What Are Intermediate-Severity Forest Disturbances and Why Are They Important?
Abstract
:1. Introduction
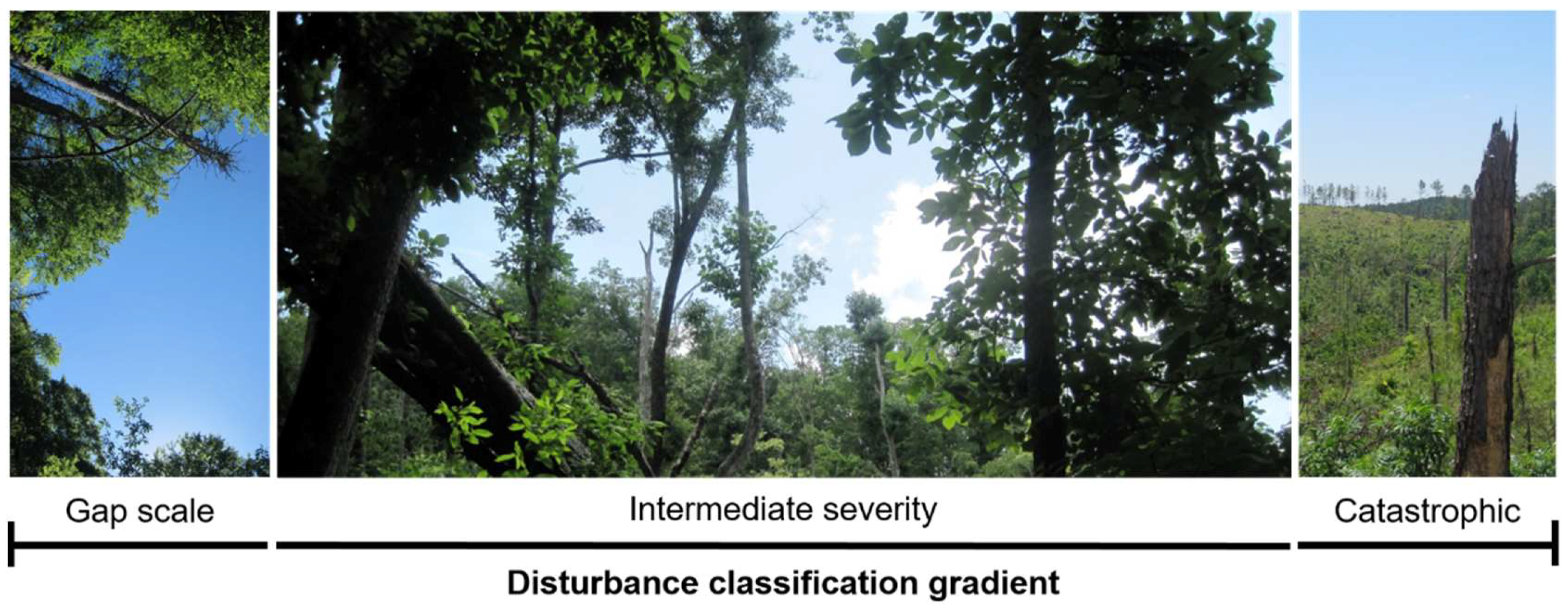
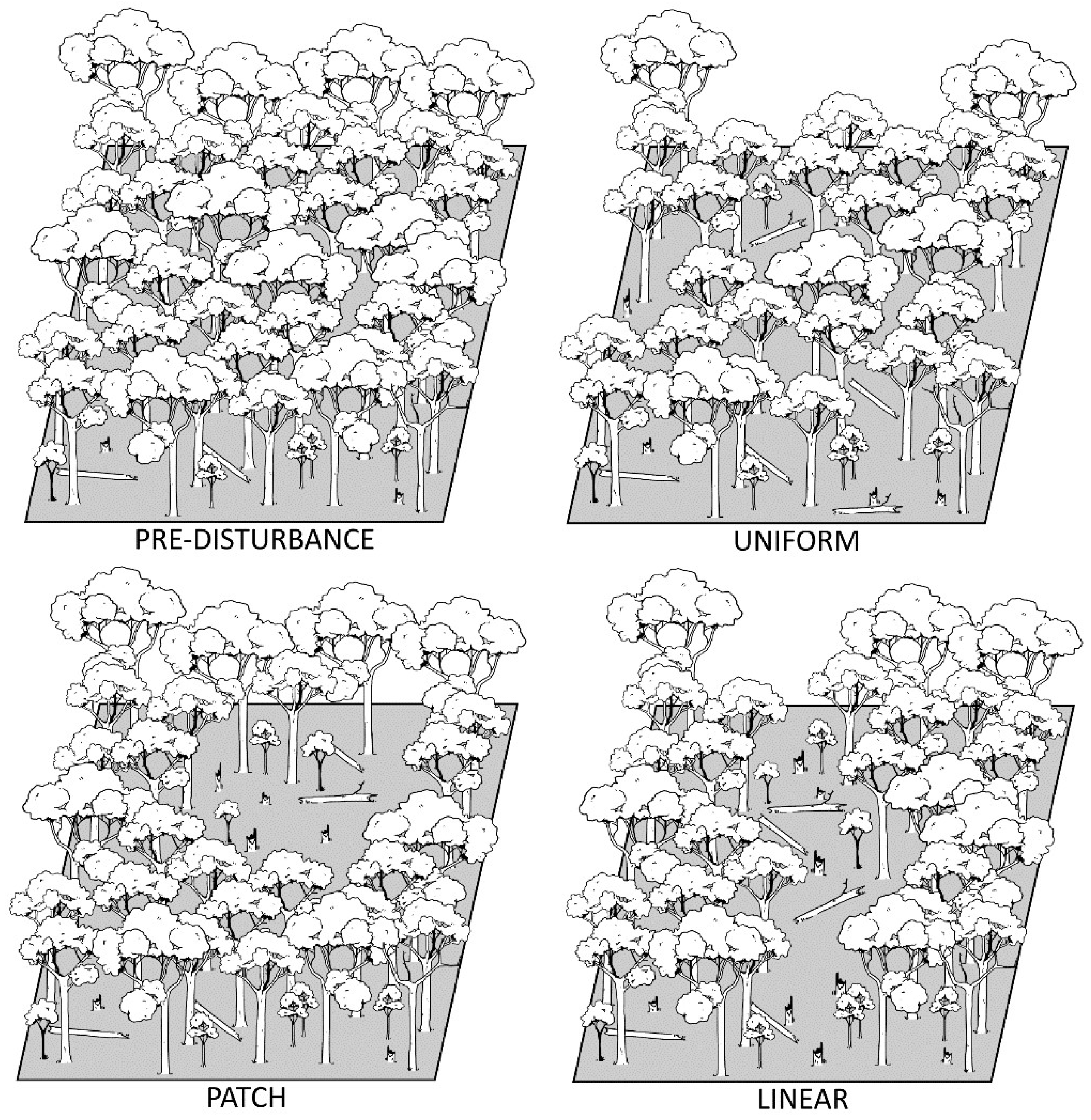
2. What Are Intermediate-Severity Disturbances?
2.1. Gap to Intermediate Disturbances
2.2. Intermediate to Catastrophic Disturbances
3. What Causes Intermediate-Severity Disturbances?
4. How Frequent Are Intermediate-Severity Disturbances?
5. How May These Events Influence Our Management?
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- White, P.S.; Jentsch, A. The search for generality in studies of disturbance and ecosystem dynamics. Prog. Bot. 2001, 62, 399–450. [Google Scholar]
- Amario, B.D.; Barr, A.G.; Barr, J.G.; Black, T.A.; Bracho, R.; Brown, M.; Chen, J.; Clark, K.L.; Davis, K.J.; Desai, A.R.; et al. Ecosystem carbon dioxide fluxes after disturbance in forests of North America. J. Geophys. Res. 2010, 115, G00K02. [Google Scholar] [CrossRef]
- Goetz, S.J.; Bond-Lamberty, B.; Law, B.E.; Hicke, J.A.; Huang, C. Observations and assessment of forest carbon dynamics following disturbance in North America. J. Geophys. Res. 2012, 117, G02022. [Google Scholar] [CrossRef]
- Johnstone, J.F.; Allen, C.D.; Franklin, J.F.; Frelich, L.E.; Harvey, B.J.; Higuera, P.E.; Mack, M.C.; Meentemeyer, R.K.; Metz, M.R.; Perry, G.L.W.; et al. Changing disturbance regimes, ecological memory, and forest resilience. Front. Ecol. Environ. 2016, 14, 369–378. [Google Scholar] [CrossRef]
- White, P.S.; Pickett, S.T.A. Natural disturbance and patch dynamics: An introduction. In The Ecology of Natural Disturbance and Patch Dynamics; Pickett, S.T.A., White, P.S., Eds.; Academic Press: San Diego, CA, USA, 1985; pp. 3–13. [Google Scholar]
- Oliver, C.D.; Larson, B.C. Forest Stand Dynamics; Wiley: New York, NY, USA, 1996. [Google Scholar]
- Runkle, J.R. Disturbance regimes in temperate forests. In The Ecology of Natural Disturbance and Patch Dynamics; Pickett, S.T.A., White, P.S., Eds.; Academic Press: New York, NY, USA, 1985; pp. 17–33. [Google Scholar]
- Yomamoto, S. Forest gap dynamics and tree regeneration. J. For. Res. 2000, 5, 223–229. [Google Scholar] [CrossRef]
- Hart, J.L. Gap-scale disturbances in central hardwood forests with implications for management. In Natural Disturbances and Historic Range of Variation: Type, Frequency, Severity, and Post-Disturbance Structure in Central Hardwood Forests USA; Greenberg, C.H., Collins, B.S., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 33–47. [Google Scholar]
- Foster, D.R.; Knight, D.H.; Franklin, J.F. Landscape patterns and legacies resulting from large, infrequent forest disturbances. Ecosystems 1998, 1, 497–510. [Google Scholar] [CrossRef]
