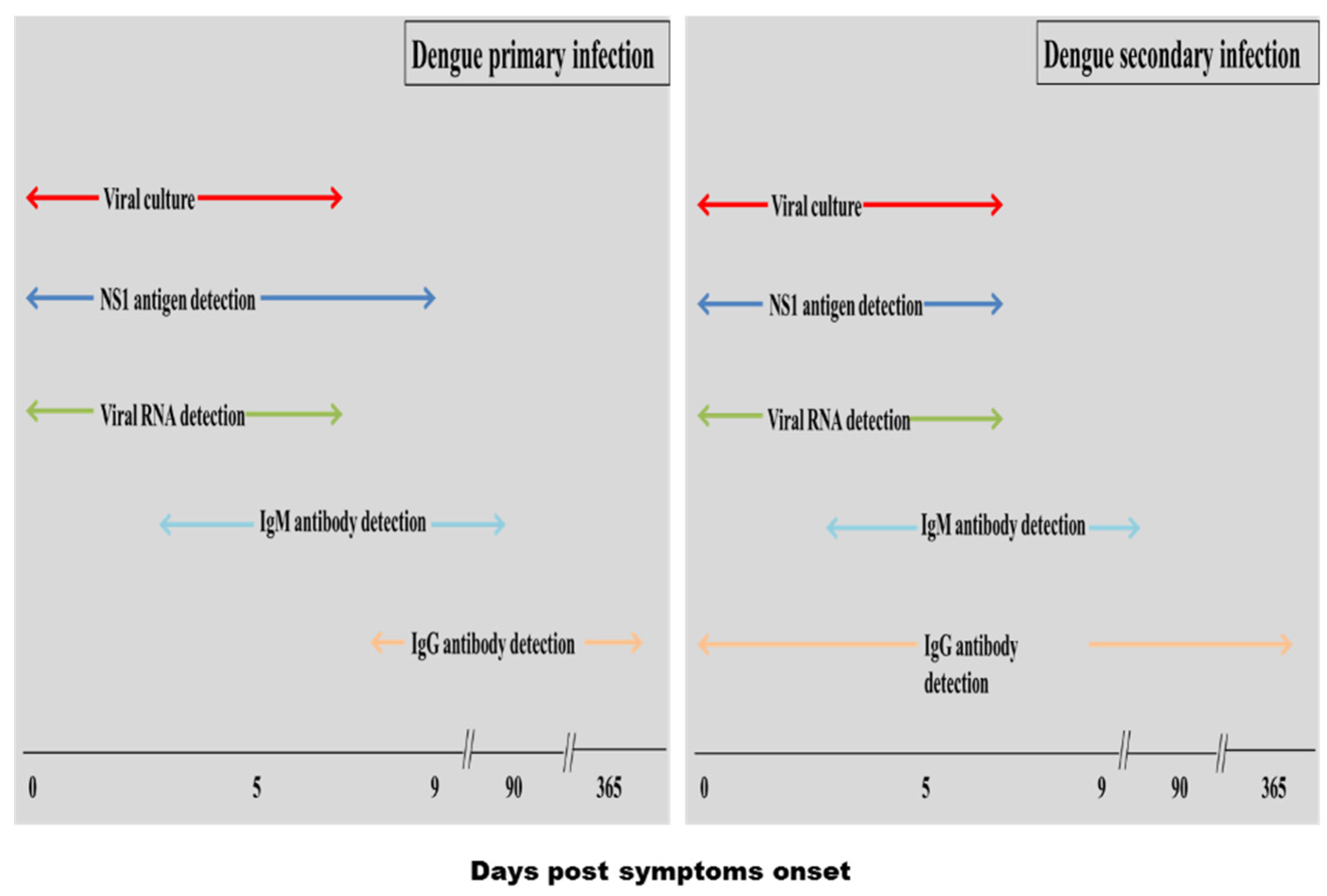
5.1. Conventional Diagnosis Methods
5.1.1. Viral Culture
5.1.2. Nucleic Acid Amplification
5.1.3. Serological Diagnosis
HI Assay
Detection of Immunoglobulin M (IgM) Antibody
| Test format | Brand | Assay time | Sensitivity | Specificity | Ref. |
|---|---|---|---|---|---|
| ELISA | Dengue Fever Virus IgM Capture, Focus Diagnostics | 225 | 98.6 | 79.9 | [46] |
| Pathozyme M Dengue, Omega | 120 | 61.5 | 84.6 | [46] | |
| Pathozyme M Dengue Capture, Omega | 110 | 83.5 | 86.5 | [45] | |
| Dengue IgM Capture, Panbio | 130 | 89.5 87.6 | 861 88.1 | [45,47] | |
| SD Dengue IgM Capture, Standard Diagnostics | 130 | 84.9 | 97.3 | [47] | |
| InBios IgM ELISA | 90 | 88.7 | 93.1 | [48] | |
| Rapid test | Panbio Dengue Duo Cassette (IgM/IgG) | 15 | 77.8 92.1 | 90.6 62.2 | [46,49] |
| Hapalyse dengue-M PA kit, Pentax | 90 | 97.7 | 76.6 | [46] | |
| SD Bioline Dengue IgG/IgM | 15–20 | 60.9 87.3% | 90 86.8 | [46,49] | |
| Dengue check WB, Zephyr (IgM/IgG) | 15 | 20.5 | 86.7 | [46] |
Detection of IgG Antibody
Detection of DENV NS1 Antigen
| Test format | Brand | Assay time (Min) | Sensitivity % | Specificity % | Ref. |
|---|---|---|---|---|---|
| ELISA | Platelia Dengue NS1 Ag Kit, Biorad | 140 | 89.4 83.6 | 97.4 98.7 | [26,57] |
| DENV Detect NS1 ELISA, Inbios | 111 | 95.9 | 100 | [57] | |
| Pan-E Dengue Early ELISA, Panbio | 160 | 85.5 72.3 | 95.5 100 | [26,57] | |
| SD Dengue NS1 Ag ELISA | 160 | 76.7 | 98.31 | [58] | |
| Rapid test | Dengue NS1 Detect , Inbios | 30 | 86.0 76.5 | 100 97.4 | [57,59] |
| Biorad NS1 Ag Strip | 15–30 | 72.8 79.1 | 100 100 | [57,59] | |
| Panbio NS1 Ag Strip | 15 | 71.9 | 95 | [57] | |
