Arbuscular Mycorrhizal Fungal Communities Are Influenced by Host Tree Species on the Loess Plateau, Northwest China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Sampling Design
2.3. Evaluation of Mycorrhizal Colonization
2.4. Quantification and Identification of AM Fungal Spores
2.5. Analysis of Soil Physicochemical Properties
2.6. Statistical Analysis
3. Results
3.1. Rhizosphere Soil Properties
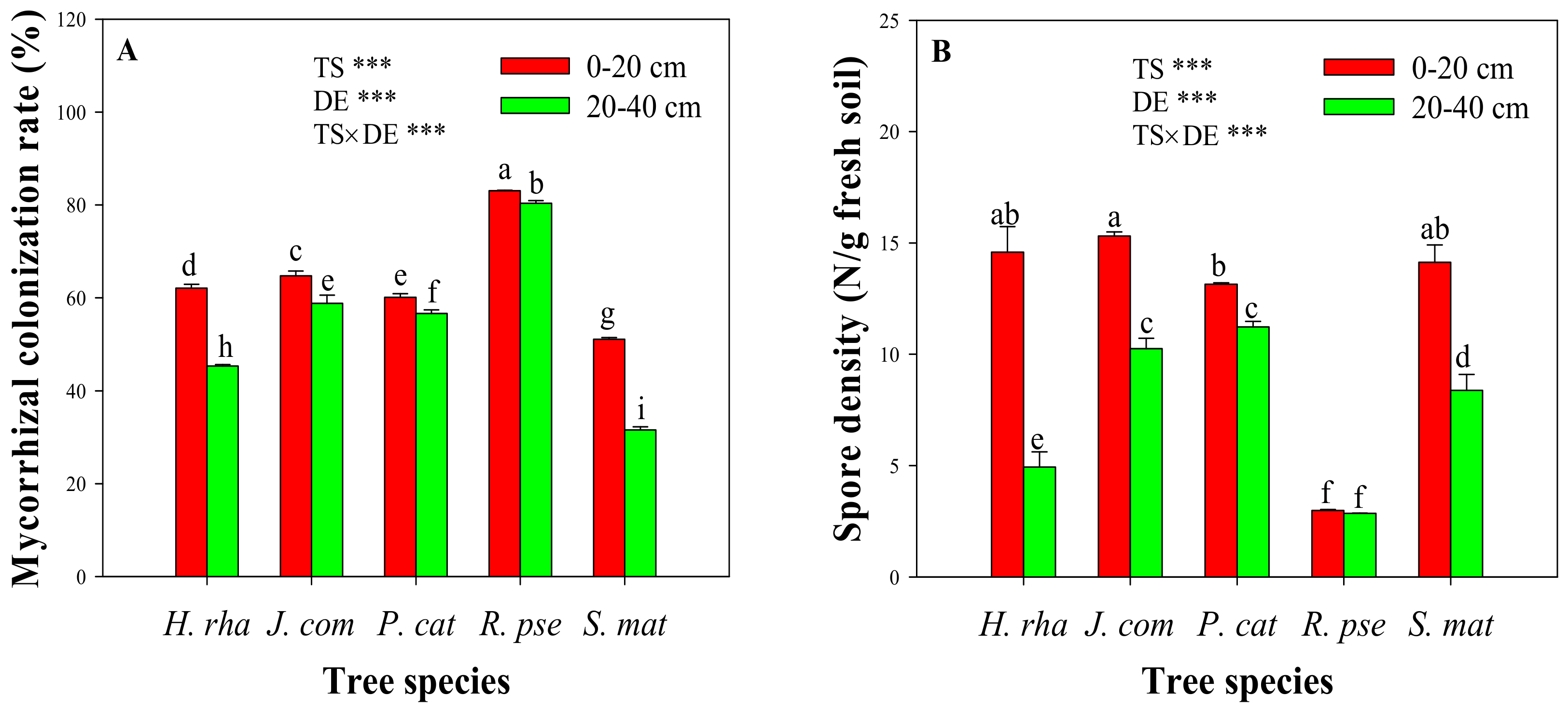
3.2. Mycorrhizal Colonization and Spore Density
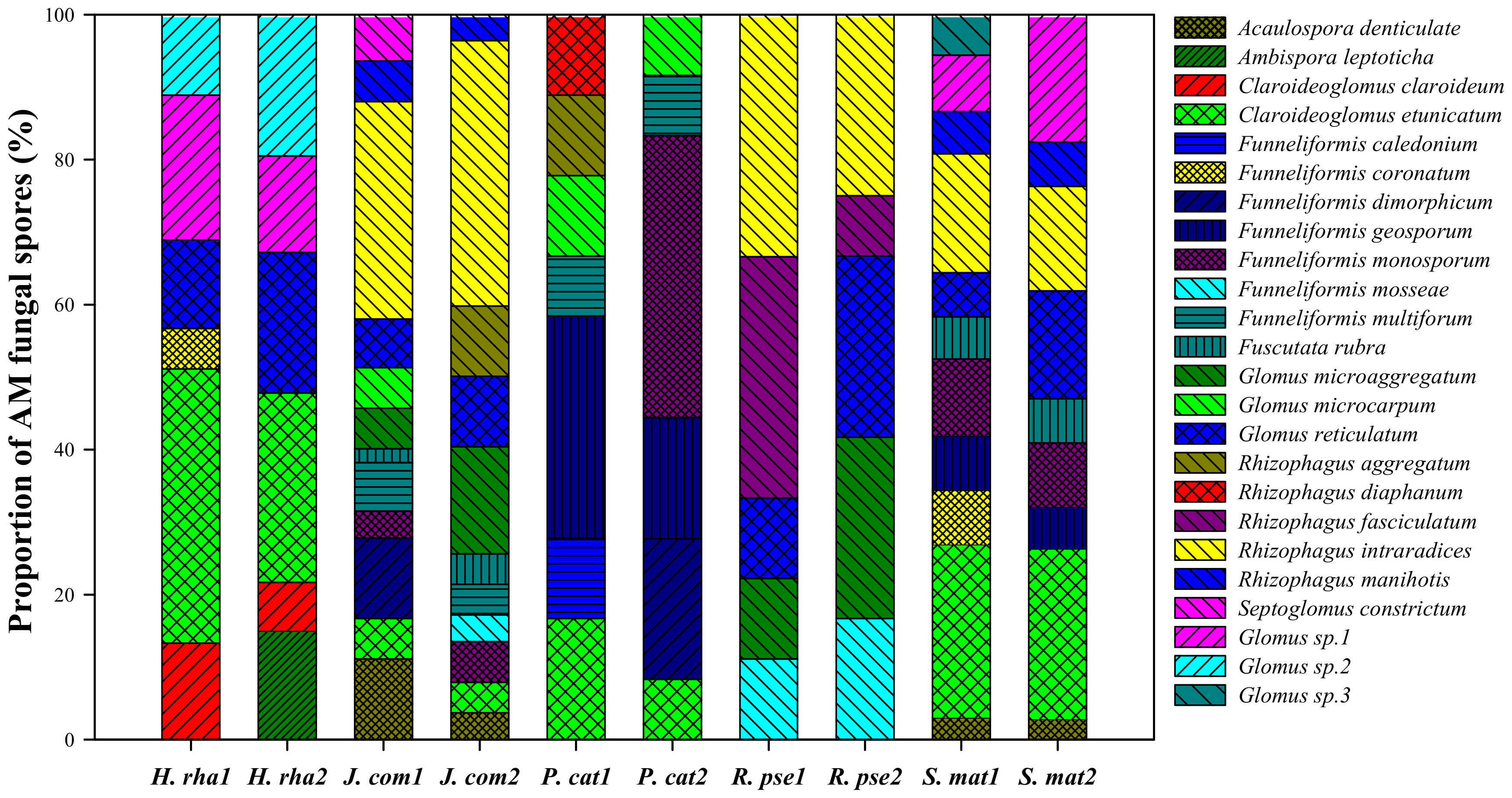
3.3. Composition of AM Fungal Communities
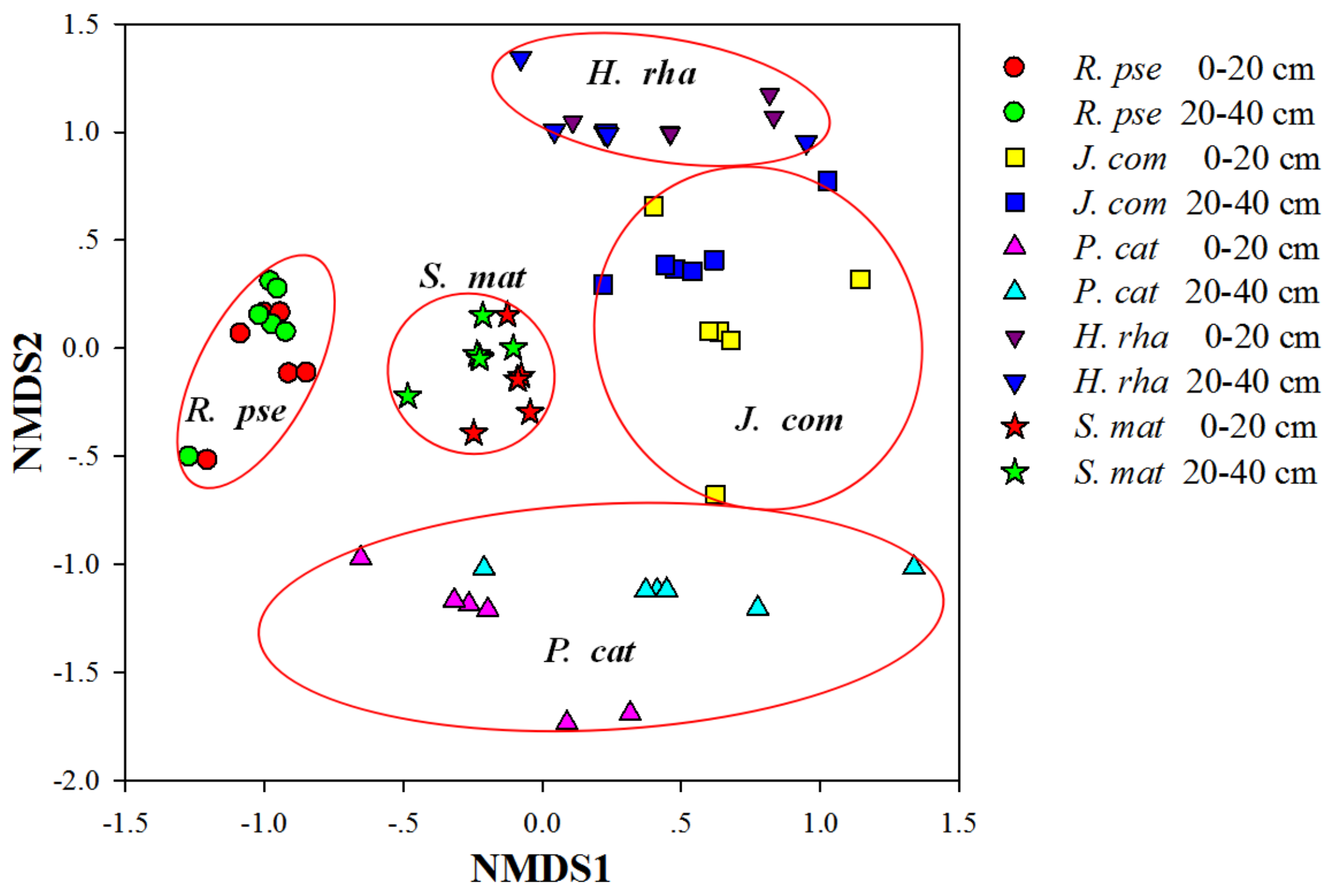
3.4. Diversity of AM Fungal Communities
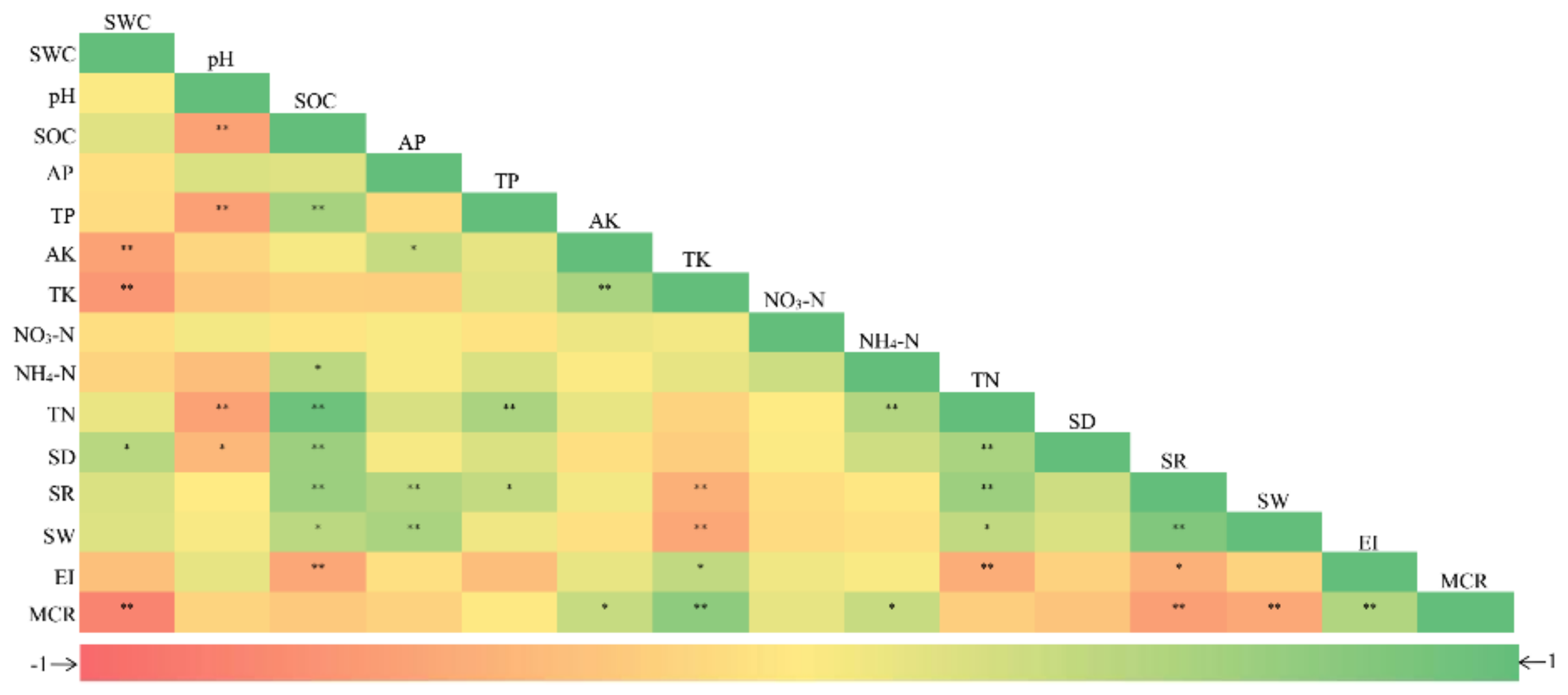
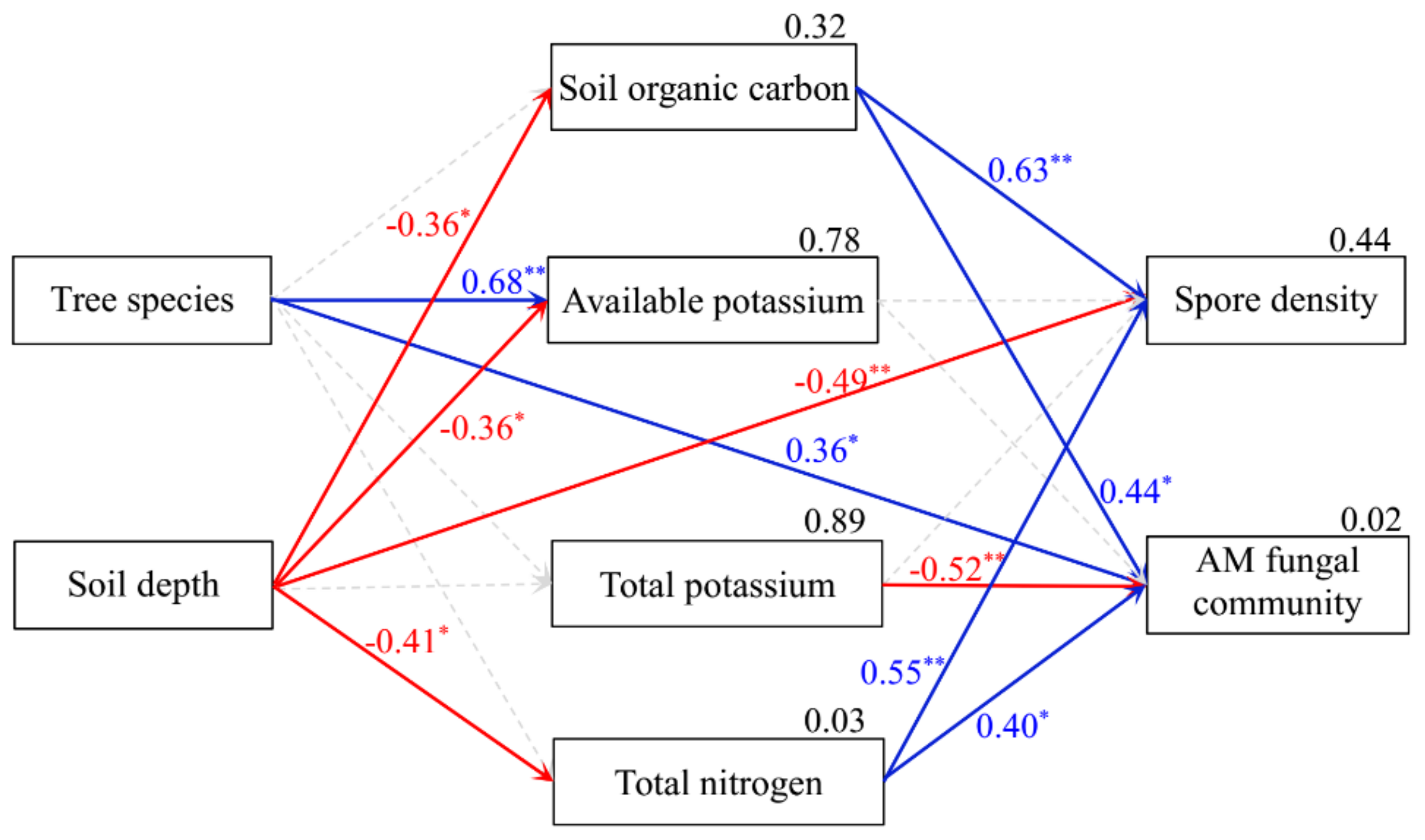
3.5. Relations between AM Fungal Diversity and Soil Properties
4. Discussion
4.1. Effects of Tree Species on AM Fungi
4.2. Effects of Soil Depth on AM Fungi
4.3. Effects of Soil Properties on AM Fungi
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Wang, L.; Su, J.Y. The soil water condition of a typical agroforestry system under the policy of northwest China. Forests 2018, 9, 730. [Google Scholar] [CrossRef]
- Yang, Y.T.; Guan, H.D.; Okke, B.; Mcvicar, T.R.; Long, D.; Shilong, P.; Wei, L.; Liu, B.; Zhao, J.; Craig, T.S. Contrasting responses of water use efficiency to drought across global terrestrial ecosystems. Sci. Rep. 2016, 6, 23284. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.Q.; Zhang, X.Y.; Fang, Z.W.; Wu, Y.Q.; Tao, J. Physiological and transcriptomic analysis of tree peony (Paeonia section Moutan DC.) in response to drought stress. Forests 2019, 10, 135. [Google Scholar] [CrossRef]
- Guo, W.; Zhao, R.X.; Yang, H.J.; Zhao, J.; Zhang, J. Using native plants to evaluate the effect of arbuscular mycorrhizal fungi on revegetation of iron tailings in grasslands. Biol. Fert. Soils 2013, 49, 617–626. [Google Scholar] [CrossRef]
- Kimura, A.C.; Scotti, M.R. Soil aggregation and arbuscular mycorrhizal fungi as indicators of slope rehabilitation in the São Francisco river basin (Brazil). Soil Water Res. 2016, 11, 114–123. [Google Scholar] [CrossRef]
