Early Differential Responses of Co-dominant Canopy Species to Sudden and Severe Drought in a Mediterranean-climate Type Forest
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Area
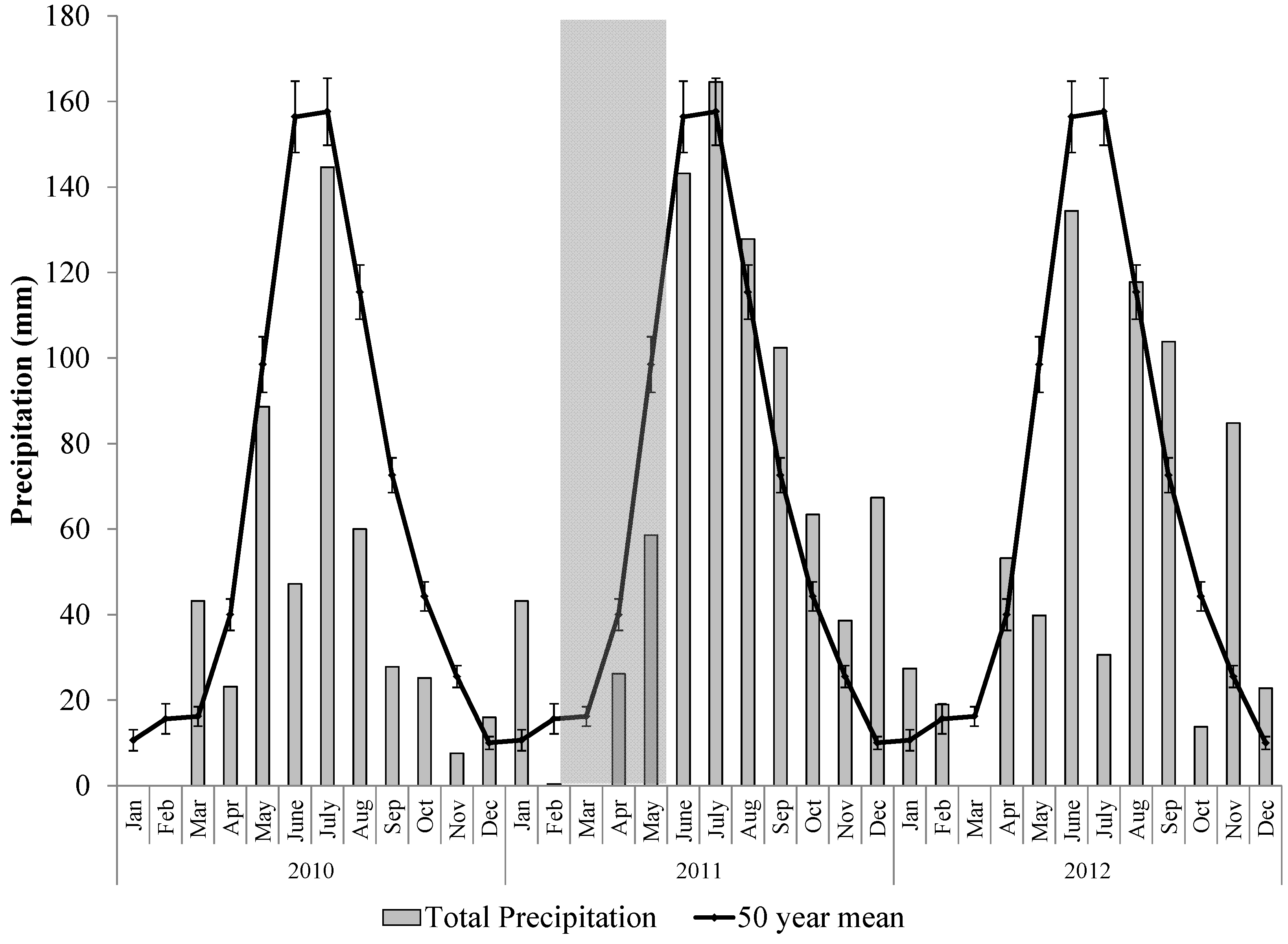
2.2. The 2011 Drought Event in the Northern Jarrah Forest
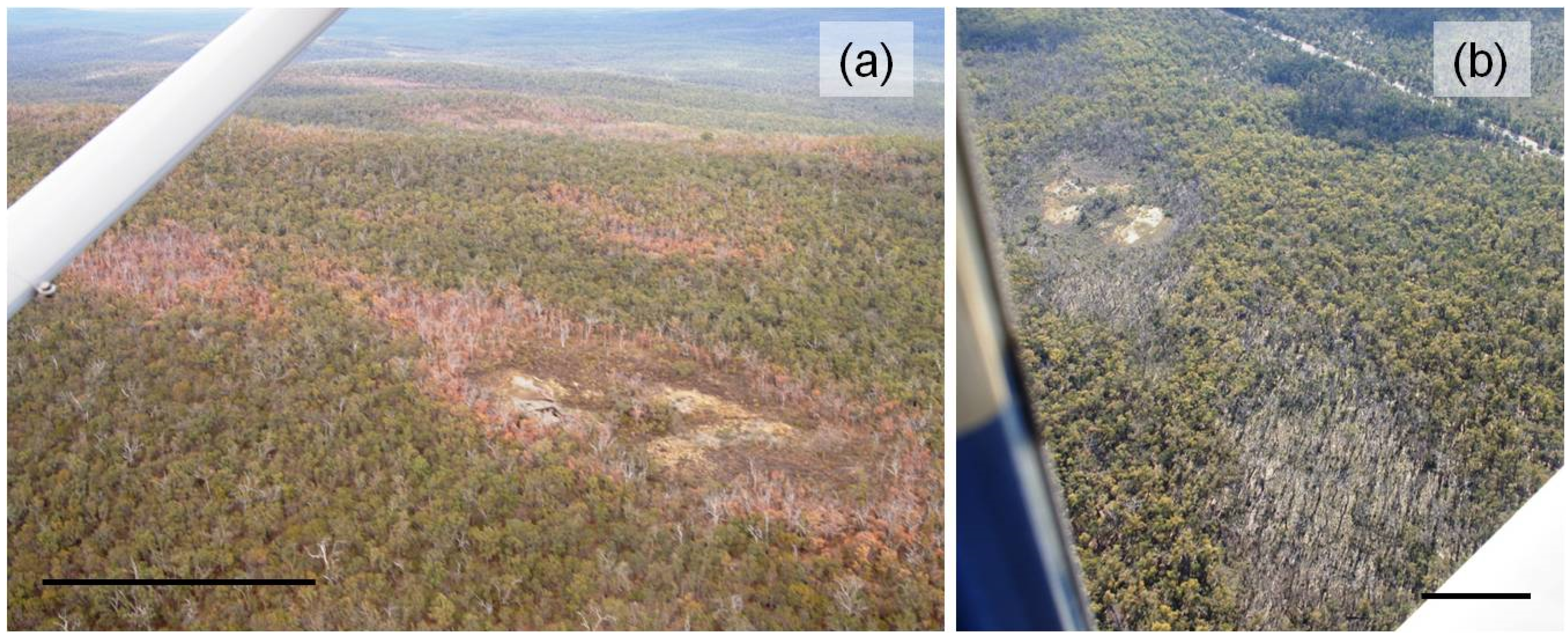
2.3. Site Selection
2.4. Sampling
2.5. Data Analysis
3. Results and Discussion

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- De Dios, V.R.; Fischer, C.; Colinas, C. Climate change effects on mediterranean forests and preventive measures. New For. 2007, 33, 29–40. [Google Scholar] [CrossRef]
- Bernstein, L.B.P.; Canziani, O. Intergovernmental Panel on Climate Change: Fourth Assessment Report; World Meterological Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Kane, J.M.; Kolb, T.E.; McMillin, J.D. Stand-scale tree mortality factors differ by site and species following drought in southwestern mixed conifer forests. For. Ecol. Manag. 2014, 330, 171–182. [Google Scholar] [CrossRef]
- Ganey, J.L.; Vojta, S.C. Tree mortality in drought-stressed mixed-conifer and ponderosa pine forests, Arizona, USA. For. Ecol. Manag. 2011, 261, 162–168. [Google Scholar] [CrossRef]
- Girard, F.; Vennetier, M.; Guibal, F.; Corona, C.; Ouarmim, S.; Herrero, A. Pinus halepensis Mill. crown development and fruiting declined with repeated drought in Mediterranean France. Eur. J. For. Res. 2012, 131, 919–931. [Google Scholar] [CrossRef]
- Matusick, G.; Ruthrof, K.X.; Hardy, G. Drought and heat triggers sudden and severe dieback in a dominant Mediterranean-type woodland species. Open J. For. 2012, 2, 183–186. [Google Scholar] [CrossRef]
