Soils Carbon Stocks and Litterfall Fluxes from the Bornean Tropical Montane Forests, Sabah, Malaysia
Abstract
:1. Introduction
2. Materials and Methods
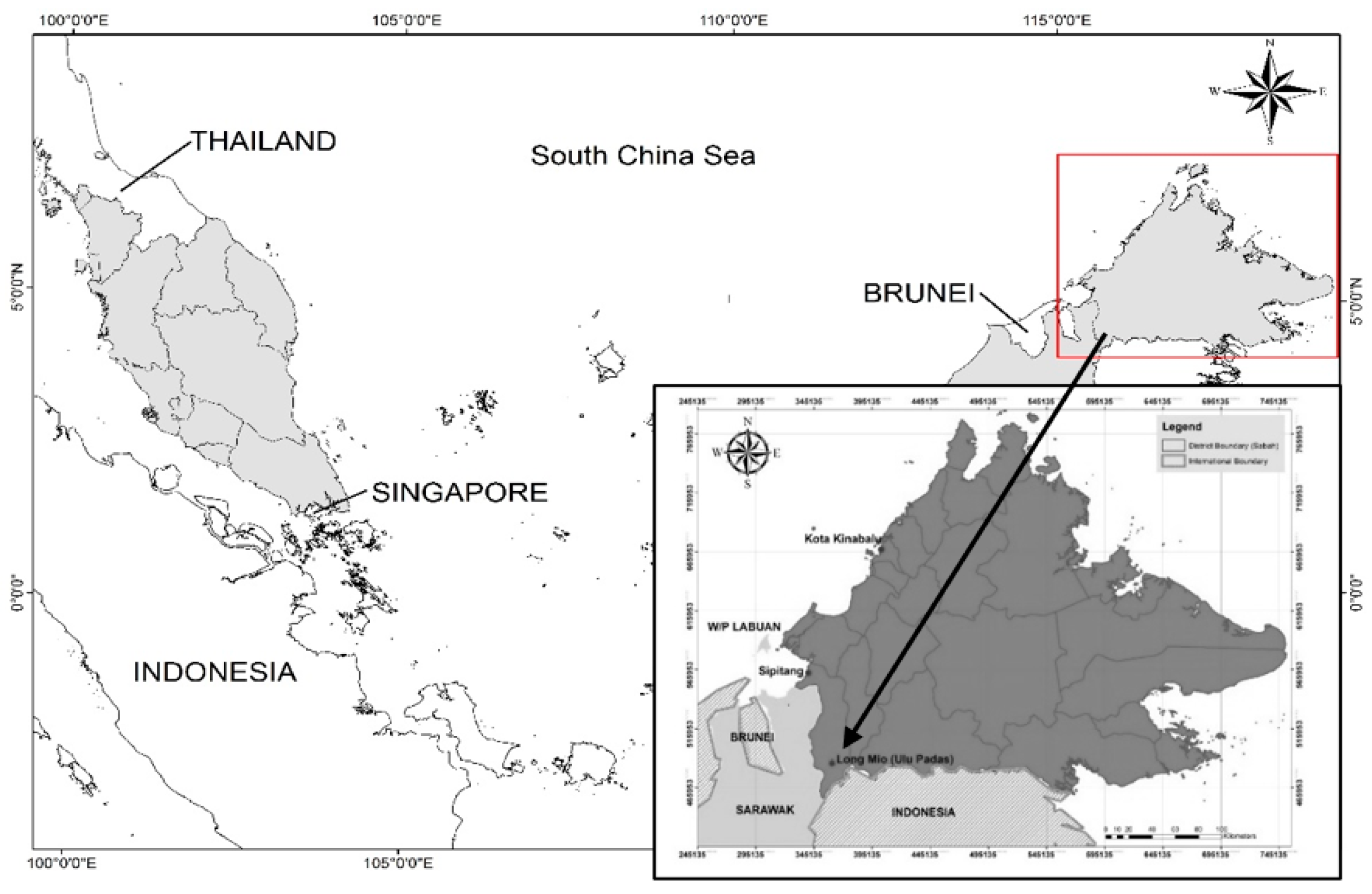
2.1. Study Area
2.2. Field Data Collection
2.3. Laboratory Analysis
2.3.1. Soil Analysis
2.3.2. Plant Samples Analysis
2.3.3. Carbon Analysis
2.4. Statistical Analysis
3. Results
3.1. Soil Physicochemical Properties
3.2. Organic Layer
3.3. Litterfall Production
3.4. Soil Carbon Stock
3.5. Total Carbon Stock (Organic Layer, Litterfall, and Soil Carbon Stock)
4. Discussion
4.1. Soil Physicochemical Properties
4.2. Litterfall and the Organic Layer
4.3. Soil Carbon Stock
4.4. Total Carbon Stock of Litterfall, Organic Layer, and Soil Carbon Pools
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Green, C.; Byrne, K.A. Biomass: Impact on carbon cycle and greenhouse gas emissions. In Encyclopedia of Energy; Cleveland, C.J., Ed.; Elsevier: Amsterdam, The Netherlands, 2004; pp. 223–236. [Google Scholar]
- Live Science. Available online: https://www.livescience.com/28698-facts-about-carbon.html (accessed on 23 March 2021).
- Reichle, D.E. The global carbon cycle and the biosphere. In The Global Carbon Cycle and Climate Change; Reichle, D.E., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 183–208. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO). Global Forest Resources Assessment 2020: Main Report; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- FAO; UNEP. The State of the World’s Forest 2020. Forests, Biodiversity and People; FAO: Rome, Italy; UNEP: Nairobi, Kenya, 2020. [Google Scholar] [CrossRef]