- Fisseha, A.; Tamrat, B.; Emiru, B. The potential role of arbuscular mycorrhizal fungi in the restoration of degraded lands. Front. Microbiol. 2016, 7, 1095. [Google Scholar]
- Mukerji, K.G.; Chamola, B.P.; Singh, J. Mycorrhizal Biology; Kluwer Academic: Dordrecht, The Netherlands; Plenum Publishers: New York, NY, USA, 2000. [Google Scholar]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: London, UK; New York, NY, USA, 2008; pp. 1–145. [Google Scholar]
- Gong, M.G.; Tang, M.; Chen, H.; Zhang, Q.M.; Xu, H.; Zheng, C.L. Effects of Glomus mosseae and Rhizobium on the growth of black locust seedlings and the quality of weathered soft rock soils in the Loess Plateau, China. Ann. Microbiol. 2012, 62, 1579–1586. [Google Scholar] [CrossRef]
- Gong, M.G.; Tang, M.; Zhang, Q.M.; Feng, X.X. Effects of climatic and edaphic factors on arbuscular mycorrhizal fungi in the rhizosphere of Hippophae rhamnoides in the Loess Plateau, China. Acta Ecol. Sin. 2012, 32, 62–67. [Google Scholar] [CrossRef]
- Wang, K.; He, X.L.; Xie, L.L.; Zhao, L.L. Arbuscular mycorrhizal fungal community structure and diversity are affected by host plant species and soil depth in the Mu Us Desert, northwest China. Arid Land Res. Manag. 2018, 32, 198–211. [Google Scholar] [CrossRef]
- Peña-Venegas, C.P.; Kuyper, T.W.; Davison, J.; Jairus, T.; Vasar, M.; Stomph, T.J.; Struik, P.C.; Öpik, M. Distinct arbuscular mycorrhizal fungal communities associate with different manioc landraces and Amazonian soils. Mycorrhiza 2019, 29, 263–275. [Google Scholar] [CrossRef] [Green Version]
- He, F.; Tang, M.; Zhong, S.L.; Yang, R.; Huang, L.; Zhang, H.Q. Effects of soil and climatic factors on arbuscular mycorrhizal fungi in rhizosphere soil under Robinia pseudoacacia in the Loess Plateau, China. Eur. J. Soil Sci. 2016, 67, 847–856. [Google Scholar] [CrossRef]
- Faggioli, V.; Menoyo, E.; József, G.; Kemppainen, M.; Pardo, A.; Salazar, M.J.; Becerra, A.G. Soil lead pollution modifies the structure of arbuscular mycorrhizal fungal communities. Mycorrhiza. 2019, 29, 363–373. [Google Scholar] [CrossRef]
- Gai, J.P.; Tian, H.; Yang, F.Y.; Christie, P.; Li, X.L.; Klironomos, J.N. Arbuscular mycorrhizal fungal diversity along a Tibetan elevation gradient. Pedobiologia 2012, 55, 145–151. [Google Scholar] [CrossRef]