- Turner, M.G.; Dale, V.H. Comparing large, infrequent disturbances: What have we learned? Ecosystems 1998, 1, 493–496. [Google Scholar] [CrossRef]
- Lorimer, C.G. Historical and ecological roles of disturbance in eastern North American forests: 9000 years of change. Wildl. Soc. Bull. 2001, 29, 425–439. [Google Scholar]
- Seymour, R.S.; White, A.S.; deMaynadier, P.G. Natural disturbance regimes in northeastern North America—Evaluating silvicultural systems using natural scales and frequencies. For. Ecol. Manag. 2002, 155, 357–367. [Google Scholar] [CrossRef]
- Hanson, J.J.; Lorimer, C.G. Forest structure and light regimes following moderate wind storms: Implications for multi-cohort management. Ecol. Appl. 2007, 17, 1325–1340. [Google Scholar] [CrossRef] [PubMed]
- White, S.D.; Hart, J.L.; Schweitzer, C.J.; Dey, D.C. Altered structural development and accelerated succession from intermediate-scale wind disturbance in Quercus stands on the Cumberland Plateau, USA. For. Ecol. Manag. 2015, 336, 52–64. [Google Scholar] [CrossRef]
- Keeley, J.E. Fire intensity, fire severity and burn severity: A brief review and suggested usage. Int. J. Wildland Fire 2009, 18, 116–126. [Google Scholar] [CrossRef]
- Turner, M.G. Disturbance and landscape dynamics in a changing world. Ecology 2010, 91, 2833–2849. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhang, G.; Wang, G.G.; Yan, Q.; Lu, D.; Li, X.; Zheng, X. On the size of forest gaps: Can their lower and upper limits be objectively quantified? Agric. For. Meteorol. 2015, 213, 64–76. [Google Scholar] [CrossRef]
- Cowden, M.M.; Hart, J.L.; Schweitzer, C.J.; Dey, D.C. Effects of intermediate-scale wind disturbance on composition, structure, and succession in Quercus stands: Implications for natural disturbance-based silviculture. For. Ecol. Manag. 2014, 330, 240–251. [Google Scholar] [CrossRef]
- Schliemann, S.A.; Bockheim, J. Methods for studying tree-fall gaps: A review. For. Ecol. Manag. 2011, 261, 1143–1151. [Google Scholar] [CrossRef]
- Barden, L.S. Forest development in canopy gaps of a diverse hardwood forest of the southern Appalachian Mountains. Oikos 1981, 37, 205–209. [Google Scholar] [CrossRef]
- Runkle, J.R. Patterns of disturbance in some old-growth mesic forests of the eastern North America. Ecology 1982, 63, 1533–1546. [Google Scholar] [CrossRef]
- Coates, K.D. Tree recruitment in gaps of various sizes, clearcuts, and undisturbed mixed forest of interior British Columbia, Canada. For. Ecol. Manag. 2002, 155, 387–398. [Google Scholar] [CrossRef]
- Gagnon, J.L.; Jokela, E.J.; Moser, W.K.; Huber, D.A. Characteristics of gaps and natural regeneration in mature longleaf pine flatwoods ecosystems. For. Ecol. Manag. 2004, 187, 373–380. [Google Scholar] [CrossRef]
