The Composition and Height of Saplings Capturing Silvicultural Gaps at Two Long-Term Experiments in Managed Northern Hardwood Forests
Abstract
:1. Introduction
2. Methods
2.1. Study Areas
2.1.1. Divide Canopy Gap Study
2.1.2. Yellow Birch Legacy-Tree Project
2.2. Field Sampling
2.3. Data Analysis
3. Results
3.1. Study Sites and Initial Conditions
3.2. The Composition of Gap-Capturing Saplings
3.3. Sapling Height
3.4. Spatial Patterning of Gap-Capturing Saplings
4. Discussion
4.1. Composition
4.2. Legacy-Tree Effects
4.3. Harvest Methods
4.4. Herbivory
4.5. Management History
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pretzsch, H. Diversity and productivity in forests: Evidence from long-term experimental plots. In Forest Diversity and Function; Springer: Berlin, Germany, 2005; pp. 41–64. [Google Scholar]
- Millar, C.I.; Stephenson, N.L.; Stephens, S.L. Climate change and forests of the future: Managing in the face of uncertainty. Ecol. Appl. 2007, 17, 2145–2151. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Crowther, T.W.; Picard, N.; Wiser, S.; Zhou, M.; Alberti, G.; Schulze, E.D.; McGuire, A.D.; Bozzato, F.; Pretzsch, H. Positive biodiversity-productivity relationship predominant in global forests. Science 2016, 354, aaf8957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comita, L.S.; Queenborough, S.A.; Murphy, S.J.; Eck, J.L.; Xu, K.; Krishnadas, M.; Beckman, N.; Zhu, Y. Testing predictions of the Janzen–Connell hypothesis: A meta-analysis of experimental evidence for distance-and density-dependent seed and seedling survival. J. Ecol. 2014, 102, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, J.F.; Allen, C.D.; Franklin, J.F.; Frelich, L.E.; Harvey, B.J.; Higuera, P.E.; Mack, M.C.; Meentemeyer, R.K.; Metz, M.R.; Perry, G.L. Changing disturbance regimes, ecological memory, and forest resilience. Front. Ecol. Environ. 2016, 14, 369–378. [Google Scholar] [CrossRef]
- Tews, J.; Brose, U.; Grimm, V.; Tielbörger, K.; Wichmann, M.; Schwager, M.; Jeltsch, F. Animal species diversity driven by habitat heterogeneity/diversity: The importance of keystone structures. J. Biogeogr. 2004, 31, 79–92. [Google Scholar] [CrossRef]
- Kernaghan, G. Mycorrhizal diversity: Cause and effect? Pedobiologia 2005, 49, 511–520. [Google Scholar] [CrossRef]
- Nowacki, G.J.; Abrams, M.D. The demise of fire and “mesophication” of forests in the eastern United States. BioScience 2008, 58, 123–138. [Google Scholar] [CrossRef]
- Schulte, L.A.; Mladenoff, D.J.; Crow, T.R.; Merrick, L.C.; Cleland, D.T. Homogenization of northern US Great Lakes forests due to land use. Landsc. Ecol. 2007, 22, 1089–1103. [Google Scholar] [CrossRef]
- Williams, M. Americans and Their Forests: A Historical Geography; Cambridge University Press: Cambridge, UK, 1992. [Google Scholar]
- Crow, T.R.; Buckley, D.S.; Nauertz, E.A.; Zasada, J.C. Effects of management on the composition and structure of northern hardwood forests in Upper Michigan. For. Sci. 2002, 48, 129–145. [Google Scholar]
- O’Hara, K.L. The historical development of uneven-aged silviculture in North America. Forestry 2002, 75, 339–346. [Google Scholar] [CrossRef]
- Eyre, F.H.; Zillgitt, W.M. Partial Cuttings in Northern Hardwoods of the Lake States: Twenty-Year Experimental Results; US Dept. of Agriculture: Washington, DC, USA, 1953.
- Arbogast, C. Marking Guides for Northern Hardwoods under the Selection System; Service, U.F., Ed.; Lake States Forest Experiment Station: St. Paul, MN, USA, 1957. [Google Scholar]
- Seymour, R.S.; Guldin, J.; Marshall, D.; Palik, B. Large-scale, long-term silvicultural experiments in the United States: Historical overview and contemporary examples. Allg. Forst Jagdztg. 2006, 177, 104–112. [Google Scholar]
- Wisconsin Department of Natural Resources. Silvicultural Handbook; Wisconsin Dept. of Natural Resources: Madison, WI, USA, 2013; Volume 24315, pp. 2114–2116. [Google Scholar]
- Nyland, R.D. Selection system in northern hardwoods. J. For. 1998, 96, 18–21. [Google Scholar]
- Pond, N.C.; Froese, R.E.; Nagel, L.M. Sustainability of the selection system in northern hardwood forests. For. Sci. 2014, 60, 374–381. [Google Scholar] [CrossRef]
- Woods, K.D. Long-term change and spatial pattern in a late-successional hemlock–northern hardwood forest. J. Ecol. 2000, 88, 267–282. [Google Scholar] [CrossRef]
- Leak, W. Species composition and structure of a northern hardwood stand after 61 years of group/patch selection. North J. Appl. 1999, 16, 151–153. [Google Scholar] [CrossRef]
- Leak, W.B. Regeneration of patch harvests in even-aged northern hardwoods in New England. North. J. Appl. For. 2003, 20, 188–189. [Google Scholar]
- Webster, C.R.; Lorimer, C.G. Single-tree versus group selection in hemlock-hardwood forests: Are smaller openings less productive? Can. J. For. Res. 2002, 32, 591–604. [Google Scholar] [CrossRef]
- Dale, M.E.; Smith, H.C.; Pearcy, J.N. Size of Clearcut Opening Affects Species Composition, Growth Rate, and Stand Characteristics; Res. Pap. NE-698; US Department of Agriculture, Forest Service, Northeastern Forest Experiment Station: Radnor, PA, USA, 1995; p. 21.