- Matusick, G.; Ruthrof, K.X.; Brouwers, N.C.; Dell, B.; Hardy, G.S. Sudden forest canopy collapse corresponding with extreme drought and heat in a mediterranean-type eucalypt forest in Southwestern Australia. Eur. J. For. Res. 2013, 132, 497–510. [Google Scholar] [CrossRef]
- Bates, B.C.; Hope, P.; Ryan, B.; Smith, I.; Charles, S. Key findings from the indian ocean climate initiative and their impact on policy development in Australia. Clim. Chang. 2008, 89, 339–354. [Google Scholar] [CrossRef]
- Doblas-Miranda, E.; Martínez-Vilalta, J.; Lloret, F.; Álvarez, A.; Ávila, A.; Bonet, F.J. Reassessing global change research priorities in mediterranean terrestrial ecosystems: How far have we come and where do we go from here? Glob. Ecol. Biogeogr. 2015, 24, 25–43. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef] [PubMed]
- Lloret, F.; Escudero, A.; Iriondo, J.M.; Martinez-Vilalta, J.; Valladares, F. Extreme climatic events and vegetation: The role of stabilizing processes. Glob. Chang. Biol. 2012, 18, 797–805. [Google Scholar] [CrossRef]
- Slik, J.W.F. El nino droughts and their effects on tree species composition and diversity in tropical rain forests. Oecologia 2004, 141, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Suarez, M.L.; Kitzberger, T. Recruitment patterns following a severe drought: Long-term compositional shifts in Patagonian forests. Can. J. For. Res. 2008, 38, 3002–3010. [Google Scholar] [CrossRef]
- Allen, C.D.; Breshears, D.D. Drought-induced shift of a forest-woodland ecotone: Rapid landscape response to climate variation. Proc. Natl. Acad. Sci. 1998, 95, 14839–14842. [Google Scholar] [CrossRef] [PubMed]
- Chmura, D.J.; Anderson, P.D.; Howe, G.T.; Harrington, C.A.; Halofsky, J.E.; Peterson, D.L.; Shaw, D.C.; St Clair, J.B. Forest responses to climate change in the northwestern United States: Ecophysiological foundations for adaptive management. For. Ecol. Manag. 2011, 261, 1121–1142. [Google Scholar] [CrossRef]