- Zakaria, M.; Rajpar, M.N.; Ozdemir, I.; Rosli, Z. Fauna Diversity in Tropical Rainforest: Threats from Land-Use Change. In Tropical Forest–The Challenges of Maintaining Ecosystem Services While Managing Landscape; Blanco, J.A., Chang, S.C., Lo, Y.H., Eds.; IntechOpen: London, UK, 2016. [Google Scholar]
- Houghton, R.A. The Contemporary Carbon Cycle. In Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 399–435. [Google Scholar] [CrossRef]
- Sadono, R.; Pujiono, E.; Lestari, L. Land Cover Changes and Carbon Storage Before and After Community Forestry Program in Bleberan village, Gunungkidul, Indonesia, 1999–2018. Forest Sci. Technol 2020, 16, 134–144. [Google Scholar] [CrossRef]
- Leifeld, J.; Bassin, S.; Fuhrer, J. Carbon Stock in Swiss Agricultural Soils Predicted by Land use, Soil Characteristics and Altitude. Agric. Ecosyst. Environ. 2005, 105, 255–266. [Google Scholar] [CrossRef]
- Xiao, C. Soil Organic Carbon Storage (Sequestration) Principles and Management: Potential Role for Recycled Organic Materials in Agricultural Soils of Washington State; Washington State Department of Ecology: Olympia, WA, USA, 2015.
- Ioki, K.; Tsuyuki, T.; Phua, M.H.; Wong, W.V.C.; Johari, S.A.; Korom, A.; James, D.; Saito, H.; Takao, G. Evaluation of the similarity in tree community composition in a tropical rainforest using airborne LiDAR data. Remove Sens. Environ. 2016, 173, 304–313. [Google Scholar] [CrossRef]
- Raihan, A.; Said, M.N.M.; Abdullah, S.M.S. Climate change mitigation options in the Forestry Sector of Malaysia. J. Kejuruter. 2018, 1, 89–98. [Google Scholar] [CrossRef]
- EC. Thematic Strategy for Soil Protection (COM 2006, 231 Final). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52006DC0231 (accessed on 10 March 2021).