- Hubbell, S.P.; Foster, R.B.; O’Brien, S.T.; Harms, K.E.; Condit, R.; Wechsler, B.; Wright, S.J.; Loo de Lao, S. Light-gap disturbances, recruitment limitation, and tree diversity in a neotropical forest. Science 1999, 283, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Rentch, J.S.; Fajvan, M.A.; Hicks, R.R., Jr. Oak establishment and canopy accession strategies in five old-growth stands in the Central Hardwood forest region. For. Ecol. Manag. 2003, 184, 285–297. [Google Scholar] [CrossRef]
- Hart, J.L.; Grissino-Mayer, H.D. Gap-scale disturbance processes in secondary hardwood stands on the Cumberland Plateau, Tennessee, USA. Plant Ecol. 2009, 201, 131–146. [Google Scholar] [CrossRef]
- Curzon, M.T.; Keeton, W.S. Spatial characteristics of canopy disturbance in riparian old-growth hemlock-northern hardwood forests, Adirondack Mountains, New York, USA. Can. J. For. Res. 2010, 40, 13–25. [Google Scholar] [CrossRef]
- Rentch, J.S.; Schuler, T.M.; Nowacki, G.W.; Beane, N.R.; Ford, W.M. Canopy gap dynamics of second-growth red spruce-northern hardwood stands in West Virginia. For. Ecol. Manag. 2010, 260, 1921–1929. [Google Scholar] [CrossRef]
- Richards, J.D.; Hart, J.L. Canopy gap dynamics and development patterns in secondary Quercus stands on the Cumberland Plateau, Alabama, USA. For. Ecol. Manag. 2011, 262, 2229–2239. [Google Scholar] [CrossRef]
- Kneeshaw, D.D.; Bergeron, Y. Canopy gap characteristics and tree replacement in the southeastern boreal forest. Ecology 1998, 79, 783–794. [Google Scholar] [CrossRef]
- Spies, T.A.; Franklin, J.F.; Klopsch, M. Canopy gaps in Douglas-fir forests of the Cascade Mountains. Can. J. For. Res. 1990, 20, 649–658. [Google Scholar] [CrossRef]
- Malcolm, D.C.; Mason, W.L.; Clarke, G.C. The transformation of conifer forests in Britain—regeneration, gap size and silvicultural systems. For. Ecol. Manag. 2001, 151, 7–23. [Google Scholar] [CrossRef]
- Nyland, R.D. Silviculture: Concepts and Applications; McGraw-Hill: New York, NY, USA, 2016. [Google Scholar]
- Dey, D.C.; Brissette, J.C.; Schweitzer, C.J.; Guldin, J.M. Silviculture of forests in the eastern United States. In Cumulative Watershed Effects of Fuel Management in the Eastern United States; LaFayette, R., Brooks, M.T., Potyondy, J.P., Audin, L., Krieger, S.L., Trettin, C.C., Eds.; Gen. Tech. Rep. SRS-161; U.S. Department of Agriculture, Forest Service, Southern Research Station: Asheville, NC, USA, 2012; pp. 7–40. [Google Scholar]
- Schmidt, W.C.; Shearer, R.C.; Roe, A.L. Ecology and Silviculture of Western Larch Forests; U.S. Department of Agriculture, Forest Service: Washington, DC, USA, 1976.
- Seidel, K.W. Regeneration in Mixed Conifer and Douglas-Fir Shelterwood Cuttings in the Cascade Range of Washington; Res. Pap. RP-PNW-314; U.S. Department of Agriculture, Forest Service, Pacific: Portland, OR, USA, 1983.
