Abstract
Fraxinus chinensis subsp. rhynchophylla (Oleaceae), hereafter F. rhynchophylla, is an important timber species in northeast China; however, little is known about its seed dormancy and germination, which hinders regeneration of the species from seeds for reforestation and conservation. Our aim was to determine the class of seed dormancy and how to break it. Studies were conducted to determine the permeability of the seed coat to water, changes in embryo development during cold stratification and effects of cold stratification on germination over a range of temperatures. The seeds were water-permeable, and the embryo was fully developed and filled the embryonic cavity. Cold stratification at 5 °C for 8 weeks was effective in breaking dormancy; thus, we conclude that the seeds have nondeep physiological dormancy (PD). As cold stratification time was increased, the ability of seeds to germinate at low temperatures (e.g., 10 °C and 15 °C) increased, indicating the presence of Type 2 nondeep PD, in which the minimum temperature for germination decreases during dormancy-break. Nondormant seeds germinated to high percentages and rates at constant temperatures of 25 °C (germination percentage was 63%) and at alternating temperature regimes of 35 °C/25 °C, 30 °C/15 °C, 25 °C/20 °C and 20 °C/10 °C (germination percentage was 66%, 67%, 65% and 66%, respectively). To produce seedlings, we recommend 8 weeks of cold stratification at 5 °C before sowing the seeds at temperatures ranging from 15 °C to 30 °C.
1. Introduction
Within the broad context of regeneration of forest trees from seeds, there is a great need to determine the dormancy-breaking and germination requirements of seeds, thereby ensuring that high percentages of the collected seeds will produce normal, healthy seedlings [1]. Seed dormancy refers to the failure of viable seeds to germinate under suitable environmental conditions due to internal reasons, while seed germination refers to a series of orderly physiological and morphogenetic processes starting from imbibition [2]. In working with seeds for which little or no information is available, the first step is to determine if the freshly-matured seeds are dormant or nondormant, and if dormant what class of dormancy do they have [3]. The five classes of dormancy are morphological dormancy (MD), morphophysiological dormancy (MPD), physiological dormancy (PD), physical dormancy (PY) and combinational dormancy (PY + PD) [4,5]. In the temperate zone of the world, most forest trees produce seeds that have PD [6,7,8], in which the seed coat is water permeable, but the fully developed embryo has a physiological inhibiting mechanism of germination [2,3,4].
The types of PD are deep PD, intermediate PD and nondeep PD [2,5,8]. GA3 can promote germination of seeds with nondeep PD, may or may not promote germination of seeds with intermediate PD, and does not promote germination of seeds with deep PD. Embryos excised from seeds with nondeep and intermediate PD produce normal seedlings, but those from seeds with deep PD either do not grow or the seedlings are dwarfed. Cold and/or warm stratification can break dormancy nondeep, intermediate and deep PD, depending on the species. To break dormancy, a few days to a few months of treatment are required for seeds with nondeep PD, 1~6 months for seeds with intermediate PD and several months to 1~2 years for seeds with deep PD.
The temperature range for germination is widened in some seeds with nondeep PD as dormancy-break proceeds. The only way to determine if newly harvested seeds are dormant or ND is to test them over a range of temperatures. Seeds that fail to germinate at any test conditions are classified as dormant, while those that germinate over the range of conditions are ND. However, if fresh seeds germinate over a narrow range of conditions and then after a dormancy-breaking treatment germinate over wide range of conditions, they are classified as being conditional dormant (CD) at maturity. Only seeds with nondeep PD have dormancy cycling, which is a change from dormancy to non-dormancy (ND) and from ND back to dormancy in response to the annual seasonal changes in environmental conditions [9,10,11]. As seeds cycle between dormancy and nondormancy, they pass through conditional dormancy.
Seed dormancy does not suddenly begin, nor does it suddenly end. During the period of dormancy loss in some species with nondeep PD, the range of temperatures over which seeds can germinate changes. Some seeds exhibit an increase in the maximum temperature (Type 1) or a decrease in the minimum temperature (Type 2), others show an increase in maximum temperature and a decrease in minimum temperature (Type 3) [12], and still others only germinate at relatively high temperatures (Type 4) or low temperatures (Type 5) when they become non-dormant [13].