- Greiner, L.; Keller, A.; Gret-Regamey, A.; Papritz, A. Soil function assessment: Review of methods for quantifying the contributions of soil to ecosystem services. Land Use Policy 2017, 69, 224–237. [Google Scholar] [CrossRef]
- Jónsson, J.Ö.G.; Davíðsdóttir, B.; Nikolaidis, N.P. Valuation of soil ecosystem services. In Advances in Agronomy; Banwart, S.A., Sparks, D.L., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 142, pp. 353–384. [Google Scholar]
- Gaudel, G.; Zhang, W.H.; Dang, Q.H.; Le, T.H. The global terrestrial carbon stocks, status of carbon in forest and shrubland of Nepal, and relationship between carbon stock and diversity. Int. J. Res. 2016, 31, 620–634. [Google Scholar]
- FAO; ITPS. Status of the World’s Soil resources–Main Report; Food and Agriculture Organization of the United Nations and Intergovernmental Technical Panel on Soils: Rome, Italy, 2015. [Google Scholar]
- Ronald, V. Threats to Soils: Global Trends and Perspective; Global Soil Partnership Food and Agriculture Organization of the United Nations: Bonn, Germany, 2017; pp. 1–27. [Google Scholar]
- Ontl, T.A.; Schulte, L.A. Soil carbon storage. Nat. Educ. Knowl. 2012, 3, 35–47. [Google Scholar]
- Trivedi, P.; Singh, B.P.; Singh, B.K. Soil Carbon: Introduction, Importance, Status, Threat, and Mitigation. In Soil Carbon Storage; Singh, B.K., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 1–28. [Google Scholar]
- FAO; ITPS; GSBI; CBD; EC. State of Knowledge of Soil Biodiversity. Status, Challenges, and Potentialities, Report 2020; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Langer, A.; Samejima, H.; Ong, R.C.; Titin, J.; Kitayama, K. Integration of Carbon Conservation Into Sustainable Forest Management Using High Resolution Satellite ImageneryL: A Case Study in Sabah, Malaysian Borneo. Int. J. Appl. Earth Obs. Geoinf. 2012, 18, 305–312. [Google Scholar] [CrossRef]
- Wong, C.J.; James, D.; Besar, N.A.; Kamlun, K.U.; Tangah, J.; Tsuyuki, S.; Phua, M.H. Estimating Mangrove Above-Ground Biomass Loss Due to Deforestation in Malaysian Northern Borneo Between 2000 and 2015 Using SRTM and Landsat Images. Forests 2020, 11, 1018. [Google Scholar] [CrossRef]
- Broich, M.; Hansen, M.; Potapov, P.V.; Wimberly, M.C. Patterns of Tree-Cover Loss Along the Indonesia-Malaysia Border on Borneo. Int. J. Remote Sens. 2013, 34, 5748–5760. [Google Scholar] [CrossRef]
- Loh, H.Y.; James, D.; Ioki, K.; Wong, W.V.C.; Tsuyuki, S.; Phua, M.H. Aboveground Biomass Changes in Tropical Montane Forest of Northern Borneo Estimated Using Spaceborne and Airborne Digital Elevation Data. Forests 2020, 12, 3677. [Google Scholar] [CrossRef]
- Chen, S.; Arrouays, D.; Angers, D.A.; Martin, M.P.; Walter, C. Soil Carbon Stocks Under Different Land Uses and the Applicability of the Carbon Saturation Concept. Soil Tillage Res. 2019, 188, 53–58. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A Large and Persistent Carbon Sink in the World’s Forests. Science 2011, 333, 988–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdullahi, A.A.; Siwar, C.; Ismail, S.M.; Isahak, A. A Review on Carbon Sequestration in Malaysian Forest Soils: Opportunities and Barriers. Int. J. Soil Sci. 2015, 10, 17–27. [Google Scholar] [CrossRef]
- Poeplau, C.; Don, A. Sensitivity of Soil Organic Carbon Stocks and Fractions to Different Land-use Changes Across Europe. Geoderma 2013, 192, 189–201. [Google Scholar] [CrossRef]
- Ngo, K.M.; Turner, B.L.; Muller-Landau, H.C.; Davies, S.J.; Larjavaara, M.; Hassa, N.F.; Lum, S. Carbon Stocks in primary and Secondary Tropical Forests in Singapore. For. Ecol. Manag. 2013, 296, 81–89. [Google Scholar] [CrossRef]
- Abdullahi, A.C.; Siwar, C.; Shaharudin, M.I.; Anizan, I. The Effects of Forest Type and Land Use on Soil Carbon Stock in Malaysian Dipterocarps Forests. Int. J. Adv. Appl. Sci. 2018, 5, 11–17. [Google Scholar] [CrossRef]
- Malaysian Meterological Department. (Selangor, Malaysia). Weather forecast (2018–2019). Unpublished Report.