- Barnett, J.P.; Baker, J.B. Regeneration methods. In Forest Regeneration Manual; Duryea, M.L., Dougherty, P.M., Eds.; Springer: Dordrecht, The Netherlands, 1991; pp. 3–50. [Google Scholar]
- Brockway, D.G.; Outcalt, K.W. Gap-phase regeneration in longleaf pine wiregrass ecosystems. For. Ecol. Manag. 1998, 106, 125–139. [Google Scholar] [CrossRef]
- Voicu, M.F.; Comeau, P.G. Microclimatic and spruce growth gradients adjacent to young aspen stands. For. Ecol. Manag. 2006, 221, 13–26. [Google Scholar] [CrossRef]
- Walters, M.B.; Farinos, E.J.; Willis, J.L.; Gottschalk, K.W. Managing for diversity: Harvest gap size drives complex light, vegetation, and deer herbivory impacts on tree seedlings. Ecosphere 2016, 7, e01397. [Google Scholar] [CrossRef]
- De Montigny, L.E.; Smith, N.J. The effects of gap size in a group selection silvicultural system on the growth response of young, planted Douglas-fir: A sector plot analysis. Forestry 2017, 90, 426–435. [Google Scholar] [CrossRef]
- Flower, C.E.; Knight, K.S.; Gonzalez-Meler, M.A. Impacts of the emerald ash borer (Agrilus planipennis. Fairmaire) induced ash (Fraxinus spp.) mortality on forest carbon cycling and successional dynamics in the eastern United States. Biol. Invasions 2013, 15, 931–944. [Google Scholar] [CrossRef]
- Keever, C. Present composition of some stands of the former oak-chestnut forest in the southern Blue Ridge Mountains. Ecology 1953, 34, 44–54. [Google Scholar] [CrossRef]
- Ellison, A.M.; Bank, M.S.; Clinton, B.D.; Colburn, E.A.; Elliott, K.; Ford, C.R.; Foster, D.R.; Kloeppel, B.D.; Knoepp, J.D.; Lovett, G.M.; et al. Loss of foundation species: Consequences for the structure and dynamics of forested ecosystems. Front. Ecol. Environ. 2005, 3, 479–486. [Google Scholar] [CrossRef]
- Hart, J.L.; van de Gevel, S.L.; Grissino-Mayer, H.D. Forest dynamics in a natural area of the southern Ridge and Valley, Tennessee. Nat. Areas J. 2008, 28, 275–289. [Google Scholar] [CrossRef]
- van de Gevel, S.L.; Hart, J.L.; Spond, M.D.; White, P.B.; Sutton, M.N.; Grissino-Mayer, H.D. American chestnut to northern red oak: Forest dynamics in an old-growth forest in the Blue Ridge Mountains, USA. Botany 2012, 90, 126–1276. [Google Scholar] [CrossRef]
- Axelson, J.N.; Alfaro, R.I.; Hawkes, B.C. Changes in stand structure in uneven-aged lodgepole pine stands impacted by mountain pine beetle epidemics and fires in central British Columbia. For. Chron. 2010, 87–99. [Google Scholar] [CrossRef]
- Dordel, J.; Feller, M.C.; Simard, Z.W. Effects of mountain pine beetle (Dendroctonus ponderosae Hopkins) infestations on forest stand structure in southern Canadian Rocky Mountains. For. Ecol. Manag. 2008, 255, 3563–3570. [Google Scholar] [CrossRef]
- Ocasio-Morales, R.G.; Tsopelas, P.; Harrington, T.C. Origin of Ceratocytis platani on native Platanus orientalis in Greece and its impact on natural forests. Plant Dis. 2007, 91, 901–904. [Google Scholar] [CrossRef]
- Swetnam, T.W.; Lynch, A.M. A tree-ring reconstruction of western spruce budworm history in the southern Rocky Mountains. For. Sci. 1989, 35, 962–986. [Google Scholar]
- Klooster, W.S.; Herms, D.A.; Knight, K.S.; Herms, C.P.; McCullough, D.G.; Smith, A.; Gandhi, K.J.K.; Cardina, J. Ash (Fraxinus spp.) mortality, regeneration, and seed bank dynamics in mixed hardwood forests following invasion by emerald ash borer (Agrilus planipennis). Biol. Invasions 2014, 16, 859–873. [Google Scholar] [CrossRef]
- Kolka, R.K.; D’Amato, A.W.; Wagenbrenner, J.W.; Slesak, R.A.; Pypker, T.G.; Youngquist, M.B.; Grinde, A.R.; Palik, B.J. Review of ecosystem level impacts of emerald ash borer on black ash wetlands: What does the future hold? Forests 2018, 9, 179. [Google Scholar] [CrossRef]
- Hancock, J.E.; Arthur, M.A.; Weathers, K.C.; Lovett, G.M. Carbon cycling along a gradient of beech bark disease impact in the Catskill Mountains, New York. Can. J. For. Res. 2008, 38, 1267–1274. [Google Scholar] [CrossRef]
- Mueller, R.C.; Scudder, C.M.; Porter, M.E.; Trotter, R.T.; Gehring, C.A.; Whitman, T.G. Differential tree mortality in response to severe drought: Evidence for long-term vegetation shifts. J. Ecol. 2005, 93, 1085–1093. [Google Scholar] [CrossRef]
- Elliot, K.J.; Swank, W.T. Impacts of drought on tree mortality and growth in a mixed hardwood forest. J. Veg. Sci. 1994, 5, 229–236. [Google Scholar] [CrossRef] [Green Version]
- Cosgriff, R.J.; Nelson, J.C.; Yin, Y. Floodplain forest response to large-scale flood disturbance. Trans. Ill. State Acad. Sci. 2007, 100, 47–70. [Google Scholar]
- Sakai, T.; Tanaka, H.; Shibata, M.; Suzuki, W.; Nomiya, H.; Kanazashi, T.; Iida, S.; Nakashizuka, T. Riparian disturbance and community structure of a Quercus-Ulmus forest in central Japan. Plant Ecol. 1999, 140, 99–109. [Google Scholar] [CrossRef]
- Covey, K.R.; Barrett, A.L.; Ashton, M.S. Ice storms as a successional pathway for Fagus grandifolia advancement in Quercus rubra dominated forests of southern New England. Can. J. For. Res. 2015, 45, 1628–1635. [Google Scholar] [CrossRef]
- Rebertus, A.J.; Shifley, S.R.; Richards, R.H.; Roovers, L.M. Ice storm damage to an old-growth oak-hickory forest in Missouri. Am. Midl. Nat. 1997, 137, 48–61. [Google Scholar] [CrossRef]
