Abstract
Drought stress can dramatically impair woody plant growth and restrict the geographical distribution of many tree species. To better understand the dynamics between the response and mechanism of Cupressus gigantea to drought and post-drought recovery, a comparative analysis was performed, relying on physiological measurements, RNA sequencing (RNA-Seq) and two-dimensional gel electrophoresis (2-DE) proteins. In this study, the analyses revealed that photosynthesis was seriously inhibited, while osmolyte contents, antioxidant enzyme activity and non-enzymatic antioxidant contents were all increased under drought stress in seedlings. Re-watering led to a recovery in most of the parameters analyzed, mainly the photosynthetic parameters and osmolyte contents. Transcriptomic and proteomic profiling suggested that most of the differentially expressed genes (DEGs) and differentially expressed proteins (DEPs) were specifically altered, and a few were consistently altered. Drought induced a common reduction in the level of DEGs and DEPs associated with photosynthesis. Notably, DEGs and DEPs involved in reactive oxygen species (ROS) scavenging, such as ascorbate oxidase and superoxide dismutase (SOD), showed an inverse pattern under desiccation. This study may improve our understanding of the underlying molecular mechanisms of drought resistance in C. gigantea and paves the way for more detailed molecular analysis of the candidate genes.
1. Introduction
Environmental stresses are known to adversely affect plants’ growth and distribution [1]. Among these many adverse factors, drought is a serious detrimental environmental factor constraining seed germination, plant growth and the economic value of crops [2]. However, being sessile organisms, plants are unable to escape environmental stresses and thus have evolved sophisticated strategies to acclimate to dehydration, including morphological, anatomical, physiological and molecular adaptive strategies [3]. Accordingly, the initial response of plants is closely related to the reduction in water evaporation, and consequent reductions in photosynthesis, transpiration, stomatal closure and the accumulation of osmolytes [4]. Of these, the decline in photosynthetic process under drought stress is mainly attributed to stomatal closure, reductions in CO2 fixation and disturbances in photosynthetic electron transport (PET) [5]. PET is composed of multi-subunit protein complexes, such as Photosystems I and II (PSI, PSII), the cytochrome b6/f complex (Cytb6/f) and ATPase synthase, which absorb light energy and transduce solar energy into chemical energy [6]. Among these, PSII functions as a water-plastoquinone oxidoreductase embedded within the thylakoid membrane, which contains a series of peripheral light-harvesting complexes (LHC), and is vital to the initiation of photosynthesis and electron transport [7]. The Cyt b6/f complex, a rate-limiting step in photosynthesis, is involved the linear electron transport from PSII to PSI, giving rise to the production of ATP and NADPH [8]. Accumulation of some osmolytes such as proline and soluble sugar can play a prominent roles in preventing membrane disintegration and enzyme inactivation under water deficit [9].
When a reduction in water evaporation is insufficient to mitigate the stress stimulus, plants mainly respond to dehydration by activating a defense system of enzymatic and non-enzymatic antioxidants to cope with the overaccumulation of reactive oxygen species (ROS) [10]. Superoxide dismutase (SOD) is the first line of plant ROS defense, catalyzing the conversion of oxygen ions (O2·−) to oxygen (O2) and hydrogen peroxide (H2O2), which is then eliminated by the coordinated action of peroxidase (POD), catalase (CAT), ascorbate peroxidase (APX) and the AsA-GSH cycle [11]. Additionally, some enzymes, including APX, glutathione reductase (GR), dehydroascorbate reductase (DHAR) and monodehydroascorbate reductase (MDHAR), maintain the balance of AsA-GSH [12]. In addition, numerous drought-related transcripts and proteins are induced, which involve signaling transduction, activation/regulation of transcription, antioxidant capacity and ROS scavengers [13]. Thus, joint transcriptomic and proteomic profiling is considered to be effective and acceptable for disentangling the sophisticated processes of plants’ responses to water deficit at the molecular levels [14,15].
More recently, to understand such complex regulatory mechanisms, integrated multi-omics approaches (e.g., transcriptomics, proteomics and metabonomics) have been applied because multi-omics techniques offer powerful ways to reveal the relationships between genotype and phenotype. For example, comparative proteomic and transcriptomic analyses have provided insight into the formation mechanisms of seed size in castor bean [16]. Similarly, comparative transcriptomic and proteomic analyses were performed to determine the effect of pigment content on drought resistance in a wheat mutant [17]. In maize, an integrated transcriptomic, proteomic and metabolomic analysis found that its UV-B stress response involves signal transduction and signal molecules [18]. Dry-farm plants are promising candidates for studies on drought-related genes, proteins and metabolites [19]. While candidate genes and proteins have been discovered for wheat, rice, and soybean under dehydration conditions, no such reports are yet available for C. gigantea [20,21,22].
Being a rare tree species that is remarkably drought-tolerant, Cupressus gigantea W.C. Chen et L.K. Fu has high ecological and medicinal values due to its extensive use in afforestation, traditional Tibetan medicine and the construction industry [23]. It was added to the Red List of Threatened Species of the International Union for Conservation of Nature (IUCN), given the slow natural regeneration of this species, the anthropogenic disturbances it faces and its geographic isolation [24]. To date, studies on C. gigantea have mainly focused on its genetic diversity and population structure [25], total protein extraction [26], photosynthetic capacity [27], determination of the complete chloroplast genome [28] and comprehensive transcriptome characterization [29]. Nevertheless, to our best knowledge, no study has comprehensively investigated the molecular mechanisms enabling C. gigantea to tolerate drought. Overall, the mining of genes and proteins related to drought is an indispensable step towards deciphering the adaptive mechanisms of C. gigantea.
In this study, a cross-disciplinary approach combining classical physiological measurements, RNA sequencing (RNA-Seq) and two-dimensional gel electrophoresis (2-DE) was carried out to gain insight into the responses of C. gigantea to drought stress. This study identified differentially expressed genes (DEGs) and differentially expressed proteins (DEPs) linked to the tree’s drought stress responses, which are of great and timely importance for understanding the mechanisms by which C. gigantea ameliorates the effects of water deficit at the physiological and molecular levels.
2. Materials and Methods
2.1. Plant Material and Drought Treatments
In this study, we selected C. gigantea from the Tibet Agriculture and Animal Husbandry College (Linzhi, Tibet, China). Experiments were conducted at the Northeast Forestry University of (Harbin, Heilongjiang, China). Three-year-old seedlings were sown in plastic pots (25 cm in diameter and 25 cm high) containing a 1:1 mix of perlite and soil. The soil was humus soil, with a pH in H2O of 4.5–5.5, an organic matter content of 12.5 g/kg, and available nitrogen (N), phosphorous (P) and potassium (K) contents of 100, 40 and 90 mg/kg, respectively. All seedlings were grown in a greenhouse at a temperature of 17 °C during the night and 22 °C during the daytime with a relative humidity of 60% and a 12 h photoperiod. Three-year-old seedlings were exposed to the drought treatment (DTs), in which irrigation of the plants was suspended for 21 consecutive days (hereafter referred to as DT0, DT7, DT14 and DT21). At the end of the stress treatment, the seedlings were re-watered over a period of 7 days (hereafter referred to as RW). Well-watered control plant seedlings (CKs) irrigated every 7 days served as the control group (hereafter referred to as CK0, CK7, CK14, CK21 and CK28). Leaves were collected from DT and CK plants. Three biological replicates were used for the total RNA and protein extractions, with another 5 biological replicates measured for their photosynthesis and physiology parameters.
2.2. Physiological Measurements
To evaluate the physiological changes in C. gigantea under drought stress, the net photosynthetic rate (Pn), stomatal conductance (Gs), intercellular CO2 concentration (Ci) and transpiration rate (Tr) of the leaves were measured using a Li-6400 portable photosynthesis system (LI-COR Inc, Lincoln, NE, USA) at 9:00–11:00 a.m. The CO2 concentration, stimulation light intensity, and gas flow rate were set as 450 μmol·mol−1, 1000 μmol·m−2·s−1 and 500 μmol·s−1, respectively. Five plants were chosen from the control and drought-stressed groups, and each plant was measured 5 times.
2.3. Biochemical Analysis
The leaves were infiltrated with 0.1 mg/mL 3, 3′-diaminobenzidin (DAB) (Sigma, USA) in 50 mM Tris-acetate buffer (pH 5.0) for H2O2 staining or with 0.1 mg/mL nitro blue tetrazolium (NBT) (Sigma, USA) in 25 mM K-HEPES (pH 7.6) for superoxide staining, according to the method described by Chen [30].
Proline from the leaves was extracted by the method described by Zhang [31]. The leaves (0.3 g) were extracted in 4 mL of 1% sulfosalicylic acid. After centrifugation at 6000 g for 5 min, 1 mL of the supernatant was added to 1.5 mL of glacial acetic acid and 2 mL of a ninhydrin solution. The mixture was incubated at 100 °C for 30 min. After cooling, the contents were separated with 2 mL toluene, and the optical density was measured at 520 nm.
The soluble sugars of the leaves were analyzed by the anthrone method as described by Zhao [32]. Tissue oven-dried at 65 °C for 24 h (100 mg) was added to 10 mL of 80% ethanol and incubated in a thermostat water bath at 90 °C for 10 min, then the supernatant was collected. The pellet was extracted again as described above, and the supernatant was obtained and combined with the previous aliquot. After adding 3.5 mL of anthrone reagent to 0.1 mL of the supernatant, the mixture was heated in a thermostat water bath at 90 °C for 10 min. After the mixture had cooled to room temperature, the absorbance was measured at 620 nm.
The SOD (EC 1.15.1.1) activity of the leaves was detected with NBT [33]. To measure enzyme activity, leaves (0.3 g) were ground to a fine powder in liquid nitrogen and dissolved in 4 mL of potassium phosphate buffer (PBS) (50 mM, pH = 7.8). The assay mixture contained 50 mM PBS (pH = 7.8), 195 mM methionine, 0.3 mM ethylene diamine tetra-acetic acid, 1.125 mM NBT, 70 μL of the extracting solution and 60 μM riboflavin. Enzyme activity was detected at 560 nm by a spectrophotometer.