- World Weather Online. Long Miau Monthly Climate Average. Available online: https://www.worldweatheronline.com/long-miau-weather/sabah/my.aspx (accessed on 28 April 2021).
- Shukla, A.; Panchal, H.; Mishra, M.; Patel, P. Soil moisture estimation using Gravimetric Technique and FDR Probe Technique: A comparative analysis. Am. Int. J. Res. Form. Appl. Nat. Sci. 2014, 8, 89–92. [Google Scholar]
- Day, R.P. Particle Fractionation and Particle-Size Analysis. In Methods of Soil Analysis; Black, C.A., Ed.; Agronomy: Madison, WI, USA, 1965; pp. 545–567. [Google Scholar]
- Allison, L.E. Organic Carbon. In Methods of Soil Analysis; Agronomy: Madison, WI, USA, 1965; pp. 1367–1378. [Google Scholar]
- Hossain, M.; Aziz, C.; Saha, M. Relationship between soil physic-chemical properties and total viable bacterial counts in Sunderban mangrove forest, Bangladesh. Dhaka Univ. J. Biol. Sci. 2012, 21, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Culman, S.; Mann, M.; Brown, C. Calculating cation exchange capacity, base saturation, and calcium saturation. Agric. Nat. Resour. 2019, 81, 6. Available online: https://ohioline.osu.edu/factsheet/anr-81 (accessed on 28 April 2021).
- Kueh, R.J.H.; Majid, N.M.; Ahmed, O.H.; Gandaseca, S. Assessment of carbon stock in chronosequence rehabilitated tropical forest stands in Malaysia. J. For. Environ. Sci. 2016, 32, 302–310. [Google Scholar] [CrossRef]
- Hong, L.C.; Hemati, Z.H.; Zakaria, R.M. Carbon Stock Evaluation of Selected mangrove Forests in Peninsular Malaysia and its potential Market Value. J. Environ. Sci. Manag. 2017, 20, 77–87. [Google Scholar]
- Hazra, A.; Gogtay, N. Biostatistics series module 3: Comparing groups: Numerical variables. Indian J. Dermatol. 2016, 61, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Voltr, V.; Menšík, L.; Hlisnikovský, E.; Pospíšilová, L. The soil Organic Matter in Connection with Soil Properties and Soil Inputs. Agronomy 2021, 11, 779. [Google Scholar] [CrossRef]
- Martuti, N.K.T.; Setyowati, D.L.; Nugraha, S.B.; Mutiatari, D.P. Carbon stock potency of mangrove ecosystem at Tapak Sub-village Semarang, Indonesia. AACL Bioflux 2017, 10, 1524–1533. [Google Scholar]
- Suhaili, N.S.; Fei, J.L.J.; Sha’ari, F.W.; Idris, M.I.; Hatta, S.M.; Kodoh, J.; Besar, N.A. Carbon stock estimation of mangrove forest in Sulaman Lake Forest Reserve, Sabah, Malaysia. Biodiversitas J. Biol. Divers. 2020, 21, 5657–5664. [Google Scholar]
- Zhang, Y.-Y.; Wu, W.; Liu, H. Factors affecting variations of soil pH in different horizons in hilly regions. PLoS ONE 2019, 14, e0218563. [Google Scholar] [CrossRef]