| SD Dengue Duo | 15–20 | 70.6 72.4 | 100 73.4 | [26,57] |
5.2. Biosensor Based Methods for Dengue
5.2.1. Electrochemical Biosensor
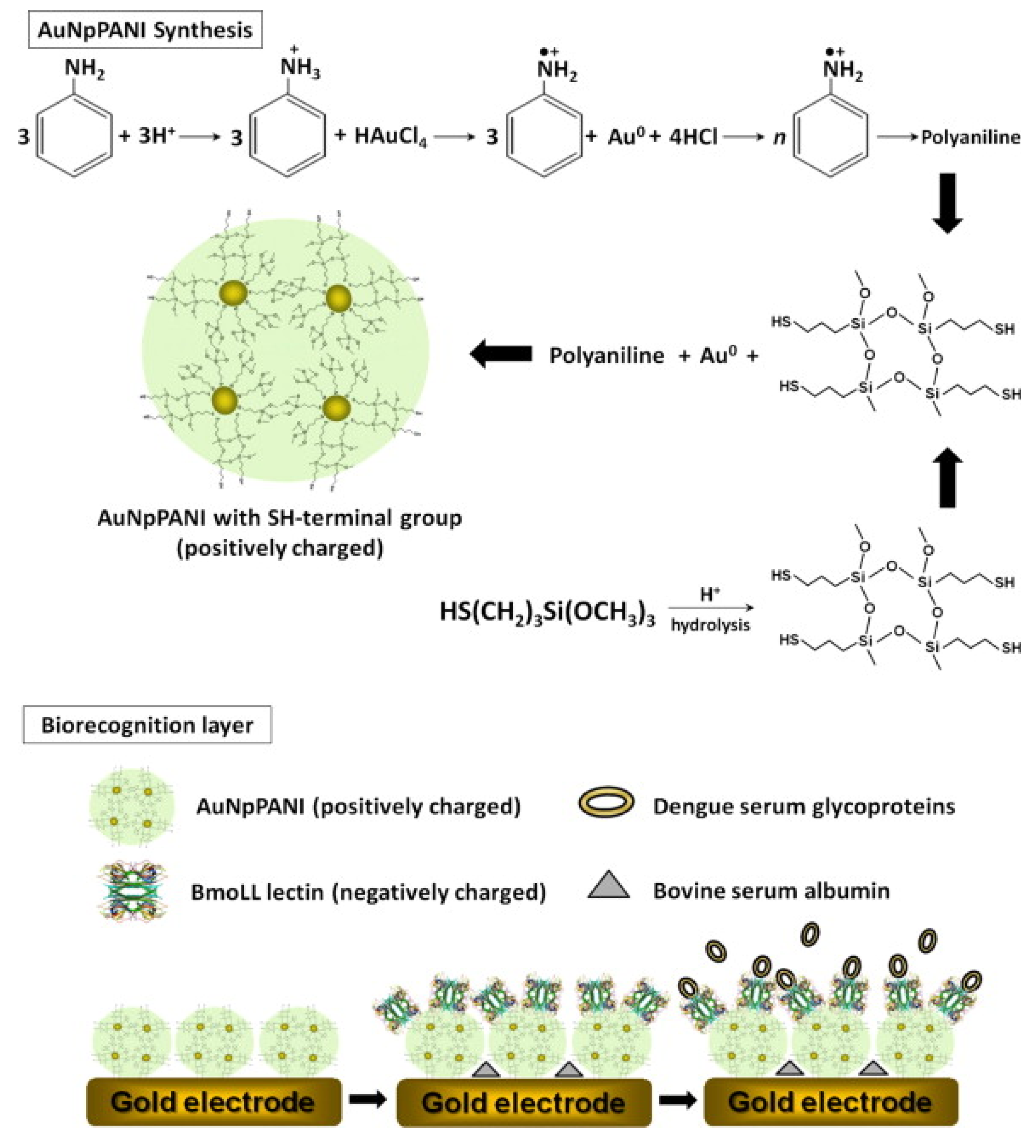
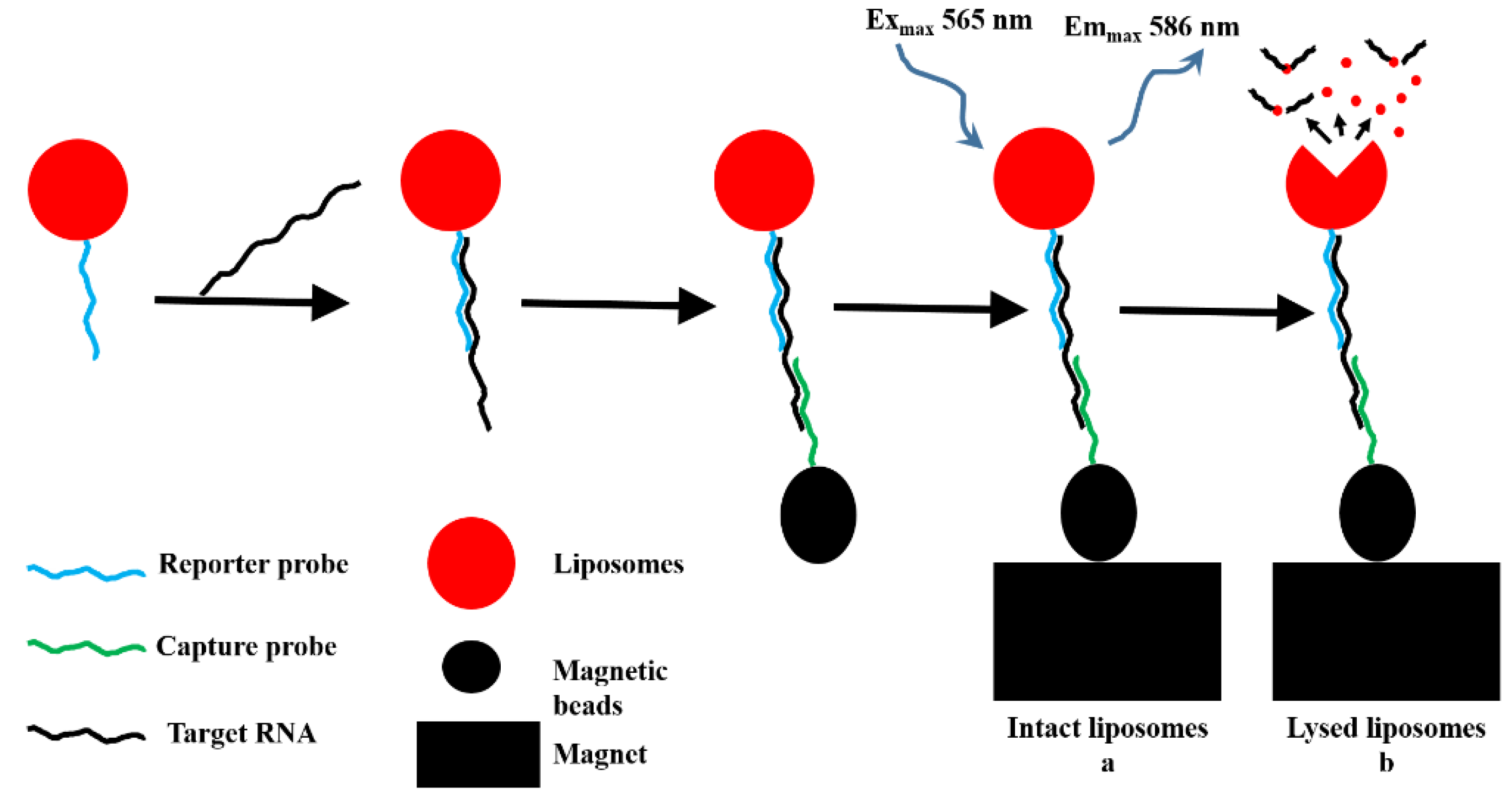
5.2.2. Piezoelectric Biosensor
5.2.3. Optical Biosensor
| Transduction mechanism | Analyte (target) | Limit of detection (LOD) | Assay time (min.) | Detection in real sample/spiked sample | Ref. |
|---|---|---|---|---|---|
| Electrochemical | RNA | 0.01 μM | – | – | [77] |
| SG | 80 dilution Fold | – | serum | [63] | |
| SG | 1:150 | – | serum | [65] | |
| NS1 | 0.33 ngm·L−1 | – | serum | [66] | |
| NS1 | 0.12 ngm·L−1 | spiked serum | [55] | ||
| SG | 80-dilution fold | – | serum | [70] | |
| Viral particle | 1 PFU·mL−1 | 50 | – | [75] | |
| Viral particle | 0.230 and 0.710 PFU·mL−1 | 40 | spiked serum | [76] | |
| RNA | 0.92 nM | – | – | [78] | |
| cDNA | 9.55 × 10−12 M | – | serum | [80] | |
| cDNA | 2.7 × 10−12 M | – | – | [81] | |
| NS1 | 0.09 µg·mL−1 | – | [71] | ||
| Piezoelectric | NS1 | 0.740 µg·mL−1 | serum | [83] | |
| Envelope protein | 1.727 µg·mL−1 | serum | [83] | ||
| cDNA | 2 PFU·mL−1 | 90 | spiked blood | [85] | |
| NS1 | 1–10 µg·L−1 | 20–30 | blood | [84] | |
| NS1 | 0.05 μg·mL−1 | 30–60 | – | [11] | |
| optical | RNA | 10 pmol·L−1 | – | – | [87] |
| RNA | 0.125·nM | 20 | – | [88] | |
| cDNA | 100 PFU·mL−1 | – | serum | [89] | |
| IgM antibody | 1:106 dilution | serum | [28] | ||
| IgM antibody | 12 pg/mm2 | – | blood plasma | [12] |
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; Myers, M.F.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Gubler, D.J. Dengue/dengue haemorrhagic fever: History and current status. Novartis Found. Symp. 2006, 277, 3–16. [Google Scholar] [PubMed]