POD (EC 1.11.1.7) and CAT (EC 1.11.1.6) activity was measured in leaves according to the method of Kosar et al. [34]. The activity of POD was assayed in a mix containing 50 μL of the extracting solution, 50 mM PBS, 14 μL guaiacol and 19 μL H2O2 (30%, v/v). Enzyme activity was measured at 470 nm. The activity of CAT was assayed in a mix containing 0.2 mL of the extracting solution, 50 mM PBS (50 mM, pH = 7.8), 500 μL water and 10 mM H2O2, and the decrease in absorbance at 240 nm was monitored for 3 min.
The contents of ascorbate and glutathione were estimated in leaves according to the protocol of Sun et al. [35]. The activity of ASA was assayed in a mix containing 0.1 mL of the extracting solution, 0.7 mL ddH2O, 10% TCA, 44% H3PO4, 4% 2,2′-dipyridyl and 3% FeCl3, then the mixture was heated in a thermostat water bath at 37 °C for 60 min. After the mixture had cooled to room temperature, the absorbance was measured at 525 nm. The activity of ASA + DHA was assayed in a mix containing 0.1 mL of the extracting solution, 0.5 mL ddH2O, 10 mM DTT, 10% TCA, 5% N-ethylmaleimide, 44% H3PO4, 4% 2,2′-dipyridyl and 3% FeCl3, then the mixture was heated in a thermostat water bath at 37 °C for 60 min. After the mixture had cooled to room temperature, the absorbance was measured at 525 nm. The activity of GSH was assayed in a mix containing 0.2 mL of the extracting solution, 150 mM (pH = 7.4) NaH2PO4 and 0.6 mM DTNB, then the mixture was heated in a thermostat water bath at 30 °C for 5 min. After the mixture had cooled to room temperature, the absorbance was measured at 412 nm. The activity of GSH + GSSG was assayed in a mix containing 0.2 mL of the extracting solution, 50 mM (pH = 7.4) PBS, 0.6 mM DTNB, 2 mM NADPH and 2 U GR, then the mixture was heated in a thermostat water bath at 25 °C for 10 min. After the mixture had cooled to room temperature, the absorbance was measured at 412 nm.
The activity of APX (EC 1.11.1.11), DHAR, GR and MDHAR was determined in leaves as described previously [36]. To measure the enzyme activity, leaves (0.3 g) were ground to a fine powder in liquid nitrogen and dissolved in 4 mL of a potassium phosphate buffer (PBS) (50 mM, pH = 7.8). To measure APX, the extraction solution was added to a mix containing 50 mM PBS (50 mM, pH = 7.8), 5 mM ASA and 20 mM H2O2, and the decrease in absorbance at 290 nm was monitored for 3 min. To measure DHAR, the extraction solution was added to a mix containing 50 mM PBS (50 mM, pH = 7.8), 0.5 mM DHA and 5 mM GSH, and the decrease in absorbance at 265 nm was monitored for 3 min. To measure GR, the extraction solution was added to a mix containing 50 mM PBS (50 mM, pH = 7.8), 2 mM NaDPH and 10 mM GSSG, and the decrease in absorbance at 340 nm was monitored for 3 min. To measure MDHAR, the extraction solution was added to a mix containing 50 mM PBS (pH = 7.8), 0.1 mM ASA and 0.55 U AAO, and the decrease in absorbance at 340 nm was monitored for 3 min.
2.4. Protein Preparation and Quantitative Proteome Analysis
In each sample, protein was extracted from the leaves using the TCA/acetone method [37]. Plant tissue (2 g) frozen by liquid nitrogen was ground into powder, then suspended in 4 mL of a cold extraction buffer (0.5 M Tris-HCl (SRL, India) pH = 7.5, 0.7 M sucrose (Himedia, India)) for protein solubilization. Samples were incubated overnight at 4 °C, after which an equal volume of phenol saturated with Tris-HCl (pH = 7.5) was added to each sample. The homogenate was centrifuged (5000× g at 4 °C for 30 min) and the clear white pellet was washed three times with ice-cold acetone (Himedia, Mumbai, India) at −20 °C, followed by centrifugation at 5000× g for 5 min. The pellet was then freeze-dried and stored at −80 °C. The protein powder was dissolved in a rehydration buffer (8 M urea (Sigma, St. Louis, MO, USA), 2% (w/v) CHAPS (Sigma, St. Louis, MO, USA), 50 mM DTT (Sigma, St. Louis, MO, USA), and 0.2% (v/v) Biolyte (Bio-Rad, Hercules, CA, USA)) for 1 h at 37 °C. After centrifugation, the protein concentration of the supernatant was determined by the Bradford assay (Bio-Rad, Hercules, CA, USA).
The 2-DE was carried out according to the methodology of Wang et al. [38]. First, the immobilized pH gradient (IPG) strips [pH 4–7, 13 cm, (GE Healthcare, Chicago, IL, USA)] were separated. Next, 1.5 mg of the protein sample was applied to each IPG strip and this was covered with 800 μL of mineral oil. The voltages used were as follows: 30 V for 13 h, 100 V for 1 h, 500 V for 1 h, 1000 V for 1 h and 3000 V for 1 h, and then 8000 V for 7 h. After focusing, the strips were equilibrated in Equilibration Buffer I (0.1 g DTT and 10 mL of a SDS balancing buffer) and in Equilibration Buffer II (0.15 g iodoacetamide (IAM) (Sigma, St. Louis, MO, USA) and 10 mL of a SDS balancing buffer). After 15 min, the strips were washed with a SDS balancing buffer. The equilibrated strips were analyzed by 12.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis. The electrophoresis was run on Electrophoreses Power Supply EPS 601 (GE Healthcare, Chicago, IL, USA) at a constant current of 2 W for 30 min and then modulated to 8 W. The gels were visualized by staining them overnight with colloidal Coomassie brilliant blue R-250. Stained gels were scanned on an Image Scanner II (GE Healthcare, Chicago, IL, USA), and the captured images were analyzed in Melanie 7.0 software (GeneBio, Geneva, Switzerland). Three independent replicated gels were analyzed and characterized. Protein spot detection was based on a fold-change of ≥2 or ≤0.5, for which a threshold of p ≤ 0.05 was used to distinguish the differentially expressed protein spots.
The protein spots were carefully excised from the gels. The gel spots were washed twice for 20 min with deionized water, then incubated and dehydrated with acetonitrile (ACN). Proteins were digested for 18 h at 37 °C in 10 μL of a trypsin solution (15 ng μL−1). Next, the supernatants were collected, and the gel spots were extracted twice with a 50 μL extraction buffer (50% ACN (Sigma, St. Louis, MO, USA) and 5% TFA) for 1 h at 37 °C. The extractions and the trypsin supernatant of the gel spots were combined and then vacuum-dried. The peptides’ mass spectra were detected via matrix-assisted laser desorption ionization time-of-flight/time-of-flight (MALDI-TOF/TOF) (ABI 4700, AB Systems, CA, USA) and the proteins were identified using the UniProt database (http://www.uniprot.org, accessed on 22 February 2020). The gene ontology of identified proteins was determined using the Blast2Go v2.3.6, with the Target P program used for their functional classification.
2.5. RNA-Seq Library Construction and Transcriptome Analysis
The RNA-Seq libraries’ preparation and their sequencing were carried out in leaves by Novogene equipment (Beijing, China). Briefly, RNA degradation and contamination were monitored on 1% agarose gels, RNA purity was checked using a NanoPhotometer® spectrophotometer (Implen, Westlake Village, CA, USA) and RNA integrity was assessed using the RNA Nano 6000 Assay Kit of the Bioanalyzer 2100 system (Agilent Technologies, Palo Alto, CA, USA), with 1 μg RNA per sample used as the input material for RNA sample preparation. The sequencing libraries were generated using the NEBNext® UltraTM RNA Library Prep Kit for Illumina® (NEB, Ipswich, MA, USA), following the manufacturer’s recommendations, with unique index codes added to attribute the sequences to each sample. The clustering of the index-coded samples was performed on a cBot Cluster Generation System, using TruSeq PE Cluster Kit v3-cBot-HS (Illumina, San Diego, CA, USA), according to the manufacturer’s instructions. After cluster generation, the libraries of the preparations were sequenced on an Illumina NovaSeq platform, from which 150-bp paired-end reads were generated. Both the reference genome and the gene model annotation files were downloaded from the genome website directly. Through use of Hisat2 v2.0.5 (Johns Hopkins University, Baltimore, MD, USA), the index of the reference genome was built, and paired-end clean reads were aligned to it. The clean data were deposited in NCBI Sequence Read Archive (SRA; https://www.ncbi.nlm.nih.gov/sra, accessed on 18 July 2021) under the accession number SRR15183944-SRR15183948.
Gene ontology (GO) enrichment analysis of the DEGs was implemented by the R package ‘clusterProfiler’, which corrected any gene length bias. GO terms with a corrected p-value of <0.05 were considered to be significantly enriched by the DEGs. The Kyoto Encyclopedia of Genes and Genomes (KEGG) is a database resource for understanding the high-level functions and utilities of biological systems (http://www.genome.jp/kegg, accessed on 22 March 2020). The clusterProfiler package in R was used to statistically test for the enrichment of DEGs in the KEGG pathways.
2.6. Quantitative Real-Time PCR (RT-PCR)
Total RNA was extracted by using the OmniPlant RNA Kit (DNase I) (Cowin Biosciences, Beijin, China). A summary of the procedure is as follows: the samples were individually milled in a mortar with liquid nitrogen and then incubated with 500 μL of RLS Buffer. The samples were centrifuged at 12,000 rpm for 2 min at 4 °C, and a half volume of absolute ethanol was added to the supernatant after centrifuging, then 52 μL RNase-Free Water, 8 μL 10 × Reaction Buffer and 20 μL DNase I was then added to the spin column after incubating at room temperature for 15 min. The samples were centrifuged at 12,000 rpm for 2 min at 4 °C, then the precipitates were washed in RNA Wash Buffer II and dried. RNA was dissolved with RNase-Free Water, then 0.5 μg of total RNA was used to synthesize the first-strand cDNA by using the ReverTra Ace® qPCR RT Master Mix with the gDNA Remover (Toyobo, Osaka, Japan). Amplifications were performed on a Roche 480 PCR System (Roche, Rotkreuz, Switzerland) with a THUNDERBIRD® qPCR Mix (Toyobo, Osaka, Japan). The primer sequences used for the real-time qPCR are listed in Table S7.