- Lovelock, C.E.; Andersen, K.; Morton, J.B. Arbuscular mycorrhizal communities in tropical forests are affected by host tree species and environment. Oecologia 2003, 135, 268–279. [Google Scholar] [CrossRef]
- Jian, S.Q.; Wu, Z.N.; Hu, C.H.; Zhang, X.L. Sap flow in response to rainfall pulses for two shrub species in the semiarid Chinese Loess Plateau. J. Hydrol. Hydromec. 2016, 64, 121–132. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Miao, H.T.; Huang, Z.; Cui, Z.; He, H.H.; Zheng, J.Y.; Han, F.P.; Chang, X.F.; Wu, G.L. Soil water depletion patterns of artificial forest species and ages on the Loess Plateau (China). For. Ecol. Manag. 2018, 417, 137–143. [Google Scholar] [CrossRef]
- Yang, Y.R.; Song, Y.Y.; Scheller, H.V.; Ghosh, A.; Ban, Y.H.; Chen, H.; Tang, M. Community structure of arbuscular mycorrhizal fungi associated with Robinia pseudoacacia in uncontaminated and heavy metal contaminated soils. Soil Biol. Biochem. 2015, 86, 146–158. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Gerdemann, J.W.; Nicolson, T.H. Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans. Br. Mycol. Soc. 1963, 46, 235–244. [Google Scholar] [CrossRef]
- Morton, J.B. Taxonomy of VA mycorrhizal fungi: Classification, nomenclature, and identification. Mycotaxon 1988, 32, 267–324. [Google Scholar]
- Oehl, F.; Sieverding, E.; Palenzuela, J.; Ineichen, K.; Silva, G.A. Advances in Glomeromycota taxonomy and classification. IMA Fungus 2011, 2, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Oehl, F.; Silva, G.A.; Goto, B.T.; Maia, L.C.; Sieverding, E. Glomeromycota: Two new classes and a new order. Mycotaxon 2011, 116, 365–379. [Google Scholar] [CrossRef]
- Oehl, F.; Silva, G.A.; Goto, B.T.; Sieverding, E. Glomeromycetes: Three new genera and glomoid species reorganized. Mycotaxon 2011, 116, 75–120. [Google Scholar] [CrossRef]
- Redecker, D.; Schüßler, A.; Stockinger, H.; Stürmer, S.L.; Morton, J.B.; Walker, C. An evidence-based consensus for the classification of arbuscular mycorrhizal fungi (Glomeromyota). Mycorrhiza 2013, 23, 515–531. [Google Scholar] [CrossRef]