Fraxinus chinensis subsp. rhynchophylla (Hance) A.E.Murray (Oleaceae), hereafter, Fraxinus rhynchophylla, is an economically important, hardwood broad-leaved tree in the mountainous region of northeast China [14]. It grows on slopes, riverbanks and roadsides at elevations up to 1500 m a.s.l. Its broad distribution in China includes the provinces of Gansu, Hebei, Heilongjiang, Henan, Jilin, Liaoning, Shaanxi, Shandong and Shanxi, and it also grows in Korea and Russia. F. rhynchophylla wood and various products are in short supply and expensive in the domestic and international markets. It is the preferred tree species for constructing shelter forests and soil and water conservation forests, and it also is cultivated as an afforestation tree, such as border trees and courtyard trees. The wood is hard and fine-grained and can be used to produce high-grade furniture and instruments. Its bark can be used as medicine, and the leaves are used to produce livestock feed [15,16,17,18,19,20]. Previous forestry research has paid little attention to regeneration of F. rhynchophylla from seeds, and the studies that have been done resulted in low germination percentages and irregularity in time of seedling emergence [20]. Thus, seeds of this species are dormant, but the class of dormancy has not been identified.
Previous research on seed dormancy and germination in the genus Fraxinus has revealed that seeds of many species have PD, for example Fraxinus americana L. [21], F. angustifolia Vahl [22], F. dipetala Hook. & Arn. [23], F. floribunda C.K.Schneid. [24], F. hookeri Wenz. [24], F. hubeiensis S.Z.Qu, C.B.Shang & P.L.Su [25], F. latifolia Benth. [23], F. ornus L. [6], F. pennsylvanica Marshall [26], F. velutina Torr. [23] and F. xanthoxyloides (G.Don) Wall. ex A.DC. [24]. Current year seeds of F. americana L. have mature embryos, and the main reason for dormancy is the mechanical stress of seed coat and inhibition substances in coat and endosperm [27]. F. hubeiensis seeds were water-permeable, and in vitro embryos could germinate normally. Seed cotyledons were intact, and embryos fully developed [25], but inhibitory substances in the embryo and endosperm, and mechanical stress inhibited embryo elongation [28]. Mature seeds of F. pennsylvanica have fully developed embryos, and cold stratification at 5 °C could effectively promote seed germination [29]. However, some species of Fraxinus produce seeds that have MPD, for example F. excelsior L. [24], F. mandschurica var. japonica [30] and F. nigra Marshall [24]. F. excelsior seeds were water-permeable [31], and the embryos were significantly grown; germination could occur as endogenous inhibitors decreased during cold stratification at 5 °C. Some studies suggested that the non-germination of seed embryos was caused by mechanical stress of pericarp, seed coat and endosperm. Thus F. excelsior seeds had embryo dormancy, which deepened during embryo formation and disappeared during after-ripening [32]. F. mandshurica also is an economically important, hardwood broad-leaved tree in the mountainous region of northern China. The seeds of F. mandshurica have MPD and require 12 weeks 18 °C warm stratification and 10 weeks 5 °C cold stratification to release dormancy; the appropriate temperature for germination is 10 °C [33,34]. Dormancy of F. mandschurica var. japonica seeds is related to an immature seed embryo and the mechanical stress of seed endosperm and coat, which controlled the growth of the embryo and germination of the seed [35]. F. nigra seeds did not germinate well under cold stratification, which might be caused by mechanical stress of external structure [36], and this phenomenon plays an important role in controlling germination of deeply dormant seeds of Fraxinus [37]. Further, it should be noted that a few species such as F. formosana Hayata and F. lanuginosa Koidz [24] have ND seeds.
The broad objective of our research was to determine the class of dormancy in seeds of F. rhynchophylla and how to break it. Since the species of Fraxinus with MPD belong to the section of the genus called Fraxinus, and F. chinensis belongs to section Ornus of the genus which has PD [38], we hypothesize that seeds of F. rhynchophylla have PD. To determine the kind of dormancy in seeds of F. rhynchophylla, we determined water permeability of the seed coat, if the embryo was underdeveloped (i.e., grows inside the seed prior to radicle emergence) and the effects of cold stratification on germination over a range of temperatures.
