1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the airborne viral pathogen that causes the pandemic coronavirus disease of 2019 (COVID-19). The COVID-19 pandemic has resulted in significant worldwide deaths, and the search for strategies to reduce the incidence and clinical severity of the disease continues. The clinical spectrum of COVID-19 ranges from mild, self-limiting respiratory tract illness to severe progressive pneumonia. In addition, many people do not fully recover from the initial respiratory illness and go on to suffer from post-COVID-19 syndrome referred to as long-COVID. The most common symptoms of long-COVID are fatigue, shortness of breath, tightness of the chest, racing heart, difficulty concentrating and brain fog, loss of smell and taste, loss of appetite, hair loss, difficulty sleeping, anxiety and depression [
1]. Many of those who suffer from long-COVID had mild initial symptoms and were not hospitalised. The effects of long-COVID are currently associated with a chronic aberrant immune response. There is an urgent need to develop strategies to prevent SARS-CoV-2 infection to eliminate the potential adverse events that infection can cause.
Astodrimer sodium is a large (3–4 nm, ~16.5 kDa), negatively charged, highly-branched dendrimer that has significant antiviral and virucidal activity against different isolates of SARS-CoV-2 in vitro [
2]. Its mechanism of action is primarily to act as a barrier to block the binding of the highly positively charged SARS-CoV-2 spike protein to its receptor-complex, which includes the angiotensin converting enzyme 2 (ACE2) receptor. Many viruses, including SARS-CoV-2 [
3], utilise negatively charged heparan sulphate proteoglycans (HSPGs) protruding from the cell membrane to potentially bind and guide, or “surf”, the virus to its specific cellular receptor. Astodrimer sodium interferes with these early virus entry steps to block infection with a range of viruses in vitro [
4,
5] and in vivo [
6,
7]. The size and negative charge of astodrimer sodium mean that it is not systemically absorbed following topical application to mucosal epithelia [
8,
9].
SARS-CoV-2 receptors and coreceptors have been shown to be highly expressed in nasal epithelial cells [
10], such that intranasally administered therapeutic modalities could be effective in helping to prevent spread of infection of the virus. Astodrimer sodium has been formulated as a nasal spray with the intention of being applied in the nasal cavity to help reduce exposure to infectious viral load, thereby helping to protect from infection with SARS-CoV-2 and other respiratory viruses.
Animal models of SARS-CoV-2 replication and pathogenesis provide an opportunity to study aspects of the disease that are not easily investigated in humans. SARS-CoV-2 infection via the nasal inoculation of K18-hACE2 transgenic mice, a previously established model for the investigation of SARS-CoV infection [
11], has been shown to cause SARS-CoV-2 viral load-dependent respiratory illness, with a subset of animals developing brain infection that also contributes to death [
12]. The tissue distribution of infectious virus and viral genomic RNA confirms the extensive infection of the lung and brain. In a high viral load (10
5 plaque forming units [PFU] intranasal dose) infection of mice, small amounts of SARS-CoV-2 RNA were also detected in other tissues including the liver, small intestine, heart, spleen, kidney, large intestine and colon, indicating the low-level distribution of the virus via blood. The histological examination of these tissues identified that the liver was the only other tissue that displayed detectable evidence of the disease [
12]. Cytokine, chemokine and innate immune transcripts are elevated in the livers of SARS-CoV-2 infected hACE2 mice, which is indicative of an active immune response to a pathogen [
12]. Liver enzyme abnormalities are found in up to 50% of COVID-19 patients, and the virus infection of hepatocytes has been described [
13].
The current study extends the established antiviral and virucidal activity of astodrimer sodium against SARS-CoV-2 in vitro to evaluate, in a recognised in vivo challenge model of infection, the ability of the compound in a nasal spray formulation to protect against and reduce the severity of SARS-CoV-2 infection via the upper respiratory tract of K18-hACE2 mice.
The results of the study demonstrated that astodrimer sodium 1% nasal spray formulation demonstrated protective effects in K18-hACE2 mice challenged with SARS-CoV-2 infection via the upper respiratory tract, inhibiting SARS-CoV-2 replication and significantly reducing infectious viral load and the production of pro-inflammatory cytokines.
2. Materials and Methods
2.1. Animals
Transgenic mice expressing human ACE2 (hACE2) under the control of the cytokeratin 18 promoter (K18) were described in McCray et al., 2007 [
11]. The K18 promoter primarily directs gene expression in numerous epithelial-lined tissues [
14] and neurons [
15]. The SARS-CoV-2 infection of the K18-hACE2 mice has been described in the literature [
16,
17,
18]. These mice are congenic on the C57BL/6 background.
Six-to-eight-week-old K18-hACE2 mice (male or female) were purchased from The Jackson Laboratory (Bar Harbor, ME, USA; Stock No. 034860) and assessed for ill health upon arrival. The animals underwent acclimatisation for 1–2 weeks and were housed individually to minimize the risk of cross infection. The mice were kept by the Department of Animal Resources at The Scripps Research Institute (TSRI) (La Jolla, CA, USA) vivarium and the study was conducted in strict accordance with protocols approved by TSRI Ethics Committee, the Institutional Animal Care and Use Committee (Protocol Number: 20-0007, approved 12 June 2020), and with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (NIH).
Animals were maintained under isoflurane anaesthesia for dosing and virus inoculation and were returned to their housing for recovery.
2.2. SARS-CoV-2 and Cells
The 2019n-CoV/US-WA1/2020 strain of SARS-CoV-2 was used in these studies (obtained from BEI Resources (Manassas, VA, USA), National Institute of Allergy and Infectious Disease (NIAID), NIH: SARS-Related Coronavirus 2, Isolate USA-WA1/2020, recombinant infectious clone with nanoluciferase gene (icSARS-CoV-2-nLuc), NR-54003). The virus was passaged in Vero E6 cells (CRL-1586™, ATCC, Washington, DC, USA). The Vero E6 cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% foetal bovine serum (FBS).
