The Prospect of Physiological Events Associated with the Micropropagation of Eucalyptus sp.
Abstract
:1. Introduction
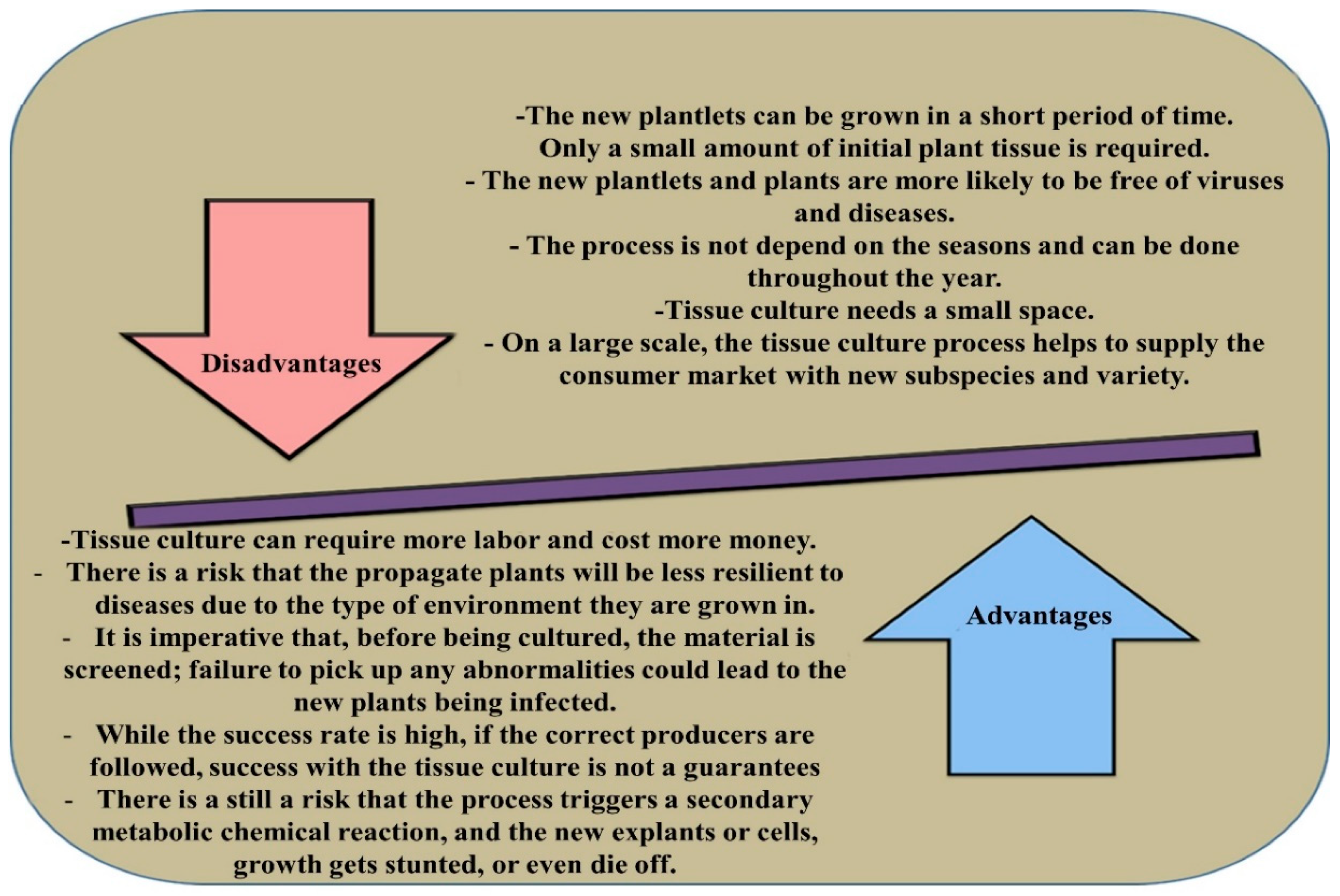
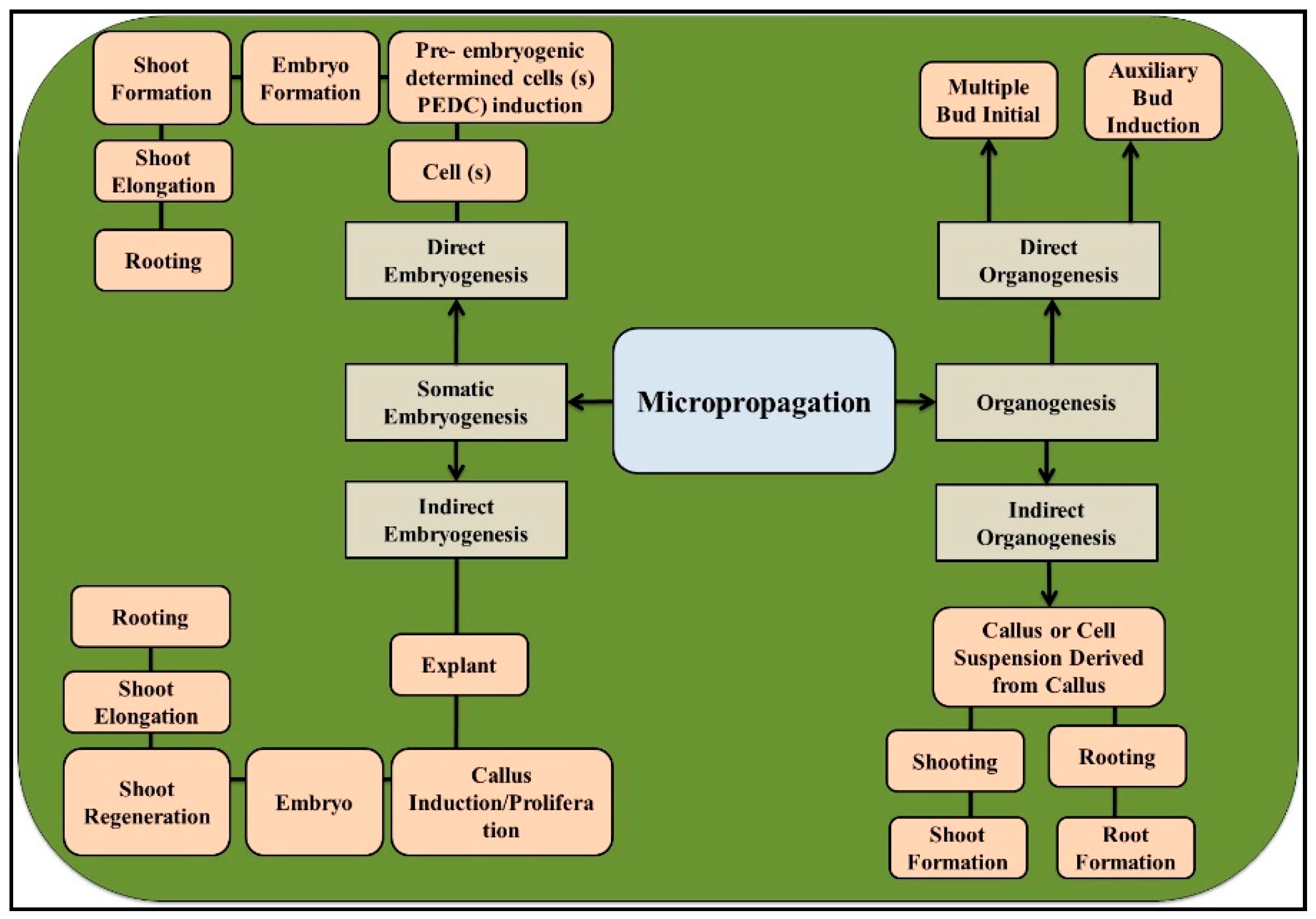
2. General Features of Micropropagation and Their Applications in Eucalyptus
2.1. Organogenesis
2.2. Somatic Embryogenesis
3. Importance of Eucalyptus Root Architecture and Behaviour
4. The Relationship between Root Structure and Shoot System in Eucalyptus
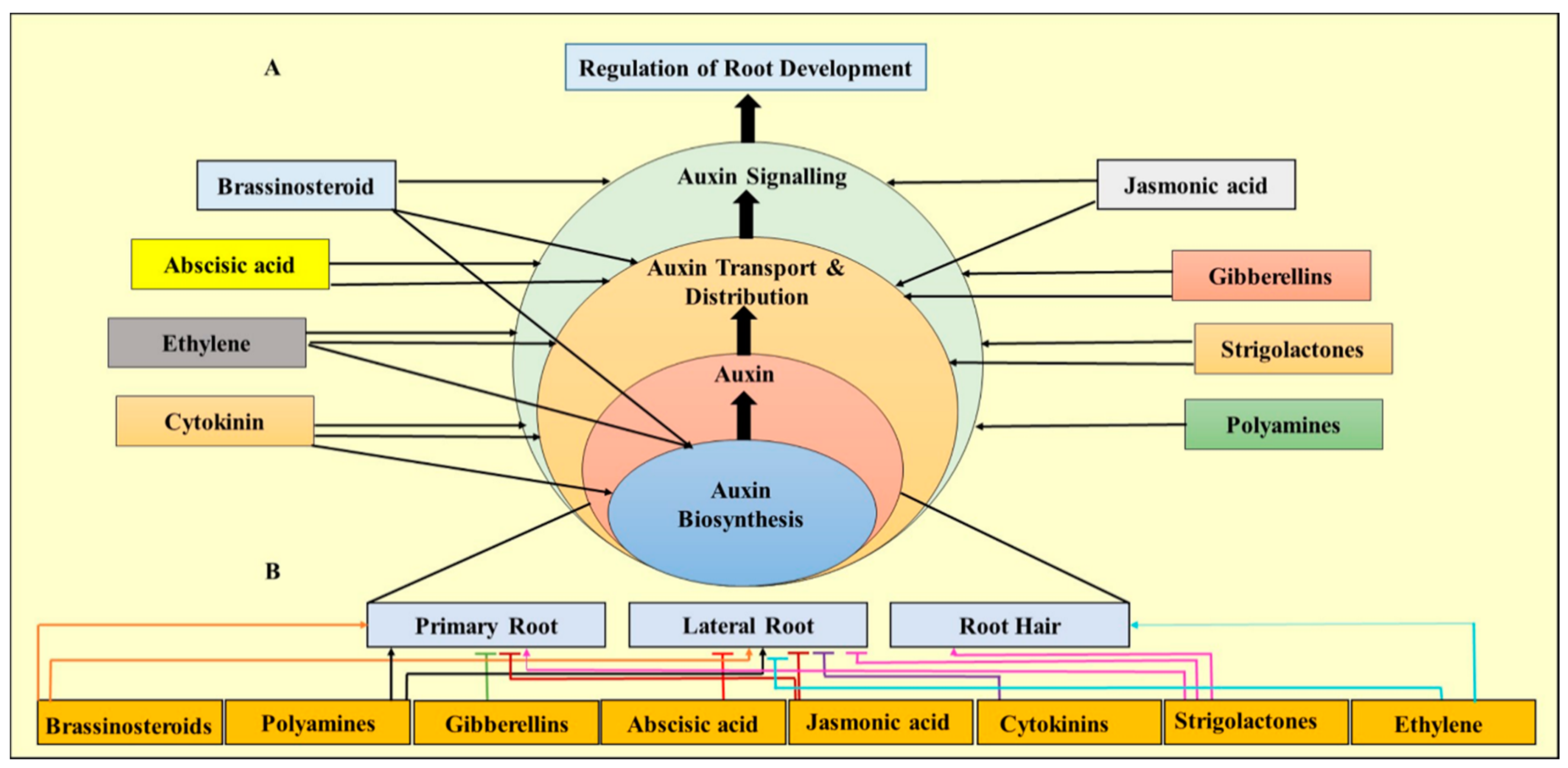
5. Role of Plant Growth Regulators (PGRs) in Micropropagation of Eucalyptus
5.1. Role of Auxins on Root Structure and Formation
5.2. Effect of Ethylene on Root Architecture
5.3. Role of Cytokinins in Micropropagation of Eucalyptus
5.4. Role of Auxins in Somatic Embryogenesis
6. Influence of Environmental and External Factors on Micropropagation of Eucalyptus
6.1. Effect of Media
6.2. Importance of Nitrogen, Calcium, Boron, and Colchicine on Micropropagation of Eucalyptus
6.3. Effect of Carbohydrate
6.4. Light and Radiation Effect
7. Regeneration and Acclimatisation of Eucalyptus
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Batista, T.R.; Mendonase, E.G.; Padua, M.S.; Stein, V.C.; Paiva, L. Morpho and cytological differentiation of calli of Eucalyptus grandis x Eucalyptus urophylla during somatic embryogenesis. Braz. Arch. Biol. Technol. 2018, 61, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Merkle, S.A.; Nairn, C.J. Hardwood tree biotechnology. In Vitro Cell. Dev. Biol. Plant. 2005, 41, 602–619. [Google Scholar] [CrossRef]
- Nogueira, M.C.D.J.A.; de Araujo, V.A.; Vasconcelos, J.S.; Christoforo, A.L.; Lahr, F.A.R. Sixteen properties of Eucalyptus Tereticornis wood for structural uses. Bioscience 2020, 36, 449–457. [Google Scholar] [CrossRef] [Green Version]
- Rezende, G.D.S.; de Resende, M.D.V.; de Assis, T.F. Eucalyptus breeding for clonal forestry. In Challenges and Opportunities for the World’s Forests in the 21st Century; Springer: Berlin/Heidelberg, Germany, 2014; pp. 393–424. [Google Scholar]
- Benra, F.; Nahuelhual, L.; Gaglio, M.; Gissi, E.; Aguayo, M.; Jullian, C.; Bonn, A. Ecosystem services tradeoffs arising from non-native tree plantation expansion in southern Chile. Landsc. Urban Plan. 2019, 190, 1–19. [Google Scholar] [CrossRef]
- Dhakad, A.K.; Pandey, V.V.; Beg, S.; Rawat, J.M.; Singh, A. Biological, medicinal and toxicological significance of Eucalyptus leaf essential oil: A review. J. Sci. Food Agric. 2018, 98, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, H.; Allsopp, M.H.; Canavan, S.; Cheek, M.; Geerts, S.; Geldenhuys, C.J.; Harding, G.; Hurley, B.P.; Jones, W.; Keet, J.H. Eucalyptus camaldulensis in South Africa- “past, present, future”. Trans. R. Soc. S. Afr. 2020, 75, 1–22. [Google Scholar] [CrossRef]
- Kerk, N.M.; Jiang, K.; Feldman, L.J. Auxin metabolism in the root apical meristem. Plant Physiol. 2000, 122, 925–932. [Google Scholar] [CrossRef] [Green Version]
- Stokes, A.; Atger, C.; Bengough, A.G.; Fourcaud, T.; Sidle, R.C. Desirable plant root traits for protecting natural and engineered slopes against landslides. Plant Soil 2009, 324, 1–30. [Google Scholar] [CrossRef]
- Monteuuis, O. Vegetatively propagating forest trees. In Proceedings of the Fourth International Conference of the IUFRO Unit 2.09.02 on “Development and Application of Vegetative Prpagation Technologiesd in Plantation Forestry Cope with a Changing Climate and Environemnt”, La Plata, Argentina, 19–23 September 2016; pp. 37–57. [Google Scholar]
- Naidoo, S.; Slippers, B.; Plett, J.M.; Coles, D.; Oates, C.N. The road to resistance in forest trees. Front. Plant Sci. 2019, 10, 273. [Google Scholar] [CrossRef] [Green Version]
- Nakhooda, M.; Watt, M.P.; Mycock, D. The choice of auxin analogue for in vitro root induction influences post-induction root development in Eucalyptus grandis. Turk. J. Agric. For. 2014, 38, 258–266. [Google Scholar] [CrossRef]
- Pinto, G.; Park, Y.; Neves, L.; Araujo, C.; Santos, C. Genetic control of somatic embryogenesis induction in Eucalyptus globulus Labill. Plant Cell Rep. 2008, 27, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.M. An updated overview of advances in somatic embryogenesis in forest trees. In Plantation Technology in Tropical Forest Science; Springer: Berlin/Heidelberg, Germany, 2006; pp. 113–122. [Google Scholar]
- Hussain, A.; Qarshi, I.A.; Nazir, H.; Ullah, I. Plant Tissue Culture: Current Status and Opportunities. Recent Advances in Plant In Vitro Culture; Leva, A., Ed.; InTech: London, UK, 2012; pp. 1–28. [Google Scholar]
- Girijashankar, V.; Sharma, K.; Balakrishna, P.; Seetharama, N. Direct somatic embryogenesis and organogenesis pathway of plant regeneration can seldom occur simultaneously within the same explant of sorghum. J. SAT Agric. Res. 2007, 3, 1–3. [Google Scholar]
- Stewart, C.N., Jr. Plant Biotechnology and Genetics: Principles, Techniques, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Johns, A.E. Lessons for Plant Micropropagation; Educreation Publishing: Chhattisgarh, India, 2019; p. 85. [Google Scholar]
- Trueman, S.J.; Hung, C.D.; Wendling, I. Tissue culture of Corymbia and Eucalyptus. Forests 2018, 9, 84. [Google Scholar] [CrossRef] [Green Version]
- Kendurkar, S.V.; Rangaswamy, M. in vitro Approaches for the Improvement of Eucalyptus. In Biotechnologies of Crop Improvement; Springer: Berlin/Heidelberg, Germany, 2018; pp. 159–214. [Google Scholar]
- Arya, I.; Chauhan, S.S.S.; Arya, S. Micropropagation of superior Eucalyptus hybrids FRI-5 (Eucalyptus camaldulensis Dehn x E. tereticornis Sm) and FRI-14 (Eucalyptus torelliana FV Muell x E. citriodora Hook): A commercial multiplication and field evaluation. Afr. J. Biotechnol. 2009, 8, 5718–5726. [Google Scholar]
- Prakash, M.; Gurumurthi, K. Effects of type of explant and age, plant growth regulators and medium strength on somatic embryogenesis and plant regeneration in Eucalyptus camaldulensis. Plant Cell Tissue Organ Cult. 2010, 100, 13–20. [Google Scholar] [CrossRef]
- Guerra, M.; Fraga, H.; Vieira, L.; Ree, J.; Heringer, A.; Maldonado, S.B. Fundamentals, advances and applications of somatic embryogenesis in selected Brazilian native species. Acta Hortic. 2016, 1113, 1–12. [Google Scholar] [CrossRef]