- Turcotte, R.M.; Elliott, T.R.; Fajvan, M.A.; Park, Y.L.; Snider, D.A.; Tobin, P.C. Effects of ice storm damage on hardwood survival and growth in Ohio. North. J. Appl. For. 2012, 29, 53–59. [Google Scholar]
- Lafon, C.W. Forest disturbance by ice storms in Quercus forests of the southern Appalachian Mountains. Ecoscience 2006, 13, 30–43. [Google Scholar] [CrossRef]
- Whitney, H.E.; Johnson, W.C. Ice storms and forest succession in southwestern Virginia. Bull. Torrey Bot. Club 1984, 111, 429–437. [Google Scholar] [CrossRef]
- McIntosh, A.C.S.; Macdonald, S.E. Potential for lodgepole pine regeneration after mountain pine beetle attack in newly invaded Alberta stands. For. Ecol. Manag. 2013, 295, 11–19. [Google Scholar] [CrossRef]
- Fajvan, M.A.; Wood, J.M. Stand structure and development after gypsy moth defoliation in the Appalachian Plateau. For. Ecol. Manag. 1996, 89, 79–88. [Google Scholar] [CrossRef]
- Kayes, L.J.; Tinker, D.B. Forest structure and regeneration following a mountain pine beetle epidemic in southeastern Wyoming. For. Ecol. Manag. 2012, 263, 57–66. [Google Scholar] [CrossRef]
- Seiwa, K.; Miwa, Y.; Akasaka, S.; Kanno, H.; Tomita, M.; Saitoh, T.; Ueno, N.; Kimura, M.; Hasegawa, Y.; Konno, M.; et al. Landslide-facilitated species diversity in a beech-dominated forest. Ecol. Res. 2013, 28, 29–41. [Google Scholar] [CrossRef]
- Miles, D.W.R.; Swanson, F.J. Vegetation composition on recent landslides in the Cascade Mountains of western Oregon. Can. J. For. Res. 1986, 16, 739–744. [Google Scholar] [CrossRef]
- Fulè, P.Z.; Course, J.E.; Heinlein, T.A.; Moore, M.M.; Covington, W.; Verkamp, G. Mixed-severity fire regime in a high-elevation forest of Grand Canyon, Arizona, USA. Landsc. Ecol. 2003, 18, 465–486. [Google Scholar] [CrossRef]
- Taylor, A.H.; Skinner, C.N. Fire history and landscape dynamics in a late-successional reserve, Klamath Mountains, California, USA. For. Ecol. Manag. 1998, 111, 285–301. [Google Scholar] [CrossRef]
- Lentile, L.B.; Smith, F.W.; Shepperd, W.D. Patch structure, fire-scar formation, and tree regeneration in a large mixed-severity fire in the South Dakota Black Hills, USA. Can. J. For. Res. 2005, 35, 2875–2885. [Google Scholar] [CrossRef]
- Mortenson, L.A.; Hughes, R.F.; Friday, J.B.; Keith, L.M.; Barbosa, J.M.; Friday, N.J.; Liu, Z.; Sowards, T.G. Assessing spatial distribution, stand impacts and rate of Ceratocystis fimbriata induced ‘ōhi’a (Metrosideros polymorpha) mortality in tropical wet forest, Hawai’I.; Island, USA. For. Ecol. Manag. 2016, 377, 83–92. [Google Scholar] [CrossRef]
- Cox, L.E.; Hart, J.L.; Dey, D.C.; Schweitzer, C.J. Composition, structure, and intra-stand spatial patterns along a disturbance severity gradient in a Quercus stand. For. Ecol. Manag. 2016, 381, 305–317. [Google Scholar] [CrossRef]
- Holzmueller, E.J.; Gibons, D.J.; Suchecki, P.F. Accelerated succession following an intense wind storm in an oak-dominated forest. For. Ecol. Manag. 2012, 279, 141–146. [Google Scholar] [CrossRef]
- Altman, J.; Dolezal, J.; Cerny, T.; Song, J.S. Forest response to increasing typhoon activity on the Korean peninsula: Evidence from oak tree-rings. Glob. Chang. Biol. 2013, 19, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Myster, R.W.; Malahy, M.P. Tornado effects on damage, resprouting and spatial heterogeneity in the Cross Timbers ecotone of Oklahoma, USA. J. Plant Ecol. 2010, 3, 157–163. [Google Scholar] [CrossRef] [Green Version]
- Nagel, T.A.; Diaci, J. Intermediate wind disturbance in an old-growth beech-fir forest in southeastern Slovenia. Can. J. For. Res. 2006, 36, 629–638. [Google Scholar] [CrossRef]
- Peterson, C.J. Consistent influence of tree diameter and species on damage in nine eastern North America tornado blowdowns. For. Ecol. Manag. 2007, 250, 96–108. [Google Scholar] [CrossRef]
- Peterson, C.J.; Rebertus, A.J. Tornado damage and initial recovery in three adjacent, lowland temperate forests in Missouri. J. Veg. Sci. 1997, 8, 559–564. [Google Scholar] [CrossRef]
- Woods, K.D. Intermediate disturbance in a late-successional hemlock-northern hardwood forest. J. Ecol. 2004, 92, 464–476. [Google Scholar] [CrossRef] [Green Version]
- Nagel, T.A.; Svoboda, M.; Diaci, J. Regeneration patterns after intermediate wind disturbance in an old-growth Fagus-Abies forest in southeastern Slovenia. For. Ecol. Manag. 2006, 226, 268–278. [Google Scholar] [CrossRef]
- Shankman, D. Channel migration and vegetation patterns in the southeastern Coastal Plain. Conserv. Biol. 1993, 7, 176–183. [Google Scholar] [CrossRef]
- Lafon, C.W. Ice storms in central hardwood forests: The disturbance regime, spatial patterns, and vegetation influences. In Natural Disturbances and Historic Range of Variation: Type, Frequency, Severity, and Post-Disturbance Structure in Central Hardwood Forests USA; Greenberg, C.H., Collins, B.S., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 147–166. [Google Scholar]
- Agee, J.K. The landscape ecology of Western forest fire regimes. Northwest Sci. 1998, 72, 24–34. [Google Scholar]