The quantitation of viral inoculum generated in vitro and the amount of virus in tissue homogenates at the end of the study were determined by plaque assay in Vero E6 cells. Viral genomes in serum and tissue homogenates were detected by a quantitative reverse transcriptase polymerase chain reaction (qRT-PCR).
2.3. Antiviral and Virucidal Efficacy Studies Experimental Design
2.3.1. Intranasal Administration of Astodrimer Sodium 1% Nasal Spray Formulation
Two groups (Groups 1.1 and 1.2) of three animals each were used to determine the antiviral efficacy of astodrimer sodium 1% nasal spray formulation compared with the placebo (phosphate buffered saline [PBS]), administered via intranasal administration, against SARS-CoV-2 infection in vivo. The animals in Groups 1.1 and 1.2 were inoculated via intranasal administration with 25 µL per nostril of a virus suspension containing 50 PFU/25 µL of SARS-CoV-2 (total challenge: 102 PFU). The animals were treated with PBS (Group 1.1) or 1% astodrimer sodium nasal spray formulation (Group 1.2) via intranasal administration, 25 µL/nostril (total volume of 50 µL), for 7 days. Astodrimer sodium 1% nasal spray or PBS was administered on Day 0, 5 min prior to and 5 min after the virus inoculation, and then once daily on Days 1–6.
2.3.2. Intranasal and Intratracheal Administration of Astodrimer Sodium 1% Nasal Spray Formulation