- Kundu, S.; Gantait, S. Fundamental facets of somatic embryogenesis and its applications for advancement of peanut biotechnology. In Biotechnologies of Crop Improvement; Springer: Berlin/Heidelberg, Germany, 2018; pp. 267–298. [Google Scholar]
- Nazir, M.; Sadat, S.; Soltani Howyzeh, M. The effect of different hormone combinations on direct and indirect somatic embryogenesis in Agave americana. Plant Physiol. 2019, 9, 2739–2747. [Google Scholar]
- Ochatt, S.J.; Abirached-Darmency, M. The underlying processes governing seed size plasticity: Impact of endoploidy on seed coat development and cell expansion in Medicago truncatula. In The Model Legume Medicago truncatula, 1st ed.; de Bruijn, F.J., Ed.; John Wiley & Sons, Inc.: London, UK, 2020; pp. 99–115. [Google Scholar]
- Elmeer, K.E.S. Factors regulating somatic embryogenesis in plants. In Somatic Embryogenesis and Gene Expression; Narosa Publishing House: New Delhi, India, 2013; pp. 56–81. [Google Scholar]
- Martinez, M.T.; San Jose, M.D.C.; Arrillaga, I.; Cano, V.; Morcillo, M.; Cernadas, M.J.; Corredoira, E. Holm oak somatic embryogenesis: Current status and future perspectives. Front. Plant Sci. 2019, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Lelu-Walter, M.A.; Thompson, D.; Harvengt, L.; Sanchez, L.; Toribio, M.; Paques, L.E. Somatic embryogenesis in forestry with a focus on Europe: State-of-the-art, benefits, challenges and future direction. Tree Genet. Genom. 2013, 9, 883–899. [Google Scholar] [CrossRef]
- Moura, L.C.D.; Xavier, A.; Cruz, A.C.F.D.; Gallo, R.; Gatti, K.C.; Miranda, N.A.; Otoni, W.C. Effects of explant type, culture media and picloram and dicamba growth regulators on induction and proliferation of somatic embryos in Eucalyptus grandis x E urophylla1. Rev. Árvore 2017, 41, 1–10. [Google Scholar] [CrossRef]
- Carraro, N.; Tisdale-Orr, T.E.; Clouse, R.M.; Knoller, A.S.; Spicer, R. Diversification and expression of the PIN, AUX/LAX, and ABCB families of putative auxin transporters in Populus. Front. Plant Sci. 2012, 3, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, G.; Park, Y.S.; Loureiro, J.; Neves, L.; Araujo, C.; Silva, S.; Santos, C. Somatic embryogenesis in Eucalyptus -an update to 2009. Appl. Plant Biotechnol. In Vitro Propagat. Plant Transf. Second. Metabol. Prod. 2009, 1, 531–542. [Google Scholar]
- Hiti-Bandaralage, J.C.; Hayward, A.; Mitter, N. Micropropagation of avocado (Persea americana Mill.). Am. J. Plant Sci. 2017, 8, 2898. [Google Scholar] [CrossRef] [Green Version]
- Nourissier, S.; Monteuuis, O. in vitro rooting of two Eucalyptus urophylla x Eucalyptus grandis mature clones. In Vitro Cell. Dev. Biol. Plant. 2008, 44, 263–272. [Google Scholar] [CrossRef]
- George, E.F.; Hall, M.A.; De Klerk, G.J. Plant Propagation by Tissue Culture: Volume 1. The Background; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Nakhooda, M.; Jain, S.M. A review of Eucalyptus propagation and conservation. Propag. Ornam. Plants. 2016, 16, 101–119. [Google Scholar]
- Mokotedi, M.E.; Watt, M.; Pammenter, N. Analysis of differences in field performance of vegetatively andseed-propagated Eucalyptus varieties II: Vertical uprooting resistance. South For. 2010, 72, 31–36. [Google Scholar] [CrossRef]
- Geiss, G.; Gutierrez, L.; Bellini, C. Adventitious root formation: New insights and perspectives. Annu. Rev. Plant Biol. 2018, 37, 127–156. [Google Scholar]
- Da Costa, C.T.; De Almeida, M.R.; Ruedell, C.M.; Schwambach, J.; Maraschin, F.D.S.; Fett-Neto, A.G. When stress and development go hand in hand: Main hormonal controls of adventitious rooting in cuttings. Front. Plant Sci. 2013, 4, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilasboa, J.; Da Costa, C.T.; Fett-Neto, A.G. Rooting of eucalypt cuttings as a problem-solving oriented model in plant biology. PBMB 2019, 146, 85–97. [Google Scholar] [CrossRef]
- Druege, U.; Franken, P.; Hajirezaei, M.R. Plant hormone homeostasis, signaling, and function during adventitious root formation in cuttings. Front. Plant Sci. 2016, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Nakhooda, M.; Watt, M. Adventitious root formation in Eucalyptus: The role of phytohormones. Acta Hortic. 2015, 1155, 505–512. [Google Scholar]
- Wang, H.; Inukai, Y.; Yamauchi, A. Root development and nutrient uptake. Crit. Rev. Plant Sci. 2006, 25, 279–301. [Google Scholar] [CrossRef]
- Nakhooda, M.; Watt, M.P.; Mycock, D. The properties and interaction of auxins and cytokinins influence rooting of shoot cultures of Eucalyptus. Afr. J. Biotechnol. 2012, 11, 16568–16578. [Google Scholar]
- Rashotte, A.M.; Brady, S.R.; Reed, R.C.; Ante, S.J.; Muday, G.K. Basipetal auxin transport is required for gravitropism in roots of Arabidopsis. Plant Physiol. 2000, 122, 481–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolan, L.; Davies, J. Cell expansion in roots. Curr. Opin. Plant Biol. 2004, 7, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Nakhooda, M.; Watt, M.P.; Mycock, D. Auxin stability and accumulation during in vitro shoot morphogenesis influences subsequent root induction and development in Eucalyptus grandis. Plant Growth Regul. 2011, 65, 263–271. [Google Scholar] [CrossRef]
- Mokany, K.; Raison, R.J.; Prokushkin, A.S. Critical analysis of root: Shoot ratios in terrestrial biomes. Glob. Chang. Biol. 2006, 12, 84–96. [Google Scholar] [CrossRef]
- Jacobs, D.; Salifu, K.; Seifert, J. Relative contribution of initial root and shoot morphology in predicting field performance of hardwood seedlings. New For. 2005, 30, 235–251. [Google Scholar] [CrossRef]
- Gregory, P.J. Plant Roots: Growth, Activity and Interactions with the Soil; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Nic-Can, G.I.; Loyola-Vargas, V.M. The role of the auxins during somatic embryogenesis. In Somatic Embryogenesis: Fundamental Aspects and Applications; Springer: Berlin/Heidelberg, Germany, 2016; pp. 171–182. [Google Scholar]
- Moura, L.C.D.; Xavier, A.; Cruz, A.C.F.D.; Gallo, R.; Miranda, N.A.; Otoni, W.C. Auxin pulse in the induction of somatic embryos of Eucalyptus. Rev. Árvore 2019, 43, 1–12. [Google Scholar] [CrossRef]