- Schenck, N.C.; Pérez, Y. Manual for the Identification of VA Mycorrhizal Fungi; Synergistic Publications: Gainesville, FL, USA, 1990. [Google Scholar]
- Shannon, C.E. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949; pp. 10–14. [Google Scholar]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Abdelhalim, T.S.; Finckh, M.R.; Babiker, A.G.; Oehl, F. Species composition and diversity of arbuscular mycorrhizal fungi in White Nile state, Central Sudan. Arch. Agron. Soil Sci. 2013, 60, 377–391. [Google Scholar] [CrossRef]
- Gardner, W.H.; Klute, A. Water content. In Methods of Soil Analysis; Klute, A., Ed.; American Society of Agronomy: Madison, WI, USA, 1986; pp. 493–544. [Google Scholar]
- Peech, M. Hydrogen-ion activity. In Methods of Soil Analysis; Black, C.A., Ed.; American Society of Agronomy: Madison, WI, USA, 1965; pp. 914–926. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon and organic matter. In Methods of Soil Analysis; Page, A.L., Ed.; American Society of Agronomy and Soil Science Society of America: Madison, WI, USA, 1982; pp. 539–579. [Google Scholar]
- Bremner, J.M.; Mulvaney, C.S. Total nitrogen. In Methods of Soil Analysis; Page, A.L., Ed.; American Society of Agronomy and Soil Science Society of America: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Raave, H.; Keres, I.; Kauer, K.; Nõges, M.; Rebane, J.; Tampere, M.; Loit, E. The impact of activated carbon on NO3−-N, NH4+-N, P and K leaching in relation to fertilizer use. Eur. J. Soil Sci. 2014, 65, 120–127. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis; Prentice-Hall, Inc.: Englewood Cliffs, NJ, USA, 1958. [Google Scholar]
- Page, A.L. Chemical and microbiological properties. In Methods of Soil Analysis; Page, A.L., Ed.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1982; pp. 625–641. [Google Scholar]
- Kindt, R.; Coe, R. Tree Diversity Analysis: A Manual and Software for Common Statistical Methods for Ecological and Biodiversity Studies; World Agroforestry Centre: Nairobi, Kenya, 2005. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B. Vegan: Community ecology Package. R package Version 2.0–10. Available online: http://CRAN.R-project.org/package=vegan (accessed on 20 September 2013).