- Klausmeyer, K.; Shaw, M.R. Climate change, habitat loss, protected areas and the climate adaptation potential of species in Mediterranean ecosystems worldwide. PLoS ONE 2009, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pausas, J.G.; Carbo, E.; Caturla, R.N.; Gil, J.M.; Vallejo, R. Post-fire regeneration patterns in the eastern Iberian Peninsula. Acta Oecol. 1999, 20, 499–508. [Google Scholar] [CrossRef]
- Rundel, P.W. Disturbance in Mediterranean-climate shrublands and woodlands. In Ecosystems of Disturbed Ground; Walker, L.R., Ed.; Elsevier: New York, NY, USA, 1999; pp. 271–285. [Google Scholar]
- Bond, W.J.; Midgley, J.J. Ecology of sprouting in woody plants: The persistence niche. Trends Ecol. Evol. 2001, 16, 45–51. [Google Scholar] [CrossRef]
- Clarke, P.J.; Lawes, M.J.; Midgley, J.J.; Lamont, B.B.; Ojeda, F.; Burrows, G.E.; Enright, N.J.; Knox, K.J.E. Resprouting as a key functional trait: How buds, protection and resources drive persistence after fire. New Phytol. 2013, 197, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Regelbrugge, J.C.; Conard, S.G. Modeling tree mortality following wildfire in Pinus ponderosa forests in the centre Sierra Nevada of California. Int. J. Wildl. Fire 1993, 3, 139–148. [Google Scholar] [CrossRef]
- Moreno, J.M.; Oechel, W.C. Fire intensity and herbivory effects on postfire resprouting of Adenostoma fasciculatum in southern California chaparral. Oecologia 1991, 85, 429–433. [Google Scholar] [CrossRef]
- Midgley, G. Why the world’s vegetation is not totally dominated by resprouting plants; because resprouters are shorter than reseeders. Ecography 1996, 19, 92–95. [Google Scholar] [CrossRef]