- Arno, S.F.; Parsons, D.J.; Keane, R.E. Mixed-severity fire regimes in the northern Rocky Mountains: Consequences of fire exclusion and options for the future. In Wilderness Science in a Time of Change Conference; U.S. Department of Agriculture Forest Service, Rocky Mountain Research Station: Ogden, UT, USA, 2000; pp. 225–232. [Google Scholar]
- Heyerdahl, E.K.; Loehman, R.A.; Falk, D.A. Mixed-severity fire in lodgepole pine dominated forests: Are historical regimes sustainable on Oregon’s Pumice Plateau, USA? Can. J. For. Res. 2014, 44, 593–603. [Google Scholar] [CrossRef]
- Mark, A.F.; Scott, G.A.M.; Sanderson, F.R.; James, P.W. Forest succession on landslides above Lake Thomson, Fiordland. N. Z. J. Bot. 1964, 2, 60–89. [Google Scholar] [CrossRef]
- Wooten, R.M.; Witt, A.C.; Miniat, C.F.; Hales, T.C.; Aldred, J.L. Frequency and magnitude of historical selected landslide events in the southern Appalachian Highlands of North Carolina and Virginia: Relationships to rainfall, geological, and ecohydrological controls, and effects. In Natural Disturbances and Historic Range of Variation: Type, Frequency, Severity, and Post-Disturbance Structure in Central Hardwood Forests USA; Greenberg, C.H., Collins, B.S., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 203–262. [Google Scholar]
- Buma, B. Disturbance interactions: Characterization, prediction, and the potential for cascading effects. Ecosphere 2015, 6, 70. [Google Scholar] [CrossRef]
- Harvey, B.J.; Donata, D.C.; Romme, W.H.; Turner, M.G. Influence of recent bark beetle outbreak on fire severity and postfire tree regeneration in montane Douglas-fir forests. Ecology 2013, 94, 2475–2486. [Google Scholar] [CrossRef] [PubMed]
- Agne, M.C.; Shaw, D.C.; Woolley, T.J.; Queijeiro-Bolanõs, M.E. Effects of dwarf mistletoe on stand structure of lodgepole pine forests 21–28 years post-mountain pine beetle epidemic in central Oregon. PLoS ONE 2014, 9, e107532. [Google Scholar] [CrossRef] [PubMed]
- Hart, J.L.; Cox, L.E. Incorporating intermediate-severity disturbances in oak stand development. Forests 2017, 8, 284. [Google Scholar] [CrossRef]
- Peterson, C.J.; Cannon, J.B.; Godfrey, C.M. First steps in defining the wind disturbance regime in central hardwood forests. In Natural Disturbances and Historic Range of Variation: Type, Frequency, Severity, and Post-Disturbance Structure in Central Hardwood Forests USA; Greenberg, C.H., Collins, B.S., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 89–122. [Google Scholar]
- Nowacki, G.J.; Abrams, M.D. Radial-growth averaging criteria for reconstructing disturbance histories from presettlement-origin oaks. Ecol. Monogr. 1997, 67, 225–249. [Google Scholar] [CrossRef]
- Ruffner, C.M.; Abrams, M.D. Relating land-use history and climate to the dendroecology of a 326-year old Quercus prinus talus slope forest. Can. J. For. Res. 1998, 28, 347–358. [Google Scholar] [CrossRef]
- Lafon, C.W.; Speer, J.H. Using dendrochronology to identify major ice storm events in oak forests of southwestern Virginia. Clim. Res. 2002, 20, 41–54. [Google Scholar] [CrossRef] [Green Version]
- Hart, J.L.; Grissino-Mayer, H.D. Vegetation patterns and dendroecology of a mixed hardwood forest on the Cumberland Plateau: Implications for stand development. For. Ecol. Manag. 2008, 255, 1960–1975. [Google Scholar] [CrossRef]
- Hart, J.L.; Clark, S.L.; Torreano, S.J.; Buchanan, M.L. Composition, structure, and dendroecology of an old-growth Quercus forest on the tablelands of the Cumberland Plateau, USA. For. Ecol. Manag. 2012, 266, 11–24. [Google Scholar] [CrossRef]
- Hart, J.L.; Cowden, M.M.; Torreano, S.J.; Vestal, P.R. Disturbance, succession, and structural development of an upland hardwood forest on the Interior Low Plateau, Tennessee. Nat. Areas J. 2015, 35, 557–573. [Google Scholar] [CrossRef]
- Ford, S.A.; Kleinman, J.S.; Hart, J.L. Spatial patterns of canopy disturbance, structure, and species composition in a multi-cohort hardwood stand. Forests 2017, 8, 93. [Google Scholar] [CrossRef]
- Zielonka, T.; Holeksa, J.; Fleischer, P.; Kapusta, P. A tree-ring reconstruction of wind disturbances in a forest of the Slovakian Tatra Mountains, Western Carpathians. J. Veg. Sci. 2010, 21, 31–42. [Google Scholar] [CrossRef]
- Svoboda, M.; Janda, P.; Nagel, T.A.; Fraver, S.; Rejzek, J.; Bače, R. Disturbance history of an old-growth sub-alpine Picea abies stand in the Bohemian Forest, Czech Republic. J. Veg. Sci. 2012, 23, 86–97. [Google Scholar] [CrossRef]
- Splechtna, B.E.; Georg, G.; Black, B.A. Disturbance history of a European old-growth mixed-species forest—A spatial dendro-ecological analysis. J. Veg. Sci. 2005, 16, 511–522. [Google Scholar]
- Nagel, T.A.; Levanic, T.; Diaci, J. A dendroecological reconstruction of disturbance in an old-growth Fagus-Abies forest in Slovenia. Ann. For. Sci. 2007, 64, 891–897. [Google Scholar] [CrossRef]
- Trotsiuk, V.; Hobi, M.L.; Commarmot, B. Age structure and disturbance dynamics of the relic virgin beech forest Uholka (Ukrainian Carpathians). For. Ecol. Manag. 2012, 265, 181–190. [Google Scholar] [CrossRef]
- Abrams, M.D.; van de Gevel, S.L.; Dodson, R.C.; Copenheaver, C.A. The dendroecology and climatic impacts for old-growth white pine and hemlock on the extreme slopes of the Berkshire Hills, Massachusetts, USA. Can. J. Bot. 2000, 78, 851–861. [Google Scholar]