2. Materials and Methods
2.1. Plant Material
The germination unit of F. rhynchophylla is a fruit called a samara, in which the true seed (with embryo and endosperm) is covered by the pericarp that is expanded into a wing on one end. Mature fruits (hereafter seeds) of F. rhynchophylla collected in 2018 from trees growing in Jilin Province, China, were purchased from Pinnong Forest Flower Lawn Seed Distribution Company, and they were stored in woven cloth bags at low temperature (−20 °C) for a year. Seed purity was 95.7%, water content 8.4%, 1000-seed weight 32.93 g and viability 85.0%.
2.2. Water Uptake Test
Water uptake was determined for eight replicates of 200 seeds each. Half of the seeds were intact and half had the seed wing removed. The seeds were placed in beakers of distilled water at ambient room temperature. After 0, 12, 24, 36, 48, 60, 72, 84 and 96 h of incubation, seeds were removed and dried with filter paper and weighed. Percent water uptake was calculated as follow:
where Ws was increase in mass of the seeds, Wh seed mass after a given interval of imbibition, and Wi initial seed mass at 0 h.
2.3. Cold Stratification
Seeds were soaked in water at room temperature for 72 h and disinfected with 0.5% potassium permanganate solution for 60 min. Then, the seeds were washed with running water and surface water was drained from them. Sterilized seeds were placed in sterilized white plastic boxes without substrate and cold stratified in darkness at 5 °C for 12 weeks. However, seeds were periodically examined in room light, at which time they were sprayed with distilled water to keep them moist, and rotten ones, which were covered with fungi or softening, were removed.
2.4. Developmental Status of Seed Embryo
To determine if the embryo grew during cold stratification, 30 seeds were selected for determination of the embryo length (E): seed length (S) ratio after 0, 2, 4, 6 and 8 weeks at 5 °C. We measured the S and E with vernier calipers accurate to 0.01 mm, and calculated the E:S, then, the 30 seeds were randomly divided into three replicates. The endosperm (including seed coat) and seed embryo were separated and dried in an oven at 105 °C for 24 h, after which they were weighed with an electronic balance. Then, the embryo weight ratio (embryo:seed dry weight ratio) was calculated.
2.5. Germination Test
To determine the temperature for germination, seeds that have not been stratified and those cold stratified for 4 and 8 weeks were tested for germination in the dark at 28 temperatures: constant temperature of 5 °C, 10 °C, 15 °C, 20 °C, 25 °C, 30 °C and 35 °C, and alternating (12 h/12 h) temperature regimes of 35 °C/30 °C, 35 °C/25 °C, 35 °C/20 °C, 35 °C/15 °C, 35 °C/10 °C, 35 °C/5 °C, 30 °C/25 °C, 30 °C/20 °C, 30 °C/15 °C, 30 °C/10 °C, 30 °C/5 °C, 25 °C/20 °C, 25 °C/15 °C, 25 °C/10 °C, 25 °C/5 °C, 20 °C/15 °C, 20 °C/10 °C, 20 °C/5 °C, 15 °C/10 °C, 15 °C/5 °C and 10 °C/5 °C. For each test temperature four replicates with 25 seeds each were used. The seeds were removed from 5 °C, washed with running water and sown in 9-cm-diameter Petri dishes on filter paper moistened with distilled water. The germination test was for 30 days, and seeds were monitored daily in natural light. Germination was defined as radicle emergence through the seed coat for more than 2 mm. The germination percentage (GP), germination index (GI) and mean germination time (MGT) were calculated as follows:
where Nt is germination time and Ng number of germinated seeds per day corresponding to Nt.
2.6. Statistical Analyses
Data were subjected to statistical analysis using SPSS 19.0, Statistical evaluation was conducted using analysis of variance, and significant data were analyzed further using Duncan multiple comparison. Plot using SigmaPlot 12.5.
3. Results
3.1. Seeds Water Permeability
Maximum water absorption of the de-winged seeds (66.1%) was after 96 h, but there was no significant increase after 24 h. Maximum water absorption of intact seeds (151.4%) was after 96 h, but there was no significant increase after 84 h (Figure 1).
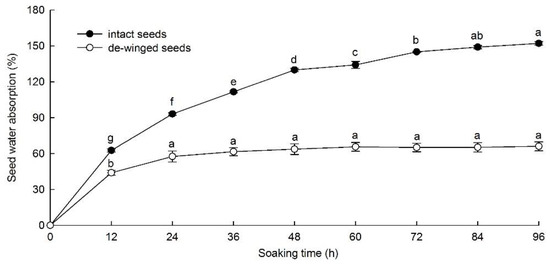
Figure 1.