- Keen, Y.C.; Jalloh, M.B.; Ahmed, O.H.; Sudin, M.; Besar, N.A. Soil organic matter and related soil properties in forest, grassland, and cultivated land use types. Int. J. Phys. Sci. 2011, 6, 7410–7415. [Google Scholar] [CrossRef]
- Navarro-Pedreño, J.; Almendro-Candel, M.B.; Zorpas, A.A. The increase of Soil Organic Matter Reduces Global Warming, Myth or Reality? Sci 2021, 3, 18. [Google Scholar] [CrossRef]
- Rennenberg, H.; Dannenmann, M. Nitrogen Nutritionof Trees in Temperate Forests-The Significance of Nitrogen Availability in the Pedosphere and Atmosphere. Forests 2015, 6, 2820–2835. [Google Scholar] [CrossRef]
- Amooh, M.K.; Bonsu, M. Effects of Soil Texture and Organic Matter on Evaporative Loss of Soil. J. Gl. Agric. Ecol. 2015, 3, 152–161. [Google Scholar]
- Jeyanny, V.; Husni, M.H.A.; Wan Rasidah, K.; Siva Kumar, B.; Arifin, A.; Kamarul Hisyam, M. Carbon Stocks in Different Carbon Pools of a tropical Lowland Forest and a Montane Forest with Varying Topography. J. Trop. For. Sci. 2014, 26, 560–571. [Google Scholar]
- Besar, N.B.; Suardi, H.; Phua, M.H.; James, D.; Mokhtar, M.B.; Ahmed, M.F. Carbon stock and sequestration potential of an agroforestry system in Sabah, Malaysia. Forests 2020, 11, 210. [Google Scholar] [CrossRef] [Green Version]
- Soewandita, H. Studi Kesuburan Tanah dan Analisis Kesesuaian Lahan Untuk Tanaman Perkebunan di Kabupaten Bengkalis. J. Sains Dan Teknol. Indones. 2008, 10, 128–133. [Google Scholar]
- Millar, N.; Baggs, E.M. Chemical Composition, or Quality, of Agroforestry Residues Influences N2O Emissions After Their Addition to Soil. Soil Biol. Biochem. 2004, 36, 935–943. [Google Scholar] [CrossRef]
- Perumal, M.; Wasli, M.E.; Lat, J. Effects of Enrichment Planting on the Soil Physicochemical Properties at Reforestation Sites Planted with Shorea macrophylla de Vriese in Sempadi Forest Reserve, Sarawak, Malaysia. In Proceedings of the International Symposium on Frontier in Tropical Forest Research: Progress in Joints Projects between Forest Department Sarawak and the Japan Research Consortium for Tropical Forests in Sarawak, Kuching, Sarawak, Malaysia, 21 September 2015; Forest Department Sarawak (FDS) and Japan Research Consortium for Tropical Forest in Sarawak (JRCTS): Kuching, Sarawak, Malaysia, June 2016; pp. 19–29. [Google Scholar]
- Harris, N.; Gibbes, S.; Potapov, P. Intact Forest Landscapes Matter for Climate Change. Here Are 3 Reasons Why. Available online: https://www.wri.org/blog/2017/01/intact-forest-landscapes-matter-climate-change-here-are-3-reasons-why (accessed on 11 September 2021).