Two groups (Groups 2.1 and 2.2) of three animals each were used to determine the antiviral efficacy of astodrimer sodium 1% nasal spray formulation compared with placebo (PBS), administered via intranasal and intratracheal administration, against SARS-CoV-2 infection in vivo. The animals in Groups 2.1 and 2.2 were inoculated via intranasal administration with 25 µL per nostril of a virus suspension containing 50 PFU/25 µL of SARS-CoV-2 (total challenge: 102 PFU). The animals were treated with PBS (Group 2.1) or 1% astodrimer sodium nasal spray formulation (Group 2.2) via intranasal (25 µL/nostril, total volume of 50 µL) and intratracheal (2 µL/g body weight) administration for 7 days. Intratracheal administration was performed by inserting a catheter down the mouth and throat. Astodrimer sodium 1% nasal spray or PBS was administered intranasally on Day 0, 5 min prior to and 5 min after virus inoculation, and then once daily on Days 1–6. Astodrimer sodium 1% nasal spray or PBS was administered intratracheally on Day 0, 5 min prior to virus inoculation, and then once daily on Days 1–6.
2.3.3. Virucidal Intervention with Astodrimer Sodium 1% Nasal Spray Formulation
Two groups (Groups 3.1 and 3.2) of three animals each were used to assess the virucidal activity of astodrimer sodium 1% nasal spray formulation and its ability to reduce replication in vivo following the pretreatment of the virus with the product. The virus (3 × 102 PFU) was incubated with 100 µL astodrimer sodium 1% nasal spray formulation or PBS for 60 min at 37 °C. Immediately following the 60 min incubation period, the mixture was pelleted through a 20% sucrose cushion to remove unbound astodrimer sodium and thereby neutralise its effects. The pelleted virus was then resuspended in 50 µL of PBS and administered intranasally (25 µL/nostril). No further product was administered to these animals.