Water absorption (mean ± standard error) of intact and de-winged F. rhynchophylla seeds from 0 to 96 h. Different lowercase letters indicate significant differences between different soaking time for the same treatment showed by Duncan multiple comparison (p < 0.05).
3.2. Embryo Developmental Status
There were no significant differences in E, E:S ratio or embryo:seed dry weight ratio of F. rhynchophylla seeds after different periods of cold stratification. The E:S ratio remained stable at about 94%, which means the embryo almost completely filled the embryonic cavity. Embryo:seed dry weight ratio remained stable at about 33% (Table 1).

Table 1.
Changes of F. rhynchophylla seed embryo development during cold stratification.
3.3. Germination of Cold Stratified Seeds at Different Temperatures
3.3.1. Germination of Seeds after Different Cold Stratification Time
To explore the suitable cold stratification time for dormancy-breaking of F. rhynchophylla seeds, we conducted the germination of seeds with 4/8 weeks and 8/12 weeks stratification by two-factor variance analysis. The results showed that cold stratification time had significant effects on the GP and GI between seeds at 4 weeks/8 weeks (p < 0.01), while it had no significant differences between seeds at 8 weeks/12 weeks. Thus, cold stratification for 8 weeks could finish dormancy-breaking of F. rhynchophylla seeds effectively (Table 2).

Table 2.
Variance analysis of the effects of cold stratification and temperature on germination of F. rhynchophylla seeds.
3.3.2. Germination of Non-Stratified Seeds at Different Temperatures
The GP, GI and MGT of F. rhynchophylla seeds differed significantly with temperature (p < 0.05) (Table 3).

Table 3.
Germination (mean ± standard error) of non-stratified F. rhynchophylla seeds at different temperatures.
With increasing constant temperature, taking 25 °C as the turning point, the GP and GI first increased and then decreased, while MGT, taking 30 °C as the turning point, first decreased and then increased. For non-stratified seeds, 25 °C and 30 °C were suitable germination temperatures followed by 20 °C and 35 °C, but 5 °C~15 °C inhibited germination (Table 3).
Most GP, GI and MGT values were better at alternating temperatures than at constant temperatures. However, the GP, GI and MGT at 30 °C/10 °C and the GP and GI at 25 °C/5 °C were slightly less than at constant temperatures, but the difference was not significant (Table 3).
The mean value of the GP of seeds cultured at 25 °C (at alternating temperatures, included 25 °C/20 °C, 25 °C/15 °C, 25 °C/10 °C and 25 °C/5 °C) was slightly lower than 25 °C at constant temperature. Germination of seeds was highest when the difference between the high and low temperature (H-L D-value) was 15 °C (the GP was 57.8, the GI was 1.3 and the MGT was 14.2 d). The GP was around 50%, except when the H-L D-value was 5 °C (Table 3).
3.3.3. Germination after 4 Weeks Cold Stratification at Different Temperatures
The GP, GI and MGT of F. rhynchophylla seeds after 4 weeks cold stratification differed significantly with temperature (p < 0.05) (Table 4).

Table 4.
Germination (mean ± standard error) of F. rhynchophylla seeds at different temperatures after 4 weeks cold stratification.
With increasing constant temperature, GP and GI, taking 30 °C as the turning point, first increased and then decreased. Except for 5 °C, the GP was about 50 and the GI was about 1.0. MGT was relatively short from 15 °C to 30 °C, which was about 13 d. For seeds that received 4 weeks cold stratification, 30 °C, 25 °C and 10 °C were suitable germination temperatures followed by 20 °C, 35 °C and 15 °C, while 5 °C inhibited germination (Table 4).
Most GP, GI and MGT values were better at alternating temperatures than at constant temperatures. Only the GP at 35 °C/10 °C was lower than at constant temperatures, but the difference was not significant (Table 4).
Mean values of the GP were higher at alternating temperatures than at constant temperatures. Germination of seeds was best when the H-L D-value was 15 °C (the GP was 73.3, the GI was 1.9, and the MGT was 11.5 d). All GPs were above 50% under all H-L D-value (Table 4).