- Feher, A.; Pasternak, T.P.; Dudits, D. Transition of somatic plant cells to an embryogenic state. Plant Cell Tissue Organ Cult. 2003, 74, 201–228. [Google Scholar] [CrossRef]
- Aloni, R.; Aloni, E.; Langhans, M.; Ullrich, C. Role of cytokinin and auxin in shaping root architecture: Regulating vascular differentiation, lateral root initiation, root apical dominance and root gravitropism. Ann. Bot. 2006, 97, 883–893. [Google Scholar] [CrossRef]
- Swarup, R.; Bennett, M.J. Root gravitropism. Annu. Rev. Plant Biol. 2018, 37, 157–174. [Google Scholar] [CrossRef]
- Woodward, A.W.; Bartel, B. Auxin: Regulation, action, and interaction. Ann. Bot. 2005, 95, 707–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, E.A.; Gibson, R.; Wightman, F. Biosynthesis and metabolism of indol-3yl-acetic acid: I. The native indoles of barley and tomato shoots. JExB 1972, 23, 152–170. [Google Scholar] [CrossRef]
- Tromas, A.; Perrot-Rechenmann, C. Recent progress in auxin biology. Comptes Rendus Biol. 2010, 333, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Ludwig-MUller, J. Indole-3-butyric acid in plant growth and development. Plant Growth Regul. 2000, 32, 219–230. [Google Scholar] [CrossRef]
- Wiesman, Z.; Riov, J.; Epstein, E. Characterization and rooting ability of indole-3-butyric acid conjugates formed during rooting of mung bean cuttings. Plant Physiol. 1989, 91, 1080–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epstein, E.; Ludwig-Muller, J. Indole-3-butyric acid in plants: Occurrence, synthesis, metabolism and transport. Physiol. Plant 1993, 88, 382–389. [Google Scholar] [CrossRef]
- Kevers, C.; Hausman, J.F.; Faivre-Rampant, O.; Dommes, J.; Gaspar, T. What we have learned about the physiology of in vitro adventitious rooting of woody plants and how it is relates to improvements in the practice. In Adventitious Root Formation of Forest Trees and Horticultural Plants—From Genes to Applications; Niemi, K., Ed.; Research Signpost: Trivandrum, India, 2009; pp. 400–417. [Google Scholar]
- George, E.F.; Hall, M.A.; De Klerk, G.J. Plant tissue culture procedure-background. In Plant Propagation by Tissue Culture; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1–28. [Google Scholar]
- Verstraeten, I.; Schotte, S.; Geelen, D. Hypocotyl adventitious root organogenesis differs from lateral root development. Front. Plant Sci. 2014, 5, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Won, C.; Shen, X.; Mashiguchi, K.; Zheng, Z.; Dai, X.; Cheng, Y.; Kasahara, H.; Kamiya, Y.; Chory, J.; Zhao, Y. Conversion of tryptophan to indole-3-acetic acid by TRYPTOPHAN AMINOTRANSFERASES OF ARABIDOPSIS and YUCCAs in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 18518–18523. [Google Scholar] [CrossRef] [Green Version]
- Rambaran, N. Survival and rooting of selected vegetatively propagated Eucalyptus clones in relation to supplied auxin. In Life Sciences, College of Agriculture, Engineering and Scienc; University of KwaZulu-Natal: Durban, South Africa, 2013; p. 84. [Google Scholar]
- Irvani, N.; Solouki, M.; Omidi, M.; Zare, A.; Shahnazi, S. Callus induction and plant regeneration in Dorem ammoniacum D., an endangered medicinal plant. Plant Cell Tissue Organ Cult 2010, 100, 293–299. [Google Scholar] [CrossRef]
- Traas, J. Organogenesis at the shoot apical meristem. Plants 2019, 8, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayala, P.G.; Brugnoli, E.A.; Luna, C.V.; Gonzalez, A.M.; Pezzutti, R.; Sansberro, P.A. Eucalyptus nitens plant regeneration from seedling explants through direct adventitious shoot bud formation. Trees 2019, 33, 1667–1678. [Google Scholar] [CrossRef]
- Baltierra, X.C.; Montenegro, G.; De Garcia, E. Ontogeny of in vitro rooting processes in Eucalyptus globulus. In Vitro Cell. Dev. Biol. Plant 2004, 40, 499–503. [Google Scholar] [CrossRef]
- Pernisova, M.; Klma, P.; Horak, J.; Valkova, M.; Malbeck, J.; Soucek, P.; Reichman, P.; Hoyerova, K.; Dubova, J.; Friml, J. Cytokinins modulate auxin-induced organogenesis in plants via regulation of the auxin efflux. Proc. Natl. Acad. Sci. USA 2009, 106, 3609–3614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Y.H.; Liu, Y.B.; Zhang, X.S. Auxin-cytokinin interaction regulates meristem development. Mol. Plant 2011, 4, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Moore, I. Gravitropism: Lateral thinking in auxin transport. Curr. Biol. 2002, 12, R452–R454. [Google Scholar] [CrossRef] [Green Version]
- Muday, G.K. Auxins and tropisms. J. Plant Growth Regul. 2001, 20, 226–243. [Google Scholar] [CrossRef]
- Geldner, N.; Friml, J.; Stierhof, Y.D.; JUrgens, G.; Palme, K. Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 2001, 413, 425–428. [Google Scholar] [CrossRef]
- Aumond, M.L., Jr.; de Araujo, A.T., Jr.; de Oliveira Junkes, C.F.; de Almeida, M.R.; Matsuura, H.N.; de Costa, F.; Fett-Neto, A.G. Events associated with early age-related decline in adventitious rooting competence of Eucalyptus globulus Labill. Front. Plant Sci. 2017, 8, 1734. [Google Scholar] [CrossRef] [Green Version]
- Guan, L.; Murphy, A.S.; Peer, W.A.; Gan, L.; Li, Y.; Cheng, Z.M. Physiological and molecular regulation of adventitious root formation. Crit. Rev. Plant Sci. 2015, 34, 506–521. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, C.; Liu, W.; Tang, Y.; Qi, H.; Chen, H.; Cao, S. The alcohol dehydrogenase gene family in melon (Cucumis melo L.): Bioinformatic analysis and expression patterns. Front. Plant Sci. 2016, 7, 670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douady, S.; Couder, Y. The F-box protein TIR1 is an auxin receptor. Nature 1992, 68, 441–445. [Google Scholar]