- Ndakara, O.E. Litterfall and Nutrient Returns in Isolated Stands of Persea gratissima (Avocado Pear) in the Rainforest Zone of Southern Nigeria. Ethiopian J. Environ. Stud. Mngmt. 2011, 4, 42–50. [Google Scholar] [CrossRef]
- Köhler, L.; Hölscher, D.; Leuschner, C. High Litterfall in Old-growth and Secondary Upper Montane Forest of Costa Rica. Plant Ecol. 2008, 199, 163–173. [Google Scholar] [CrossRef] [Green Version]
- Park, B.B.; Rahman, A.; Han, S.H.; Youn, W.B.; Hyun, H.J.; Hernandez, J.; An, J.Y. Carbon and Nutrients Inputs by Litterfall in Evergreen and Deciduous Forests in Korea. Forests 2020, 11, 143. [Google Scholar] [CrossRef] [Green Version]
- Hertel, D.; Moser, G.; Culmsee, H.; Erasmi, S.; Horna, V.; Schuldt, B.; Leuschner, C. Below- and Above-ground Biomass and Net Primary Production in a Paleotropical Natural Forest (Sulawesi, Indonesia) as compared to Neotropical Forests. For. Ecol. Manag. 2009, 258, 1904–1912. [Google Scholar] [CrossRef]
- Jafarov, E.; Schaefer, K. The Importance of a Surface Organic Layer in Simulating Permafrost Thermal and Carbon Dynamics. Cryosphere 2016, 10, 465–475. [Google Scholar] [CrossRef] [Green Version]
- Xiao, S.; Zhang, J.; Duan, J.; Liu, H.; Wang, C.; Tang, C. Soil Organic Carbon Sequestration and Active Carbon Component Changes Following Different Vegetation Restoration Ages on Severely Eroded Red Soils in Subtropical China. Forests 2020, 11, 1304. [Google Scholar] [CrossRef]


| Land Use | Depth (cm) | Sand (%) | Silt (%) | Clay (%) | Texture |
|---|---|---|---|---|---|
| Intact Forest | 0–5 | 44 ± 4.35 | 20 ± 2.06 | 35 ± 4.11 | Clay Loam |
| 5–10 | 44 ± 3.86 | 19 ± 1.17 | 37 ± 5.26 | Clay Loam | |
| 10–30 | 43 ± 3.40 | 18 ± 3.08 | 38 ± 4.10 | Clay Loam | |
| Logged-Over Forest | 0–5 | 41 ± 7.90 | 18 ± 2.01 | 42 ± 9.04 | Clay |
| 5–10 | 44 ± 7.69 | 12 ± 3.76 | 43 ± 11.06 | Clay | |
| 10–30 | 44 ± 8.69 | 16 ± 3.22 | 40 ± 9.02 | Clay | |
| Plantation Forest | 0–5 | 56 ± 4.81 | 19 ± 4.63 | 24 ± 1.46 | Sandy Clay Loam |
| 5–10 | 67 ± 2.97 | 11 ± 2.22 | 21 ± 2.75 | Sandy Clay Loam | |
| 10–30 | 69 ± 2.62 | 15 ± 1.77 | 15 ± 2.86 | Sandy Loam |
| Soil Properties | Depth (cm) | Land Use | ||
|---|---|---|---|---|
| Intact Forest | Logged-Over Forest | Plantation Forest | ||
| Moisture content (%) | 0–5 | 6.00 ± 0.62a | 8.00 ± 1.81a | 7.25 ± 2.28a |
| 5–10 | 3.27 ± 0.31a | 4.84 ± 0.66a | 3.13 ± 0.90a | |
| 10–30 | 2.71 ± 0.34ab | 3.75 ± 0.40a | 1.35 ± 0.53b | |
| Bulk Density (g cm−3) | 0–5 | 0.90 ± 0.04a | 0.83 ± 0.08a | 0.87 ± 0.05a |