3.3.4. Germination after 8 Weeks Cold Stratification at Different Temperatures
The GP, GI and MGT of F. rhynchophylla seeds after 8 weeks cold stratification differed significantly with temperature (p < 0.05) (Table 5).

Table 5.
Germination (mean ± standard error) of F. rhynchophylla seeds at different temperatures after 8 weeks cold stratification.
At constant temperature from 10 °C to 30 °C, all GPs were above 50 and highest at 25 °C (68.0). From 15 °C to 30 °C, GIs were all above 2.1 and highest at 30 °C (2.5), while MGTs were all below 13 d and shortest at 30 °C (10.5 d). For seeds that received 8 weeks cold stratification, 15 °C~30 °C were suitable germination temperatures followed by 10 °C and 35 °C, while 5 °C inhibited germination (Table 5).
All GP, GI and MGT values at alternating temperatures were better or had no significant difference compared with those at constant temperatures, except for the GI and MGT at 10 °C/5 °C (Table 5).
Mean values of the GP and GI were lower at alternating temperatures than at constant temperatures of 15 °C (only GP), 25 °C and 30 °C, thus after dormancy break seeds germinated well when incubated at constant temperature. The GP of seeds was best at 15 °C (57.9) of H-L D-value, and the GP at all H-L D-value was above 50% and below 60% (Table 5).
3.3.5. Germination of Seeds at Constant Temperatures after Cold Stratification
Seeds incubated at 25 °C had a relatively stable GP during stratification, and 8 weeks stratification increased the GI and shortened the MGT. Seeds incubated at 30 °C had a relatively stable GP and MGT during stratification, and 8 weeks stratification increased the GI. Seeds incubated at 10 °C and 35 °C had a relatively stable MGT during stratification, and 4 weeks stratification increased the GI, while 8 weeks increased the GP. Four weeks stratification increased the GI, while eight weeks increased the GP and shortened MGT of seeds incubated at 15 °C and 20 °C. Eight weeks stratification increased the GP of seeds incubated at 5 °C but not the GI (Table 3, Table 4 and Table 5).
At constant temperatures, the GP of F. rhynchophylla seeds after 4 weeks cold stratification (expect 25 °C), and the GI of seeds after 8 weeks cold stratification were significantly higher than those for non-stratified seeds, and the MGT of seeds after 8 weeks cold stratification was significantly shorter than that of non-stratified seeds in 15 °C~30 °C. That is, cold stratification increased germination percentages and rates. Taking 25 °C as the turning point, the germination of non-stratified seeds first increased and then decreased with increasing temperature. Stratified seeds germinated well at 10 °C~30 °C (Table 3, Table 4 and Table 5).
4. Discussion
Our hypothesis that seeds of F. rhynchophylla have PD is supported by the results. The seeds are water-permeable and thus do not have PY [13]. Furthermore, the embryo of mature seeds is fully differentiated and developed, and embryo growth as well as dry material accumulation does not occur inside the seed prior to radicle emergence. Consequently, the seeds do not have MD or MPD [39]. During the cold stratification treatment at 5 °C, a small number of seeds germinated between weeks 8 and 12. Since cold stratification increased the germination percentage and rate and widened the temperature range for germination of seeds, we conclude that 8 weeks cold stratification at 5 °C broke the dormancy of the seeds [10]. Since cold stratification increased the germination percentage and rate, we conclude that the seeds have PD, following the seed dormancy classification scheme by Baskin and Baskin [5]. Thus, F. rhynchophylla can be added to the list of Fraxinus species, mentioned in the Introduction, whose seeds have PD.
Since more than half the F. rhynchophylla seeds germinated at 25 °C, 30 °C, and a series of multiple alternating temperature such as 30 °C/15 °C and 25 °C/15 °C, but later germinated at low temperatures following cold stratification, we conclude that most of the non-stratified seeds were conditionally dormant [11]. Compared with non-stratified seeds, the temperature range for germination of cold stratified seeds widened from 25 °C~30 °C to 10 °C~30 °C, indicating that the non-stratified F. rhynchophylla seeds had CD [5]. Both newly harvested seeds of Celastrus rosthornianus [40] and Manglietia grandis [41] had CD. The GP of the former was above 50% at 10 °C~20 °C, low at 25 °C (12.2%), and seeds did not germinate at 30 °C. However, after being buried in sand at 5 °C or 20 °C/10 °C, the GP at 30 °C reached 75% and 70%, respectively. The GP of the latter was 36% at 20 °C/10 °C, and after GA3 treatment reached 84%. Stratification or GA3 treatment significantly widened the temperature range for germination of the seeds [42]. The seeds of Corispermum lehmannianum [43] and Pedicularis myriophylla [44] also had CD.