2.7. Statistical Analysis
The data were subject to analysis of variance (ANOVA) (p < 0.05). Multiple comparisons of the treatment effects were analyzed using the least significant difference (LSD). All the tests were performed using SPSS 22.0 software (Windows, USA). All figures were developed using GraphPad Prism 8.2 (GraphPad Software, CA, USA) and Adobe Illustrator CC2018 (Adobe, CA, USA).
3. Results
3.1. Drought Stress Induced Growth and Physiological Changes in C. gigantea
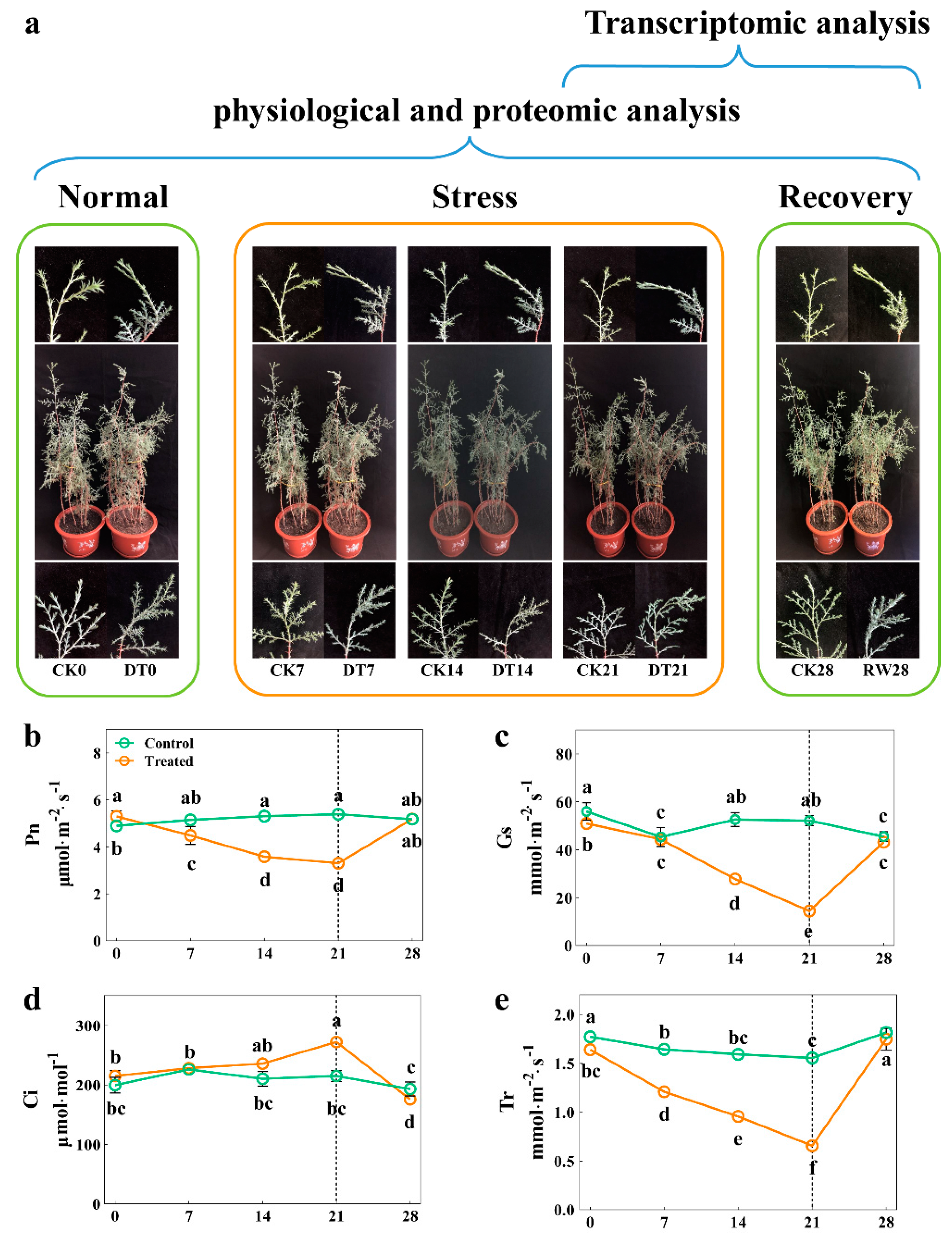
To investigate the morphological and physiological response mechanisms of C. gigantea under drought stress, 3-year-old seedlings were planted in the same environment and then subjected to a drought treatment for 3 weeks, then re-watered for 1 week (Figure 1a). At the onset of stress, no significant differences were observed in plant growth. After 21 days, seedlings in the drought treatment had leaves and stems that were badly curled and wilted when compared with those of the control group. Interestingly, the drought-stressed seedlings were able to largely restore their viability during the re-watering period. Upon closer examination, the seedlings’ Pn, Gs, and Tr significantly decreased by 38.8%, 72.3% and 57.9%, respectively, whereas Ci gradually increased by 20.9% after completing the drought treatment compared with the control (p < 0.05). Surprisingly, RW induced a rapid recovery in C. gigantea, reaching parameters similar to those recorded in the control group (Figure 1b–e), indicating that photosynthesis was inhibited by drought.
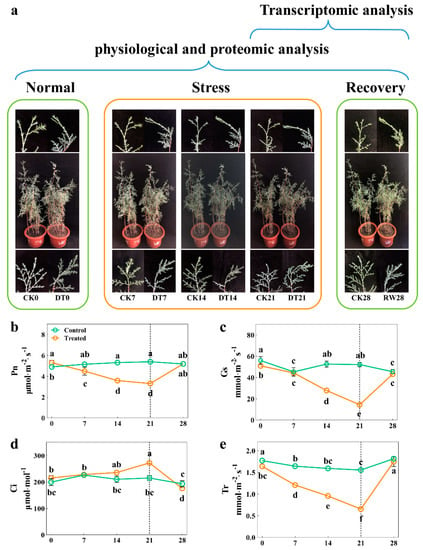
Figure 1.
The effect of drought stress on the morphological and photosynthesis traits of C. gigantea. (a) Photographs showing the phenotypes of the seedlings over time. (b–e) Line charts showing the physiological changes corresponding to Pn, Gs, Tr, Ci, respectively. Values are the means ± SD (n = 5). Columns headed by different letters denote means differing significantly from one another (p < 0.05).
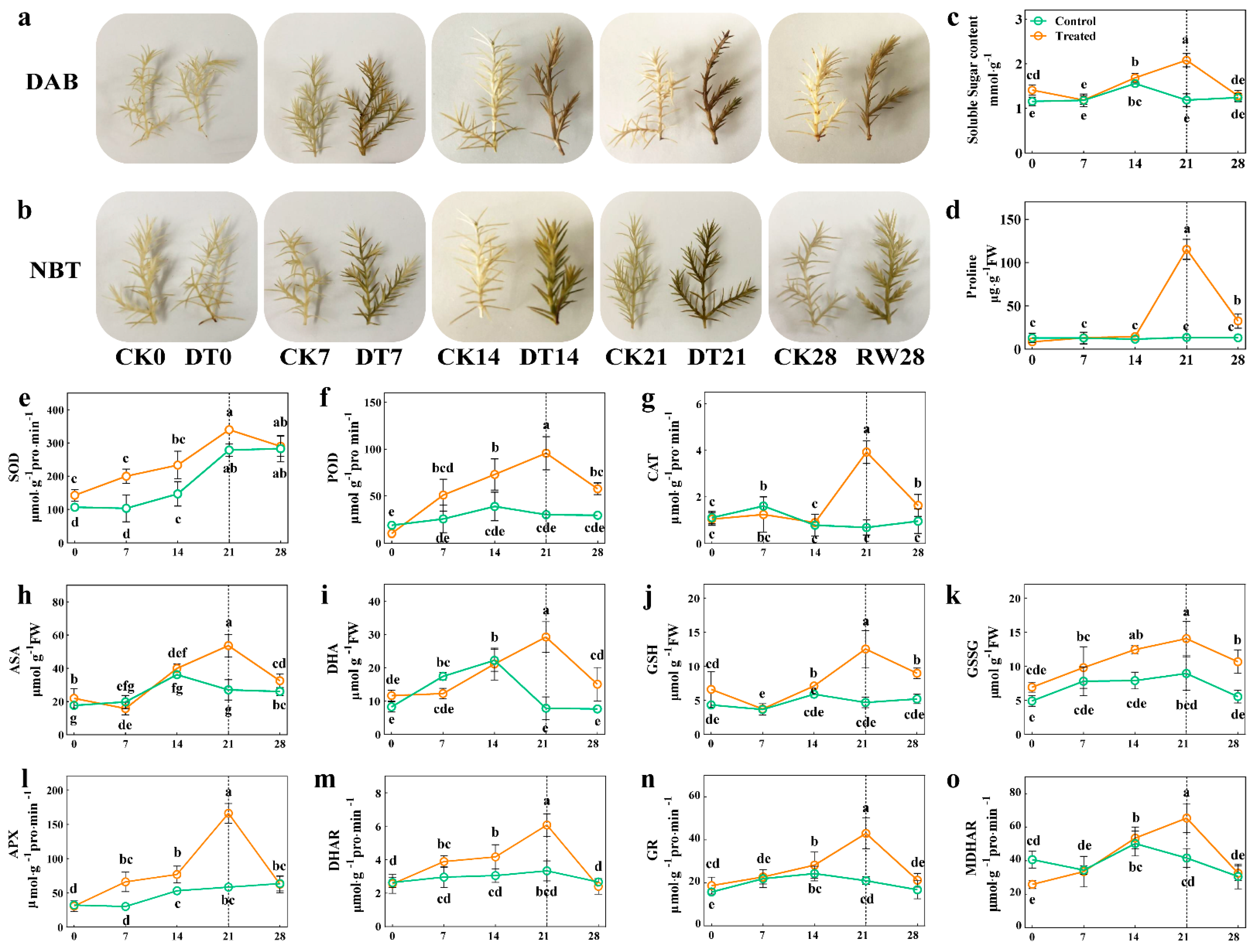
Physiological indexes such as histochemical staining, soluble sugar content, proline content and antioxidant enzymes are commonly used to evaluate the stress resistance capacity of plants in response to drought stress. Here, DAB and NBT histochemical staining analyses were conducted to measure the H2O2 and O2− contents. Under normal conditions, there was no apparent difference between the treatment and control; however, during prolonged drought stress, the DAB and NBT staining intensities of the treatment plants’ leaves were stronger than those of the control leaves (Figure 2a,b). Importantly, we found that the proline and soluble sugar contents were higher in the seedlings under the drought stress treatment than in the control (Figure 2c,d). Furthermore, sharp increases of 17.9%, 68.6% and 82.6%, respectively, were observed in SOD, POD and CAT activity (p < 0.05) after 21 days of the drought treatment (Figure 2e–g), indicating that C. gigantea promoted its ROS scavenging by modulating the activity of key antioxidant enzymes, such as SOD, POD and CAT. Similarly, non-enzymatic antioxidant contents, namely those of ascorbic acid (ASA), dehydroascorbic acid (DHA), reduced glutathione (GSH) and oxidized glutathione (GSSG), were starkly increased under the drought stress treatment relative to the control seedlings (p < 0.05, Figure 2h–k). The antioxidant enzyme activities of APX, DHAR, GR and MDHAR were further analyzed; this showed that they increased in content after the drought treatment (p < 0.05), and their accumulation was highest at 21 days of the drought treatment (p < 0.05, Figure 2i–o). Subsequent to rehydration of the seedlings’, the parameters of those in the treatment returned to similar levels to those of the control group. Overall, these results revealed that seedlings of C. gigantea enhanced their drought resistance by increasing their osmolyte content, activating the antioxidant system and maintaining ROS homeostasis.