- Mcclure, J.W.; Lee, T.D. Small-Scale Disturbance in a Northern Hardwoods Forest-Effects on Tree Species Abundance and Distribution. Can. J. For. Res. 1993, 23, 1347–1360. [Google Scholar] [CrossRef]
- Kern, C.C.; Burton, J.I.; Raymond, P.; D’Amato, A.W.; Keeton, W.S.; Royo, A.A.; Walters, M.B.; Webster, C.R.; Willis, J.L. Challenges facing gap-based silviculture and possible solutions for mesic northern forests in North America. For. Int. J. For. Res. 2017, 90, 4–17. [Google Scholar] [CrossRef]
- Clark, J.; Beckage, B.; Camill, P.; Cleveland, B.; HilleRisLambers, J.; Lichter, J.; McLachlan, J.; Mohan, J.; Wyckoff, P. Interpreting recruitment limitation in forests. Am. J. Bot. 1999, 86, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Bolton, N.W.; D’Amato, A.W. Regeneration responses to gap size and coarse woody debris within natural disturbance-based silvicultural systems in northeastern Minnesota, USA. For. Ecol. Manag. 2011, 262, 1215–1222. [Google Scholar] [CrossRef]
- Webster, C.R.; Lorimer, C.G. Minimum opening sizes for canopy recruitment of midtolerant tree species: A retrospective approach. Ecol. Appl. 2005, 15, 1245–1262. [Google Scholar] [CrossRef]
- Bataineh, M.; Kenefic, L.; Weiskittel, A.; Wagner, R.; Brissette, J. Influence of partial harvesting and site factors on the abundance and composition of natural regeneration in the Acadian Forest of Maine, USA. For. Ecol. Manag. 2013, 306, 96–106. [Google Scholar] [CrossRef]
- Willis, J.L.; Walters, M.B.; Farinosi, E. Local seed source availability limits young seedling populations for some species more than other factors in northern hardwood forests. For. Sci. 2016, 62, 440–448. [Google Scholar] [CrossRef]
- Cole, W.G.; Lorimer, C.G. Probabilities of small-gap capture by sugar maple saplings based on height and crown growth data from felled trees. Can. J. For. Res. 2005, 35, 643–655. [Google Scholar] [CrossRef]
- Webster, C.R.; Dickinson, Y.L.; Burton, J.I.; Frelich, L.E.; Jenkins, M.A.; Kern, C.C.; Raymond, P.; Saunders, M.R.; Walters, M.B.; Willis, J.L. Promoting and maintaining diversity in contemporary hardwood forests: Confronting contemporary drivers of change and the loss of ecological memory. For. Ecol. Manag. 2018, 421, 98–108. [Google Scholar] [CrossRef]
- McClure, J.W.; Lee, T.D.; Leak, W.B. Gap capture in northern hardwoods: Patterns of establishment and height growth in four species. For. Ecol. Manag. 2000, 127, 181–189. [Google Scholar] [CrossRef]
- Klingsporn, S.; Webster, C.R.; Bump, J.K. Influence of legacy-tree retention on group-selection opening persistence. For. Ecol. Manag. 2012, 286, 121–128. [Google Scholar] [CrossRef]
- Nyland, R.D. Even-to uneven-aged: The challenges of conversion. For. Ecol. Manag. 2003, 172, 291–300. [Google Scholar] [CrossRef]
- Lorimer, C.G. Relative effects of small and large disturbances on temperate hardwood forest structure. Ecology 1989, 70, 565–567. [Google Scholar] [CrossRef]
- Marshall, R. The Growth of Hemlock Before and After Release from Suppression; Harvard Forest: Petersham, MA, USA, 1927. [Google Scholar]
- Webster, C.R.; Jensen, N.R. A shift in the gap dynamics of Betula alleghaniensis in response to single-tree selection. Can. J. For. Res. 2007, 37, 682–689. [Google Scholar] [CrossRef]
- Marquis, D.A. Prunus serotina Ehrh. black cherry. Silv. N. Am. 1990, 2, 594–604. [Google Scholar]
- Poznanovic, S.K.; Poznanovic, A.J.; Webster, C.R.; Bump, J.K. Spatial patterning of underrepresented tree species in canopy gaps 9 years after group selection cutting. For. Ecol. Manag. 2014, 331, 1–11. [Google Scholar] [CrossRef]
- Weigel, D.R.; Parker, G.R. Tree regeneration response to the group selection method in southern Indiana. North J. Appl. 1997, 14, 90–94. [Google Scholar] [CrossRef]
- Lu, D.; Wang, G.G.; Yan, Q.; Gao, T.; Zhu, J. Effects of gap size and within-gap position on seedling growth and biomass allocation: Is the gap partitioning hypothesis applicable to the temperate secondary forest ecosystems in Northeast China? For. Ecol. Manag. 2018, 429, 351–362. [Google Scholar] [CrossRef]
- Poulson, T.L.; Platt, W.J. Gap light regimes influence canopy tree diversity. Ecology 1989, 70, 553–555. [Google Scholar] [CrossRef]
- Kern, C.C.; Reich, P.B.; Montgomery, R.A.; Strong, T.F. Do deer and shrubs override canopy gap size effects on growth and survival of yellow birch, northern red oak, eastern white pine, and eastern hemlock seedlings? For. Ecol. Manag. 2012, 267, 134–143. [Google Scholar] [CrossRef]
- Soil Survey Staff. Web Soil Survey; Natural Resources Conservation Service, United States Department of Agriculture: Lincoln, NE, USA, 2017. Available online: https://websoilsurvey.sc.egov.usda.gov/ (accessed on 30 January 2019).