- Blacksell, S.D. Commercial dengue rapid diagnostic tests for point-of-care application: Recent evaluations and future needs? J. Biomed. Biotechnol. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.; Salas, R.; Vickers, I.; Heslop, O.; Smikle, M. Dengue virus serotypes in Jamaica, 2003–2007. West Indian Med. J. 2011, 60, 114–119. [Google Scholar] [PubMed]
- Chen, W.H.; Hsu, I.; Sun, Y.C.; Wang, Y.K.; Wu, T.K. Immunocapture couples with matrix-assisted laser desorption/ionization time-of-flight mass spectrometry for rapid detection of type 1 dengue virus. J. Chromatogr. A 2013, 1288, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Chawla, P.; Yadav, A.; Chawla, V. Clinical implications and treatment of dengue. Asian Pac. J. Trop. Med. 2014, 7, 169–178. [Google Scholar] [CrossRef]
- Barrero, P.R.; Mistchenko, A.S. Complete genome sequencing of dengue virus type 1 isolated in Buenos Aires, Argentina. Virus Res. 2004, 101, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Rodenhuis-Zybert, I.A.; Wilschut, J.; Smit, J.M. Dengue virus life cycle: Viral and host factors modulating infectivity. Cell. Mol. Life Sci. 2010, 67, 2773–2786. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A. Dengue Fever Transmission; News Medical: London, UK, 2015. [Google Scholar]
- CDC Epidemiology—Dengue. Available online: http://www.cdc.gov/dengue/epidemiology/ (accessed on 6 June 2015).