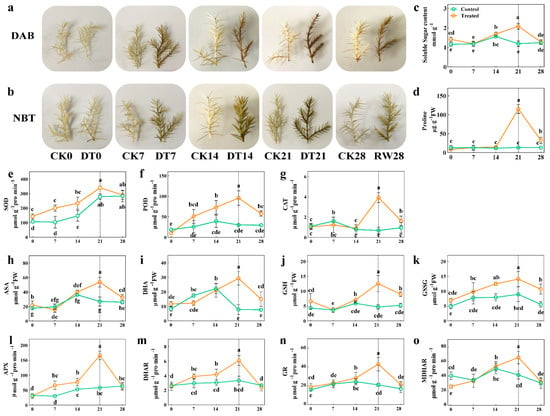
Figure 2.
The effect of drought stress on the physiological indexes of C. gigantea. (a,b) Photographs showing DAB and NBT histochemical staining of the seedlings over time. (c–o) Line charts showing the physiological changes corresponding to soluble sugar, proline, superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), ascorbic acid (ASA), dehydroascorbic acid (DHA), reduced glutathione (GSH), oxidized glutathione (GSSG), ascorbate peroxidase (APX), dehydroascorbate reductase (DHAR), glutathione reductase (GR) and monodehydroascorbate reductase (MDHAR), respectively. Values are the means ± SD (n = 3). Columns headed by different letters denote means differing significantly from one another (p < 0.05).
3.2. Characterization and Functional Distribution of the C. gigantea Leaf Proteome
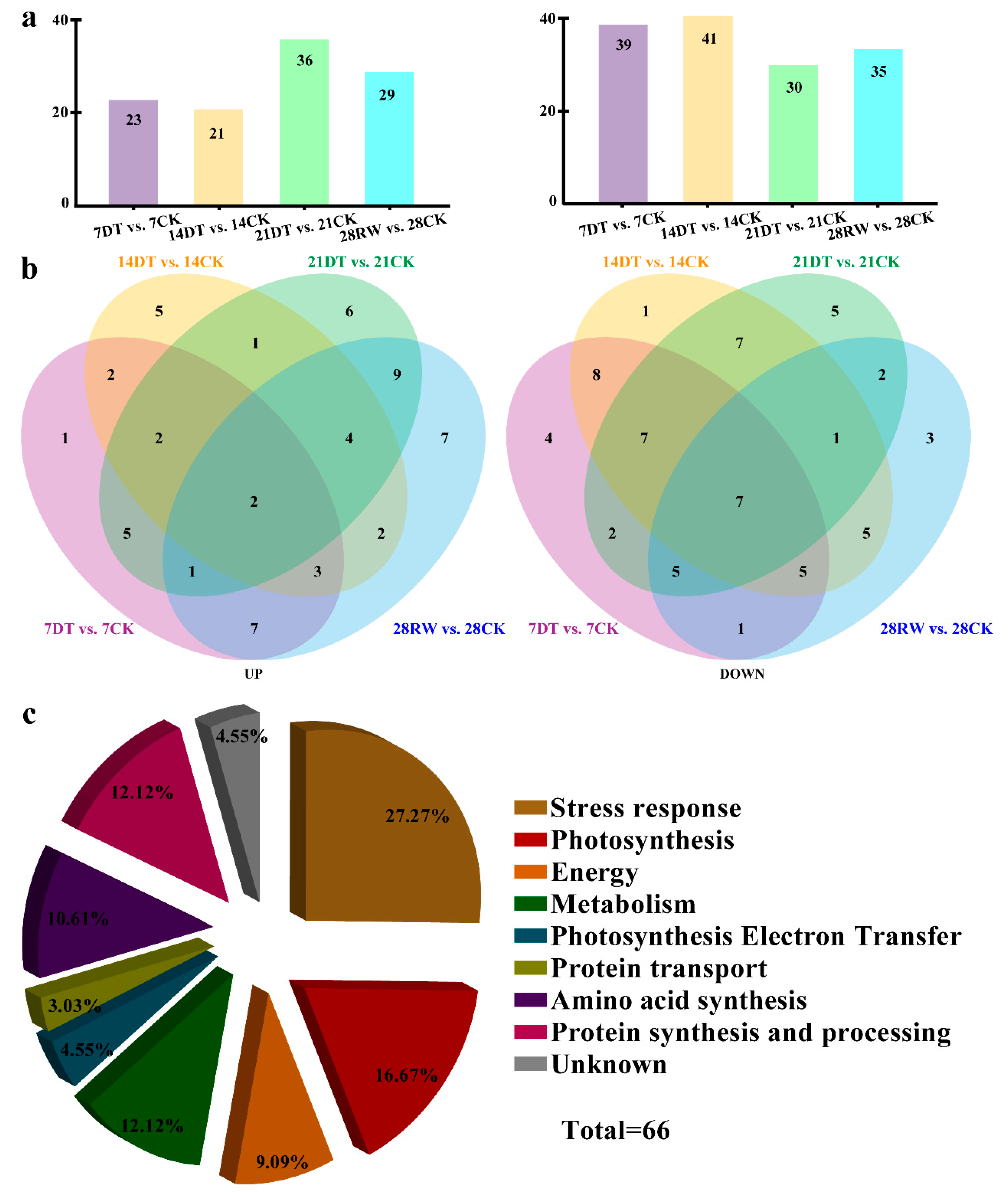
To identify the drought-induced proteins of C. gigantea, we performed a 2-DE analysis coupled with MALDI-TOF/TOF at different time points (7, 14, 21, and 28 days) in seedlings under drought stress. Total protein extracts were separated in all samples, with a pI range of 4–7 and a molecular mass range of 14.4–116 kDa. All these protein spots were localized and detected on Coomassie brilliant blue-stained gels. Accordingly, 66 protein spots changed significantly in abundance between the drought-stressed and control seedlings of C. gigantea (Figure S1). Temporally, of these, 26 (7 days), 16 (14 days), 21 (21 days) and 3 (28 days) protein spots were found to be altered by drought stress.
In total, 66 DEPs were identified in the control vs. treatment comparison: 61 (i.e., 23 upregulated and 39 downregulated) in the 7DT vs. 7CK comparison, 62 (21 upregulated and 41 downregulated) in the 14DT vs. 14CK comparison, 66 (36 upregulated and 30 downregulated) in the 21DT vs. 21CK comparison and 64 (29 upregulated and 35 downregulated) in the 28RW vs. 28CK comparison (Figure 3a,b). In addition, there were four proteins (Spots 6, 10, 11 and 17) whose expression was significantly upregulated by drought, but was upregulated/unchanged during the recovery period. Conversely, the expression of two proteins (Spots 36 and 39) was upregulated/unchanged by drought yet downregulated during the recovery phase of the seedlings (Figure S2).
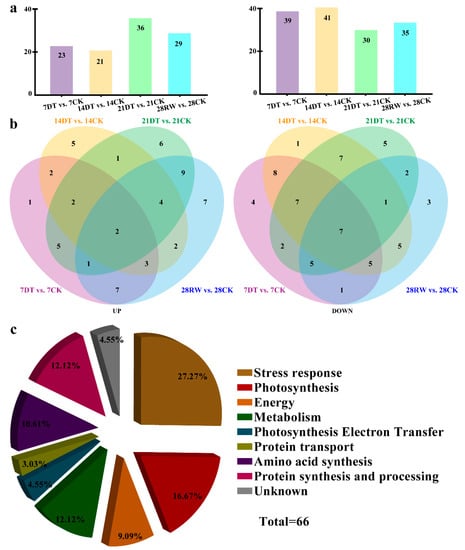
Figure 3.
Identification and functional classification analysis of the proteomics data from C. gigantea seedlings. (a) Bar graph showing the upregulated and downregulated differentially expressed proteins (DEPs) for each pairwise comparison. (b) Venn diagrams depicting the overlaps of upregulated and downregulated differentially expressed proteins (DEPs) across four comparisons. The total number of differentially expressed proteins (DEPs) is provided in parentheses. (c) Functional categorization of the differentially expressed proteins (DEPs) identified in C. gigantea in response to drought.
The experimental MW and pI values of the protein spots were then estimated and compared with the theoretical counterparts of the corresponding proteins. As Table S1 shows, most of the experimental values matched up well with the theoretical ones, indicating unambiguous identifications. According to their biological function, these identified proteins were classified into nine major groups: stress response (27.27%), photosynthesis (16.67%), energy (9.09%), metabolism (12.12%), photosynthesis electron transfer (4.55%), protein transport (3.03%), amino acid synthesis (10.61%), protein synthesis and processing (12.12%), and unknown (4.55%) under the drought stress treatment (Figure 3c).