- Grones, P.; Chen, X.; Simon, S.; Kaufmann, W.A.; De Rycke, R.; Nodzynski, T.; Zazimalova, E.; Friml, J. Auxin-binding pocket of ABP1 is crucial for its gain-of-function cellular and developmental roles. JExB 2015, 66, 5055–5065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheng, L.; Hu, X.; Du, Y.; Zhang, G.; Huang, H.; Scheres, B.; Xu, L. Non-canonical WOX11-mediated root branching contributes to plasticity in Arabidopsis root system architecture. Development 2017, 144, 3126–3133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, K.I. The interaction and integration of auxin signaling components. Plant Cell Physiol. 2012, 53, 965–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Almeida, M.R.; Ruedell, C.M.; Ricachenevsky, F.K.; Sperotto, R.A.; Pasquali, G.; Fett-Neto, A.G. Reference gene selection for quantitative reverse transcription-polymerase chain reaction normalization during in vitro adventitious rooting in Eucalyptus globulus Labill. BMC Mol. Biol. 2010, 11, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ruedell, C.M.; de Almeida, M.R.R.; Schwambach, J.L.; Posenato, C.F.; Fett-Neto, A.G. Pre and post-severance effects of light quality on carbohydrate dynamics and microcutting adventitious rooting of two Eucalyptus species of contrasting recalcitrance. Plant Growth Regul. 2013, 69, 235–245. [Google Scholar] [CrossRef]
- Aggarwal, D.; Kumar, A.; Sharma, J.; Reddy, M.S. Factors affecting micropropagation and acclimatization of an elite clone of Eucalyptus tereticornis Sm. In Vitro Cell Dev Biol. 2012, 48, 521–529. [Google Scholar] [CrossRef]
- Silva de Oliveira, L.; Brondani, G.E.; Batagin-Piotto, K.D.; Calsavara, R.; Gonçalves, A.N.; de Almeida, M. Micropropagation of Eucalyptus cloeziana mature trees. Aust. For. 2015, 78, 219–231. [Google Scholar] [CrossRef]
- Brondani, G.E.; Dutra, L.F.; Wendling, I.; Grossi, F.; Hansel, F.A.; Araujo, M.A. Micropropagation of an Eucalyptus hybrid (Eucalyptus benthamii x Eucalyptus dunnii). Acta Sci. Agron. 2011, 3, 655–663. [Google Scholar] [CrossRef] [Green Version]
- Ricci, A.; Bertoletti, C. Urea derivatives on the move: Cytokinin-like activity and adventitious rooting enhancement depend on chemical structure. Plant Biol. 2009, 11, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Rolli, E.; Incerti, M.; Brunoni, F.; Vicini, P.; Ricci, A. Structure-activity relationships of N-phenyl-N’-benzothiazol-6-ylurea synthetic derivatives: Cytokinin-like activity and adventitious rooting enhancement. Phytochemistry 2012, 74, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Romanov, G.A.; Lomin, S.N.; Schmilling, T. Cytokinin signaling: From the ER or from the PM? That is the question! New Phytol. 2018, 218, 41–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.; Ouyang, L.; Li, Z.; Zeng, F. A urea-type cytokinin, 2-Cl-PBU, stimulates adventitious bud formation of Eucalyptus urophylla by repressing transcription of rboh1 gene. Plant Cell Tissue Organ Cult. 2014, 119, 359–368. [Google Scholar] [CrossRef]
- Lee, S.H.; Tewari, R.K.; Hahn, E.J.; Paek, K.Y. Photon flux density and light quality induce changes in growth, stomatal development, photosynthesis and transpiration of Withania somnifera (L.) Dunal. plantlets. Plant Cell Tissue Organ Cult. 2007, 90, 141–151. [Google Scholar] [CrossRef]
- Li, X.-S.; Wang, D.-H. Suppression of thermogenic capacity during reproduction in primiparous Brandt’s voles (Microtus brandtii). J. Therm. Biol. 2005, 30, 431–436. [Google Scholar] [CrossRef]
- Huang, Z.; Li, H. Control of oxidative stress by a combination of PBU, BAP and DMTU enhances adventitious shoot formation in Eucalyptus urophylla. Plant Cell Tissue Organ Cult. 2020, 141, 533–541. [Google Scholar] [CrossRef]
- Huang, Z.C.; Zeng, F.H.; Lu, X.Y. Efficient regeneration of Eucalyptus urophylla from seedling-derived hypocotyls. Biol. Plantarum 2010, 54, 131–134. [Google Scholar] [CrossRef]
- Brunoni, F.; Rolli, E.; Dramis, L.; Incerti, M.; Abarca, D.; Pizarro, A.; Diaz-Sala, C.; Ricci, A. Adventitious rooting adjuvant activity of 1, 3-di (benzo [d] oxazol-5-yl) urea and 1, 3-di (benzo [d] oxazol-6-yl) urea: New insights and perspectives. Plant Cell Tissue Organ Cult. 2014, 118, 111–124. [Google Scholar] [CrossRef]
- Oberschelp, G.P.J.; Goncalves, A.N.; Meneghetti, E.C.; Graner, A.M.; de Almeida, M. Eucalyptus dunnii Maiden plant regeneration via shoot organogenesis on a new basal medium based on the mineral composition of young stump shoots. In Vitro Cell. Dev. Biol. Plant 2015, 51, 626–636. [Google Scholar] [CrossRef]
- Overvoorde, P.; Fukaki, H.; Beeckman, T. Auxin control of root development. Cold Spring Harbor Perspect. Biol. 2010, 2, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- PamfilL, D.; Bellini, C. Auxin control in the formation of adventitious roots. Not. Bot. Horti Agrob. Cluj Napoca 2011, 39, 307–316. [Google Scholar]
- Feher, A. Why somatic plant cells start to form embryos? In Somatic Embryogenesis; Springer: Berlin/Heidelberg, Germany, 2005; pp. 85–101. [Google Scholar]
- Picoli, E.D.T. Growth regulators pulsing pre-treatment effect on the Eucalyptus grandis shoot and adventitious root morphogenesis. Plant Cell Cult. Micropropag. 2006, 2, 20–28. [Google Scholar]
- Prakash, M.; Gurumurthi, K. Somatic embryogenesis and plant regeneration in Eucalyptus tereticornis Sm. Curr. Sci. 2005, 88, 1311–1316. [Google Scholar]
- Esposito-Polesi, N.P.; de Oliveira, L.S.; Baccarin, F.J.B.; de Almeida, C.V.; de Almeida, M. Different culture conditions applied to in vitro shoot multiplication of two Eucalyptus benthamii explant sources. J. For. Res. 2020, 31, 857–869. [Google Scholar] [CrossRef]
- De Mattos Barretto, V.C.; Valeri, S.V.; de Arruda Silveira, R.L.V.; Takahashi, E.N. Efficiency of boron use in the growth of Eucalyptus clones in pots. Sci. For. For. Sci. 2007, 76, 21–33. [Google Scholar]
- De Moura, L.C.; Xavier, A.; da Cruz, A.C.F.; Batista, D.S.; Gallo, R.; Miranda, N.A.; Otoni, W.C. Effect of calcium, BAP and putrescine on somatic embryo induction in juvenile explants of Eucalyptus grandis × E. urophylla hybrids. Aust. J. Crop Sci. 2019, 13, 513. [Google Scholar] [CrossRef]
- Kaur, G.; Sharma, A.; Guruprasad, K.; Pati, P.K. Versatile roles of plant NADPH oxidases and emerging concepts. Biotechnol. Adv. 2014, 32, 551–563. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; Van Breusegem, F. ROS signaling: The new wave? Trends Plant Sci. 2011, 16, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Gu, Q.; Zhu, M. The involvement of hydrogen peroxide and antioxidant enzymes in the process of shoot organogenesis of strawberry callus. Plant Sci. 2003, 165, 701–707. [Google Scholar] [CrossRef]