- Teles, F.S. Biosensors and rapid diagnostic tests on the frontier between analytical and clinical chemistry for biomolecular diagnosis of dengue disease: A review. Anal. Chim. Acta 2011, 687, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.R.; Krupin, O.; Sekaran, S.D.; Mahamd Adikan, F.R.; Berini, P. Serological Diagnosis of Dengue Infection in Blood Plasma Using Long-Range Surface Plasmon Waveguides. Anal. Chem. 2014, 86, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Simmons, C.P.; Farrar, J. Changing Patterns of Dengue Epidemiology and Implications for Clinical Management and Vaccines. PLoS Med. 2009, 6, e1000129. [Google Scholar] [CrossRef] [PubMed]
- Jahan, F. Dengue Fever (DF) in Pakistan. Asia Pac. Fam. Med. 2011, 10. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.L. Global dengue epidemiology trends. Rev. Inst. Med. Trop. Sao Paulo 2012, 54 (Suppl. 18), S5–S6. [Google Scholar] [CrossRef] [PubMed]
- Murray, N.E.; Quam, M.B.; Wilder-Smith, A. Epidemiology of dengue: Past, present and future prospects. Clin. Epidemiol. 2013, 5, 299–309. [Google Scholar] [PubMed]
- Chakravarti, A.; Matlani, M.; Kashyap, B.; Kumar, A. Awareness of changing trends in epidemiology of dengue fever is essential for epidemiological surveillance. Indian J. Med. Microbiol. 2012, 30, 222–226. [Google Scholar] [PubMed]
- Cafferata, M.L.; Bardach, A.; Rey-Ares, L.; Alcaraz, A.; Cormick, G.; Gibbons, L.; Romano, M.; Cesaroni, S.; Ruvinsky, S. Dengue Epidemiology and Burden of Disease in Latin America and the Caribbean: A Systematic Review of the Literature and Meta-Analysis. Value Health Reg. Issues 2013, 2, 347–356. [Google Scholar] [CrossRef]
- The Geographic Distribution of Dengue Fever and the Potential Influence of Global Climate Change. Available online: http://journal.tropika.net/scielo.php?script=sci_arttext&pid=S2078-86062010005000001&lng=es&nrm=iso&tlng=es (accessed on 1 April 2015).