3.3. Characterization and Functional Distribution of C. gigantea’s Leaf Transcriptome
To further investigate the regulatory network of C. gigantea’s response to drought stress, cDNA libraries were built using leaves from seedlings under the two treatments: severe stress (21 days) and re-watering (28 days), and these were sequenced on an Illumina NovaSeq platform (Illumina Inc, San Diego, CA, USA). High-quality reads of the treatment and control samples were acquired after implementing data-cleaning procedures (Table S2). The length distribution of the assembled transcripts of C. gigantea was obtained (Figure S3a). To validate the accuracy and reproducibility of our RNA-Seq data, we selected 15 DEGs for a follow-up qRT-PCR; their gene expression levels as inferred by RNA-Seq were strongly correlated with those from the qRT-PCR (R2 = 0.92; Figure S3c).
Many DEGs were identified via two pairwise comparisons between the treatment and control (i.e., 21DT vs. 21CK, and 28RW vs. 28CK). Under severe stress, 215 genes were expressed differentially in 21DT vs. 21CK, including 36 upregulated genes and 84 downregulated genes, while 442 genes were expressed differentially under re-watering in 28R vs. 28CK, including 250 upregulated and 97 downregulated genes (p < 0.05, fold-change ≥ 2). Another 30 upregulated and 65 downregulated genes overlapped in the pairwise comparisons between 21DT and 21CK, and between 28RW and 28CK (Table S3, Figure S3b).
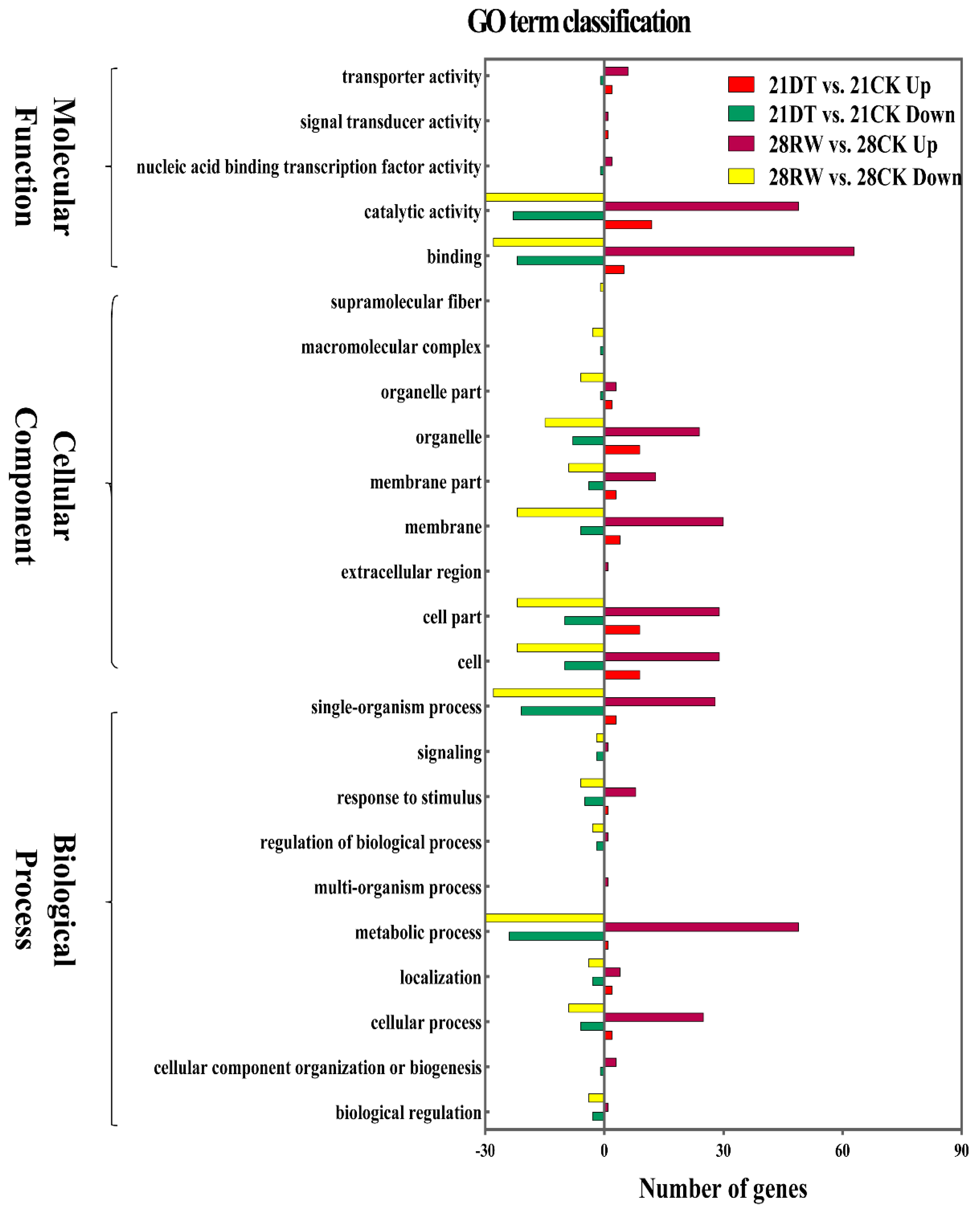
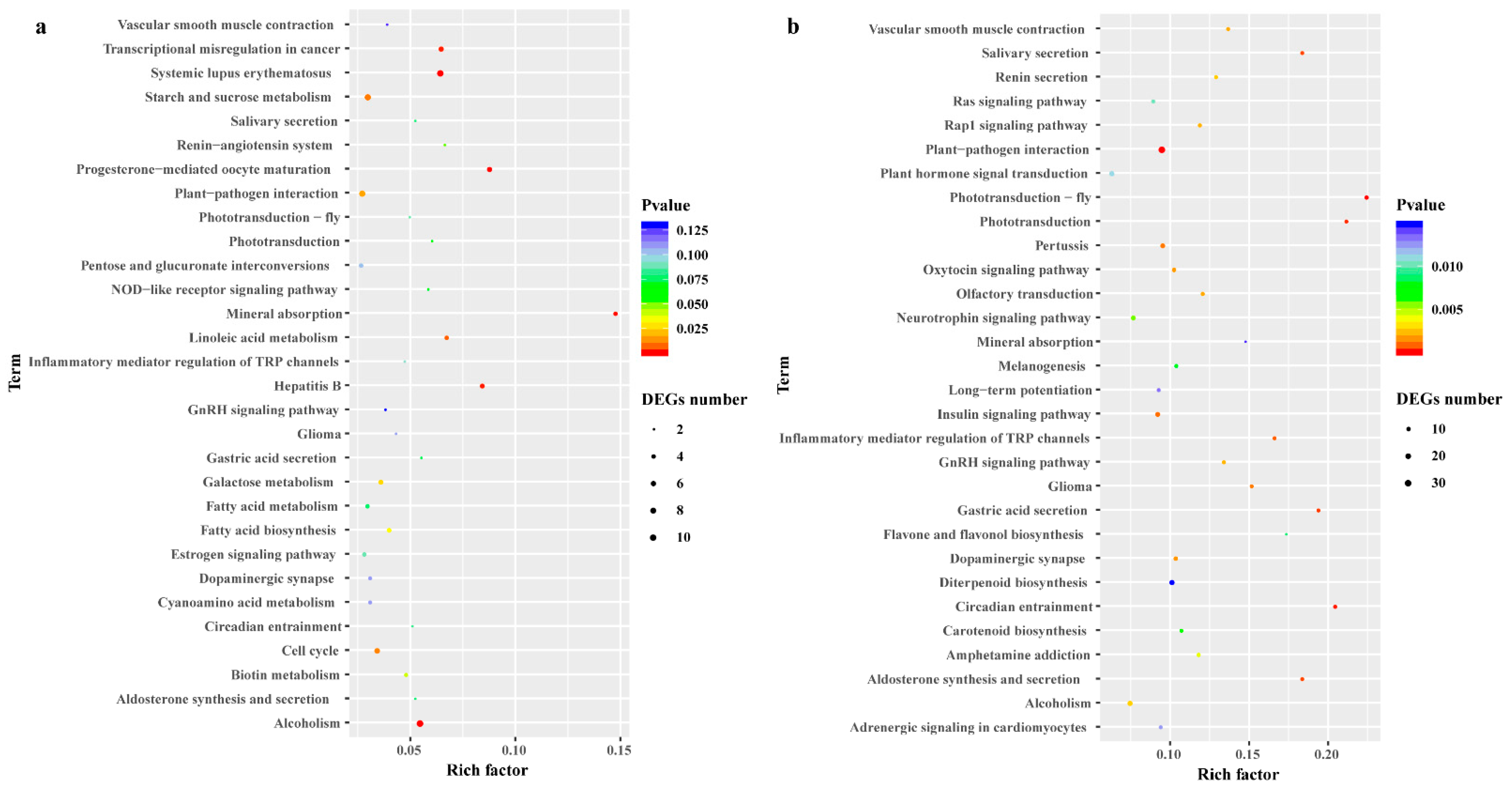
To illustrate the biological functions and pathways of the DEGs, GO annotation (at p < 0.05) was performed to analyze the drought-responsive genes in C. gigantea (Table S4), and 24 enriched GO terms were subsequently selected (Table S4, Figure 4). According to the GO classification graphic, “GO:0050896: response to stimulus”, “GO:0016020: membrane” and “GO:0003824: catalytic activity” were highly enriched during both severe drought stress and re-watering of the seedlings, suggesting that the DEGs identified are likely involved in the modulation of ROS scavenging. All DEGs were further examined for KEGG pathway enrichment (Table S5, Figure 5). The top 30 enriched KEGG pathways of DEGs between the 21DT and 21CK, and between the 28RW and 28CK groups are shown in Figure 5a,b. The pathway of phototransduction, starch and sucrose metabolism, as well as plant hormone signal transduction, were also significantly enriched for DEGs between 21DT and 21CK, and between 28RW and 28CK. Accordingly, we performed a careful manual annotation of our list of DEGs to identify candidate genes that were directly involved in ROS scavenging and photosynthesis. Based on a combination of the GO and KEGG pathway results, we identified 12 such candidate DEGs (Table S6). According to our manual annotation, eight DEGs could be assigned generically to ROS scavenging, with another four DEGs assigned to photosynthesis; this suggested that these 12 candidate DEGs are probably key regulatory components of the drought stress response in C. gigantea.