- Kotar, J.; Burger, T.L.; Kovach, J.A. A Guide to Forest Communities and Habitat Types of Northern Wisconsin; University of Wisconsin-Madison, Department of Forestry Ecology and Management: Madison, WI, USA, 2002. [Google Scholar]
- Arguez, A.; Durre, I.; Applequist, S.; Squires, M.; Vose, R.; Yin, X.; Bilotta, R. NOAA’s U.S. Climate Normals (1981–2010); NOAA National Centers for Environmental Information: Asheville, NC, USA, 2010. [CrossRef]
- Rhemtulla, J.M.; Mladenoff, D.J.; Clayton, M.K. Legacies of historical land use on regional forest composition and structure in Wisconsin, USA (mid-1800s–1930s–2000s). Ecol. Appl. 2009, 19, 1061–1078. [Google Scholar] [CrossRef]
- Kern, C.C.; D’Amato, A.W.; Strong, T.F. Diversifying the composition and structure of managed, late-successional forests with harvest gaps: What is the optimal gap size? For. Ecol. Manag. 2013, 304, 110–120. [Google Scholar] [CrossRef]
- Erdmann, G.G. Developing quality in second-growth stands. In The Northern Hardwood Resource: Management and Potential; Mroz, G.D., Reed, D.D., Eds.; Michigan Technological University: Houghton, MI, USA, 1986; pp. 206–222. [Google Scholar]
- Kotar, J.; Burger, T.L. A Guide to Forest Communities and Habitat Types of Michigan; University of Wisconsin-Madison, Department of Forestry Ecology and Management: Madison, WI, USA, 2003. [Google Scholar]
- Reed, D.D.; Holmes, M.J.; Johnson, J.A. A 22-year study of stand development and financial return in northern hardwoods. North J. Appl. 1986, 3, 35–38. [Google Scholar] [CrossRef]
- Bourdo, E.A.J.; Johnson, J.A. Practical Small Woodlot Management; Michigan College of Mining and Technology: L’Anse, MI, USA, 1959. [Google Scholar]
- Neuendorff, J.K.; Nagel, L.M.; Webster, C.R.; Janowiak, M.K. Stand structure and composition in a northern hardwood forest after 40 years of single-tree selection. North J. Appl. 2007, 24, 197–202. [Google Scholar] [CrossRef]
- Schwartz, J.W.; Nagel, L.M.; Webster, C.R. Effects of uneven-aged management on diameter distribution and species composition of northern hardwoods in Upper Michigan. For. Ecol. Manag. 2005, 211, 356–370. [Google Scholar] [CrossRef]
- Usda, N. The PLANTS Database; National Plant Data Team: Greensboro, NC, USA, 2019. Available online: http://plants.usda.gov (accessed on 29 April 2019).
- Shields, J.M.; Webster, C.R.; Nagel, L.M. Factors influencing tree species diversity and Betula alleghaniensis establishment in silvicultural openings. Forestry 2007, 80, 293–307. [Google Scholar] [CrossRef] [Green Version]
- Burns, R.M.; Honkala, B.H. Silvics of North America: 1. Conifers; 2. Hardwoods; U.S. Department of Agriculture, Forest Service: Washington, DC, USA, 1990; Volume 2, p. 877.
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package, R Package Version 2.5-4. 2019. Available online: https://CRAN.R-project.org/package=vegan (accessed on 6 May 2019).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- McCune, B.G.; Grace, J.J.B. Analysis of Ecological Communities; MJM Software Design: Gleneden Beach, OR, USA, 2002. [Google Scholar]
- Pinheiro, J.; Bates, D.; Sarkar, D.; Team, R.C. nlme: Linear and Nonlinear Mixed Effects Models, 3.1-129. 2019. Available online: https://CRAN.R-project.org/package=nlme (accessed on 13 May 2019).
- Lenth, R. emmeans: Estimated Marginal Means, Aka Least-Squares Means, R Package Version 1.3.3. 2019. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 5 August 2019).
- Zuur, A.F.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology in R.; Springer Science+Business Media, LLC: Berlin, Germany, 2009. [Google Scholar]
- Akima, H.; Gebhardt, A. akima: Interpolation of Irregularly and Regularly Spaced Data, 0.6-2. 2016. Available online: https://CRAN.R-project.org/package=akima (accessed on 27 April 2019).