- Bhattacharya, M.; Maitra, S.; Ganguly, A.; Bhattacharya, A.; Sinha, A. Dengue: A Growing Menace—A Snapshot of Recent Facts, Figures & Remedies. Int. J. Biomed. Sci. 2013, 9, 61–67. [Google Scholar] [PubMed]
- Teng, A.K.; Singh, S. Epidemiology and new initiatives in the prevention and control of dengue in Malaysia. Dengue Bull 2001, 25, 7–12. [Google Scholar]
- Gubler, D.J. Dengue, urbanization and globalization: The unholy trinity of the 21(st) century. Trop. Med. Health 2011, 39, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Darwish, N.T.; Alias, Y.B.; Khor, S.M. An introduction to dengue-disease diagnostics. TrAC Trends Anal. Chem. 2015, 67, 45–55. [Google Scholar] [CrossRef]
- Tricou, V.; Vu, H.T.; Quynh, N.V.; Nguyen, C.V.; Tran, H.T.; Farrar, J.; Wills, B.; Simmons, C.P. Comparison of two dengue NS1 rapid tests for sensitivity, specificity and relationship to viraemia and antibody responses. BMC Infect. Dis. 2010, 10, 142. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.-L.; King, C.-C.; Chao, D.-Y.; Wu, H.-L.; Chang, G. Laboratory diagnosis of dengue virus infection: Current and future perspectives in clinical diagnosis and public health. J. Microbiol. Immunol. Infect. 2005, 38, 5–16. [Google Scholar] [PubMed]
- Rathakrishnan, A.; Sekaran, S.D. New development in the diagnosis of dengue infections. Expert Opin. Med. Diagn. 2013, 7, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.F.; Ooi, E.E. Diagnosis of dengue: An update. Expert Rev. Anti. Infect. Ther. 2012, 10, 895–907. [Google Scholar] [CrossRef] [PubMed]
- Atias, D.; Liebes, Y.; Chalifa-Caspi, V.; Bremand, L.; Lobel, L.; Marks, R.S.; Dussart, P. Chemiluminescent optical fiber immunosensor for the detection of IgM antibody to dengue virus in humans. Sens. Actuators B Chem. 2009, 140, 206–215. [Google Scholar] [CrossRef]
- Kong, Y.Y.; Thay, C.H.; Tin, T.C.; Devi, S. Rapid detection, serotyping and quantitation of dengue viruses by TaqMan real-time one-step RT-PCR. J. Virol. Methods 2006, 138, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Castro-Jorge, L.A.; Machado, P.R.L.; Fávero, C.A.; Borges, M.C.; Passos, L.M.R.; de Oliveira, R.M.; Fonseca, B.A.L. Clinical evaluation of the NS1 antigen-capture ELISA for early diagnosis of dengue virus infection in Brazil. J. Med. Virol. 2010, 82, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- Fry, S.R.; Meyer, M.; Semple, M.G.; Simmons, C.P.; Sekaran, S.D.; Huang, J.X.; McElnea, C.; Huang, C.-Y.; Valks, A.; Young, P.R.; et al. The Diagnostic Sensitivity of Dengue Rapid Test Assays is Significantly Enhanced by Using a Combined Antigen and Antibody Testing Approach. PLoS Negl. Trop. Dis. 2011, 5, e1199. [Google Scholar] [CrossRef] [PubMed]
- Kittigul, L.; Suankeow, K. Use of a rapid immunochromatographic test for early diagnosis of dengue virus infection. Eur. J. Clin. Microbiol. Infect. Dis. 2002, 21, 224–226. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, I.T.; Silva, B.V.; Peres, N.G.; Moura, P.; Sotomayor, M.D.; Guedes, M.I.; Dutra, R.F. A disposable chitosan-modified carbon fiber electrode for dengue virus envelope protein detection. Talanta 2012, 91, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.; de Vries, P.; Hoang, L.; Phan, G.; Le, H.; Tran, B.; Vo, C.; Nguyen, N.; Kager, P.; Nagelkerke, N.; et al. Enzyme-linked immunoassay for dengue virus IgM and IgG antibodies in serum and filter paper blood. BMC Infect. Dis. 2006, 6, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Dengue Guidelines for Diagnosis, Treatment, Prevention and Control; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Teles, F.R.; Prazeres, D.M.; Lima-Filho, J.L. Trends in dengue diagnosis. Rev. Med. Virol. 2005, 15, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, D.W.; Nisalak, A.; Solomon, T.; Kalayanarooj, S.; Kneen, R.; Cuzzubho, A.; Devine, P. Rapid serologic diagnosis of dengue virus infection using a commercial capture ELISA that distinguishes primary and secondary infections. Am. J. Trop. Med. Hyg. 1999, 60, 693–698. [Google Scholar] [PubMed]
- Peeling, R.W.; Artsob, H.; Pelegrino, J.L.; Buchy, P.; Cardosa, M.J.; Devi, S.; Enria, D.A.; Farrar, J.; Gubler, D.J.; Guzman, M.G.; et al. Evaluation of diagnostic tests: Dengue. Nat. Rev. Microbiol. 2010, 8, S30–S38. [Google Scholar] [CrossRef] [PubMed]
- Subedi, D.; Taylor-Robinson, A.W. Laboratory diagnosis of dengue infection: Current techniques and future strategies. Open J. Clin. Diagn. 2014, 2014. [Google Scholar] [CrossRef]