- Tibok, A.; Blackhall, N.; Power, J.; Davey, M. Optimized plant regeneration from callus derived from seedling hypocotyls of Eucalyptus urophylla. Plant Sci. 1995, 110, 139–145. [Google Scholar] [CrossRef]
- Pinto, G.; Araujo, C.; Santos, C.; Neves, L. Plant regeneration by somatic embryogenesis in Eucalyptus spp.: Current status and future perspectives. South For. 2013, 75, 59–69. [Google Scholar] [CrossRef]
- Ribeiro, C.W.; Korbes, A.P.; Garighan, J.A.; Jardim-Messeder, D.; Carvalho, F.E.; Sousa, R.H.; Caverzan, A.; Teixeira, F.K.; Silveira, J.A.; Margis-Pinheiro, M. Rice peroxisomal ascorbate peroxidase knockdown affects ROS signaling and triggers early leaf senescence. Plant Sci. 2017, 263, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, R.; Rios, D.; Aviles, F.; Sainchez-Olate, M. in vitro multiplication of Eucalyptus globulus by temporary immersion system. Bosque 2011, 32, 147–154. [Google Scholar] [CrossRef]
- Grzyb, M.G.; Mikula, A. Explant type and stress treatment determine the uni-and multicellular origin of somatic embryos in the tree fern Cyathea delgadii Sternb. Plant Cell Tissue Organ Cult. 2019, 136, 221–230. [Google Scholar] [CrossRef] [Green Version]
- Anjum, N.A.; Sharma, P.; Gill, S.S.; Hasanuzzaman, M.; Khan, E.A.; Kachhap, K.; Mohamed, A.A.; Thangavel, P.; Devi, G.D.; Vasudhevan, P. Catalase and ascorbate peroxidase-representative H2O2-detoxifying heme enzymes in plants. Environ. Sci. Pollut. Res. 2016, 23, 19002–19029. [Google Scholar] [CrossRef]
- Pandey, S.; Fartyal, D.; Agarwal, A.; Shukla, T.; James, D.; Kaul, T.; Negi, Y.K.; Arora, S.; Reddy, M.K. Abiotic stress tolerance in plants: Myriad roles of ascorbate peroxidase. Front. Plant Sci. 2017, 8, 581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sukweenadhi, J.; Kim, Y.J.; Rahimi, S.; Silva, J.; Myagmarjav, D.; Kwon, W.S.; Yang, D.C. Overexpression of a cytosolic ascorbate peroxidase from Panax ginseng enhanced salt tolerance in Arabidopsis thaliana. Plant Cell Tissue Organ Cult. 2017, 129, 337–350. [Google Scholar] [CrossRef]
- Foreman, J.; Demidchik, V.; Bothwell, J.H.; Mylona, P.; Miedema, H.; Torres, M.A.; Linstead, P.; Costa, S.; Brownlee, C.; Jones, J.D. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 2003, 422, 442–446. [Google Scholar] [CrossRef]
- Rajesh, M.; Radha, E.; Sajini, K.; Anitha, K. Polyamine-induced somatic embryogenesis and plantlet regeneration in vitro from plumular explants of dwarf cultivars of coconut (Cocos nucifera). Indian J. Agric. Sci. 2014, 84, 527–530. [Google Scholar]
- Corredoira, E.; Merkle, S.; Martinez, M.; Toribio, M.; Canhoto, J.; Correia, S.; Ballester, A.; Vieitez, A. Non-zygotic embryogenesis in hardwood species. Crit. Rev. Plant Sci. 2019, 38, 29–97. [Google Scholar] [CrossRef]
- Feher, A. Somatic embryogenesis-stress-induced remodeling of plant cell fate. Biochim. Biophys. Acta Gene Regul. Mech. 2015, 1849, 385–402. [Google Scholar] [CrossRef] [PubMed]
- Chieng, L.M.; Chen, T.; Sim, S.; Goh, D.K. Induction of organogenesis and somatic embryogenesis of Gonystylus bancanus (Miq.) Kurz (Ramin) In Sarawak. Kuching Sarawak For. Corpor. ITTO 2014, 1, 1–28. [Google Scholar]
- Corredoira, E.; Ballester, A.; Ibarra, M.; Vieitez, A. Induction of somatic embryogenesis in leaf and shoot apex explants from adult trees of the genus Eucalyptus. In Proceedings of the Third International Conference of the IUFRO unit 2.09. 02: Somatic Embryogenesis and Other Vegetative Propagation Technologies, Vitoria-Gasteiz, Spain, 18–12 September 2014; p. 66. [Google Scholar]
- Corredoira, E.; Ballester, A.; Ibarra, M.; Vieitez, A. Induction of somatic embryogenesis in explants of shoot cultures established from adult Eucalyptus globulus and E. salignaa × E. maidenii trees. Tree Physiol. 2015, 35, 678–690. [Google Scholar] [CrossRef]
- Shirin, F.; Rana, P. in vitro plantlet regeneration from nodal explants of field-grown culms in Bambusa glaucescens Willd. Plant Biotechnol. Rep. 2007, 1, 141–147. [Google Scholar] [CrossRef]
- Kothari, S.; Agarwal, K.; Kumar, S. Inorganic nutrient manipulation for highly improved in vitro plant regeneration in finger millet- Eleusine coracana (L.) Gaertn. In Vitro Cell. Dev. Biol. Plant. 2004, 40, 515–519. [Google Scholar] [CrossRef]
- Debergh, P.; Maene, L. A scheme for commercial propagation of ornamental plants by tissue culture. Sci. Hortic. 1981, 14, 335–345. [Google Scholar] [CrossRef]
- Dibax, R.; Quisen, R.C.; Bona, C.; Quoirin, M. Plant regeneration from cotyledonary explants of Eucalyptus camaldulensis Dehn and histological study of organogenesis in vitro. Braz. Arch. Biol. Technol. 2010, 53, 311–318. [Google Scholar] [CrossRef] [Green Version]
- Radmann, E.B.; Bianchi, V.J.; Souza, T.M.; Fachinello, J.C.; de Oliveria, R.P. Influencia da composiao do meio de cultivo e do tipo de explante na micropropagaation do porta-enxerto de Prunus sp. GXN-9. Sci. Agraria 2009, 10, 095–101. [Google Scholar] [CrossRef] [Green Version]
- Oberschelp, G.P.J.; Goncalves, A.N. Assessing the effects of basal media on the in vitro propagation and nutritional status of Eucalyptus dunnii Maiden. In Vitro Cell. Dev. Biol. Plant. 2016, 52, 28–37. [Google Scholar] [CrossRef]
- Brondani, G.E.; de Wit Ondas, H.W.; Baccarin, F.J.B.; Goncalves, A.N.; de Almeida, M. Micropropagation of Eucalyptus benthamii to form a clonal micro-garden. In Vitro Cell. Dev. Biol. Plant. 2012, 48, 478–487. [Google Scholar] [CrossRef]
- Glocke, P.; Delaporte, K.; Collins, G.; Sedgley, M. Micropropagation of juvenile tissue of Eucalyptus erythronema × Eucalyptus stricklandii cv.’urrbrae gem’. In Vitro Cell. Dev. Biol. Plant. 2006, 42, 139–143. [Google Scholar] [CrossRef]
- Lanoue, J.; Leonardos, E.D.; Grodzinski, B. Effects of light quality and intensity on diurnal patterns and rates of photo-assimilate translocation and transpiration in tomato leaves. Front. Plant Sci. 2018, 9, 756. [Google Scholar] [CrossRef] [Green Version]
- Lima, L.; Seabra, A.; Melo, P.; Cullimore, J.; Carvalho, H. Post-translational regulation of cytosolic glutamine synthetase of Medicago truncatula. JExB 2006, 57, 2751–2761. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Ziv, M. The effect of ancymidol on hyperhydricity, regeneration, starch and antioxidant enzymatic activities in liquid-cultured Narcissus. Plant Cell Rep. 2001, 20, 22–27. [Google Scholar] [CrossRef]