- Baddeley, A.; Rubak, E.; Turner, R. Spatial Point Patterns: Methodology and Applications with R; Chapman and Hall/CRC: London, UK, 2015. [Google Scholar]
- Besag, J. Discussion of Dr Ripley’s paper. J. R. Stat. Soc. Ser. B 1977, 39, 193–195. [Google Scholar]
- Ripley, B.D. Modelling spatial patterns. J. R. Stat. Soc. Ser. B (Methodol.) 1977, 39, 172–192. [Google Scholar] [CrossRef]
- Falk, K.J.; Elliott, K.A.; Burke, D.M.; Nol, E. Early seedling response to group selection harvesting in a northern hardwood forest. For. Chron. 2010, 86, 100–109. [Google Scholar] [CrossRef] [Green Version]
- Knapp, S.P.; Webster, C.R.; Kern, C.C. Can group selection with legacy retention change compositional trajectories in conventionally managed hardwoods? For. Ecol. Manag. 2019, 448, 174–186. [Google Scholar] [CrossRef]
- Klingsporn Poznanovic, S.K.; Webster, C.R.; Bump, J.K. Maintaining mid-tolerant tree species with uneven-aged forest management: 9-year results from a novel group-selection experiment. Forestry 2013, 86, 555–567. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Lu, D.; Zhang, W. Effects of gaps on regeneration of woody plants: A meta-analysis. J. For. Res. 2014, 25, 501–510. [Google Scholar] [CrossRef]
- Nagel, T.A.; Svoboda, M.; Rugani, T.; Diaci, J. Gap regeneration and replacement patterns in an old-growth Fagus–Abies forest of Bosnia–Herzegovina. Plant Ecol. 2010, 208, 307–318. [Google Scholar] [CrossRef]
- Denslow, J.S. Gap Partitioning among Tropical Rainforest Trees. Biotropica 1980, 12, 47–55. [Google Scholar] [CrossRef]
- Kaelke, C.M.; Kruger, E.L.; Reich, P.B. Trade-offs in seedling survival, growth, and physiology among hardwood species of contrasting successional status along a light-availability gradient. Can. J. For. Res. 2001, 31, 1602–1616. [Google Scholar] [CrossRef]
- York, R.A.; Battles, J.J.; Heald, R.C. Edge effects in mixed conifer group selection openings: Tree height response to resource gradients. For. Ecol. Manag. 2003, 179, 107–121. [Google Scholar] [CrossRef]
- Curzon, M.; Baker, S.; Kern, C.; Palik, B.; D’Amato, A. Influence of mature overstory trees on adjacent 12-year regeneration and the woody understory: Aggregated retention versus intact forest. Forests 2017, 8, 31. [Google Scholar] [CrossRef]
- D’Amato, A.W.; Catanzaro, P.F.; Fletcher, L.S. Early regeneration and structural responses to patch selection and structural retention in second-growth northern hardwoods. For. Sci. 2014, 61, 183–189. [Google Scholar] [CrossRef]
- Urgenson, L.S.; Halpern, C.B.; Anderson, P.D. Twelve-year responses of planted and naturally regenerating conifers to variable-retention harvest in the Pacific Northwest, USA. Can. J. For. Res. 2012, 43, 46–55. [Google Scholar] [CrossRef]
- Canham, C.D.; Denslow, J.S.; Platt, W.J.; Runkle, J.R.; Spies, T.A.; White, P.S. Light regimes beneath closed canopies and tree-fall gaps in temperate and tropical forests. Can. J. For. Res. 1990, 20, 620–631. [Google Scholar] [CrossRef]
- Gauthier, M.-M.; Lambert, M.-C.; Bédard, S. Effects of harvest gap size, soil scarification, and vegetation control on regeneration dynamics in sugar maple-yellow birch stands. For. Sci. 2016, 62, 237–246. [Google Scholar] [CrossRef]
- Godman, R.M.; Krefting, L.W. Factors important to yellow birch establishment in Upper Michigan. Ecology 1960, 41, 18–28. [Google Scholar] [CrossRef]
- Prevost, M.; Raymond, P.; Lussier, J.-M. Regeneration dynamics after patch cutting and scarification in yellow birch–conifer stands. Can. J. For. Res. 2010, 40, 357–369. [Google Scholar] [CrossRef]
- Raymond, P.; Munson, A.D.; Ruel, J.-C.; Nolet, P. Group and single-tree selection cutting in mixed tolerant hardwood–white pine stands: Early establishment dynamics of white pine and associated species. For. Chron. 2003, 79, 1093–1106. [Google Scholar] [CrossRef]
- Pellerin, M.; Saïd, S.; Richard, E.; Hamann, J.-L.; Dubois-Coli, C.; Hum, P. Impact of deer on temperate forest vegetation and woody debris as protection of forest regeneration against browsing. For. Ecol. Manag. 2010, 260, 429–437. [Google Scholar] [CrossRef]
- Krueger, L.M.; Peterson, C.J.; Royo, A.; Carson, W.P. Evaluating relationships among tree growth rate, shade tolerance, and browse tolerance following disturbance in an eastern deciduous forest. Can. J. For. Res. 2009, 39, 2460–2469. [Google Scholar] [CrossRef]
- Hurley, P.M.; Flaspohler, D. An assessment of long-term biodiversity recovery from intense and sustained deer browse on North Manitou Island, Sleeping Bear Dunes National Lakeshore. In Proceedings of the Michigan Society of American Foresters Conference, St. Ignace, MI, USA, 10 June 2005. [Google Scholar]
- Long, Z.T.; Pendergast IV, T.H.; Carson, W.P. The impact of deer on relationships between tree growth and mortality in an old-growth beech-maple forest. For. Ecol. Manag. 2007, 252, 230–238. [Google Scholar] [CrossRef]
- Matonis, M.S.; Walters, M.B.; Millington, J.D. Gap-, stand-, and landscape-scale factors contribute to poor sugar maple regeneration after timber harvest. For. Ecol. Manag. 2011, 262, 286–298. [Google Scholar] [CrossRef]
- Frawley, B.J. Michigan Deer Harvest Survey Report 2016 Seasons; Michigan Department of Natural Resources: Lansing, MI, USA, 2017. [Google Scholar]
- Frawley, B.J. Michigan Deer Harvest Survey Report 2017 Seasons; Michigan Department of Natural Resources: Lansing, MI, USA, 2018. [Google Scholar]
- Frawley, B.J.; Boon, C.E. Michigan Deer Harvest Survey Report 2015 Seasons; Michigan Department of Natural Resources: Lansing, MI, USA, 2016. [Google Scholar]
- Wallenfang, K. Forest County Buck Harvest Densities. Available online: https://dnr.wi.gov/wideermetrics/DeerStats.aspx?R=5 (accessed on 29 May 2019).