2.4. Determination of Endpoints
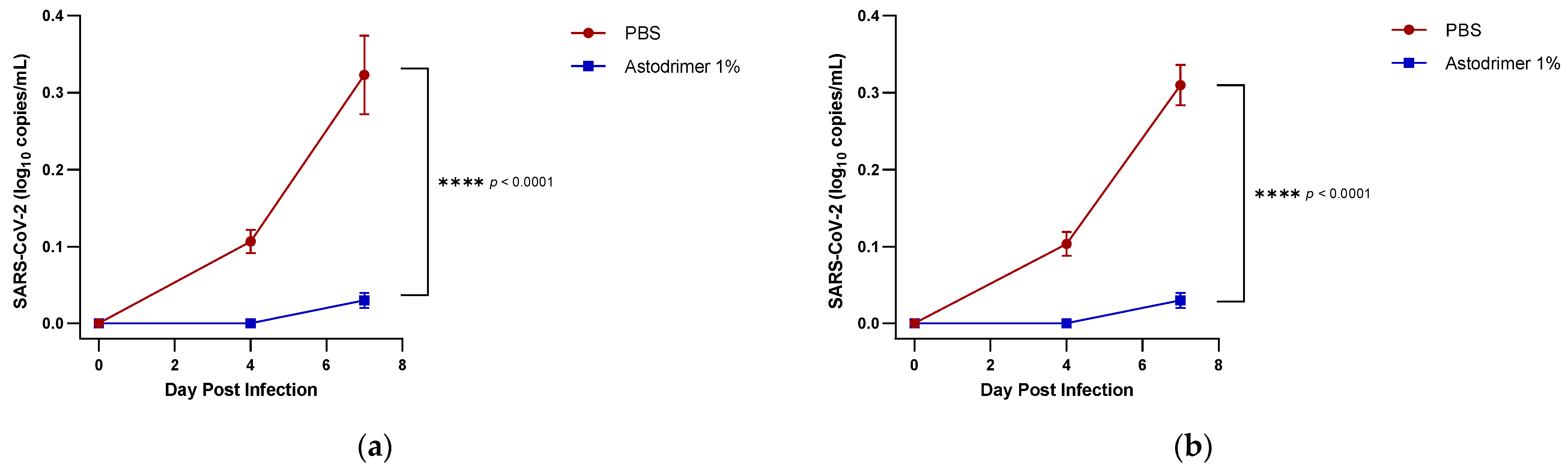
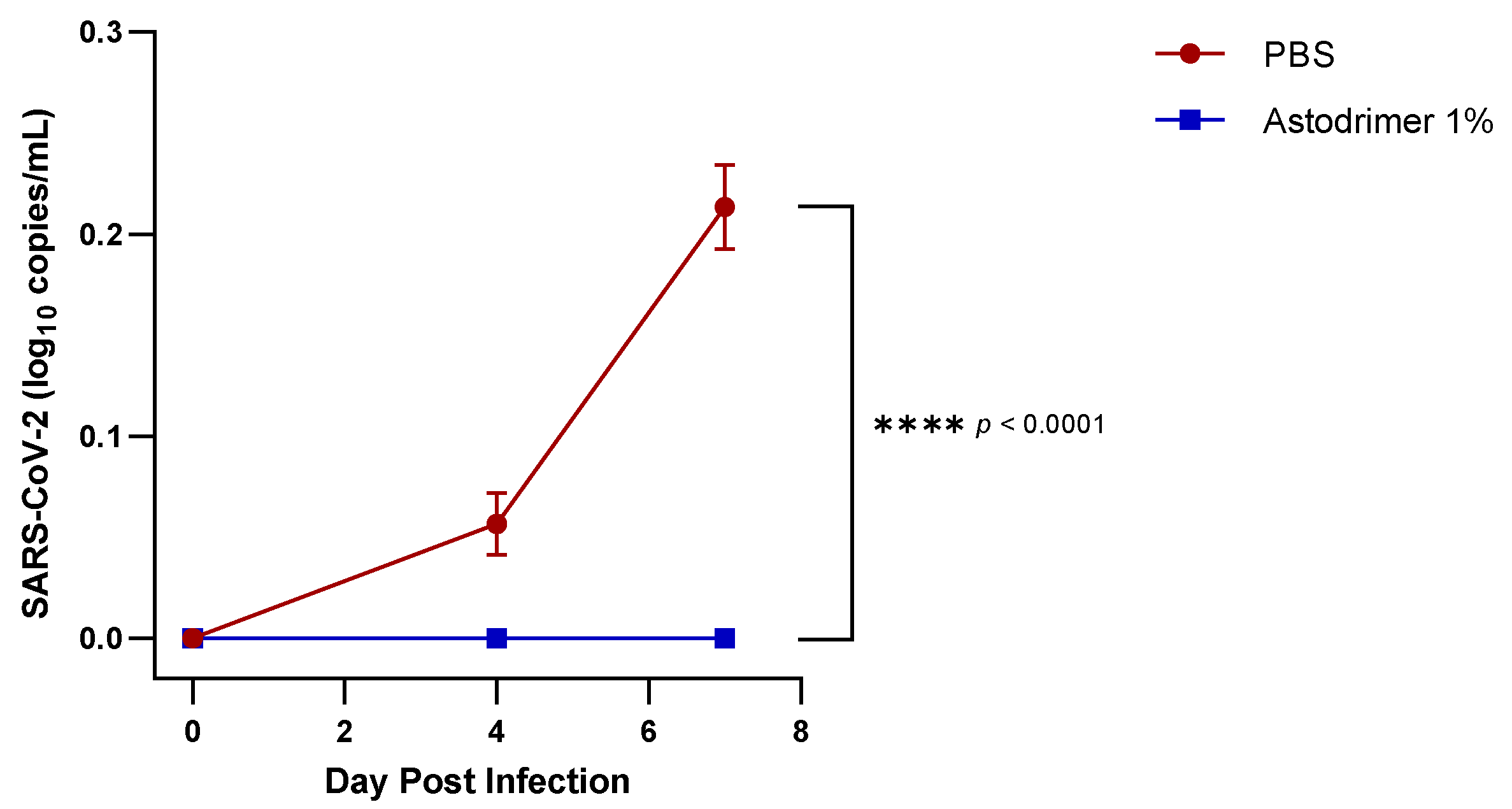
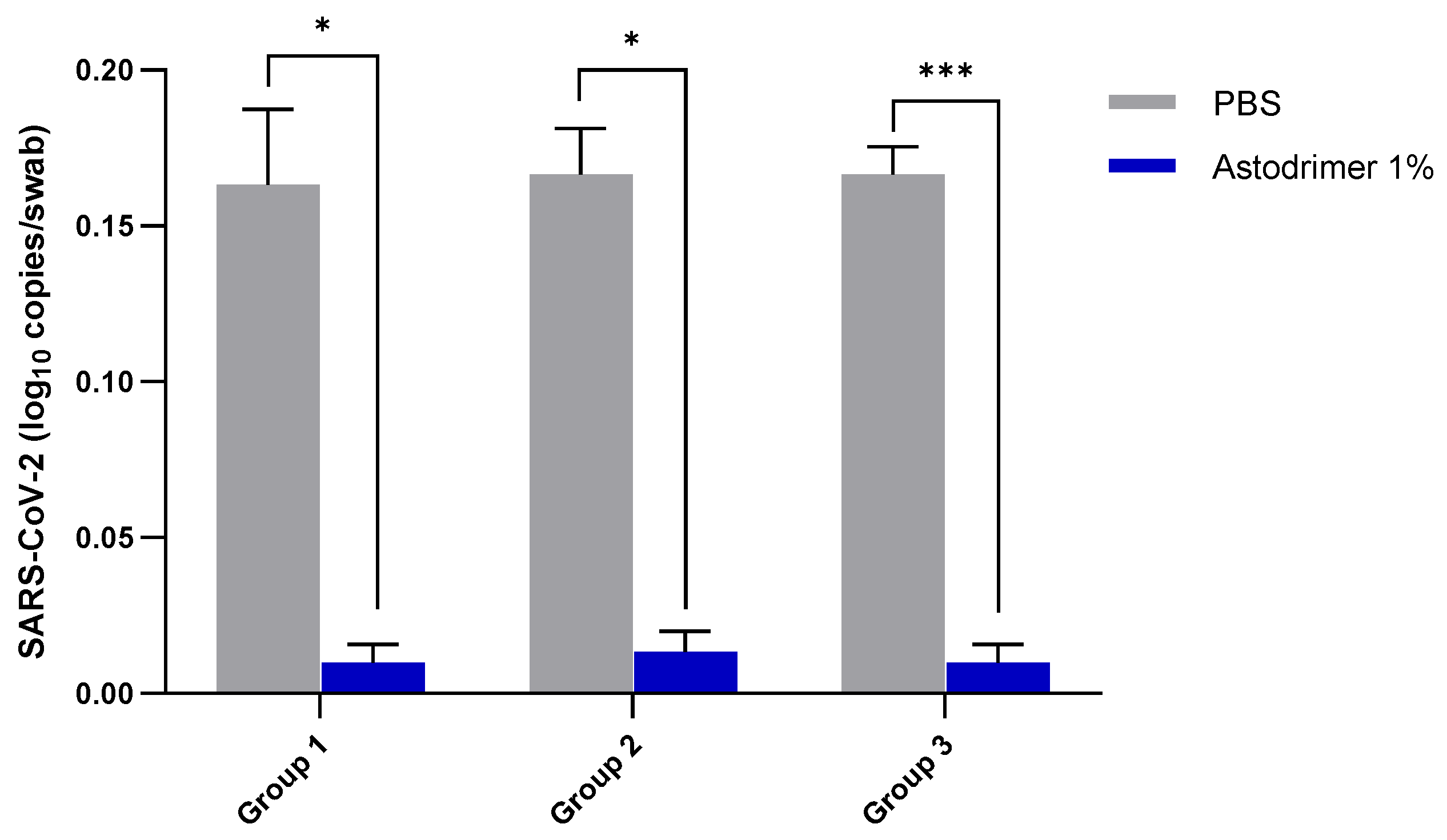
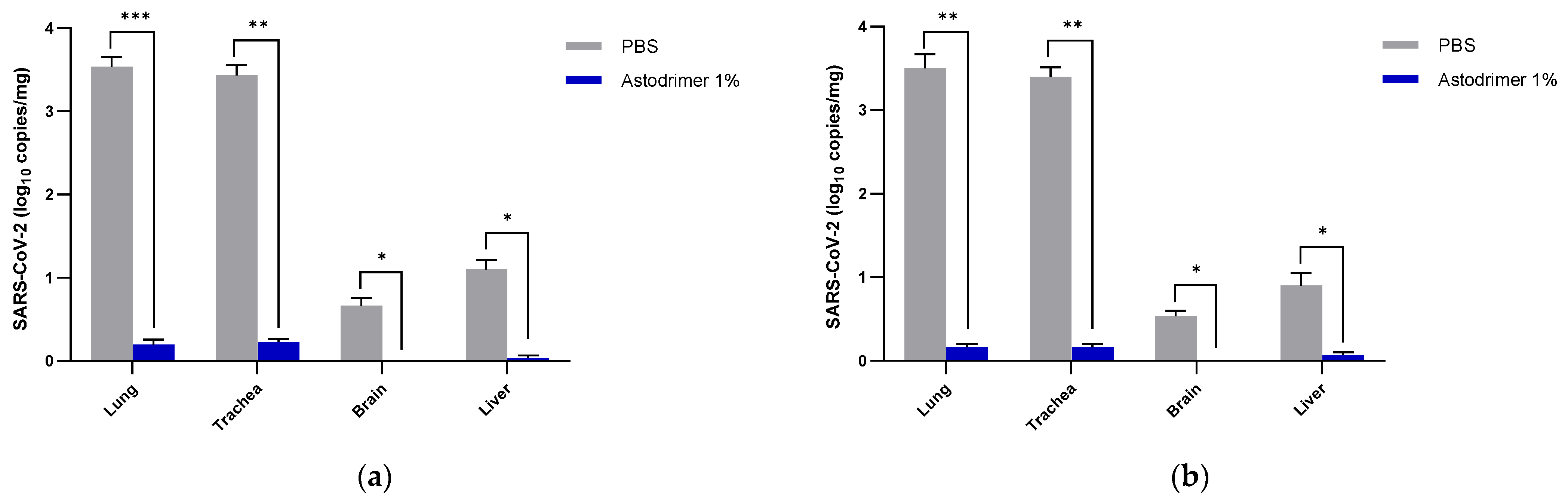
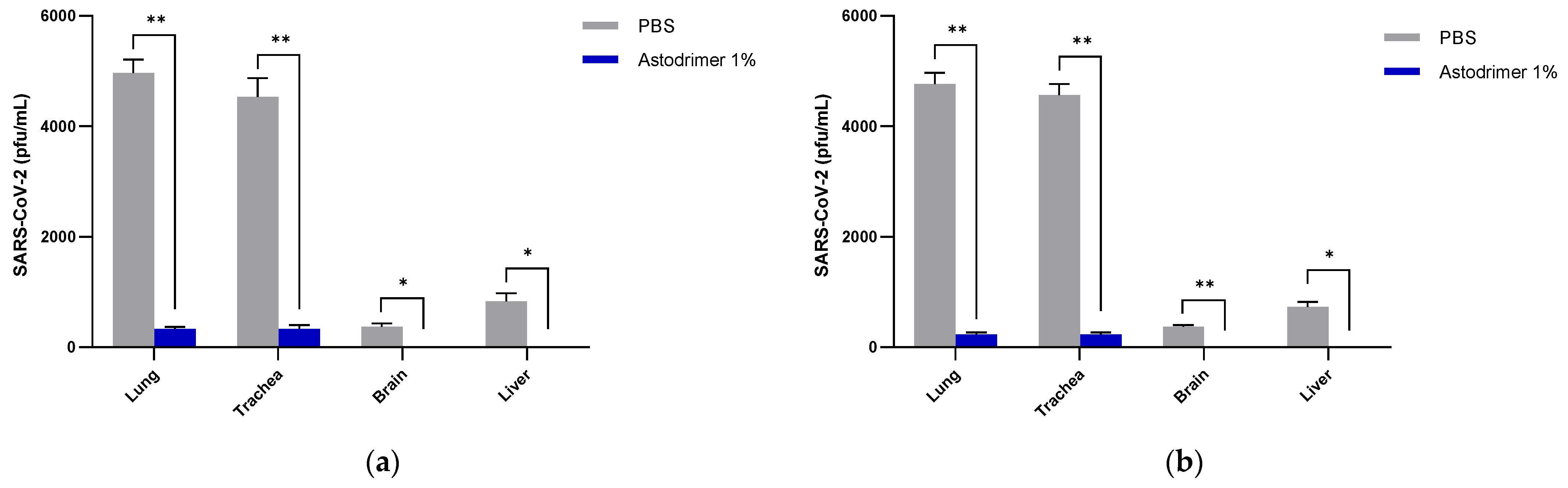
Body weight and mortality were assessed during the study. The animals were euthanised on Day 7. The status of SARS-CoV-2 infection was determined by measuring the viral load by a qRT-PCR in blood samples (on Day 0, Day 4 and Day 7) and nasal secretions (Day 7), and a qRT-PCR and plaque assay determination of the infectious viral titre of the lung, trachea, brain and liver tissue homogenates (Day 7).
To prepare tissue homogenates, 25 mg of frozen tissue was homogenised using a Bead Genie homogeniser (Scientific Industries, Bohemia, NY, USA). The tissue homogenates were transferred into pre-filled 2.0 mL tubes with stainless steel (acid-washed) homogeniser beads, Ø:2.8 mm (Stellar Scientific, Baltimore, MD, USA) and 500 µL lysis buffer with protease inhibitors. The tubes were shaken at a speed of 6 m/s for 25 s.
The serum and tissue homogenates were analysed for the highly conserved SARS-CoV-2 nucleocapsid RNA by a qRT-PCR using the method previously described by Winkler et al., 2020 [