| 5–10 | 0.99 ± 0.06a | 0.91 ± 0.13a | 1.15 ± 0.12a | |
| 10–30 | 1.33 ± 0.04a | 1.14 ± 0.11a | 1.32 ± 0.10a | |
| Soil Properties | Depth (cm) | Land Use | ||
|---|---|---|---|---|
| Intact Forest | Logged-Over Forest | Plantation Forest | ||
| pH | 0–5 | 4.31 ± 0.14a | 4.21 ± 0.18a | 4.54 ± 0.09a |
| 5–10 | 4.67 ± 0.16a | 4.50 ± 0.12a | 5.11 ± 0.38a | |
| 10–30 | 4.69 ± 0.14a | 4.68 ± 0.11a | 5.71 ± 0.48b | |
| Organic matter (%) | 0–5 | 13.48 ± 1.27a | 14.62 ± 2.20a | 14.26 ± 4.30a |
| 5–10 | 6.72 ± 0.40a | 8.53 ± 0.97a | 7.87 ± 1.24a | |
| 10–30 | 5.22 ± 0.40ab | 5.99 ± 0.53a | 3.34 ± 0.83b | |
| Total Phosphorus (meq/100 g) | 0–5 | 0.29 ± 0.03a | 0.17 ± 0.07a | 0.27 ± 0.14a |
| 5–10 | 0.86 ± 0.43a | 0.49 ± 0.24a | 0.16 ± 0.02a | |
| 10–30 | 0.26 ± 0.02a | 0.31 ± 0.21a | 0.02 ± 0.01a | |
| Land Use | Depth (cm) | Acid Cations (meq/100 g) | Base Cations (meq/100 g) | CEC (meq/100 g) | ||||
|---|---|---|---|---|---|---|---|---|
| H+ | Al3+ | Mg2+ | Ca2+ | Na+ | K+ | |||
| Intact Forest | 0–5 | 1.04 ± 0.18 | 3.97 ± 1.28 | 0.58 ± 0.12 | 2.47 ± 0.51 | 1.56 ± 0.37 | 1.12 ± 0.18 | 10.75 ± 1.73 |
| 5–10 | 0.89 ± 0.12 | 5.99 ± 1.16 | 0.97 ± 0.33 | 2.47 ± 0.65 | 1.26 ± 0.25 | 1.50 ± 0.27 | 13.09 ± 2.01 | |
| 10–30 | 0.72 ± 0.14 | 5.71 ± 0.89 | 0.93 ± 0.25 | 4.23 ± 0.93 | 1.03 ± 0.12 | 1.80 ± 0.34 | 14.43 ± 1.48 | |
| Logged-Over Forest | 0–5 | 1.47 ± 0.41 | 10.28 ± 3.91 | 0.77 ± 0.24 | 2.33 ± 1.07 | 1.43 ± 0.49 | 1.55 ± 0.46 | 17.84 ± 5.31 |
| 5–10 | 1.00 ± 0.28 | 4.86 ± 1.05 | 0.45 ± 0.06 | 1.60 ± 0.06 | 1.01 ± 0.39 | 0.70 ± 0.17 | 9.63 ± 1.37 | |
| 10–30 | 0.91 ± 0.25 | 10.68 ± 3.94 | 0.58 ± 0.18 | 1.35 ± 0.48 | 0.87 ± 0.11 | 0.75 ± 0.18 | 14.63 ± 4.82 | |
| Plantation Forest | 0–5 | 2.54 ± 0.39 | 2.91 ± 1.77 | 0.59 ± 0.24 | 2.95 ± 0.72 | 0.86 ± 0.05 | 0.87 ± 0.25 | 10.72 ± 3.32 |
| 5–10 | 0.73 ± 0.11 | 0.87 ± 0.47 | 0.28 ± 0.03 | 1.47 ± 0.03 | 0.54 ± 0.02 | 1.30 ± 1.06 | 5.18 ± 1.43 | |
| 10–30 | 0.46 ± 0.16 | 1.25 ± 0.65 | 0.25 ± 0.03 | 1.75 ± 0.46 | 0.57 ± 0.09 | 0.30 ± 0.04 | 4.58 ± 1.10 | |
| Soil Properties | Depth (cm) | Land Use | ||
|---|---|---|---|---|
| Intact Forest | Logged-Over Forest | Plantation Forest | ||
| Carbon (%) | 0–5 | 5.38 ± 1.16a | 6.30 ± 1.70a | 5.26 ± 1.16a |
| 5–10 | 2.91 ± 0.23a | 3.28 ± 1.07a | 3.75 ± 1.16a | |
| 10–30 | 2.23 ± 0.28a | 2.34 ± 0.77a | 1.86 ± 0.62a | |
| Nitrogen (%) | 0–5 | 0.41 ± 0.09a | 0.37 ± 0.08a | 0.44 ± 0.12a |
| 5–10 | 0.23 ± 0.06a | 0.24 ± 0.05a | 0.29 ± 0.12a | |
| 10–30 | 0.20 ± 0.03a | 0.11 ± 0.02a | 0.14 ± 0.08a | |