- National Operational Hydrologic Remote Sensing Center. Snow Data Assimilation System (SNODAS) Data Products at NSIDC, November 15–April 15 in 2005–2018; National Snow and Ice Data Center: Boulder, CO, USA, 2004. [Google Scholar]
- Western Regional Climate Center. Period of Record Monthly Climate Survey 1939–2016. Available online: https://wrcc.dri.edu/cgi-bin/cliMAIN.pl?wi2314 (accessed on 23 August 2019).
- Verme, L.J. Movements of white-tailed deer in upper Michigan. J. Wildl. Manag. 1973, 37, 545–552. [Google Scholar] [CrossRef]
- Witt, J.; Webster, C.; Froese, R.; Drummer, T.; Vucetich, J. Scale-dependent drivers of ungulate patch use along a temporal and spatial gradient of snow depth. Can. J. Zool. 2012, 90, 972–983. [Google Scholar] [CrossRef]
- Tahtinen, B.; Murray, B.D.; Webster, C.R.; Tarasoff, C.S.; Burton, A.J. Does ungulate foraging behavior in forest canopy gaps produce a spatial subsidy with cascading effects on vegetation? For. Sci. 2013, 60, 819–829. [Google Scholar] [CrossRef]
- Closset-Kopp, D.; Chabrerie, O.; Valentin, B.; Delachapelle, H.; Decocq, G. When Oskar meets Alice: Does a lack of trade-off in r/K-strategies make Prunus serotina a successful invader of European forests? For. Ecol. Manag. 2007, 247, 120–130. [Google Scholar] [CrossRef]