18]. Total viral RNA was extracted from serum, nasal secretions or tissue using the MagMax™ mirVana™ Total RNA Isolation Kit (ThermoFisher Scientific, Waltham, MA, USA) on the KingFisher™ Flex extraction robot (ThermoFisher Scientific, Waltham, MA, USA). The SARS-CoV-2 nucleocapsid (N) gene was reverse transcribed and amplified using the TaqMan
® RNA-to CT™ 1-Step Kit (ThermoFisher Scientific, Waltham, MA, USA). The SARS-CoV-2 N gene was detected using Forward primer: ATGCTGCAATCGTGCTACAA; Reverse primer: GACTGCCGCCTCTGCTC; Probe: /56-FAM/TCAAGGAAC/ZEN/AACATTGCCAA/3IABkFQ/. This region was included in an RNA standard to allow for copy number determination down to 10 copies per reaction. The reaction mixture contained final concentrations of primers and probe of 500 and 100 nM, respectively. The reverse transcription was performed at 48 °C for 15 min followed by 2 min at 95 °C. The amplification was achieved over 50 cycles by the following repeated cycle of 95 °C for 15 s and 60 °C for 1 min.
The quantitation of the infectious virus in the tissue homogenates was performed by plaque assay, as described in Paull et al., 2021 [
2], with Vero E6 cells as the detecting cell line.
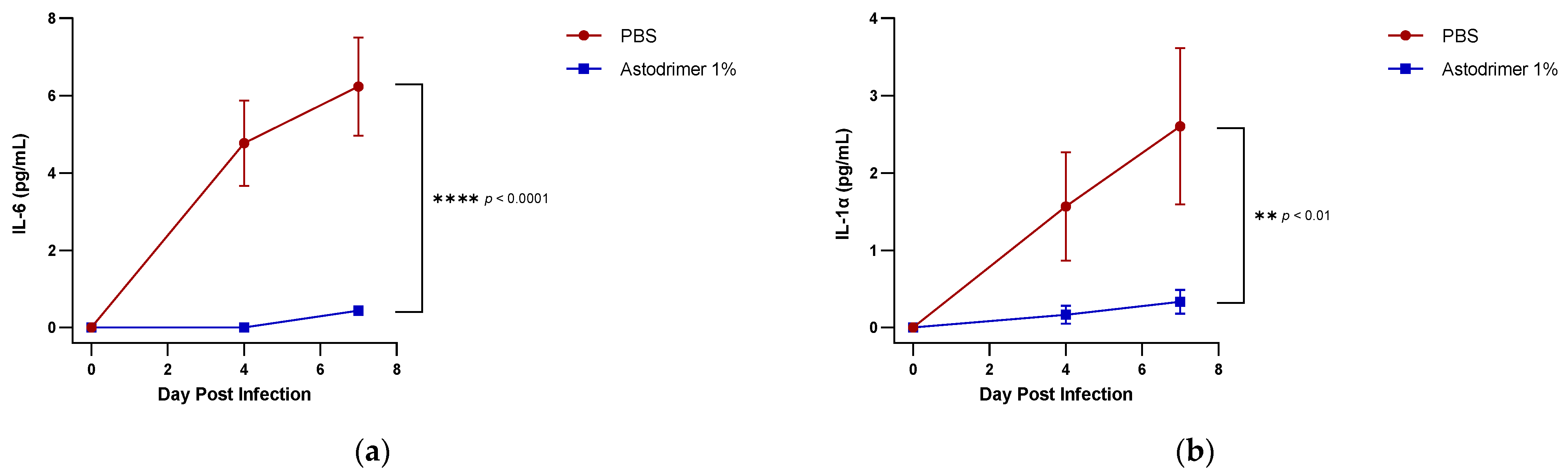
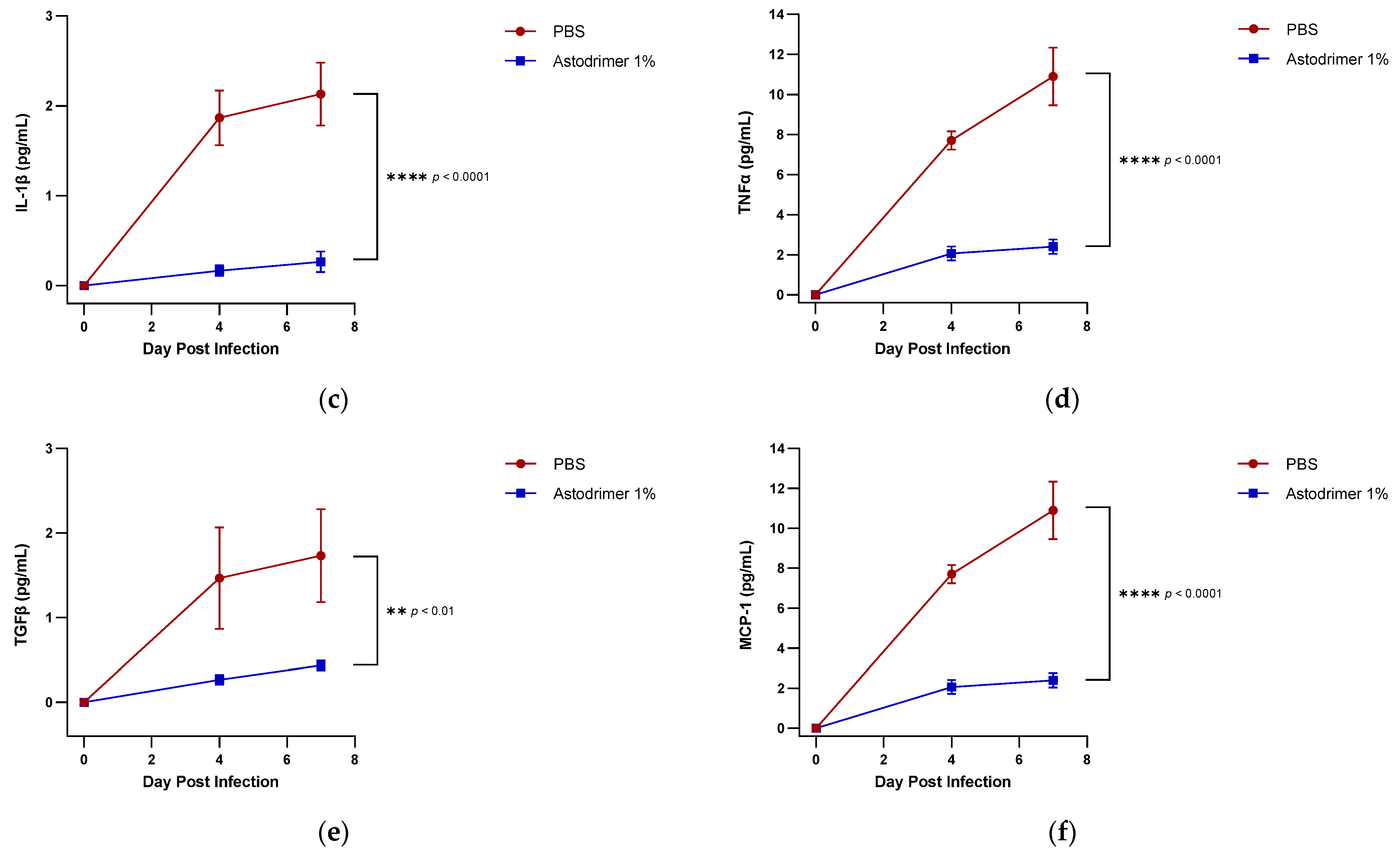
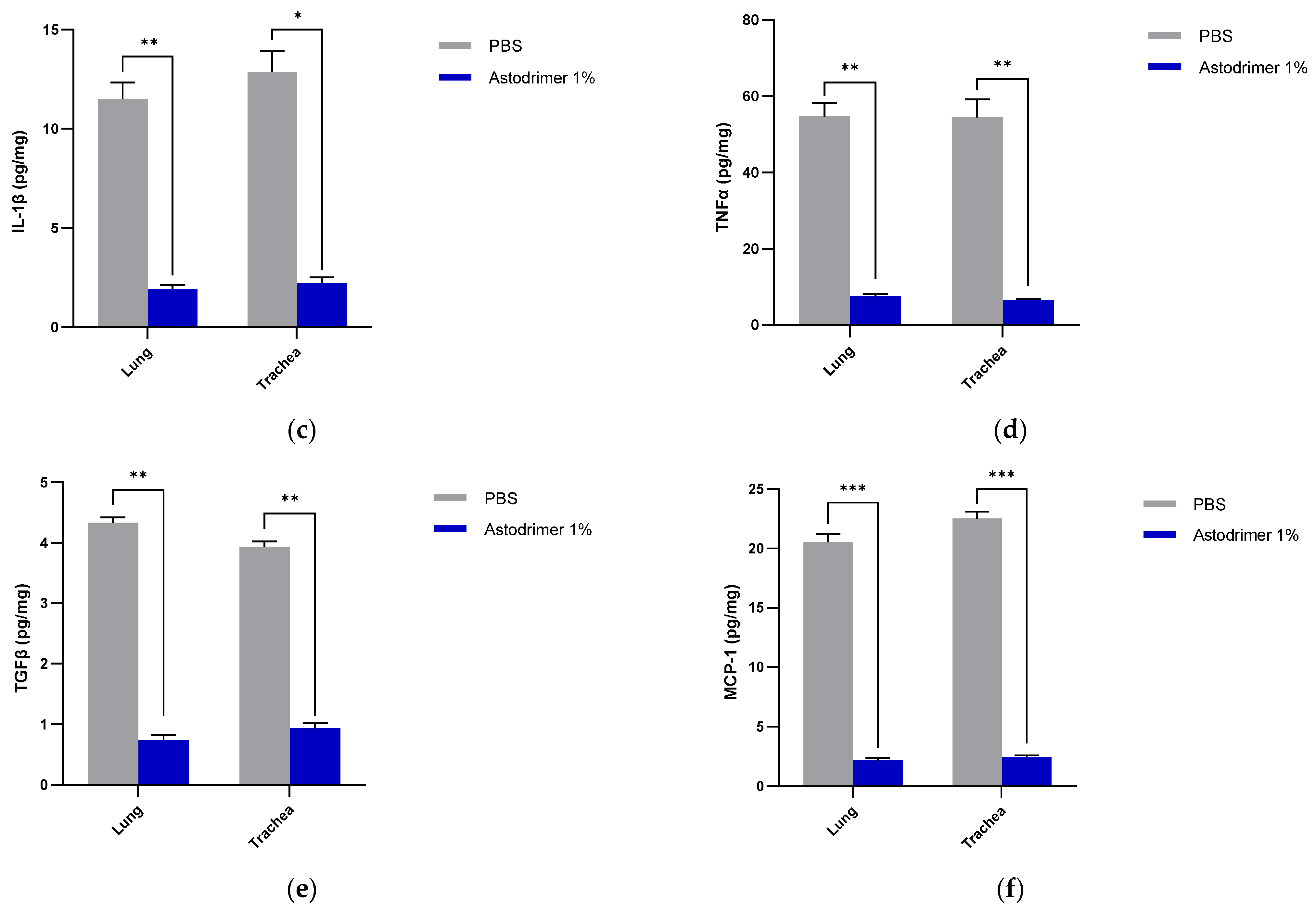
The level of inflammation in the tissues was measured by determining the number of selected cytokines and the amount of interleukin (IL)-6 (RayBio® Mouse IL-6 ELISA[ELM-IL6-1], RayBiotech Life, Peachtree Corners, GA, USA), IL-1 alpha (IL-1α) (RayBio® Mouse IL-1α ELISA [ELM-IL1a-1]), IL-1 beta (IL-1β) (RayBio® Mouse IL-1β ELISA [ELM-IL1b-1], RayBiotech