- Guarin, A.; Taylor, A.H. Drought triggered tree mortality in mixed conifer forests in Yosemite National Park, California, USA. For. Ecol. Manag. 2005, 218, 229–244. [Google Scholar] [CrossRef]
- Peñuelas, J.; Lloret, F.; Montoya, R. Severe drought effects on Mediterranean woody flora in Spain. For. Sci. 2001, 47, 214–218. [Google Scholar]
- Havel, J.J. Definition of site vegetation types. In Site-Vegetation Mapping in the Northern Jarrah Forest (Darling Range); Forests Department: Perth, Australia, 1975. [Google Scholar]
- Brouwers, N.; Matusick, G.; Ruthrof, K.; Lyons, T.; Hardy, G. Landscape-scale assessment of tree crown dieback following extreme drought and heat in a Mediterranean eucalypt forest ecosystem. Landsc. Ecol. 2013, 28, 69–80. [Google Scholar] [CrossRef]
- Dell, B.; Havel, J.J. The jarrah forest, an introduction. In The Jarrah Forest: A Complex Mediterranean Ecosystem; Dell, B., Havel, J.J., Malajczuk, N., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1989; pp. 1–10. [Google Scholar]
- Churchward, H.M.; Dimmock, G.M. The soils and landforms of the northern jarrah forest. In The Jarrah Forest: A Complex Mediterranean Ecosystem; Dell, B., Havel, J.J., Malajczuk, N., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1989; pp. 13–21. [Google Scholar]
- Gentilli, J. Climate of the jarrah forest. In The Jarrah Forest: A Complex Mediterranean Ecosystem; Dell, B., Havel, J.J., Malajczuk, N., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1989; pp. 23–40. [Google Scholar]
- Western Australia in 2010: A Very Dry Year in Southwest Western Australia. Available online: http://www.bom.gov.au/climate/current/annual/wa/archive/2010.summary.shtml (accessed on 2 January 2013).
- Perth in Summer 2010/11: Very Hot Summer in Perth. Available online: http://www.bom.gov.au/climate/current/season/wa/archive/201102.perth.shtml (accessed on 2 January 2013).