- Hupperts, S.F.; Dickinson, Y.L.; Webster, C.R.; Kern, C.C. Promoting structural and species diversity in Great Lakes northern hardwoods: A conceptual model and its application. For. Int. J. For. Res. 2018, 120, 16–25. [Google Scholar] [CrossRef]
- Tubbs, C.H. Natural Regeneration of Northern Hardwoods in the Northern Great Lakes Region; North Central Forest Experimental Station: St. Paul, MN, USA, 1977. [Google Scholar]
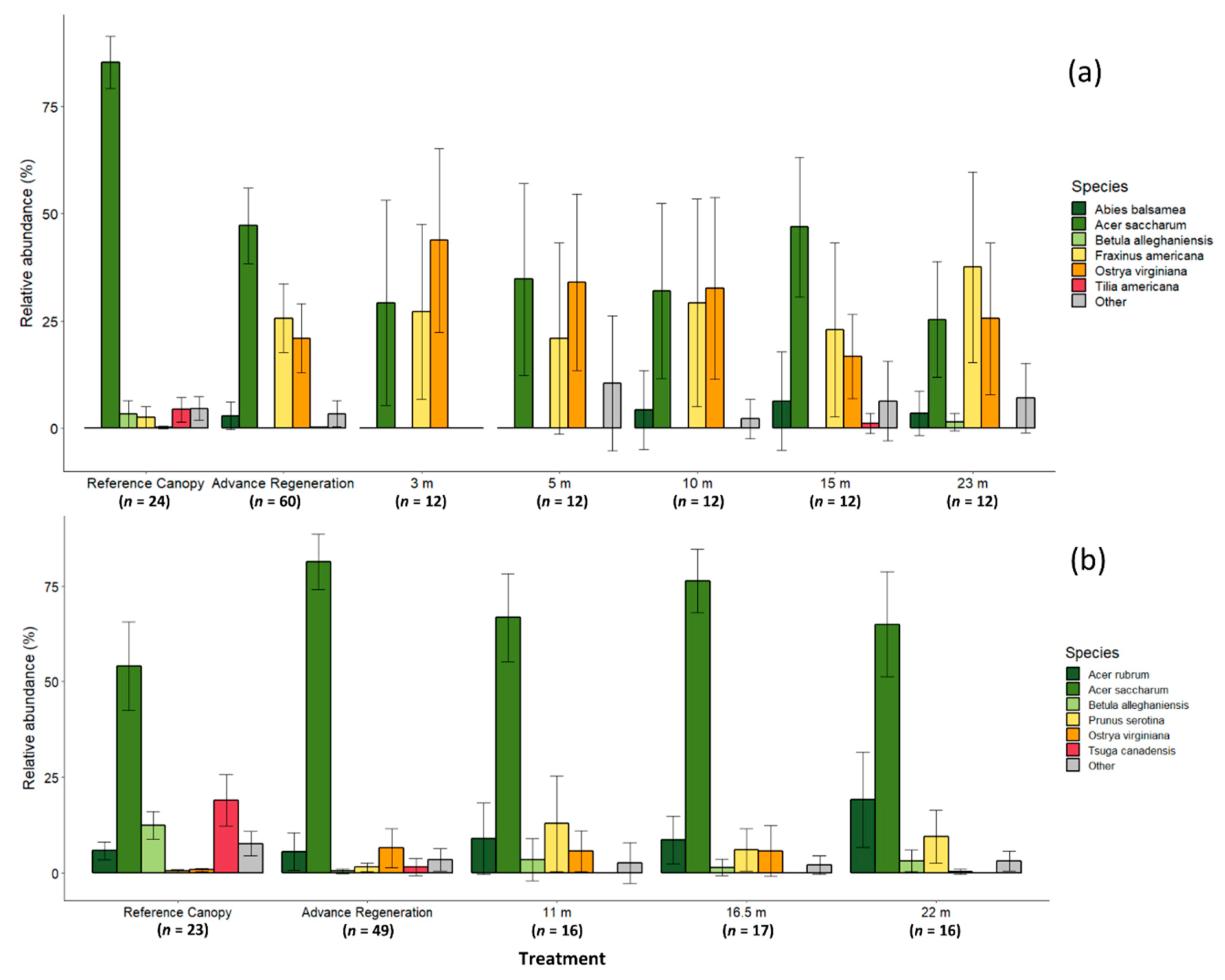
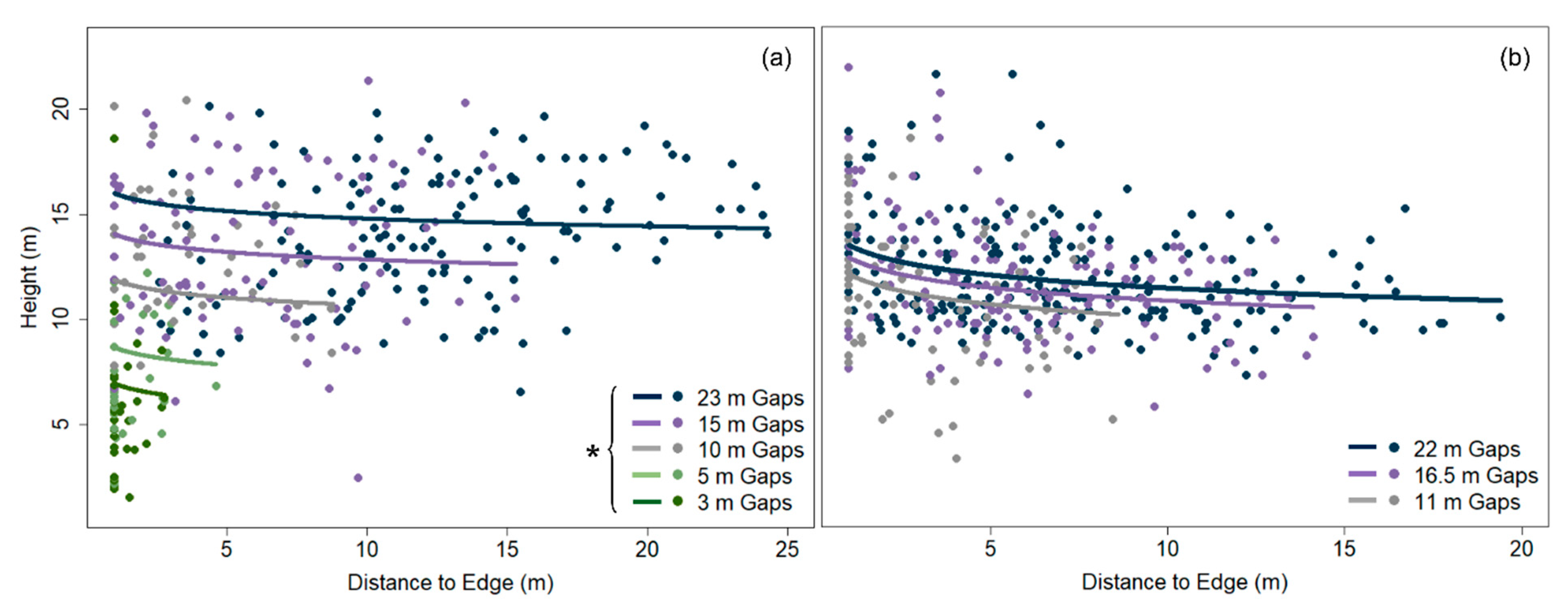
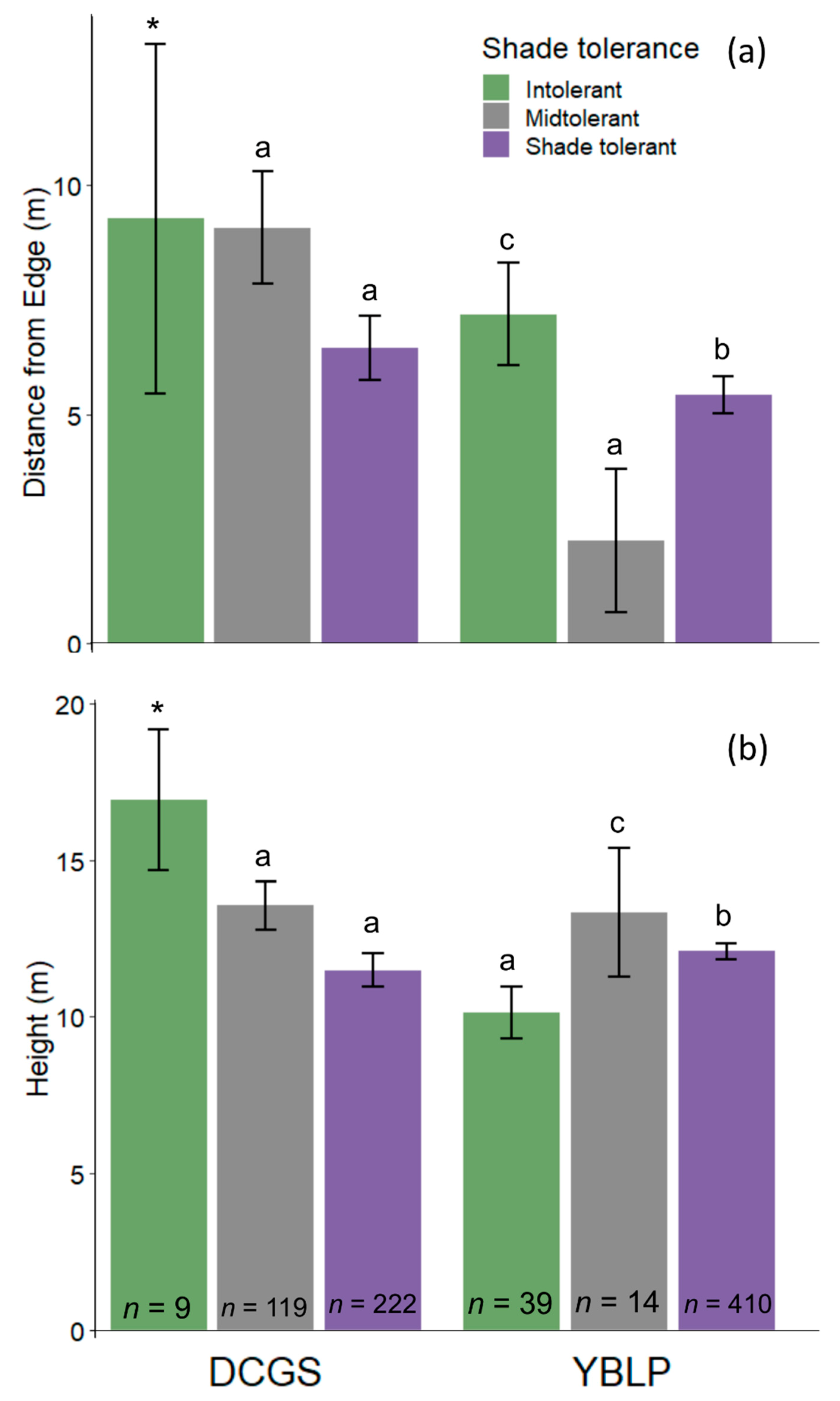
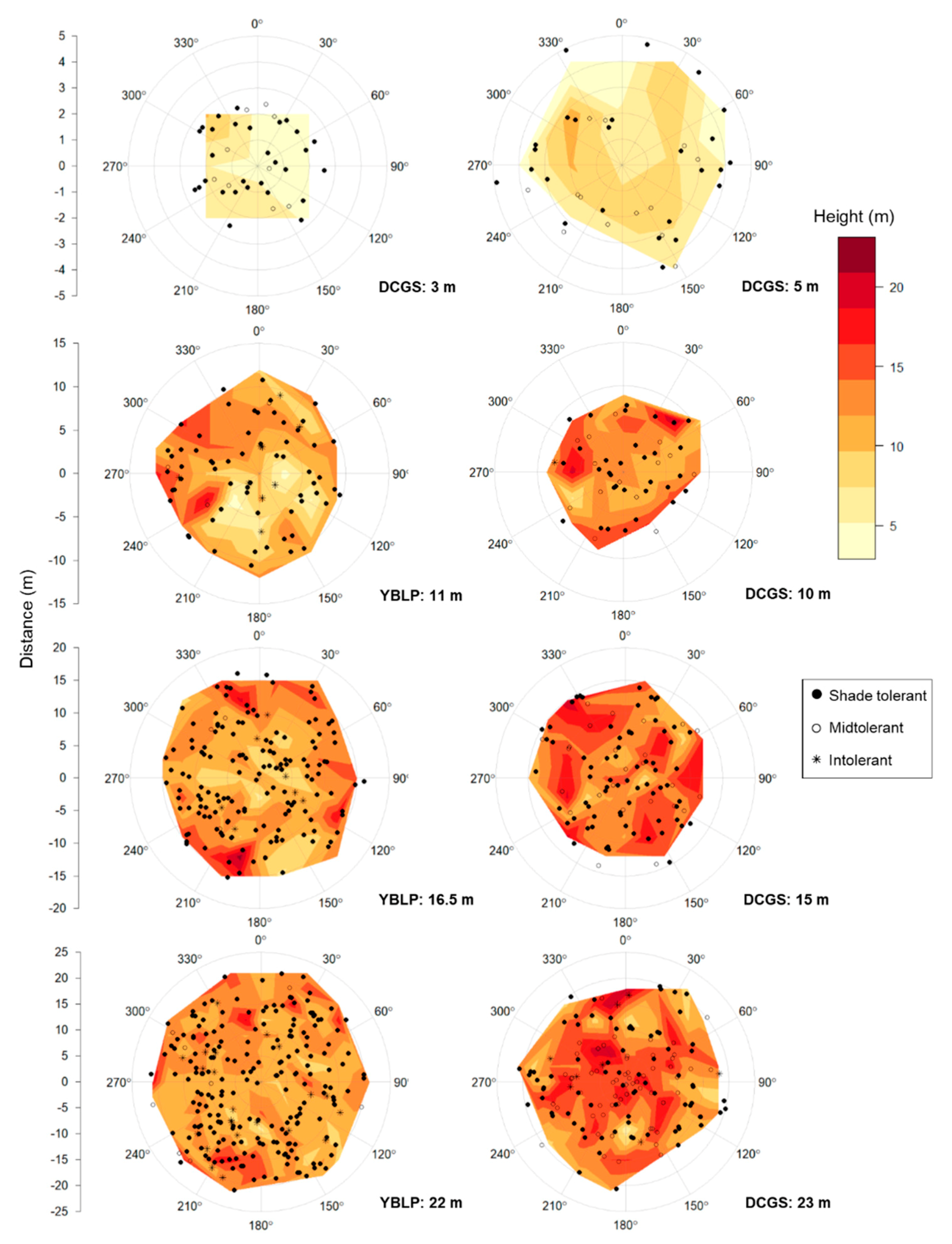
| Species | Shade Tolerance | DCGS | YBLP |
|---|---|---|---|
| Abies balsamea | Shade tolerant | X | X |
| Acer rubrum | Shade tolerant | X | X |
| Acer saccharum | Shade tolerant | X | X |
| Ostrya virginiana | Shade tolerant | X | X |
| Tilia americana | Shade tolerant | X | X |
| Tsuga canadensis | Shade tolerant | X | X |
| Betula alleghaniensis | Midtolerant | X | X |
| Corylus cornunta | Midtolerant | X | |
| Fraxinus americana | Midtolerant | X | |
| Picea glauca | Midtolerant | X | |