Life, Peachtree Corners, GA, USA), tumour necrosis factor alpha (TNFα) (RayBio® Mouse TNF-alpha ELISA [ELM-TNFa-1], RayBiotech Life, Peachtree Corners, GA, USA), transforming growth factor beta (TGFβ) (TGF-beta-1 Mouse ELISA kit [BMS608-4], ThermoFisher Scientific, Waltham, MA, USA) and monocyte chemoattractant protein (MCP)-1 (also referred to as chemokine [C-C motif] ligand 2 [CCL2]) (RayBio® Mouse MCP-1 ELISA [ELM-MCP1-1], RayBiotech Life, Peachtree Corners, GA, USA) according to their respective manufacturers’ instructions. Cytokines and chemokine were detected in serum samples collected on Days 0, 4 and 7. Day 7 tissue homogenates from trachea and lung were also used to determine the amount of cytokine and chemokine present. Briefly, the ELISA protocols were similar in that they utilised a solid-phase sandwich ELISA design. A cytokine/chemokine target antibody had been precoated to the plate. The samples were added to the wells to bind to the capture antibody. The addition of a second antibody enabled the detection of the target-antibody sandwich complex, which was quantitated using a colorimetric reporting signal that was directly proportional to the concentration in the original specimen.
2.5. Statistical Analysis
The means and standard deviations (SD) or standard errors of the mean (SEM) were calculated using GraphPad Prism 9 (GraphPad Software, San Diego, CA, USA). The blood viral load and cytokine/chemokine data were analysed by a two-way analysis of variance (ANOVA) with Bonferroni multiple