- Zheng, Y.; Chen, L.; Luo, C.Y.; Zhang, Z.H.; Wang, S.P.; Guo, L.D. Plant identity exerts stronger effect than fertilization on soil arbuscular mycorrhizal fungi in a sown pasture. Microb. Ecol. 2016, 72, 647–658. [Google Scholar] [CrossRef]
- Chen, L.H.; Hu, X.W.; Yang, W.Q.; Xu, Z.F.; Zhang, D.J.; Gao, S. The effects of arbuscular mycorrhizal fungi on sex-specific responses to Pb pollution in Populus Cathayana. Ecotox. Environ. Saf. 2015, 113, 460–468. [Google Scholar] [CrossRef]
- Wang, B.; Qiu, Y.L. Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 2006, 16, 299–363. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.G.; Mielke, L.A.; Nguyen, N.H. Ecological responses to forest age, habitat, and host vary by mycorrhizal type in boreal peatlands. Mycorrhiza 2018, 28, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Chagnon, P.L.; Bradley, R.L.; Klironomos, J.N. Trait-based partner selection drives mycorrhizal network assembly. Oikos 2015, 124, 1609–1616. [Google Scholar] [CrossRef]
- Kubisch, P.; Hertel, D.; Leuschner, C. Do ectomycorrhizal and arbuscular mycorrhizal temperate tree species systematically differ in root order-related fine root morphology and biomass? Front. Plant Sci. 2015, 6, 64. [Google Scholar] [CrossRef]
- Soudzilovskaia, N.A.; Aksenova, A.A.; Makarov, M.I.; Onipchenko, V.; Logvinenko, O.A.; Ter Braak, C.; Cornelissen, J.H.C. Legumes affect alpine tundra community composition via multiple biotic interactions. Ecosphere 2012, 3, 1–15. [Google Scholar] [CrossRef]
- Zhou, X.; Tian, L.; Zhang, J.F.; Ma, L.N.; Li, X.J.; Tian, C.J. Rhizospheric fungi and their link with the nitrogen-fixing Frankia harbored in host plant Hippophae rhamnoides L. J. Basic Microb. 2017, 57, 1–10. [Google Scholar] [CrossRef]
- Alguacil, M.M.; Díaz, G.; Torres, P.; Rodríguez-Caballero, G.; Roldán, A. Host identity and functional traits determine the community composition of the arbuscular mycorrhizal fungi in facultative epiphytic plant species. Fungal Ecol. 2019, 39, 307–315. [Google Scholar] [CrossRef]
- Engelmoer, D.J.P.; Kiers, E.T. Host diversity affects the abundance of the extraradical arbuscular mycorrhizal network. New Phytol. 2015, 205, 1485–1491. [Google Scholar] [CrossRef]