- Axelson, J.N.; Alfaro, R.I.; Hawkes, B.C. Influence of fire and mountain pine beetle on the dynamics of lodgepole pine stands in British Columbia, Canada. For. Ecol. Manag. 2009, 257, 1874–1882. [Google Scholar] [CrossRef]
- Fraver, S.; Seymour, R.S.; Speer, J.H.; White, A.S. Dendrochronological reconstruction of spruce budworm outbreaks in northern Maine, USA. Can. J. For. Res. 2007, 37, 523–529. [Google Scholar] [CrossRef]
- Boulanger, Y.; Arseneault, D.; Morin, H.; Jardon, Y.; Bertrand, P.; Dagneau, C. Dendrochronological reconstruction of spurce budworm (Choristoneura fumiferana) outbreaks in southern Quebec for the last 400 years. Can. J. For. Res. 2012, 42, 1264–1276. [Google Scholar] [CrossRef]
- Baker, P.J.; Bunyavejchewin, S.; Oliver, C.D.; Ashton, P.S. Disturbance history and historical stand dynamics of a seasonal tropical forest in western Thailand. Ecol. Monogr. 2005, 75, 317–343. [Google Scholar] [CrossRef]
- D’Amato, A.W.; Jokela, E.J.; O’Hara, K.L.; Long, J.N. Silviculture in the United States: An amazing period of change over the past 30 years. J. For. 2018, 116, 55–67. [Google Scholar] [CrossRef]
- Franklin, J.F.; Spies, T.A.; Van Pelt, R.; Carey, A.B.; Thornburgh, D.A.; Berg, D.R.; Lindenmayer, D.B.; Harmon, M.E.; Keeton, W.S.; Shaw, D.C.; et al. Disturbances and structural development of natural forest ecosystems with silvicultural implications, using Douglas-fir forests as an example. For. Ecol. Manag. 2002, 155, 399–423. [Google Scholar] [CrossRef]
- Long, J.N. Emulating natural disturbance regimes as a basis for forest management: A North American view. For. Ecol. Manag. 2009, 257, 1868–1873. [Google Scholar] [CrossRef]
- Buma, B.; Wessman, C.A. Differential species responses to compounded perturbations and implications for landscape heterogeneity and resilience. For. Ecol. Manag. 2012, 266, 25–33. [Google Scholar] [CrossRef]
- Reyer, C.P.O.; Rammig, A.; Brouwers, N.; Langerwisch, F. Forest resilience, tipping points and global change processes. J. Ecol. 2015, 103, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Trumbore, S.; Brando, P.; Hartmann, H. Forest health and global change. Science 2015, 349, 814–818. [Google Scholar] [CrossRef] [PubMed]
- DeRose, R.J.; Long, J.N. Resistance and resilience: A conceptual framework for silviculture. For. Sci. 2014, 60, 1205–1212. [Google Scholar] [CrossRef]
- Seidl, R.; Rammer, W.; Spies, T.A. Disturbance legacies increase the resilience of forest ecosystem structure, composition, and functioning. Ecol. Appl. 2014, 24, 2063–2077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantarello, E.; Newton, A.C.; Martin, P.A.; Evans, P.M.; Gosal, A.; Lucash, M.S. Quantifying resilience of multiple ecosystem services and biodiversity in a temperate forest landscape. Ecol. Evol. 2017, 7, 9661–9675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donato, D.C.; Campbell, J.L.; Franklin, J.F. Multiple successional pathways and precocity in forest development: Can some forests be born complex? J. Veg. Sci. 2012, 23, 576–584. [Google Scholar] [CrossRef]
- Meigs, G.; Keeton, W.S. Intermediate-severity wind disturbance in mature temperate forests: Legacy structure, carbon storage, and stand dynamics. Ecol. Appl. 2018, 28, 798–815. [Google Scholar] [CrossRef] [PubMed]
- Axelson, J.N.; Hawkes, B.C.; van Akker, L.; Alfaro, R.I. Stand dynamics and the mountain pine beetle—Thirty years of forest change in Waterton Lakes National Park, Alberta, Canada. Can. J. For. Res. 2018. [Google Scholar] [CrossRef]
- O’Hara, K.L. Multiaged Silviculture: Managing for Complex Forest Stand Structures; Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- O’Hara, K.L.; Ramage, B.S. Silviculture in an uncertain world: Utilizing multi-aged management systems to integrate disturbance. Forestry 2013, 86, 401–410. [Google Scholar] [CrossRef]
- Mitchell, S.J.; Beese, W.J. The retention system: Reconciling variable retention with the principles of silvicultural systems. For. Chron. 2002, 78, 397–403. [Google Scholar] [CrossRef]
- Seymour, R.S. Integrating disturbance parameters into conventional silvicultural systems: Experience from the Acadian forest of northeastern North America. In Balancing Ecosystem Values: Innovative Experiments for Sustainable Forestry; Peterson, C.E., Maguire, D.A., Eds.; U.S. Department of Agriculture Forest Service: Washington, DC, USA, 2005; pp. 41–48. [Google Scholar]
- Raymond, P.; Bèdard, S.; Roy, V.; Larouche, C.; Tremblay, S. The irregular shelterwood system: Review, classification, and potential application to forests affected by partial disturbances. J. For. 2009, 107, 405–413. [Google Scholar] [CrossRef]
- Beese, W.J.; Dunsworth, B.G.; Zielke, K.; Bancroft, B. Maintaining attributes of old-growth forests in coastal BC through variable retention. For. Chron. 2003, 79, 570–578. [Google Scholar] [CrossRef]
- O’Hara, K.L.; Nagel, L.M. The stand: Revisiting a central concept in forestry. J. For. 2013, 111, 335–340. [Google Scholar] [CrossRef]
- Franklin, J.F.; Mitchell, R.J.; Palik, B.J. Natural Disturbance and Stand Development Principles for Ecological Forestry; GTR-NRS-19; U.S. Department of Agriculture Forest Service: Newton Square, PA, USA, 2007; p. 44.