- Shu, P.-Y.; Huang, J.-H. Current Advances in Dengue Diagnosis. Clin. Diagn. Lab. Immunol. 2004, 11, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Gubler, D.J. Dengue and Dengue Hemorrhagic Fever. Clin. Microbiol. Rev. 1998, 11, 480–496. [Google Scholar] [PubMed]
- De Paula, S.O.; Fonseca, B.A. Dengue: A review of the laboratory tests a clinician must know to achieve a correct diagnosis. Braz. J. Infect. Dis. 2004, 8, 390–398. [Google Scholar] [CrossRef] [PubMed]
- WHO. Comprehensive Guidelines for Prevention and Control of Dengue and Dengue Haemorrhagic Fever; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Guzman, M.G.; Halstead, S.B.; Artsob, H.; Buchy, P.; Farrar, J.; Gubler, D.J.; Hunsperger, E.; Kroeger, A.; Margolis, H.S.; Martinez, E.; et al. Dengue: A continuing global threat. Nat. Rev. Microbiol. 2010, 8, S7-16. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.R.; Neto, J.P.; Casseb, S.M.; Nunes, K.N.; Martins, L.C.; Rodrigues, S.G.; Matheus, S.; Dussart, P.; Casseb, L.M.; Vasconcelos, P.F. Evaluation of an immunoglobulin M-specific capture enzyme-linked immunosorbent assay for rapid diagnosis of dengue infection. J. Virol. Methods 2011, 171, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Hunsperger, E.A.; Yoksan, S.; Buchy, P.; Nguyen, V.C.; Sekaran, S.D.; Enria, D.A.; Pelegrino, J.L.; Vazquez, S.; Artsob, H.; Drebot, M.; et al. Evaluation of commercially available anti-dengue virus immunoglobulin M tests. Emerg. Infect. Dis. 2009, 15, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Blacksell, S.D.; Jarman, R.G.; Gibbons, R.V.; Tanganuchitcharnchai, A.; Mammen, M.P.; Nisalak, A.; Kalayanarooj, S.; Bailey, M.S.; Premaratna, R.; de Silva, H.J.; et al. Comparison of seven commercial antigen and antibody enzyme-linked immunosorbent assays for detection of acute dengue infection. Clin. Vaccine Immunol. 2012, 19, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Welch, R.J.; Chang, G.-J.J.; Litwin, C.M. Comparison of a commercial dengue IgM capture ELISA with dengue antigen focus reduction microneutralization test and the centers for disease control dengue IgM capture-ELISA. J. Virol. Methods 2014, 195, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Dauner, A.L.; Valks, A.; Forshey, B.M.; Long, K.C.; Thaisomboonsuk, B.; Sierra, G.; Picos, V.; Talmage, S.; Morrison, A.C. Multi-country prospective clinical evaluation of two ELISAs and two rapid diagnostic tests for diagnosing dengue fever. J. Clin. Microbiol. 2015, 53, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- De Souza, V.A.U.F.; Fernandes, S.; Araújo, E.S.; Tateno, A.F.; Oliveira, O.M.; dos Reis Oliveira, R.; Pannuti, C.S. Use of an immunoglobulin G avidity test to discriminate between primary and secondary dengue virus infections. J. Clin. Microbiol. 2004, 42, 1782–1784. [Google Scholar] [CrossRef] [PubMed]
- Prince, H.E.; Yeh, C.; Lapé-Nixon, M. Utility of IgM/IgG ratio and IgG avidity for distinguishing primary and secondary dengue virus infections using sera collected more than 30 days after disease onset. Clin. Vaccine Immunol. 2011, 18, 1951–1956. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, M.T.; Braga-Neto, U.; Nogueira, R.M.R.; Marques, E.T., Jr. Reliable classifier to differentiate primary and secondary acute dengue infection based on IgG ELISA. PLoS ONE 2009, 4, e4945. [Google Scholar] [CrossRef] [PubMed]
- Libraty, D.H.; Young, P.R.; Pickering, D.; Endy, T.P.; Kalayanarooj, S.; Green, S.; Vaughn, D.W.; Nisalak, A.; Ennis, F.A.; Rothman, A.L. High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J. Infect. Dis. 2002, 186, 1165–1168. [Google Scholar] [CrossRef] [PubMed]
- Kassim, F.M.; Izati, M.N.; TgRogayah, T.; Apandi, Y.M.; Saat, Z. Use of dengue NS1 antigen for early diagnosis of dengue virus infection. Southeast Asian J. Trop. Med. Public Health 2011, 42, 562–569. [Google Scholar] [PubMed]