comparisons (GraphPad Prism 9, GraphPad Software, San Diego, CA, USA). The swab and tissue viral load, infectious viral titre and tissue cytokine/chemokine data were analysed by paired t-tests (GraphPad Prism 9, GraphPad Software, San Diego, CA, USA). Significance was established at the two-sided significance level of 0.05.
4. Discussion
The transmission of SARS-CoV-2 occurs via exposure to airborne infectious respiratory fluids released during exhalation [
19]. The amount of SARS-CoV-2 necessary to establish human infection is unknown, and exposure amounts vary based on the infectivity of the carrier and on the environmental conditions. The average viral titre in the saliva of an infectious individual was calculated as 7 × 10
6 viral RNA copies/mL [
20] and has been reported to be as high as 10
11 viral RNA copies/mL [
21]. However, virus particles in the air were calculated to be approximately 40 viral RNA copies per m
3 in medical staff areas [
22]. The actual viral load a susceptible human may receive is difficult to estimate. Mathematical modelling suggests that the number of SARS-CoV-2 particles needed to infect an individual is the equivalent of ~10 to 100 PFU. Due to the absence of specific data for SARS-CoV-2, this number was based on comparisons of infectious doses with other coronaviruses, including SARS-CoV-1, and influenza [
23]. The infectious doses of SARS-CoV-2 used in the current K18-hACE2 mice studies are comparable to N
inf used in mathematical modelling for the possible transmission of SARS-CoV-2.