- Bradford, J.B.; Betancourt, J.L.; Butterfield, B.J.; Munson, S.M.; Wood, T.E. Anticipatory natural resource science and management for a changing futurE. Front. Ecol. Environ. 2018, 16, 295–303. [Google Scholar] [CrossRef]
| Disturbance Agent | Location | Forest Type | Citation |
|---|---|---|---|
| Drought | Arizona, USA | Pinus edulis Engelm.-Juniperus monosperma (Engelm.) Sarg. | Mueller et al., 2005 [55] |
| Drought | North Carolina, USA | Mixed Quercus | Elliot and Swank 1994 [56] |
| Flood | Illinois, USA | Mixed Quercus | Cosgriff et al. 2007 [57] |
| Flood | Tochigi, Japan | Quercus mongolica Fisch. ex Ledeb. | Sakai et al., 1999 [58] |
| Ice storm | Connecticut, USA | Quercus rubra L. | Covey et al., 2015 [59] |
| Ice storm | Missouri, USA | Quercus alba L.-Quercus rubra | Rebertus et al., 1997 [60] |
| Ice storm | Ohio, USA | Mixed Quercus-Carya | Turcotte et al., 2012 [61] |
| Ice storm | Virginia, USA | Quercus prinus L. | Lafon 2006 [62] |
| Ice storm | Virginia, USA | Mixed Quercus | Whitney and Johnson 1984 [63] |
| Insect outbreak | Alberta, Canada | Pinus contorta | McIntosh and Macdonald 2013 [64] |
| Insect outbreak | British Columbia, Canada | Pinus contorta | Axelson et al., 2010 [48] |
| Insect outbreak | Pennsylvania, USA | Mixed Quercus | Fajvan and Wood 1996 [65] |
| Insect outbreak | Wyoming, USA | Pinus contorta | Kayes and Tinker 2012 [66] |
| Landslide | Miyagi, Japan | Fagus crenata Blume | Seiwa et al., 2013 [67] |
| Landslide | Oregon, USA | Psuedotsuga menziesii | Miles and Swanson 1986 [68] |
| Mixed-severity fire | Arizona, USA | Mixed Picea-Abies | Fulè et al., 2013 [69] |
| Mixed-severity fire | California, USA | Psuedotsuga menziesii | Taylor and Skinner 1998 [70] |
| Mixed-severity fire | South Dakota, USA | Pinus ponderosa Lawson & C. Lawson | Lentile et al., 2005 [71] |
| Pathogen | Hawaii, USA | Metrosideros polymorpha Gaudich. | Mortenson et al., 2016 [72] |
| Pathogen | North Carolina, USA | Mixed Quercus | Keever 1953 [44] |
| Wind event | Alabama, USA | Mixed Quercus | Cox et al., 2016 [73] |
| Wind event | Illinois, USA | Mixed Quercus | Holzmueller et al., 2012 [74] |
| Wind event | Jeju-do, South Korea | Quercus mongolica | Altman et al., 2013 [75] |
| Wind event | Oklahoma, USA | Quercus marilandica Münchh.-Quercus stellata Wangenh. | Myster and Malahy 2010 [76] |
| Wind event | Slovenia | Fagus sylvatica L.-Abies alba Mill. | Nagel and Diaci 2006 [77] |
| Wind event | Wisconsin, USA | Acer-Tsuga-Betula | Hanson and Lorimer 2007 [14] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hart, J.L.; Kleinman, J.S. What Are Intermediate-Severity Forest Disturbances and Why Are They Important? Forests 2018, 9, 579. https://doi.org/10.3390/f9090579
Hart JL, Kleinman JS. What Are Intermediate-Severity Forest Disturbances and Why Are They Important? Forests. 2018; 9(9):579. https://doi.org/10.3390/f9090579
Chicago/Turabian StyleHart, Justin L., and Jonathan S. Kleinman. 2018. "What Are Intermediate-Severity Forest Disturbances and Why Are They Important?" Forests 9, no. 9: 579. https://doi.org/10.3390/f9090579
APA StyleHart, J. L., & Kleinman, J. S. (2018). What Are Intermediate-Severity Forest Disturbances and Why Are They Important? Forests, 9(9), 579. https://doi.org/10.3390/f9090579