- Belay, Z.; Vestberg, M.; Assefa, F. Diversty and abundance of arbuscular mycorrhizal fungi across different land use types in a humid low land area of Ethiopia. Trop. Subtrop. Agroeco. 2015, 18, 47–69. [Google Scholar]
- Garbeva, J.A.; van Veen, J.A.; van Elsas, J.D. Microbial diversity in soil: Selection of microbial populations by plant and soil type and implications for disease suppressiveness. Ann. Rev. Phytopathol. 2004, 42, 243–270. [Google Scholar] [CrossRef]
- Legay, N.; Grassein, F.; Binet, M.N.; Arnoldi, C.; Personeni, E.; Perigon, S.; Polyd, F.; Pommierd, T.; Puissanta, J.; Clémenta, J.C.; et al. Plant species identities and fertilization influence on arbuscular mycorrhizal fungal colonisation and soil bacterial activities. Appl. Soil Ecol. 2016, 98, 132–139. [Google Scholar] [CrossRef]
- Shukla, A.; Vyas, D.; Anuradha, J. Soil depth: An overriding factor for distribution of arbuscular mycorrhizal fungi. J. Soil Sci. Plant. Nut. 2013, 13, 23–33. [Google Scholar] [CrossRef]
- Brady, N.C.; Weil, R.R. The Nature and Properties of Soils; Prentice-Hall Inc.: Upper Saddle River, NJ, USA, 1996. [Google Scholar]
- Verbruggen, E.; Röling, W.F.; Gamper, H.A.; Kowalchuk, G.A.; Verhoef, H.A.; van der Heijden, M.G. Positive effects of organic farming on below-ground mutualists: Large-scale comparison of mycorrhizal fungal communities in agricultural soils. New Phytol. 2010, 186, 968–979. [Google Scholar] [CrossRef] [PubMed]
- Cuenca, G.; Lovera, M. Seasonal variation anddistribution at different soil depths of arbuscular mycorrhizal fungi spores in a tropical sclerophyllous shrubland. Botany 2010, 88, 54–64. [Google Scholar] [CrossRef]
- Li, P.; Zhao, Z.; Li, Z.B.; Zhan, T.T. Characters of root biomass spatial distribution of Robinia pseudoacacia in Weibei loess areas. Ecol. Environ. 2005, 14, 405–409. [Google Scholar]
- Higo, M.; Isobe, K.; Yamaguchi, M.; Drijber, R.A.; Jeske, E.S.; Ishii, R. Diversity and vertical distribution of indigenous arbuscular mycorrhizal fungi under two soybean rotational systems. Biol. Fert. Soils 2013, 49, 1085–1096. [Google Scholar] [CrossRef]