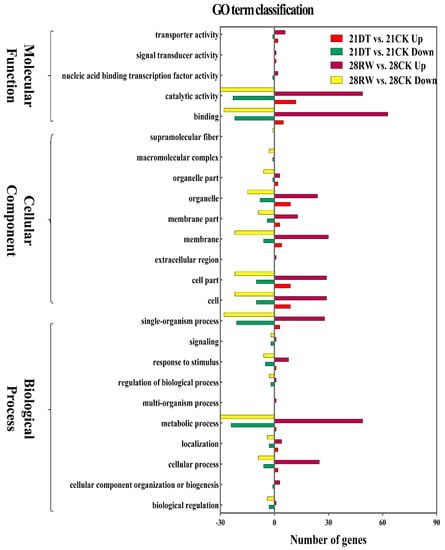
Figure 4.
Differentially expressed genes (DEGs) identified by Gene Ontology (GO) classification in C. gigantea.
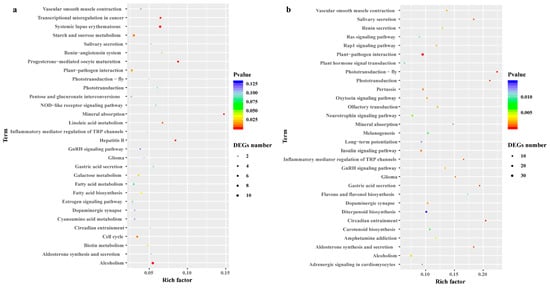
Figure 5.
Differentially expressed genes (DEGs) identified by Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis in C. gigantea. (a) Top 30 KEGG enrichment results in 21DT vs. 21CK. (b) Top 30 KEGG enrichment results in 28RW vs. 28CK.
4. Discussion
Much accumulated empirical evidence has indicated that drought can alter cell membrane fluidity, disrupt protein complexes and inhibit photosynthesis. Therefore, a comprehensive study that combined physiological measurements, RNA-Seq and 2-DE analysis was conducted here to screen out some important candidate drought-induced changes in key physiological parameters, DEGs and DEPs. Our results revealed that the transcriptional regulation of proteins related to photosynthesis and the post-transcriptional regulation of genes involved in ROS scavenging might be the reasons for drought resistance in C. gigantea.
As it is an important secondary messenger, the rapid accumulation of ROS leads to rapid stomatal closure to limit photosynthesis under conditions of water deficit [39]. A recent study found that the Pn, Gs and Tr of rubber trees decreased significantly under dehydration conditions [40]. In our study, similar results were detected in the drought-stressed seedlings (Figure 1b–e). Additionally, how a plant responds to stress not only entails changed photosynthesis but also the accumulation of osmolytes, such as soluble sugars and proline, which reflect the degree of injury [41]. We found that the content of soluble sugars and proline both enlarged as the drought stress continued (Figure 2c,d). Similar findings were reported recently for Scutellaria baicalensis Georgi [42].
In the present study, histochemical staining by both DAB and NBT together revealed a pronounced drought-induced accumulation of H2O2 and O2·− in C. gigantea leaves (Figure 2a,b). This result is consistent with earlier findings that drought promotes the generation of ROS [43]. Moreover, we demonstrated that the drought-stressed seedlings showed higher SOD, POD and CAT activity compared with the control group under drought stress (Figure 2e–g). This is consistent with another analysis of antioxidant enzymes in plants under drought conditions [44]. Similarly, drought stress significantly enhanced the contents of AsA, DHA, GSH and GSSG during drought, and these antioxidant molecules peaked under severe drought conditions (Figure 2h–o), which also is in line with prior research [45]. Compared with the control group, the characteristic withered and curved growth phenotype of the treatment seedlings suggested that C. gigantea likely relies on limited photosynthesis, the accumulation of osmolytes and ROS scavenging strategy to adapt to drought conditions.
Underlying many of these changes is the differential expression of genes in response to drought stress. Thus, both RNA-Seq and 2-DE were used to further explore the critical genes and pathways to obtain a better understanding of the molecular mechanisms underlying the response to drought. Here, we successfully quantified and compared 551 DEGs and 66 DEPs in the leaves of C. gigantea seedlings under drought stress. However, only a few genes were commonly regulated at the transcriptomic and proteomic levels, although this is consistent with a previous study [46], whose data indicated that post-transcriptional regulation plays a crucial role in the drought response of cassava plants. In the case of C. gigantea, the categories of overlapping DEGs and DEPs were most related to photosynthesis and ROS scavenging, suggesting their involvement in the drought response of this tree species.
Photosynthesis the most fundamental and intricate physiological process in plants, and it is highly susceptible to drought [47]. Consistent with previous studies of photosynthesis-related genes’ responses to drought, we found that genes involved in the Cyt b6/f complex and ATPase synthase were all downregulated after seedlings experienced the drought treatment [48]. Similarly, we found that many DEPs associated with PSII, LHC and ATP synthase were also downregulated, as inferred by the proteomic analysis (Figure S2). Similar responses in maize plants were reported recently [49]. Taken together, our results all implied that modulating PET is probably crucial to increasing the tolerance of C. gigantea to drought.
The ROS scavenging system, an important defense mechanism against drought, maintains the cellular homeostasis of ROS. Overproduction of ROS during drought not only affects the stress-related proteins but also modulates the transcription level of genes [50]. In the current work, four of these enzymes, including GST, SOD, APX and POD, were identified. Among these genes, the SOD gene was downregulated in C. gigantea seedlings under drought stress. A significant reduction in the expression of the APX gene was obtained under drought stress conditions. Notably, our proteomics data showed that the abundance of APX, SOD [Cu-Zn], ASR and GST was also enhanced in seedlings of C. gigantea to cope with the imposed drought stress. These results are consistent with the observed activities of antioxidant enzymes. These proteins could also work together to maintain the dynamic balance of ROS levels and thereby avoid the oxidative damage incurred by plants. This type of coordination occurs in alkaligrass [51], rapeseed [52] and cassava [53], perhaps due to the fact that enzyme activity is regulated by post-translational modifications [54]. Finally, POD can also contribute to how plants respond to drought stress [5]. Nevertheless, in this study, drought stress led to downregulation of POD at both the protein and gene levels, but, interestingly, the activity of POD was significantly increased by drought stress. Evidently, to explain the changes in enzyme activity and the expression of genes and proteins of C. gigantea under drought stress more precisely, further in-depth research investigations and analyses are still needed.
5. Conclusions
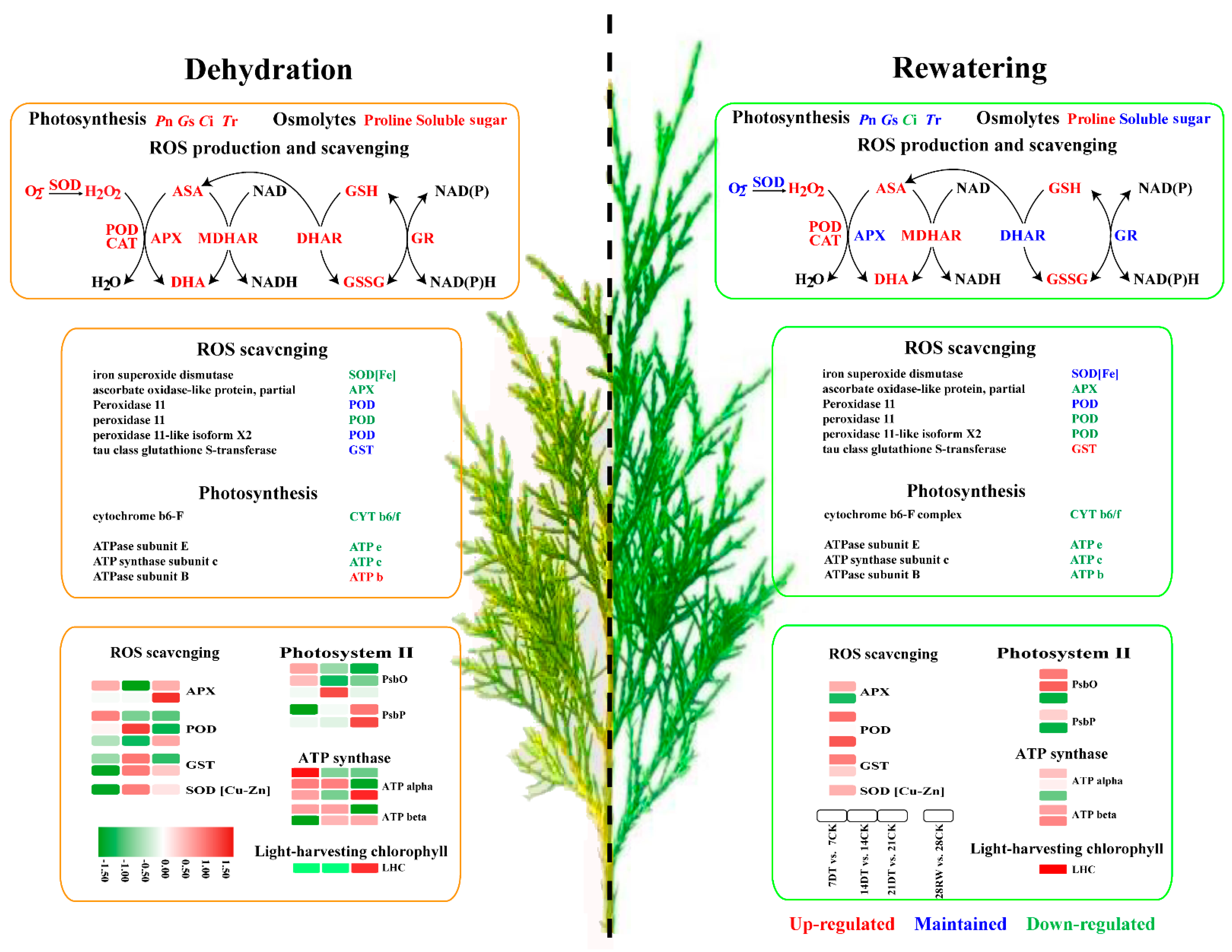
The main aim of this study was to expand our knowledge about the mechanism underlying the impact of drought stress in seedlings of C. gigantea. An attempt was made to identify the critical genes and pathways by comparing the findings from transcriptomic and proteomic analytical methods. On the basis of results, here, we propose a schematic diagram for the regulation of drought resistance in C. gigantea (Figure 6). When plants are subjected to drought, ROS induces oxidative stress and activates the ROS scavenging system. Simultaneously, oxidative stress impairs photosynthesis and inhibits PET, ultimately inhibiting plant growth. Taken together, the omics data suggest that post-transcriptional regulation of PET and ROS scavenging plays a crucial role in the drought response of C. gigantea seedlings.