- Dias, A.C.M.S.; Gomes-Filho, S.L.R.; Silva, M.M.S.; Dutra, R.F. A sensor tip based on carbon nanotube-ink printed electrode for the dengue virus NS1 protein. Biosens. Bioelectron. 2013, 44, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.D.R.Q.; Nogueira, R.M.R.; Schatzmayr, H.G.; dos Santos, F.B. Comparison of three commercially available dengue NS1 antigen capture assays for acute diagnosis of dengue in Brazil. PLoS Negl. Trop. Dis. 2010, 4, e738. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Dauner, A.L.; Mitra, I.; Forshey, B.M.; Garcia, P.; Morrison, A.C.; Halsey, E.S.; Kochel, T.J.; Wu, S.-J.L. Evaluation of Dengue NS1 Antigen Rapid Tests and ELISA Kits Using Clinical Samples. PLoS ONE 2014, 9, e113411. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.M.; Sekaran, S.D. Evaluation of a commercial SD dengue virus NS1 antigen capture enzyme-linked immunosorbent assay kit for early diagnosis of dengue virus infection. J. Clin. Microbiol. 2010, 48, 2793–2797. [Google Scholar] [CrossRef] [PubMed]
- Hermann, L.L.; Thaisomboonsuk, B.; Poolpanichupatam, Y.; Jarman, R.G.; Kalayanarooj, S.; Nisalak, A.; Yoon, I.-K.; Fernandez, S. Evaluation of a dengue NS1 antigen detection assay sensitivity and specificity for the diagnosis of acute dengue virus infection. PLoS Negl. Trop. Dis. 2014, 8, e3193. [Google Scholar] [CrossRef] [PubMed]
- Mascini, M.; Palchetti, I. Nucleic Acid Biosensors for Environmental Pollution Monitoring; Royal Society of Chemistry: London, UK, 2011. [Google Scholar]
- Sin, M.; Mach, K.E.; Wong, P.K.; Liao, J.C. Advances and challenges in biosensor-based diagnosis of infectious diseases. Expert Rev. Mol. Diagn. 2014, 14, 225–244. [Google Scholar] [CrossRef] [PubMed]
- Perumal, V.; Hashim, U. Advances in biosensors: Principle, architecture and applications. J. Appl. Biomed. 2014, 12, 1–15. [Google Scholar] [CrossRef]
- Oliveira, M.D.; Correia, M.T.; Diniz, F.B. A novel approach to classify serum glycoproteins from patients infected by dengue using electrochemical impedance spectroscopy analysis. Synth. Met. 2009, 159, 2162–2164. [Google Scholar] [CrossRef]
- Oliveira, M.D.L.; Nogueira, M.L.; Correia, M.T.S.; Coelho, L.C.B.B.; Andrade, C.A.S. Detection of dengue virus serotypes on the surface of gold electrode based on Cratylia mollis lectin affinity. Sens. Actuators B Chem. 2011, 155, 789–795. [Google Scholar] [CrossRef]
- Andrade, C.A.; Oliveira, M.D.; De Melo, C.P.; Coelho, L.C.; Correia, M.T.; Nogueira, M.L.; Singh, P.R.; Zeng, X. Diagnosis of dengue infection using a modified gold electrode with hybrid organic-inorganic nanocomposite and Bauhinia monandra lectin. J. Colloid Interface Sci. 2011, 362, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, I.T.; Guedes, M.I.F.; Sotomayor, M.D.P.T.; Yamanaka, H.; Dutra, R.F. A label-free immunosensor based on recordable compact disk chip for early diagnostic of the dengue virus infection. Biochem. Eng. J. 2012, 67, 225–230. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Hong, S.; Lee, D.; Cui, T.; Goyal, S. Carbon nanotube based sensors for the detection of viruses. Sens. Actuators B Chem. 2011, 155, 67–74. [Google Scholar] [CrossRef]
- Lee, J.E.; Seo, J.H.; Kim, C.S.; Kwon, Y.; Ha, J.H.; Choi, S.S.; Cha, H.J. A comparative study on antibody immobilization strategies onto solid surface. Korean J. Chem. Eng. 2013, 30, 1934–1938. [Google Scholar] [CrossRef]
- Luna, D.M.N.; Oliveira, M.D.L.; Nogueira, M.L.; Andrade, C.A.S. Biosensor based on lectin and lipid membranes for detection of serum glycoproteins in infected patients with dengue. Chem. Phys. Lipids 2014, 180, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Avelino, K.Y.P.S.; Andrade, C.A.S.; de Melo, C.P.; Nogueira, M.L.; Correia, M.T.S.; Coelho, L.C.B.B.; Oliveira, M.D.L. Biosensor based on hybrid nanocomposite and CramoLL lectin for detection of dengue glycoproteins in real samples. Synth. Met. 2014, 194, 102–108. [Google Scholar] [CrossRef]
- Figueiredo, A.; Vieira, N.C.S.; dos Santos, J.F.; Janegitz, B.C.; Aoki, S.M.; Junior, P.P.; Lovato, R.L.; Nogueira, M.L.; Zucolotto, V.; Guimaraes, F.E.G. Electrical Detection of Dengue Biomarker Using Egg Yolk Immunoglobulin as the Biological Recognition Element. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Parkash, O.; Yean, C.Y.; Shueb, R.H. Screen Printed Carbon Electrode Based Electrochemical Immunosensor for the Detection of Dengue NS1 Antigen. Diagnostics 2014, 4, 165–180. [Google Scholar] [CrossRef]
- Shen, W.-F.; Galula, J.U.; Chang, G.-J.J.; Wu, H.-C.; King, C.-C.; Chao, D.-Y. Improving dengue viral antigens detection in dengue patient serum specimens by a low pH glycine buffer Treatment. J. Microbiol. Immunol. Infect. 2015. [Google Scholar] [CrossRef] [PubMed]