| C:N | 0–5 | 16.81 ± 2.15 | 17.03 ± 4.38 | 11.95 ± 1.91 |
| 5–10 | 24.25 ± 8.85 | 13.67 ± 6.45 | 12.93 ± 1.67 | |
| 10–30 | 20.27 ± 4.81 | 21.27 ± 11.04 | 13.29 ± 1.58 | |
| Land Use | Biomass (Mg ha−1) | Carbon (%) | Nitrogen (%) | C:N |
|---|---|---|---|---|
| Intact Forest | 4.46 ± 0.13a | 41.40 ± 0.68a | 1.31 ± 0.08a | 31.60 ± 2.16 |
| Logged-Over Forest | 3.02 ± 0.13ab | 40.10 ± 1.33a | 1.19 ± 0.11a | 33.70 ± 4.11 |
| Plantation Forest | 1.92 ± 0.09b | 38.79 ± 4.31a | 0.95 ± 0.04a | 40.83 ± 3.95 |
| Land Use | Biomass (Mg ha−1) | Carbon (%) | Nitrogen (%) | C:N |
|---|---|---|---|---|
| Intact Forest | 6.59 ± 0.05a | 47.53 ± 0.90a | 1.19 ± 0.15a | 43.63 ± 5.71a |
| Logged-Over Forest | 6.50 ± 0.05a | 44.20 ± 2.46a | 1.40 ± 0.04a | 32.25 ± 1.71a |
| Plantation Forest | 7.41 ± 0.07a | 46.73 ± 0.27a | 1.03 ± 0.12a | 48.58 ± 5.31a |
| Land Use | Soil Carbon Stock (Mg C ha−1) | Total Soil Carbon Stock (Mg C ha−1) | ||
|---|---|---|---|---|
| 0–5 cm | 5–10 cm | 10–30 cm | ||
| Intact Forest | 23.43 ± 3.03a | 16.35 ± 1.45a | 56.64 ± 5.58a | 96.42 |
| Logged-Over Forest | 26.15 ± 8.06a | 14.14 ± 4.35a | 50.85 ± 16.24a | 91.14 |
| Plantation Forest | 23.31 ± 6.20a | 13.70 ± 1.44a | 51.91 ± 18.93a | 88.92 |
| Land Use | Carbon Pools (Mg C ha−1) | Total Carbon Stock (Mg C ha−1) | CO2 Equivalent (Mg C ha−1) | ||
|---|---|---|---|---|---|
| Organic Layer | Litterfall | Soil | |||
| Intact Forest | 2.10 ± 0.23a | 3.10 ± 0.09a | 96.42 ± 4.21a | 101.62 | 372.95 |
| Logged-Over Forest | 1.42 ± 0.23ab | 3.05 ± 0.13a | 91.14 ± 7.23a | 95.61 | 350.89 |
| Plantation Forest | 0.90 ± 0.02b | 3.48 ± 0.09a | 88.92 ± 8.35a | 93.30 | 342.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suhaili, N.S.; Mhd Hatta, S.; James, D.; Hassan, A.; Jalloh, M.B.; Phua, M.-H.; Awang Besar, N. Soils Carbon Stocks and Litterfall Fluxes from the Bornean Tropical Montane Forests, Sabah, Malaysia. Forests 2021, 12, 1621. https://doi.org/10.3390/f12121621
Suhaili NS, Mhd Hatta S, James D, Hassan A, Jalloh MB, Phua M-H, Awang Besar N. Soils Carbon Stocks and Litterfall Fluxes from the Bornean Tropical Montane Forests, Sabah, Malaysia. Forests. 2021; 12(12):1621. https://doi.org/10.3390/f12121621
Chicago/Turabian StyleSuhaili, Nurul Syakilah, Syahrir Mhd Hatta, Daniel James, Affendy Hassan, Mohamadu Boyie Jalloh, Mui-How Phua, and Normah Awang Besar. 2021. "Soils Carbon Stocks and Litterfall Fluxes from the Bornean Tropical Montane Forests, Sabah, Malaysia" Forests 12, no. 12: 1621. https://doi.org/10.3390/f12121621