- Levin, R.; Alper, Y.; Stav, R.; Watad, A.A. Methods and apparatus for liquid media and semi-automated micropropagation. Acta Hortic. 1996, 447, 659–664. [Google Scholar] [CrossRef]
- Ziv, M. The control of bioreactor environment for plant propagation in liquid culture. Acta Hortic. 1994, 393, 25–38. [Google Scholar] [CrossRef]
- Ramage, C.M.; Williams, R.R. Mineral nutrition and plant morphogenesis. In Vitro Cell. Dev. Biol. Plant. 2002, 38, 116–124. [Google Scholar] [CrossRef]
- Borges, S.R.; Xavier, A.; Oliveira, L.S.D.; Lopes, A.P.; Otoni, W.C. in vitro multiplication of hybrid clones of Eucalyptus globulus. Rev. Árvore 2011, 35, 173–182. [Google Scholar] [CrossRef] [Green Version]
- Brondani, G.E.; Araujo, M.A.D.; Alcantara, B.K.D.; Carvalho, J.G.D.; Goncalves, A.N.; Almeida, M.d. in vitro organogenesis of Eucalyptus grandis: Effects of boron and calcium. Acta Sci. Agron. 2012, 34, 403–411. [Google Scholar] [CrossRef] [Green Version]
- Hannachi, S. Morphological, Ecophysiological and Biochemical Responses to Salt Stress in Eggplant (Solanum melongena L.); Ghent University: Ghent, Belgium, 2016. [Google Scholar]
- Lanteri, M.L.; Pagnussat, G.C.; Lamattina, L. Calcium and calcium-dependent protein kinases are involved in nitric oxide-and auxin-induced adventitious root formation in cucumber. JExB 2006, 57, 1341–1351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva, A.L.L.; Tormen, G.C.R.; de Figueiredo, A.J.R.; dos Santos Ribeiro, A.L.; dos Santos, L.F.; Araujo, J.F.; Brondani, G.E. Carbohydrate sources, alanine and calcium for in vitro multiplication of Eucalyptus cloeziana F. Muell. Iheringia. Ser. Botan. 2018, 73, 329–335. [Google Scholar]
- Rasheed, M.K. Role of boron in plant growth: A review. J. Agric. Res. 2009, 47, 329–338. [Google Scholar]
- Oberschelp, G.P.J. In Vitro Propagation of Eucalyptus Dunnii Maiden: Development of a New Basal Environment and Estimation of Genetic Parameters for Morphophysiological Characteristics; Escola Superior de Agricultura Luiz de Queiroz, Universidade de Saeo Paulo: Piracicaba, Brazil, 2014. [Google Scholar]
- Tanaka, M.; Fujiwara, T. Physiological roles and transport mechanisms of boron: Perspectives from plants. Pflugers Arch. 2008, 456, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Jian, M.; Liang, L.; Pei, W.; Liu, X.; Zhang, H. Production of polyploids from cultured shoot tips of Eucalyptus globulus Labill by treatment with colchicine. Afr. J. Biotechnol. 2010, 9, 2252–2255. [Google Scholar]
- Carvalho, D.C.D.; Silva, A.L.L.D.; Schuck, M.R.; Purcino, M.; Tanno, G.N.; Biasi, L.A. Fox grape cv. Bordo (Vitis labrusca L.) and grapevine cv. Chardonnay (Vitis vinifera L.) cultivated in vitro under different carbohydrates, amino acids and 6-Benzylaminopurine levels. Braz. Arch. Biol. Technol. 2013, 56, 191–201. [Google Scholar] [CrossRef]
- Carvalho, D.; Biasi, L.; Telles, C. Organogeneie do caquizeiro ‘Fuyu’ from meristematic apps. Curr. Agric. Sci. Tech. 2004, 10, 3. [Google Scholar]
- Rai, V. Role of amino acids in plant responses to stresses. Biol. Plant. 2002, 45, 481–487. [Google Scholar] [CrossRef]
- George, E.F.; Hall, M.A.; De Klerk, G.J. The components of plant tissue culture media I: Macro-and micro-nutrients. In Plant Propagation by Tissue Culture; Springer: Berlin/Heidelberg, Germany, 2008; pp. 65–113. [Google Scholar]
- Ramsundar, P.; Guldhe, A.; Singh, P.; Bux, F. Assessment of municipal wastewaters at various stages of treatment process as potential growth media for Chlorella sorokiniana under different modes of cultivation. Bioresour. Technol. 2017, 227, 82–92. [Google Scholar] [CrossRef]
- Abiri, R.; Shaharuddin, N.A.; Maziah, M.; Yusof, Z.N.B.; Atabaki, N.; Sahebi, M.; Valdiani, A.; Kalhori, N.; Azizi, P.; Hanafi, M.M. Role of ethylene and the APETALA 2/ethylene response factor superfamily in rice under various abiotic and biotic stress conditions. Environ. Exp. Bot. 2017, 134, 33–44. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S.; Kutty, N.N.; Bera, P.; Mitra, A. Impact of light and sucrose supplementation on cellular differentiation, metabolic shift and modulation of gene expression in hairy roots of Daucus carota. Plant Cell Tissue Organ Cult. 2019, 136, 383–397. [Google Scholar] [CrossRef]
- Jung Kwak, S.S.; Kim, S.W.; Lee, H.; Choi, C.; Liu, J.R. Improvement of the catharanthine productivity in hairy root cultures of Catharanthus roseus by using monosaccharides as a carbon source. Biotechnol. Lett. 1992, 14, 695–700. [Google Scholar] [CrossRef]
- Verma, P.; Mathur, A.K. Agrobacterium tumefaciens-mediated transgenic plant production via direct shoot bud organogenesis from pre-plasmolyzed leaf explants of Catharanthus roseus. Biotechnol. Lett. 2011, 33, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Sharma, A.; Khan, S.A.; Mathur, A.K.; Shanker, K. Morphogenetic and chemical stability of long-term maintained Agrobacterium-mediated transgenic Catharanthus roseus plants. Nat. Prod. Res. 2015, 29, 315–320. [Google Scholar] [CrossRef] [PubMed]
- De Assis, T.F.; Fett-Neto, A.G.; Alfenas, A.C. Current techniques and prospects for the clonal propagation of hardwoods with emphasis on Eucalyptus. In Plantation Forest Biotechnology for the 21st Century; Research Signpost: Trivandrum, India, 2004; pp. 303–333. [Google Scholar]
- Wang, C.; Fu, L.; Tong, X.; Yang, Q.; Zhang, W. Efficient and selective conversion of sucrose to 5-hydroxymethylfurfural promoted by ammonium halides under mild conditions. Carbohydr. Res. 2012, 347, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Van Reis, R.; Zydney, A. Bioprocess membrane technology. J. Membr. Sci. 2007, 297, 16–50. [Google Scholar] [CrossRef]
- Hazarika, B. Morpho-physiological disorders in in vitro culture of plants. Sci. Hortic. 2006, 108, 105–120. [Google Scholar] [CrossRef]
- Dai, Z.W.; Meddar, M.; Renaud, C.; Merlin, I.; Hilbert, G.; Delrot, S.; Gomes, E. Long-term in vitro culture of grape berries and its application to assess the effects of sugar supply on anthocyanin accumulation. JExB 2013, 65, 4665–4677. [Google Scholar] [CrossRef]