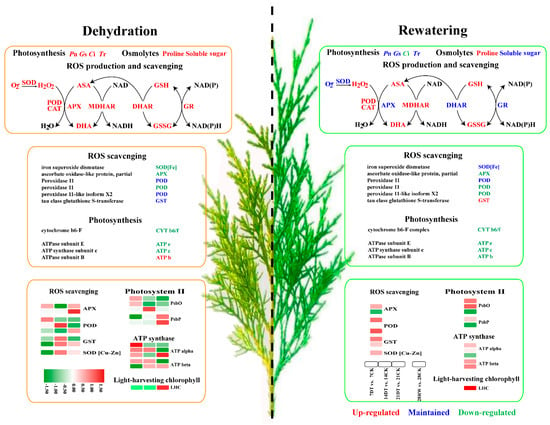
Figure 6.
Schematic diagram of drought-induced changes in the physiological parameters, genes and proteins in C. gigantea during stress and recovery.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/f13030401/s1. Figure S1: 2-DE gel images of the control and treatment seedlings under drought extracted at different time points (7 d, 14 d, 21 d and recovery). The squares represent the drought-responsive proteins identified by MS. Figure S2: Expression of DEPs in the control and drought treatment at different time points (7 d, 14 d, 21 d and recovery). Figure S3: Characteristics of C. gigantea unigenes. (a) Length frequency distribution of all unigenes. (b) Venn diagram showing the number of DEGs. (c) Correlation analysis between the transcriptome results and the qRT-PCR results, and validation of the RNA-Seq results by qRT-PCR. Table S1: The corresponding induction factor (percent volume of the spot under stress conditions/percent volume of the spots under control conditions) of drought-responsive proteins of seedlings. Table S2: Summary of unigenes identified in C. gigantea. Table S3: Summary of DEGs identified in C. gigantea. FDR ≤ 0.001 log2 fold change ≤ −2 or ≥2. Table S4: List of GO enrichment terms of DEGs in C. gigantea during drought stress. Table S5: KEGG pathway enrichment analysis of DEGs in C. gigantea during drought stress. Table S6: DEGs and DEPs involved in ROS scavenging and photosynthesis in C. giganteas during drought stress. Table S7: Primers of the genes used for qRT-PCR and the expression correlation between the RNA-seq and qRT-PCR analyses.
Author Contributions
P.L., Z.L., L.T. and F.M., conceived and designed the experiments; P.L., Z.L. and J.L. performed the experiments; P.L., Z.L. and L.X. analyzed or interpreted the data for the work; P.L., X.Z., L.T. and F.M. wrote the manuscript; P.L., X.J., L.X., G.J., X.Z. and F.M. ensure that questions related to the accuracy or integrity of any part of the work. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by the Fundamental Research Funds for the Central Universities (2572021AW15).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Sequencing data have been deposited at the Short Read Archive (SRA) database (https://www.ncbi.nlm.nih.gov/sra, accessed on 18 July 2021) under accession numbers SAMN20236055 to SAMN20236058 (Bio-Project PRJNA746687). The proteome data can be found at: https://github.com/lppaper/datebase, accessed on 18 February 2022.
Acknowledgments
We thank the reviewers and editors for their work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [Green Version]
- Kaya, C.; Senbayram, M.; Akram, N.A.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Sulfur-enriched leonardite and humic acid soil amendments enhance tolerance to drought and phosphorus deficiency stress in maize (Zea mays L.). Sci. Rep. 2020, 10, 6432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Li, Y.; Yin, Z.; Jiang, J.; Zhang, M.; Guo, X.; Ye, Z.; Zhao, Y.; Xiong, H.; Zhang, Z.; et al. OsASR5 enhances drought tolerance through a stomatal closure pathway associated with ABA and H2O2 signalling in rice. Plant Biotechnol. J. 2017, 15, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, J.; Liu, H.; Liu, N.; Shen, G.; Zhuang, H.; Wu, J. Dodder-transmitted mobile signals prime host plants for enhanced salt tolerance. J. Exp. Bot. 2020, 71, 1171–1184. [Google Scholar] [CrossRef] [PubMed]
- Talbi, S.; Antonio Rojas, J.; Sahrawy, M.; Rodriguez-Serrano, M.; Cardenas, K.E.; Debouba, M.; Maria Sandalio, L. Effect of drought on growth, photosynthesis and total antioxidant capacity of the saharan plant Oudeneya africana. Environ. Exp. Bot. 2020, 176, 104099. [Google Scholar] [CrossRef]
- Jin, H.L.; Fu, M.; Duan, Z.K.; Duan, S.J.; Li, M.S.; Dong, X.X.; Liu, B.; Feng, D.R.; Wang, J.F.; Peng, L.W.; et al. LOW PHOTOSYNTHETIC EFFICIENCY 1 is required for light-regulated photosystem II biogenesis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2018, 115, E6075–E6084. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.P.; Su, X.D.; Cao, P.; Liu, X.Y.; Chang, W.R.; Li, M.; Zhang, X.Z.; Liu, Z.F. Structure of spinach photosystem II-LHCII supercomplex at 3.2 angstrom resolution. Nature 2016, 534, 69–74. [Google Scholar] [CrossRef]
- Xiao, J.; Li, J.; Ouyang, M.; Yun, T.; He, B.; Ji, D.; Ma, J.; Chi, W.; Lu, C.; Zhang, L. DAC Is Involved in the Accumulation of the Cytochrome b(6)/f Complex in Arabidopsis. Plant Physiol. 2012, 160, 1911–1922. [Google Scholar] [CrossRef] [Green Version]
- Estravis-Barcala, M.; Gabriela Mattera, M.; Soliani, C.; Bellora, N.; Opgenoorth, L.; Heer, K.; Veronica Arana, M. Molecular bases of responses to abiotic stress in trees. J. Exp. Bot. 2020, 71, 3765–3779. [Google Scholar] [CrossRef]
- Cui, X.-Y.; Gao, Y.; Guo, J.; Yu, T.-F.; Zheng, W.-J.; Liu, Y.-W.; Chen, J.; Xu, Z.-S.; Ma, Y.-Z. BES/BZR Transcription Factor TaBZR2 Positively Regulates Drought Responses by Activation of TaGST1. Plant Physiol. 2019, 180, 605–620. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Yang, T.; Lin, Z.; Gu, B.; Xing, C.; Zhao, L.; Dong, H.; Gao, J.; Xie, Z.; Zhang, S.; et al. A WRKY transcription factor PbrWRKY53 from Pyrus betulaefolia is involved in drought tolerance and AsA accumulation. Plant Biotechnol. J. 2019, 17, 1770–1787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, C.; Zhu, Y.; Cheng, H.; Yan, H.; Zhao, L.; Tang, J.; Ma, X.; Mao, P. Nitric Oxide Regulates Seedling Growth and Mitochondrial Responses in Aged Oat Seeds. Int. J. Mol. Sci. 2018, 19, 1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaya, C.; Ashraf, M.; Wijaya, L.; Ahmad, P. The putative role of endogenous nitric oxide in brassinosteroid-induced antioxidant defence system in pepper (Capsicum annuum L.) plants under water stress. Plant Physiol. Biochem. 2019, 143, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Ricroch, A.E.; Berge, J.B.; Kuntz, M. Evaluation of Genetically Engineered Crops Using Transcriptomic, Proteomic, and Metabolomic Profiling Techniques. Plant Physiol. 2011, 155, 1752–1761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osorio, S.; Alba, R.; Damasceno, C.M.; Lopez-Casado, G.; Lohse, M.; Zanor, M.I.; Tohge, T.; Usadel, B.; Rose, J.K.; Fei, Z.; et al. Systems biology of tomato fruit development: Combined transcript, protein, and metabolite analysis of tomato transcription factor (nor, rin) and ethylene receptor (Nr) mutants reveals novel regulatory interactions. Plant Physiol. 2011, 157, 405–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, A.; Li, F.; Liu, A. Comparative proteomic and transcriptomic analyses provide new insight into the formation of seed size in castor bean. BMC Plant Biol. 2020, 20, 48. [Google Scholar] [CrossRef] [Green Version]
- Peremarti, A.; Mare, C.; Aprile, A.; Roncaglia, E.; Cattivelli, L.; Villegas, D.; Royo, C. Transcriptomic and proteomic analyses of a pale-green durum wheat mutant shows variations in photosystem components and metabolic deficiencies under drought stress. BMC Genom. 2014, 15, 125. [Google Scholar] [CrossRef]
- Casati, P.; Campi, M.; Morrow, D.J.; Fernandes, J.F.; Walbot, V. Transcriptomic, proteomic and metabolomic analysis of UV-B signaling in maize. BMC Genom. 2011, 12, 321. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Zhao, J.; He, X.; Sun, H.; Zhang, G.; Wu, F. Comparative proteomic analysis of drought tolerance in the two contrasting Tibetan wild genotypes and cultivated genotype. BMC Genom. 2015, 16, 432. [Google Scholar] [CrossRef] [Green Version]
- Koobaz, P.; Ghaffari, M.R.; Heidari, M.; Mirzaei, M.; Ghanati, F.; Amirkhani, A.; Mortazavi, S.E.; Moradi, F.; Hajirezaei, M.R.; Salekdeh, G.H. Proteomic and metabolomic analysis of desiccation tolerance in wheat young seedlings. Plant Physiol. Biochem. 2020, 146, 349–362. [Google Scholar] [CrossRef]
- Anupama, A.; Bhugra, S.; Lall, B.; Chaudhury, S.; Chugh, A. Morphological, transcriptomic and proteomic responses of contrasting rice genotypes towards drought stress. Environ. Exp. Bot. 2019, 166, 103795. [Google Scholar] [CrossRef]