However, the amount of virus given to animal models of SARS-CoV-2 is probably significantly higher than a human infectious dose and is delivered to establish a reproducible infection process in order to study virus replication and pathogenesis. The K18-hACE2 mouse model was chosen for these antiviral studies because it is a model of high SARS-CoV-2 replication and, in certain conditions, is a lethal model of SARS-CoV-2 infection. In the current study, we have achieved viral infection of the lung via intranasal dosing. Astodrimer sodium 1% is currently formulated in a nasal spray and its antiviral and virucidal against SARS-CoV-2 virus replication and pathogenesis were examined.
In these studies, the intranasal administration of astodrimer sodium 1% nasal spray as an antiviral or virucidal agent reduced viral replication in the serum, lung, trachea, brain and liver and the production of pro-inflammatory cytokines (IL-6, IL-1α, IL-1β, TNFα and TGFβ) and chemokine MCP-1 in the serum, lung and trachea at 7 days post-infection. While the complete prevention of infection was not achieved with antiviral or virucidal treatment with astodrimer sodium 1% nasal spray, the evidence supports a significant reduction in SARS-CoV-2 replication and pro-inflammatory cytokines. The severity of COVID-19 has been related to the development of a cytokine storm that is initiated and sustained by pro-inflammatory signaling that leads directly to disease progression. The experiments being reported here support the hypothesis that the intranasal administration of astodrimer sodium 1% nasal spray reduces systemic, lung and tracheal pro-inflammatory cytokine production caused by SARS-CoV-2 infection via the nasal passages. Numerous articles have described the cytokine storm related to COVID-19, but they have not always reported on the same inflammation markers. IL-6, MCP-1, TNFα and other immune markers, particularly those involved in myeloid cell recruitment and function, have been identified as markers that are important in the development of COVID-19 [
24,
25,
26].
A critique of the K18-hACE2 mouse model has been that untreated mice have higher SARS-CoV-2 infection levels of the brain and may die from brain encephalitis rather than pneumonia. In the antiviral and virucidal studies reported here, there was no detectable infectious virus in the brain when they were treated with astodrimer sodium 1% intranasally +/− intratracheal dosing. Studies performed on the autopsies of people who died due to COVID-19 have identified virus genomes, proteins and particles in the brain [
27]. One theory is that the brain is infected by virus transmitted via neurons in the olfactory bulb, located in upper nasal cavity, to the brain. Another is that it can infect the brain hematogeneously [
28,
29]. The intranasal +/− intratracheal administration of astodrimer sodium 1% nasal spray in the antiviral studies and the single virucidal dose of astodrimer sodium 1% efficiently blocks this route of viral spread, and these data may translate to protection from brain infection and its sequelae in humans, such as many of the symptoms of long-COVID.
The significant reduction in viral replication in the liver is an important finding. In up to 50% of COVID-19 cases, liver enzymes, which are used to assess liver damage, are elevated [
30]. The liver is presumably infected via the blood, and liver infection has been associated with clotting abnormalities in humans infected with SARS-CoV-2. A reduction in the virus in this organ in Groups 1.2, 2.2 and 3.2 is likely related to lower levels of disseminating virus (
Figure 1) that have reached the liver.
Although the number of animals per group is limited in this study, highly statistically significant reductions in viral load were observed. The combined data over the three groups are supportive of astodrimer sodium 1% nasal spray inactivating virus and blocking SARS-CoV-2 infection, resulting in significantly lower viral loads and infectious virus in the lung, trachea, brain and liver of K18-hACE2 mice. Astodrimer sodium 1% intranasal treatment as an antiviral or as a virucidal agent significantly reduced the titre of virus in all tissues assessed. While the antiviral and virucidal interventions did not completely prevent infection in this highly challenging model, the amount of virus that escaped the intervention was significantly lower, as determined by the viral load in serum and tissues. Furthermore, there was evidence of reduced virus shedding from the nasal cavity and transmission of the virus to the brain, presumably via olfactory bulb neurons and/or blood, as well as the infection of the liver, presumably via blood.
While this study did not investigate the toxicity of astodrimer sodium 1% nasal spray, there were no signs of toxicity observed in the K18-hACE2 mice. The safety of astodrimer sodium 1% nasal spray has been investigated separately in regulatory compliant nonclinical studies and determined to be non-irritant following repeated dosing, non-cytotoxic and non-sensitising. Astodrimer sodium was not systemically absorbed after nasal administration, so the risk of systemic toxicity is negligible. A clinical investigation in healthy volunteers has also recently been completed and showed the product to be well tolerated after dosing four times daily for 14 days, with full data to be published.
Current therapies for the prevention of COVID-19 are dominated by vaccine strategies that provide a reduction in the severity of the disease rather than absolute protection from infection and the ability to shed and transmit the virus. Complementary interventions will continue to be necessary to reduce the transmission of the virus, particularly in the current environment where the dominant variants of SARS-CoV-2 have higher transmission rates. Given the protective effects against a clinical isolate of SARS-CoV-2 observed in the current study in K18-hACE2 mice, as well as the favourable safety profile in nonclinical and clinical studies, astodrimer sodium 1% nasal spray has the potential for personal use in humans to help protect from the aggressive spread of SARS-CoV-2 infection, and it may reduce the severity or frequency of respiratory, central nervous system and gastrointestinal clinical outcomes of infection.