By definition, seeds with conditional PD have nondeep PD. Previous research showed that in vitro embryos of F. rhynchophylla removed from seeds germinate normally and 10−3 mol/L GA3 can significantly improve the GI of seeds [45] (p < 0.05), and we found that cold stratification significantly increased seed germination. Based on the seed dormancy classification scheme by Baskin and Baskin [4], the dormancy level of F. rhynchophylla seeds is non-deep PD [7,9].
Cold stratification increased the ability of seeds to germinate at low temperatures (e.g., 10 °C and 15 °C); thus, based on the seed dormancy classification scheme by Baskin and Baskin [7,9], F. rhynchophylla seeds have Type 2 nondeep PD [9,45]. Seeds of various species that grow in the temperate zone and germinate in spring have type 2 nondeep PD [9]. Dormancy in seeds of these species is broken during winter, and as dormancy break occurs, the minimum temperature at which seeds can germinate is decreased. Thus, seeds can germinate in early spring, while temperatures are relatively low. The emergence of seedlings of F. rhynchophylla and numerous other species in early spring means that the young plants have the whole frost-free season in which to grow and become established.
5. Conclusions
Seeds of F. rhynchophylla have Type 2 nondeep PD, and it can be broken by cold stratification. Because of Type 2 nondeep PD, the minimum temperature at which seeds can germinate is decreased so that it overlaps with temperatures occurring in the field in early spring. Thus, seeds in the field would be cold stratified during winter and germinate in early spring as the temperatures begin to increase. To break seed dormancy and improve seed germination vigor in production practice, we recommend that seeds be given 8 weeks of cold stratification at 5 °C before they are sown. However, the seeds should be sown at temperatures higher than 5 °C, e.g., 15 °C~30 °C for high germination percentages and rates.
Author Contributions
P.Z. and K.A. conceived and designed the study; K.A., M.Y., M.L., M.Z. and C.J. performed the experiments; K.A. and C.J. collected the data; K.A. and M.Y. analyzed the data; K.A. drafted the manuscript; C.C.B. and P.Z. revised various drafts of the manuscript; P.Z. and H.W. supervised the study. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by National Natural Science Foundation of China (31870615,31670639) and the Fundamental Research Funds for the Central Universities (2572020DR05).
Data Availability Statement
Not applicable.
Acknowledgments
Thanks to Liu Zhuolin, Lv Jintao, Zhang Yipeng, Dong Bowen, Wang Jing, Yu Xue, Zhang Zheng and Liu Ting for their help in completing experiments. Thanks to the financially from National Natural Science foundation of China (31870615, 31670639) and the Fundamental Research Funds for the Central Universities (2572020DR05).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Baskin, J.M.; Baskin, C.C. The great diversity in kinds of seed dormancy: A revision of the Nikolaeva–Baskin classification system for primary seed dormancy. Seed Sci. Res. 2021, 31, 249–277. [Google Scholar] [CrossRef]
- Finchsavage, W.; Leubnermetzger, G. Tansley review: Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef] [PubMed]
- Nikolaeva, M.G. Physiology of deep dormancy in seeds. In Seeds; NSF: Washington, DC, USA, 1969. [Google Scholar]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination, 2nd ed.; Academic Press: London, UK, 2014. [Google Scholar]