- Tripathi, P.; Rabara, R.C.; Reese, R.N.; Miller, M.A.; Rohila, J.S.; Subramanian, S.; Shen, Q.J.; Morandi, D.; Buecking, H.; Shulaev, V.; et al. A toolbox of genes, proteins, metabolites and promoters for improving drought tolerance in soybean includes the metabolite coumestrol and stomatal development genes. BMC Genom. 2016, 17, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.; Shi, D.; Wang, J.; Xu, T.; Wu, Y. Isolation and characterization of polymorphic microsatellite markers in Cupressus chenggiana S. Y. Hu (Cupressaceae). Conserv. Genet. 2008, 9, 1023–1026. [Google Scholar] [CrossRef]
- Li, S.; Qian, Z.; Fu, Y.; Zheng, W.; Li, H. Isolation and characterization of polymorphic microsatellites in the Tibetan cypress Cupressus gigantea using paired-end Illumina shotgun sequencing. Conserv. Genet. Resour. 2014, 6, 795–797. [Google Scholar] [CrossRef]
- Lu, X.; Xu, H.; Li, Z.; Shang, H.; Adams, R.P.; Mao, K. Genetic Diversity and Conservation Implications of Four Cupressus Species in China as Revealed by Microsatellite Markers. Biochem. Genet. 2014, 52, 181–202. [Google Scholar] [CrossRef]
- Yin, Z.; Wang, Y.; Wang, Y.; Sang, L.; Luo, Q.; Meng, F. Improvement on the method of the extraction of total proteins on Cupressus gigantea leaves for two-dimensional gel electrophoresis. J. Cent. South Univ. For. Technol. 2019, 39, 143–146. [Google Scholar]
- Xin, F.; Wang, Y.; Li, S.; Danzengluobu; Pubuciren. Effects of different temperatures on photosynthesis and rooting of Cupressus gigantea seedlings. J. Zhejiang Univ. Agric. Life Sci. 2019, 45, 102–108. [Google Scholar]
- Li, H.; Guo, Q.; Zheng, W. The complete chloroplast genome of Cupressus gigantea, an endemic conifer species to Qinghai-Tibetan Plateau. Mitochondrial DNA Part A 2016, 27, 3743–3744. [Google Scholar] [CrossRef]
- Zhou, S.-S.; Xing, Z.; Liu, H.; Hu, X.-G.; Gao, Q.; Xu, J.; Jiao, S.-Q.; Jia, K.-H.; Jin, Y.Q.; Zhao, W.; et al. In-depth transcriptome characterization uncovers distinct gene family expansions for Cupressus gigantea important to this long-lived species’ adaptability to environmental cues. BMC Genom. 2019, 20, 213. [Google Scholar] [CrossRef]
- Chen, K.Q.; Song, M.R.; Guo, Y.N.; Liu, L.F.; Xue, H.; Dai, H.Y.; Zhang, Z.H. MdMYB46 could enhance salt and osmotic stress tolerance in apple by directly activating stress-responsive signals. Plant Biotechnol. J. 2019, 17, 2341–2355. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.H.; Zhu, L.; Song, A.P.; Wang, H.B.; Chen, S.M.; Jiang, J.F.; Chen, F.D. Chrysanthemum (Chrysanthemum morifolium) CmICE2 conferred freezing tolerance in Arabidopsis. Plant Physiol. Biochem. 2020, 146, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xu, F.; Liu, J.; Guan, F.; Quan, H.; Meng, F. The adaptation strategies of Herpetospermum pedunculosum (Ser.) Baill at altitude gradient of the Tibetan plateau by physiological and metabolomic methods. BMC Genom. 2019, 20, 451. [Google Scholar] [CrossRef] [PubMed]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Kosar, F.; Akram, N.A.; Ashraf, M.; Ahmad, A.; Alyemeni, M.N.; Ahmad, P. Impact of exogenously applied trehalose on leaf biochemistry, achene yield and oil composition of sunflower under drought stress. Physiol. Plant 2020, 172, 317–333. [Google Scholar] [CrossRef]
- Sun, C.; Liu, L.; Yu, Y.; Liu, W.; Lu, L.; Jin, C.; Lin, X. Nitric oxide alleviates aluminum-induced oxidative damage through regulating the ascorbate-glutathione cycle in roots of wheat. J. Integr. Plant Biol. 2015, 57, 550–561. [Google Scholar] [CrossRef]
- Raja, V.; Qadir, S.U.; Alyemeni, M.N.; Ahmad, P. Impact of drought and heat stress individually and in combination on physio-biochemical parameters, antioxidant responses, and gene expression in Solanum lycopersicum. 3 Biotech 2020, 10, 208. [Google Scholar] [CrossRef]
- Cao, Y.; Luo, Q.; Tian, Y.; Meng, F. Physiological and proteomic analyses of the drought stress response in Amygdalus Mira (Koehne) Yu et Lu roots. BMC Plant Biol. 2017, 17, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.M.; Wang, M.Y.; Liu, L.; Meng, F.J. Physiological and Proteomic Responses of Diploid and Tetraploid Black Locust (Robinia pseudoacacia L.) Subjected to Salt Stress. Int. J. Mol. Sci. 2013, 14, 20299–20325. [Google Scholar] [CrossRef] [Green Version]
- Jan, S.; Abbas, N.; Ashraf, M.; Ahmad, P. Roles of potential plant hormones and transcription factors in controlling leaf senescence and drought tolerance. Protoplasma 2019, 256, 313–329. [Google Scholar] [CrossRef]
- Santos, J.O.d.; Oliveira, L.E.M.d.; Souza, T.d.; Lopes, G.M.; Coelho, V.T.; Gomes, M.P. Physiological mechanisms responsible for tolerance to, and recuperation from, drought conditions in four different rubber clones. Ind. Crop. Prod. 2019, 141, 111714. [Google Scholar] [CrossRef]
- Mohasseli, V.; Sadeghi, S. Exogenously applied sodium nitroprusside improves physiological attributes and essential oil yield of two drought susceptible and resistant specie of Thymus under reduced irrigation. Ind. Crop. Prod. 2019, 130, 130–136. [Google Scholar] [CrossRef]
- Cheng, L.; Han, M.; Yang, L.M.; Yang, L.; Sun, Z.; Zhang, T. Changes in the physiological characteristics and baicalin biosynthesis metabolism of Scutellaria baicalensis Georgi under drought stress. Ind. Crop. Prod. 2018, 122, 473–482. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Rahman, A.; Inafuku, M.; Oku, H.; Fujita, M. Exogenous nitric oxide donor and arginine provide protection against short-term drought stress in wheat seedlings. Physiol. Mol. Biol. Plants 2018, 24, 993–1004. [Google Scholar] [CrossRef]
- Li, T.; Wang, R.; Zhao, D.; Tao, J. Effects of drought stress on physiological responses and gene expression changes in herbaceous peony (Paeonia lactiflora Pall.). Plant Signal. Behav. 2020, 15, 1746034. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Zhou, Y.; Liu, M. Nitric oxide participates in the regulation of the ascorbate-glutathione cycle by exogenous jasmonic acid in the leaves of wheat seedlings under drought stress. Protoplasma 2015, 252, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Lan, P.; Li, W.; Schmidt, W. Complementary proteome and transcriptome profiling in phosphate-deficient Arabidopsis roots reveals multiple levels of gene regulation. Mol. Cell. Proteom. 2012, 11, 1156–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Begum, N.; Ahanger, M.A.; Su, Y.; Lei, Y.; Mustafa, N.S.A.; Ahmad, P.; Zhang, L. Improved Drought Tolerance by AMF Inoculation in Maize (Zea mays) Involves Physiological and Biochemical Implications. Plants 2019, 8, 579. [Google Scholar] [CrossRef] [Green Version]
- Meyer, E.; Aspinwall, M.J.; Lowry, D.B.; Palacio-Mejia, J.D.; Logan, T.L.; Fay, P.A.; Juenger, T.E. Integrating transcriptional, metabolomic, and physiological responses to drought stress and recovery in switchgrass (Panicum virgatum L.). BMC Genom. 2014, 15, 527. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Liu, C.; Zhang, D.; He, C.; Zhang, J.; Li, Z. Effects of maize organ-specific drought stress response on yields from transcriptome analysis. BMC Plant Biol. 2019, 19, 335. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Feng, Y.; Zong, Y.; Zhang, D.; Hao, X.; Li, P. Elevated CO2-induced changes in photosynthesis, antioxidant enzymes and signal transduction enzyme of soybean under drought stress. Plant Physiol. Biochem. PPB 2020, 154, 105–114. [Google Scholar] [CrossRef]
- Nemati, M.; Piro, A.; Norouzi, M.; Vahed, M.M.; Nistico, D.M.; Mazzuca, S. Comparative physiological and leaf proteomic analyses revealed the tolerant and sensitive traits to drought stress in two wheat parental lines and their F6 progenies. Environ. Exp. Bot. 2019, 158, 223–237. [Google Scholar] [CrossRef]
- Mohammadi, P.P.; Moieni, A.; Komatsu, S. Comparative proteome analysis of drought-sensitive and drought-tolerant rapeseed roots and their hybrid F1 line under drought stress. Amino Acids 2012, 43, 2137–2152. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Fu, L.; Tie, W.; Yan, Y.; Wu, C.; Hu, W.; Zhang, J. Extensive Post-Transcriptional Regulation Revealed by Transcriptomic and Proteomic Integrative Analysis in Cassava under Drought. J. Agric. Food Chem. 2019, 67, 3521–3534. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhao, Q.; Jin, Y.; Yu, J.; Yin, Z.; Chen, S.; Dai, S. Chilling-responsive mechanisms in halophyte Puccinellia tenuiflora seedlings revealed from proteomics analysis. J. Proteom. 2016, 143, 365–381. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).