- Taniguchi, T.; Usuki, H.; Kikuchi, J.; Hirobe, M.; Miki, N.; Fukuda, K.; Zhang, G.S.; Wang, L.H.; Yoshikawa, K.; Yamanaka, N. Colonization and community structure of root-associated microorganisms of Sabina vulgaris with soil depth in a semiarid desert ecosystem with shallow groundwater. Mycorrhiza 2012, 22, 419–428. [Google Scholar] [CrossRef]
- Guo, H.J.; He, X.L.; Li, Y.P. Spatial distribution of arbuscular mycorrhiza and glomalin in the rhizosphere of Caragana korshinskii Kom. in the Otindag sandy land, China. Afr. J. Microbiol. Res. 2012, 6, 5745–5753. [Google Scholar]
- Syliva, D.M.; Neal, L.H. Nitrogen affects the phosphorous response of VA mycorrhizae. New Phytol. 1990, 115, 303–310. [Google Scholar] [CrossRef]
- Khade, S.W.; Rodrigues, B.F. Spatial variations in arbuscular mycorrhizal (AM) fungi associated with Carica papaya L. in a tropical agro-based ecosystem. Biol. Agric. Hortic. 2008, 26, 149–174. [Google Scholar] [CrossRef]

| Tree Species | Depth | SWC (%) | pH | SOC (g kg−1) | AP (mg kg−1) | TP (g kg−1) | AK (mg kg−1) | TK (g kg−1) | NO3-N (mg kg−1) | NH4-N (mg kg−1) | TN (g kg−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| H. rha | 0–20 | 14.0 ± 0.8 b | 7.65 ± 0.1 a | 5.23 ± 1.70 cd | 7.40 ± 1.22 ab | 1.01 ± 0.05 ab | 11.59 ± 0.27 c | 11.14 ± 0.89 bc | 10.71 ± 0.82 a | 9.65 ± 1.28 ab | 0.24 ± 0.03 e |
| 20–40 | 15.4 ± 1.1 a | 7.66 ± 0.1 a | 4.12 ± 1.20 de | 6.38 ± 0.98 ab | 1.04 ± 0.12 ab | 11.52 ± 0.64 c | 10.63 ± 0.50 c | 10.57 ± 2.75 a | 7.07 ± 0.74 b | 0.22 ± 0.01 efg | |
| J. com | 0–20 | 11.5 ± 1.3 cd | 7.40 ± 0.1 c | 13.77 ± 2.38 a | 6.94 ± 1.96 ab | 1.55 ± 0.41 a | 12.52 ± 0.07 ab | 14.58 ± 1.13 ab | 10.48 ± 0.66 a | 10.79 ± 1.14 a | 0.84 ±0.04 a |
| 20–40 | 11.6 ± 0.2 cd | 7.51 ± 0.1 bc | 12.10 ± 1.45 ab | 5.85 ± 0.68 b | 1.57 ± 0.35 a | 11.97 ± 0.12 bc | 13.52 ± 0.83 abc | 9.43 ± 0.55 a | 6.93 ± 0.29 b | 0.77 ± 0.01 b | |
| P. cat | 0–20 | 10.3 ± 0.5 d | 7.60 ± 0.1 ab | 4.09 ± 1.05 de | 7.59 ± 0.17 ab | 1.13 ± 0.12 ab | 12.83 ± 0.05 a | 16.02 ± 3.49 a | 10.64 ± 0.37 a | 7.26 ± 1.62 b | 0.18 ± 0.01 fgh |
| 20–40 | 11.0 ± 0.3 d | 7.64 ± 0.1 ab | 3.53 ± 0.40 de | 6.36 ± 0.26 ab | 0.87 ± 0.18 b | 12.32 ± 0.24 ab | 13.04 ± 0.75 abc | 10.18 ± 0.40 a | 7.15 ± 0.58 b | 0.16 ± 0.03 gh | |