- El Far, M.; Taie, H.A. Antioxidant activities, total anthocyanins, phenolics and flavonoids contents of some sweetpotato genotypes under stress of different concentrations of sucrose and sorbitol. Aust. J. Basic Appl. Sci. 2009, 3, 3609–3616. [Google Scholar]
- Jo, E.A.; Tewari, R.K.; Hahn, E.J.; Paek, K.Y. in vitro sucrose concentration affects growth and acclimatization of Alocasia amazonica plantlets. Plant Cell Tissue Organ Cult. 2009, 96, 307. [Google Scholar] [CrossRef]
- Sircar, D.; Mukherjee, C.; Beuerle, T.; Beerhues, L.; Mitra, A. Characterization of p-hydroxybenzaldehyde dehydrogenase, the final enzyme of p-hydroxybenzoic acid biosynthesis in hairy roots of Daucus carota. Acta Physiol. Plant. 2011, 33, 2019–2024. [Google Scholar] [CrossRef]
- Welander, M.; Pawlicki, N. Carbon compounds and their influence on in vitro growth and organogenesis. In Physiology, Growth and Development of Plants in Culture; Springer: Berlin/Heidelberg, Germany, 1994; pp. 83–93. [Google Scholar]
- Wotavova-Novotna, K.; Vejsadova, H.; Kindlmann, P. Effects of sugars and growth regulators on in vitro growth of Dactylorhiza species. Biol. Plantarum 2007, 51, 198–200. [Google Scholar] [CrossRef]
- Klein, S.; Fiebig, A.; Noga, G.; Hunsche, M. Influence of light quality on leaf physiology of sweet pepper plants grown under drought. Theor. Exp. Plant Physiol. 2018, 30, 287–296. [Google Scholar] [CrossRef]
- Craig, D.S. Determining Effective Ratios of Red and Far-Red Light from Light-Emitting Diodes that Control Flowering of Photoperiodic Ornamental Crops; Michigan State University: East Lansing, MI, USA, 2012. [Google Scholar]
- Kumar, A.; Palni, L. The effect of light source and gelling agent on micropropagation of Rosa damascena Mill. and Rhynchostylis retusa (L.) Bl. J. Hortic. Sci. Biotechnol. 2003, 78, 786–792. [Google Scholar] [CrossRef]
- Sutter, E.; Langhans, R. Formation of epicuticular wax and its effect on water loss in cabbage plants regenerated from shoot-tip culture. Can. J. Bot. 1982, 60, 2896–2902. [Google Scholar] [CrossRef]
- Xia, J.; Li, Y.; Zou, D. Effects of salinity stress on PSII in Ulva lactuca as probed by chlorophyll fluorescence measurements. Aquat. Bot. 2004, 80, 129–137. [Google Scholar] [CrossRef]
- Lin, C. Blue light receptors and signal transduction. Plant Cell 2002, 14, S207–S225. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Sebastia, C.; Piche, Y.; Desjardins, Y. Water relations of whole strawberry plantlets in vitro inoculated with Glomus intraradices in a tripartite culture system. Plant Sci. 1999, 143, 81–91. [Google Scholar] [CrossRef]
- Stoneman, G. Ecology and physiology of establishment of eucalypt seedlings from seed: A review. Aust. For. 1994, 57, 11–29. [Google Scholar] [CrossRef]
- Gavira, M.; Roldan, M.D.; Castillo, F.; Moreno-Viviian, C. Regulation of NAP gene expression and periplasmic nitrate reductase activity in the phototrophic bacterium Rhodobacter sphaeroides DSM158. J. Bacteriol. 2002, 184, 1693–1702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, A.K.; Raghuram, N.; Chandok, M.R.; Das, R.; Sopory, S.K. Investigations on the nature of the phytochrome-induced transmitter for the regulation of nitrate reductase in etiolated leaves of maize. JExB 1994, 45, 485–490. [Google Scholar] [CrossRef]
- Appenroth, K.J.; Meco, R.; Jourdan, V.; Lillo, C. Phytochrome and post-translational regulation of nitrate reductase in higher plants. Plant Sci. 2000, 159, 51–56. [Google Scholar] [CrossRef]
- Jeon, M.W.; Ali, M.B.; Hahn, E.J.; Paek, K.Y. Effects of photon flux density on the morphology, photosynthesis and growth of a CAM orchid, Doritaenopsis during post-micropropagation acclimatization. Plant Growth Regul. 2005, 45, 139–147. [Google Scholar] [CrossRef]
- Raffo, A.; Mozzanini, E.; Nicoli, S.F.; Lupotto, E.; Cervelli, C. Effect of light intensity and water availability on plant growth, essential oil production and composition in Rosmarinus officinalis L. ur. Food Res. Technol. 2020, 246, 167–177. [Google Scholar] [CrossRef]
- Shen, G.; Tan, S.; Sun, X.; Chen, Y.; Li, B. Experimental Evidence for the Importance of Light on Understory Grass Communities in a Subtropical Forest. Front. Plant Sci. 2020, 11, 11–18. [Google Scholar] [CrossRef]
- Le Roux, J.J.; Van Staden, J. Micropropagation and tissue culture of Eucalyptus—A review. Tree Physiol. 1991, 9, 435–477. [Google Scholar] [CrossRef] [Green Version]
- De Fossard, R.A.; Bennett, M.T.; Gorst, J.R.; Bourne, R.A. Tissue culture propagation of Eucalyptus ficifolia F. Muell. International plant propagators’ society: Combined proceedings. Int. Plant Propag. Soc. 1987, 28, 427–435. [Google Scholar]
- Ma, C.; Deepika, R.; Myburg, A.A.; Ranik, M.; Strauss, S.H. Development of Eucalyptus tissue culture conditions for improved in vitro plant health and transformability. BMC Proc. 2011, 5, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Palhares, G.A.; Sánchez, R.R.; Ruiz, M.C.; Trina, D.P.; García, Y.G.; González-Olmedo, J.L. Effects of photomixotrophic conditions on plants of Eucalyptus urograndis propagated in temporary immersion bioreactors. Int. J. Environ. 2018, 3, 558–566. [Google Scholar] [CrossRef]
- Miranda, N.A.; Xavier, A.; Otoni, W.C.; Gallo, R.; Gatti, K.C.; de Moura, L.C.; Santos, S.S.D.O. Quality and intensity of light in the in vitro development of microstumps of Eucalyptus urophylla in a photoautotrophic system. For. Sci. 2020, 66, 1–7. [Google Scholar] [CrossRef]
- Mankessi, F.; Saya, A.; Baptiste, C.; Nourissier, S.; Monteuuis, O. in vitro rooting of genetically related Eucalyptus urophylla× Eucalyptus grandis clones in relation to the time spent in culture. Trees 2009, 23, 931–940. [Google Scholar] [CrossRef]
- Moubayidin, L.; Di Mambro, R.; Sabatini, S. Cytokinin-auxin crosstalk. Trends Plant Sci. 2009, 14, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Fei, L. Towards Automating Micropropagation: From Cells to Shoots to Plants in One Step. Ph.D. Thesis, Worcester Polytechnic Institute, Worcester, MA, USA, April 2015. [Google Scholar]