| Pinus strobus | Midtolerant | X | |
| Quercus rubra | Midtolerant | X | X |
| Ulmus americana | Midtolerant | X | X |
| Betula papyrifera | Intolerant | X | |
| Fraxinus nigra | Intolerant | X | |
| Juglans cinera | Intolerant | X | |
| Populus tremuloides | Intolerant | X | |
| Prunus serotina | Intolerant | X | X |
| Treatment | n | Line Diagram | Grouping | |||
|---|---|---|---|---|---|---|
| Divide Canopy Gap Study | ||||||
| Reference Canopy | 24 | a | ||||
| Advance Regeneration | 60 | b | ||||
| Gap-capture saplings, 3 m gap | 12 | b | ||||
| Gap-capture saplings, 5 m gap | 12 | b | ||||
| Gap-capture saplings, 10 m gap | 12 | b | ||||
| Gap-capture saplings, 15 m gap | 12 | b | ||||
| Gap-capture saplings, 23 m gap | 12 | b | ||||
| Yellow Birch Legacy Tree Project | ||||||
| Reference Canopy | 23 | a | ||||
| Advance Regeneration | 49 | b | ||||
| Gap-capture saplings, 11 m gap | 16 | c | ||||
| Gap-capture saplings, 16.5 m gap | 17 | c | ||||
| Gap-capture saplings, 22 m gap | 16 | c | ||||
| Fixed Effects | Estimate | Standard Error | df | t-Value | Pr (>|t|) | Random Effect | Std Dev of Intercept | Std Dev of Residual |
|---|---|---|---|---|---|---|---|---|