| R. pse | 0–20 | 4.5 ± 0.3 e | 7.62 ± 0.1 ab | 2.66 ± 0.28 de | 8.00 ± 0.87 ab | 1.17 ± 0.07 ab | 12.97 ± 0.05 a | 16.83 ± 0.03 a | 10.62 ± 0.43 a | 8.63 ± 0.87 ab | 0.22 ± 0.01 ef |
| 20–40 | 4.9 ± 0.8 e | 7.67 ± 0.1 a | 2.53 ± 0.62 e | 7.49 ± 2.86 ab | 1.04 ± 0.06 ab | 12.56 ± 0.04 ab | 15.15 ± 0.97 a | 10.24 ± 0.01 a | 7.96 ± 0.03 ab | 0.12 ± 0.01 h | |
| S. mat | 0–20 | 11.3 ± 0.8 cd | 7.65 ± 0.1 a | 10.44 ± 2.14 b | 10.06 ± 1.67 a | 1.29 ± 0.21 ab | 12.67 ± 0.03 ab | 10.83 ± 0.32 bc | 10.60 ± 1.08 a | 8.17 ± 0.52 ab | 0.62 ± 0.01 c |
| 20–40 | 12.6 ± 0.9 c | 7.67 ± 0.1 a | 6.69 ± 1.57 c | 9.45 ± 1.27 ab | 1.19 ± 0.10 ab | 12.36 ± 0.24 ab | 10.60 ± 0.95 c | 9.55 ± 0.77 a | 6.78 ± 2.12 b | 0.50 ± 0.01 d | |
| TS | *** | *** | *** | ** | *** | *** | *** | ns | ns | *** | |
| DE | ** | * | ** | ns | ns | *** | * | ns | *** | *** | |
| TS × DE | ns | ns | ns | ns | ns | ns | ns | ns | ns | *** | |
| Variable | R2 | F | p |
|---|---|---|---|
| SWC | 0.922 | 3.070 | 0.083 |
| pH | 0.881 | 1.932 | 0.211 |
| SOC | 0.979 | 11.952 | 0.003** |
| AP | 0.832 | 1.295 | 0.400 |
| TP | 0.934 | 3.694 | 0.055 |
| AK | 0.962 | 6.671 | 0.013* |
| TK | 0.958 | 6.950 | 0.012* |
| NO3-N | 0.686 | 0.569 | 0.847 |
| NH4-N | 0.919 | 2.953 | 0.091 |
| TN | 0.960 | 6.263 | 0.015* |
| SD | 0.951 | 5.097 | 0.025* |
| MCR | 0.920 | 3.011 | 0.087 |
| SR | 0.891 | 2.130 | 0.176 |
| SW | 0.789 | 0.974 | 0.567 |
| EI | 0.909 | 2.608 | 0.118 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, F.; Chen, H.; Tang, M. Arbuscular Mycorrhizal Fungal Communities Are Influenced by Host Tree Species on the Loess Plateau, Northwest China. Forests 2019, 10, 930. https://doi.org/10.3390/f10100930
He F, Chen H, Tang M. Arbuscular Mycorrhizal Fungal Communities Are Influenced by Host Tree Species on the Loess Plateau, Northwest China. Forests. 2019; 10(10):930. https://doi.org/10.3390/f10100930
Chicago/Turabian StyleHe, Fei, Hui Chen, and Ming Tang. 2019. "Arbuscular Mycorrhizal Fungal Communities Are Influenced by Host Tree Species on the Loess Plateau, Northwest China" Forests 10, no. 10: 930. https://doi.org/10.3390/f10100930