- Fett-Neto, A.G.; Fett, J.P.; Goulart, L.W.V.; Pasquali, G.; Termignoni, R.R.; Ferreira, A.G. Distinct effects of auxin and light on adventitious root development in Eucalyptus saligna and Eucalyptus globulus. Tree Physiol. 2001, 21, 457–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Rocha Correela, L.; Paim, D.C.; Schwambach, J.; Fett-Neto, A.G. Carbohydrates as regulatory factors on the rooting of Eucalyptus saligna Smith and Eucalyptus globulus Labill. Plant Growth Regul. 2005, 45, 63–73. [Google Scholar]
- Tournier, V.; Grat, S.; Marque, C.; El Kayal, W.; Penchel, R.; de Andrade, G.; Boudet, A.M.; Teulieres, C. An efficient procedure to stably introduce genes into an economically important pulp tree (Eucalyptus grandis × Eucalyptus urophylla). Transgenic Res. 2003, 12, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Ahad, A.; John, E.; Maqbool, A.; Malik, K.A. Development of efficient micropropagation system for E. camaldulensis with respect to age of explants. Pak. J. Agric. Sci. 2018, 55, 23–27. [Google Scholar]
- Bandyopadhyay, S.; Hamill, J.D. Ultrastructural studies of somatic embryos of Eucalyptus nitens and comparisons with zygotic embryos found in mature seeds. Ann. Bot. 2000, 86, 237–244. [Google Scholar] [CrossRef] [Green Version]
- Herve, P.; Jauneau, A.; Paqques, M.; Marien, J.N.; Boudet, A.M.; Teuliires, C. A procedure for shoot organogenesis in vitro from leaves and nodes of an elite Eucalyptus gunnii clone: Comparative histology. Plant Sci. 2001, 161, 645–653. [Google Scholar] [CrossRef]
- Gonzaez, E.R.; de Andrade, A.; Bertolo, A.L.; Lacerda, G.C.; Carneiro, R.T.; Defavari, V.A.P.; Labate, M.T.V.; Labate, C.A. Production of transgenic Eucalyptus grandis x E. urophylla using the sonication-assisted Agrobacterium transformation (SAAT) system. Funct. Plant Biol. 2002, 29, 97–102. [Google Scholar] [CrossRef]
- Girijashankar, V. Genetic transformation of Eucalyptus. Physiol. Mol. Biol. Plants 2011, 17, 9–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prakash, M.; Gurumurthi, K. Genetic transformation and regeneration of transgenic plants from precultured cotyledon and hypocotyl explants of Eucalyptus tereticornis Sm. using Agrobacterium tumefaciens. In Vitro Cell. Dev. Biol. 2009, 45, 429–434. [Google Scholar] [CrossRef]
- Trindade, M.H.M. Eucalyptus Globulus Labill: Systems for in vitro Regeneration. Ph.D. Thesis, University of Lisbon, Lisboa, Portugal, 1996. [Google Scholar]
- Muralidharan, E.; Mascarenhas, A. Somatic embryogenesis in Eucalyptus. In Somatic Embryogenesis in Woody Plants; Springer: Berlin/Heidelberg, Germany, 1995; pp. 23–40. [Google Scholar]
- Watt, M.; Blakeway, F.; Termignoni, R.; Jain, S. (Eds.) Somatic Embryogenesis in Eucalyptus Grandis and E. dunni. Somatic Embryogenesis in Woody Plants; Springer: Berlin/Heidelberg, Germany, 1999; pp. 63–78. [Google Scholar]
- Termignoni, R.R.; Wang, P.J.; Hu, C.Y. Somatic embryo induction in Eucalyptus dunnii. Plant Cell Tissue Organ Cult. 1996, 45, 129–132. [Google Scholar] [CrossRef]
- Kaeppler, S.M.; Kaeppler, H.F.; Rhee, Y. Epigenetic aspects of somaclonal variation in plants. In Plant Gene Silencing; Springer: Berlin/Heidelberg, Germany, 2000; pp. 59–68. [Google Scholar]
- Gamborg, O.L.C.; Miller, R.A.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
- Ho, C.K.; Chang, S.H.; Tsay, J.Y.; Tsai, C.J.; Chiang, V.; Chen, Z.Z. Agrobacterium tumefaciens-mediated transformation of Eucalyptus camaldulensis and production of transgenic plants. Plant Cell Rep. 1998, 17, 675–680. [Google Scholar] [CrossRef]
- Ito, K.; Doi, K.; Tatemichi, Y.; Shibata, M. Plant regeneration of Eucalypts from rotating nodule cultures. Plant Cell Rep. 1996, 16, 42–45. [Google Scholar] [CrossRef]
- Alves, E.C.S.D.C.; Xavier, A.; Otoni, W.C. Organogenesis of the leaf explant of Eucalyptus grandis x E. urophylla clones. Pesq. Agropec. Bras. 2004, 39, 421–430. [Google Scholar] [CrossRef]
- Takemori, N.; Marschner, R.; Quoirin, M.; Bona, C.; Zanette, F. Anatomical study of Racosperma (ex-Acacia) mangium tissues cultured in vitro. Braz. Arch. Biol. Technol. 2000, 43, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Stipp, L.C.L.; Mendes, B.M.J.; Piedade, S.M.S.; Rodriguez, A.P.M. in vitro morphogenesis of Cucumis melo var. inodorus. Plant Cell Tissue Organ Cult. 2001, 65, 81–89. [Google Scholar] [CrossRef]

| Species | BM | PGR or Other Supplements | Explant | Soma-Clonal | Light Conditions | Response Variation | Acclimati Sation | Other Studies | References |
|---|---|---|---|---|---|---|---|---|---|
| E. globulus | MS and B5 | PGR free, BAP, Kinetin | Secondary somatic embryo | Nd | Dark and L | Germination | no | - | [13] |
| E. globulus | B5 | NAA and BAP | Globular structures | Nd | 16 h L | Shoot proliferation | No | - | [198] |
| E. citriodora | B5 | PGR free | EC | Nd | Continous L | Emblings | Yes | Somatic embryo encapsulation | [199] |
| E. dunnii | nd | Nd | Nd | Nd | nd | Embryo maturation and germination | Nd | Nd | [200] |
| E. citriodora | B5 | PGR free | EC | Nd | Continuous L16 h L | Emblings | Yes | - | [199] |
| E. globulus | MS | PGR free | Primary somatic embryos | Nd | Dark | Emblings | no | - | [13] |
| E. dunnii | B5 | 10% coconut milk | EC | - | 16 h L | Embryos with green cotyledons | - | - | [68,201] |
| E. tereticornis | MS | PGR free | Primary somatic embryos | Nd | 16 h L | Emblings | Yes | - | [22,68,102,201] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abiri, R.; Atabaki, N.; Abdul-Hamid, H.; Sanusi, R.; Ab Shukor, N.A.; Shaharuddin, N.A.; Ahmad, S.A.; Malik, S. The Prospect of Physiological Events Associated with the Micropropagation of Eucalyptus sp. Forests 2020, 11, 1211. https://doi.org/10.3390/f11111211
Abiri R, Atabaki N, Abdul-Hamid H, Sanusi R, Ab Shukor NA, Shaharuddin NA, Ahmad SA, Malik S. The Prospect of Physiological Events Associated with the Micropropagation of Eucalyptus sp. Forests. 2020; 11(11):1211. https://doi.org/10.3390/f11111211
Chicago/Turabian StyleAbiri, Rambod, Narges Atabaki, Hazandy Abdul-Hamid, Ruzana Sanusi, Nor Aini Ab Shukor, Noor Azmi Shaharuddin, Siti Aqlima Ahmad, and Sonia Malik. 2020. "The Prospect of Physiological Events Associated with the Micropropagation of Eucalyptus sp." Forests 11, no. 11: 1211. https://doi.org/10.3390/f11111211