- Kortessis, N.; Chesson, P. Germination variation facilitates the evolution of seed dormancy when coupled with seedling competition. Theor. Popul. Biol. 2019, 130, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Tonguc, T. Dormancy level and dormancy-breaking pretreatments in seeds of Fraxinus ornus subsp. cilicica. Propag. Ornam. Plants 2013, 13, 40–45. [Google Scholar]
- Baskin, C.C.; Baskin, J.M. Chapter 3 Types of Seeds and Kinds of Seed Dormancy. In Seeds, 2nd ed.; Academic Press: New York, NY, USA, 2014; pp. 37–77. [Google Scholar]
- Finch-Savage, W.E.; Footitt, S. Seed dormancy cycling and the regulation of dormancy mechanisms to time germination in variable field environments. J. Exp. Bot. 2017, 68, 843–856. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Chapter 4 Germination Ecology of Seeds with Nondeep Physiological Dormancy. In Seeds, 2nd ed.; Academic Press: New York, NY, USA, 2014; pp. 79–117. [Google Scholar]
- Hema, S.N.D.; Steven, J.S. Variation of Seed Dormancy and Germination Ecology of Cowcockle (Vaccaria hispanica). Weed Sci. 2014, 62, 483–492. [Google Scholar]
- Vegis, A. Chapter 15 Climatic Control of Germination, Bud Break, and Dormancy. In Environmental Control of Plant Growth; Academic Press: New York, NY, USA, 1963; pp. 265–287. [Google Scholar]
- Cheng, P.; Wang, P.; Sun, J.K.; Fei, M.L.; Yang, H. Research progresses on regulation mechanisms of plant seeds dormancy and germination. J. Cent. South Univ. 2013, 33, 52–58. [Google Scholar]
- Baskin, C.C.; Baskin, J.M. Chapter 6 Germination Ecology of Seeds with Physical Dormancy. In Seeds, 2nd ed.; Academic Press: New York, NY, USA, 2014; pp. 145–185. [Google Scholar]
- Zhang, P. Seed characteristic variation and seedling growth variation among progenies from plus trees of Fraxinus rhynohophylla. China For. Sci. Technol. 2013, 2, 28–31. [Google Scholar]
- Zhang, F.; Hu, W.L.; Kong, W.X. Comparison of five broad-leaved seedlings’ physiological characteristics in natural secondary forest of eastern Liaoning. Chin. J. Ecol. 2004, 23, 106–110. [Google Scholar]
- Xiang, F.W.; Huang, Y.F.; Wang, Z.L. On the Provenance Test during Seedling Stage of Fraxinus chinensis Hance in the Southeast Mountain Area of Jilin Province. J. Beihua Univ. (Nat. Sci.) 2013, 3, 255–258. [Google Scholar]
- Sun, J.J.; Yao, C.J.; Liu, Y.A. Investigation on the Suitable Locality of Fraxinus chinensis. J. Jilin For. Univ. 1999, 15, 239–241. [Google Scholar]
- Sun, J.J.; Yao, C.J.; Jia, H.Y. The Speed-up and High Yield Breeding Technology for Fraxinus chinensis. J. Jilin For. Univ. 1999, 15, 242–244. [Google Scholar]
- Xiang, F.W.; Yao, C.J.; Liu, Y.H. On the Distribution Growth and Natural Regeneration of Fraxinus chinensis. J. Jilin For. Univ. 1997, 13, 212–215. [Google Scholar]
- Wang, X.X. Seeding and seedling raising techniques of economic tree Fraxinus rhynchophylla. For. By-Prod. Spec. China 2020, 167, 66–67. [Google Scholar]
- Ashley, J.A.; Preece, J.E. Seed cutting treatments stimulate germination and elucidate a dormancy gradient in dormant Fraxinus Americana L. and Fraxinus Pennsylvanica Marsh. Propag. Ornam. Plants 2009, 9, 121–128. [Google Scholar]
- Piotto, B. Storage of non-dormant seeds of Fraxinus angustifolia Vahl. New For. 1997, 14, 157–166. [Google Scholar] [CrossRef]
- Emery, D.E. Seed propagation of native California plants. ICSID Rev. 1988, 29, 451–473. [Google Scholar]
- Baskin, C.C.; Baskin, J.M. Chapter 9 A Geographical Perspective on Germination Ecology. In Seeds, 2nd ed.; Academic Press: New York, NY, USA, 2014; pp. 689–807. [Google Scholar]
- Ye, Y.; Wang, C.; Shi, Y. Preliminary study on cause of seed dormancy of Fraxinus hupehensis. Hubei Agric. Sci. 1999, 20, 45–47. [Google Scholar]