| Divide Canopy Gap Study | ||||||||
| Gap area | 2.108 | 0.240 | 50 | 8.766 | <0.0001 | Gap ID in Block | 2.178 | 2.382 |
| Distance to edge | −0.505 | 0.303 | 294 | −1.667 | 0.0966 | |||
| Yellow Birch Legacy Tree Project | ||||||||
| Gap area | 1.003 | 0.380 | 47 | 2.638 | 0.0113 | Gap ID | 1.237 | 2.285 |
| Distance to edge | −0.942 | 0.148 | 413 | −6.380 | <0.0001 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knapp, S.P.; Webster, C.R.; Kern, C.C. The Composition and Height of Saplings Capturing Silvicultural Gaps at Two Long-Term Experiments in Managed Northern Hardwood Forests. Forests 2019, 10, 855. https://doi.org/10.3390/f10100855
Knapp SP, Webster CR, Kern CC. The Composition and Height of Saplings Capturing Silvicultural Gaps at Two Long-Term Experiments in Managed Northern Hardwood Forests. Forests. 2019; 10(10):855. https://doi.org/10.3390/f10100855
Chicago/Turabian StyleKnapp, Samuel P., Christopher R. Webster, and Christel C. Kern. 2019. "The Composition and Height of Saplings Capturing Silvicultural Gaps at Two Long-Term Experiments in Managed Northern Hardwood Forests" Forests 10, no. 10: 855. https://doi.org/10.3390/f10100855