- Mueller, R.C.; Scudder, C.M.; Porter, M.E.; Trotter, R.T.; Gehring, C.A.; Whitham, T.G. Differential tree mortality in response to severe drought: Evidence for long-term vegetation shifts. J. Ecol. 2005, 93, 1085–1093. [Google Scholar] [CrossRef]
- Mitchell, P.J.; Benyon, R.G.; Lane, P.N.J. Responses of evapotranspiration at different topographic positions and catchment water balance following a pronounced drought in a mixed species eucalypt forest, Australia. J. Hydrol. 2012, 440, 62–74. [Google Scholar] [CrossRef]
- Fekedulegn, D.; Hicks, R.R.; Colbert, J.J. Influence of topographic aspect, precipitation and drought on radial growth of four major tree species in an Appalachian watershed. For. Ecol. Manag. 2003, 177, 409–425. [Google Scholar] [CrossRef]
- Szota, C.; Farrell, C.; Koch, J.M.; Lambers, H.; Veneklaas, E.J. Contrasting physiological responses of two co-occurring eucalypts to seasonal drought at restored bauxite mine sites. Tree Physiol. 2011, 31, 1052–1066. [Google Scholar] [CrossRef] [PubMed]
- Colquhoun, I.J.; Ridge, R.W.; Bell, D.T.; Loneragan, W.A.; Kuo, J. Comparative studies in selected species of Eucalyptus used in rehabilitation of the northern jarrah forest, Western Australia. 1 Patterns of xylem pressure potential and diffusive resistance of leaves. Aust. J. Bot. 1984, 32, 367–373. [Google Scholar] [CrossRef]
- Dell, B.; Bartle, J.R.; Tacey, W.H. Root occupation and root channels of jarrah (Eucalyptus marginata) forest. Aust. J. Bot. 1983, 31, 615–628. [Google Scholar] [CrossRef]
- Ruprecht, J.K.; Schofield, N.J. Seasonal soil-water dynamics in the jarrah forest, Western Australia. 1. Results from a hillslope transect with coarse textured soil profiles. Hydrol. Process. 1990, 4, 241–258. [Google Scholar] [CrossRef]
- Tromp-van Meerveld, H.J.; McDonnell, J.J. On the interrelations between topography, soil depth, soil moisture, transpiration rates and species distribution at the hillslope scale. Adv. Water Resour. 2006, 29, 293–310. [Google Scholar] [CrossRef]
- Seager, R.; Ting, M.F.; Held, I.; Kushnir, Y.; Lu, J.; Vecchi, G.; Huang, H.P.; Harnik, N.; Leetmaa, A.; Lau, N.C.; et al. Model projections of an imminent transition to a more arid climate in Southwestern North America. Science 2007, 316, 1181–1184. [Google Scholar] [CrossRef] [PubMed]
- Dettinger, M.D.; Cayan, D.R.; Meyer, M.K.; Jeton, A.E. Simulated hydrologic responses to climatic variations and change in the Merced, Carson and American River basins, Sierra Nevada, California, 1999–2099. Clim. Chang. 2004, 62, 283–317. [Google Scholar] [CrossRef]
- Giorgi, F.; Lionello, P. Climate change projections for the Mediterranean region. Glob. Planet. Chang. 2008, 63, 90–104. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruthrof, K.X.; Matusick, G.; Hardy, G.E.S.J. Early Differential Responses of Co-dominant Canopy Species to Sudden and Severe Drought in a Mediterranean-climate Type Forest. Forests 2015, 6, 2082-2091. https://doi.org/10.3390/f6062082
Ruthrof KX, Matusick G, Hardy GESJ. Early Differential Responses of Co-dominant Canopy Species to Sudden and Severe Drought in a Mediterranean-climate Type Forest. Forests. 2015; 6(6):2082-2091. https://doi.org/10.3390/f6062082
Chicago/Turabian StyleRuthrof, Katinka X., George Matusick, and Giles E. St. J. Hardy. 2015. "Early Differential Responses of Co-dominant Canopy Species to Sudden and Severe Drought in a Mediterranean-climate Type Forest" Forests 6, no. 6: 2082-2091. https://doi.org/10.3390/f6062082