- Allonso, D.; Meneses, M.D.F.; Fernandes, C.A.; Ferreira, D.F.; Mohana-Borges, R. Assessing Positivity and Circulating Levels of NS1 in Samples from a 2012 Dengue Outbreak in Rio de Janeiro, Brazil. PLoS ONE 2014, 9, e113634. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.S.; Ho, J.S.; Tan, C.H.; Wong, J.P.S.; Ng, L.C.; Toh, C.-S. Development of an electrochemical membrane-based nanobiosensor for ultrasensitive detection of dengue virus. Anal. Chim. Acta 2012, 725, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Peh, A.E.K.; Li, S.F.Y. Dengue virus detection using impedance measured across nanoporous aluminamembrane. Biosens. Bioelectron. 2013, 42, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Kwakye, S.; Goral, V.N.; Baeumner, A.J. Electrochemical microfluidic biosensor for nucleic acid detection with integrated minipotentiostat. Biosens. Bioelectron. 2006, 21, 2217–2223. [Google Scholar] [CrossRef] [PubMed]
- Souza, E.; Nascimento, G.; Santana, N.; Ferreira, D.; Lima, M.; Natividade, E.; Martins, D.; Lima-Filho, J. Label-free electrochemical detection of the specific oligonucleotide sequence of dengue virus type 1 on pencil graphite electrodes. Sensors 2011, 11, 5616–5629. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, H.P.O.; Oliveira, M.D.L.; de Melo, C.P.; Silva, G.J.L.; Cordeiro, M.T.; Andrade, C.A.S. An impedimetric biosensor for detection of dengue serotype at picomolar concentration based on gold nanoparticles-polyaniline hybrid composites. Colloids Surf. B Biointerfaces 2011, 86, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Rai, V.; Hapuarachchi, H.C.; Ng, L.C.; Soh, S.H.; Leo, Y.S.; Toh, C.-S. Ultrasensitive cDNA detection of dengue virus RNA using electrochemical nanoporous membrane-based biosensor. PLoS ONE 2012, 7, e42346. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Toh, C.-S. Impedimetric DNA Biosensor Based on a Nanoporous Alumina Membrane for the Detection of the Specific Oligonucleotide Sequence of Dengue Virus. Sensors 2013, 13, 7774–7785. [Google Scholar] [CrossRef] [PubMed]
- Su, C.-C.; Wu, T.-Z.; Chen, L.-K.; Yang, H.-H.; Tai, D.-F. Development of immunochips for the detection of dengue viral antigens. Anal. Chim. Acta 2003, 479, 117–123. [Google Scholar] [CrossRef]
- Wu, T.-Z.; Su, C.-C.; Chen, L.-K.; Yang, H.-H.; Tai, D.-F.; Peng, K.-C. Piezoelectric immunochip for the detection of dengue fever in viremia phase. Biosens. Bioelectron. 2005, 21, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Tai, D.-F.; Lin, C.-Y.; Wu, T.-Z.; Huang, J.-H.; Shu, P.-Y. Artificial receptors in serologic tests for the early diagnosis of dengue virus infection. Clin. Chem. 2006, 52, 1486–1491. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Chuang, Y.C.; Lu, Y.C.; Lin, H.C.; Yang, Y.L.; Lin, C.S. A method of layer-by-layer gold nanoparticle hybridization in a quartz crystal microbalance DNA sensing system used to detect dengue virus. Nanotechnology 2009, 20. [Google Scholar] [CrossRef] [PubMed]
- Abdulhalim, I.; Zourob, M.; Lakhtakia, A. Overview of optical biosensing techniques. In Handbook of Biosensors and Biochips; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar]
- Kwakye, S.; Baeumner, A. A microfluidic biosensor based on nucleic acid sequence recognition. Anal. Bioanal. Chem. 2003, 376, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Zaytseva, N.V.; Montagna, R.A.; Baeumner, A.J. Microfluidic biosensor for the serotype-specific detection of Dengue virus RNA. Anal. Chem. 2005, 77, 7520–7527. [Google Scholar] [CrossRef] [PubMed]
- Baeumner, A.J.; Schlesinger, N.A.; Slutzki, N.S.; Romano, J.; Lee, E.M.; Montagna, R.A. Biosensor for dengue virus detection: sensitive, rapid, and serotype specific. Anal. Chem. 2002, 74, 1442–1448. [Google Scholar] [CrossRef] [PubMed]
- Kumbhat, S.; Sharma, K.; Gehlot, R.; Solanki, A.; Joshi, V. Surface plasmon resonance based immunosensor for serological diagnosis of dengue virus infection. J. Pharm. Biomed. Anal. 2010, 52, 255–259. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parkash, O.; Shueb, R.H. Diagnosis of Dengue Infection Using Conventional and Biosensor Based Techniques. Viruses 2015, 7, 5410-5427. https://doi.org/10.3390/v7102877
Parkash O, Shueb RH. Diagnosis of Dengue Infection Using Conventional and Biosensor Based Techniques. Viruses. 2015; 7(10):5410-5427. https://doi.org/10.3390/v7102877
Chicago/Turabian StyleParkash, Om, and Rafidah Hanim Shueb. 2015. "Diagnosis of Dengue Infection Using Conventional and Biosensor Based Techniques" Viruses 7, no. 10: 5410-5427. https://doi.org/10.3390/v7102877