- Cram, W.H.; Lindquist, C.H. Germination of green ash is related to seed moisture content at harvest (Fraxinus pennsylvanica). For. Sci. 1982, 28, 809–812. [Google Scholar]
- Ye, J.F. Study on dormancy and seedling of Fraxinus americana. Heilongjiang Agric. Sci. 2015, 03, 61–63. [Google Scholar]
- Liu, Y.; Zhang, J.; Li, G.; Wang, Q.; Xu, S. The research for the germination characteristics of Fraxinus hupehensis Seeds. Seed. 2016, 35, 27–31. [Google Scholar]
- Tinus, R.W. Effects of de-winging, soaking, stratification, and growth regulators on germination of green ash seed. Can. J. For. Res. 1982, 12, 931–935. [Google Scholar] [CrossRef]
- Asakawa, S. Some observations on Fraxinus seeds. J. Jpn. For. Soc. 2008, 37, 1–5. [Google Scholar]
- Ferenczy, L. The dormancy and germination of seeds of the Fraxinus excelsior L. Acta Biol. 1955, 12, 17–24. [Google Scholar]
- Wagner, J. Changes in dormancy levels of Fraxinus excelsior L. embryos at different stages of morphological and physiological maturity. Trees 1996, 10, 177–182. [Google Scholar]
- Zhang, P.; Shen, H. Morphological and Physiological Changes of Manchurian Ash (Fraxinus mandshurica Rupr.) Seeds Collected at Different Developmental Periods and Its Germination Responses after Stratification. Plant Physiol. 2010, 46, 125–130. [Google Scholar]
- Ji, Y. Effects of dry storage at different stage of seed stratification on seed germination of Fraxinus mandshurica. Terr. Nat. Resour. Stu. 2019, 06, 75–77. [Google Scholar]
- Asakawa, S. Studies on the delayed germination of Fraxinus mandshurica var. japonica seeds. Bull. Gov. For. Exp. Stn. Tokyo 1956, 83, 19–28. [Google Scholar]
- Hinsinger, D.D.; Gaudeul, M.; Couloux, A.; Bousquet, J.; Frascaria-Lacoste, N. The phylogeography of Eurasian Fraxinus species reveals ancient transcontinental reticulation. Mol. Phylogenet. Evol. 2014, 77, 223–237. [Google Scholar] [CrossRef]
- Steinbauer, G.P. Dormancy and germination of Fraxinus seeds. Plant Physiol. 1935, 12, 813–824. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Chapter 5 Germination Ecology of Seeds with Morphophysiological Dormancy. In Seeds, 2nd ed.; Academic Press: New York, NY, USA, 2014; pp. 119–143. [Google Scholar]
- Dou, Q.L.; Zhang, R.B. Seed germination characteristics of Celastrus rosthornianus loes. J. Plant Physiol. 2013, 49, 75–80. [Google Scholar]
- Pan, R.; Sun, W.B. Seed Dormancy and Germination of the Critically Endangered Manglietia grandis Hu et Cheng. Plant Physiol. Commun. 2009, 45, 1089–1092. [Google Scholar]
- Zhang, L.W.; Liu, H.L.; Zhang, D.Y.; Bian, W.G. Seed dormancy release and germination characteristics of Corispermum lehmannianum Bunge, an endemic species in the Gurbantunggut desert of China. Phyton 2015, 84, 58–63. [Google Scholar]
- Sui, X.L.; Li, A.R.; Guan, K.Y. Impacts of climatic changes as well as seed germination characteristics on the population expansion of Pedicularis verticillata. Ecol. Environ. Sci. 2013, 22, 1099–1104. [Google Scholar]
- Chien, C.T.; Yang, J.C.; Lin, T.P. Seed storage behavior of Lindera communis, Lindera megaphylla, Phoebe formosana, Helicia cochinchinensis, and Helicia formosana in Taiwan. Taiwan J. For. Sci. 2004, 19, 119–131. [Google Scholar]
- Zhang, H.B.; Wang, Z.; Yang, X.; Liu, T.Y.; Zhang, P. Effect of Seed Soaking with Plant Growth Regulators and Stratification on Seed Germination of Fraxinus rhynchophylla. Heilongjiang Sci. 2022, 9, 1248–1261. [Google Scholar]
- Soltani1, E.; Baskin, C.C.; Baskin, J.M. A graphical method for identifying the six types of nondeep physiological dormancy in seeds. Plant Biol. 2017, 19, 673–682. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).