SEM Analysis of the Interfacial Transition Zone between Cement-Glass Powder Paste and Aggregate of Mortar under Microwave Curing
Abstract
:1. Introduction
2. Results
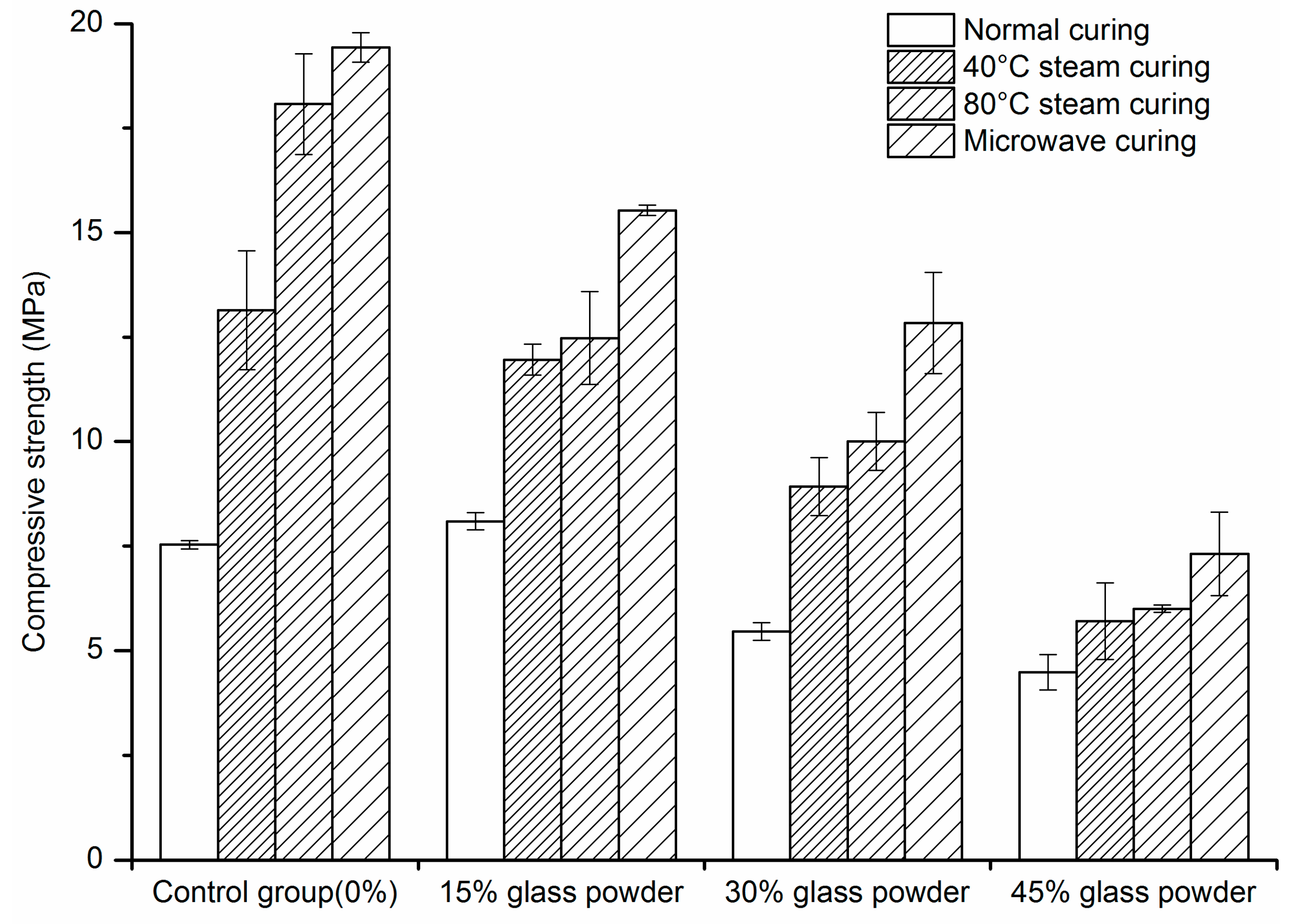
2.1. Compressive Strength
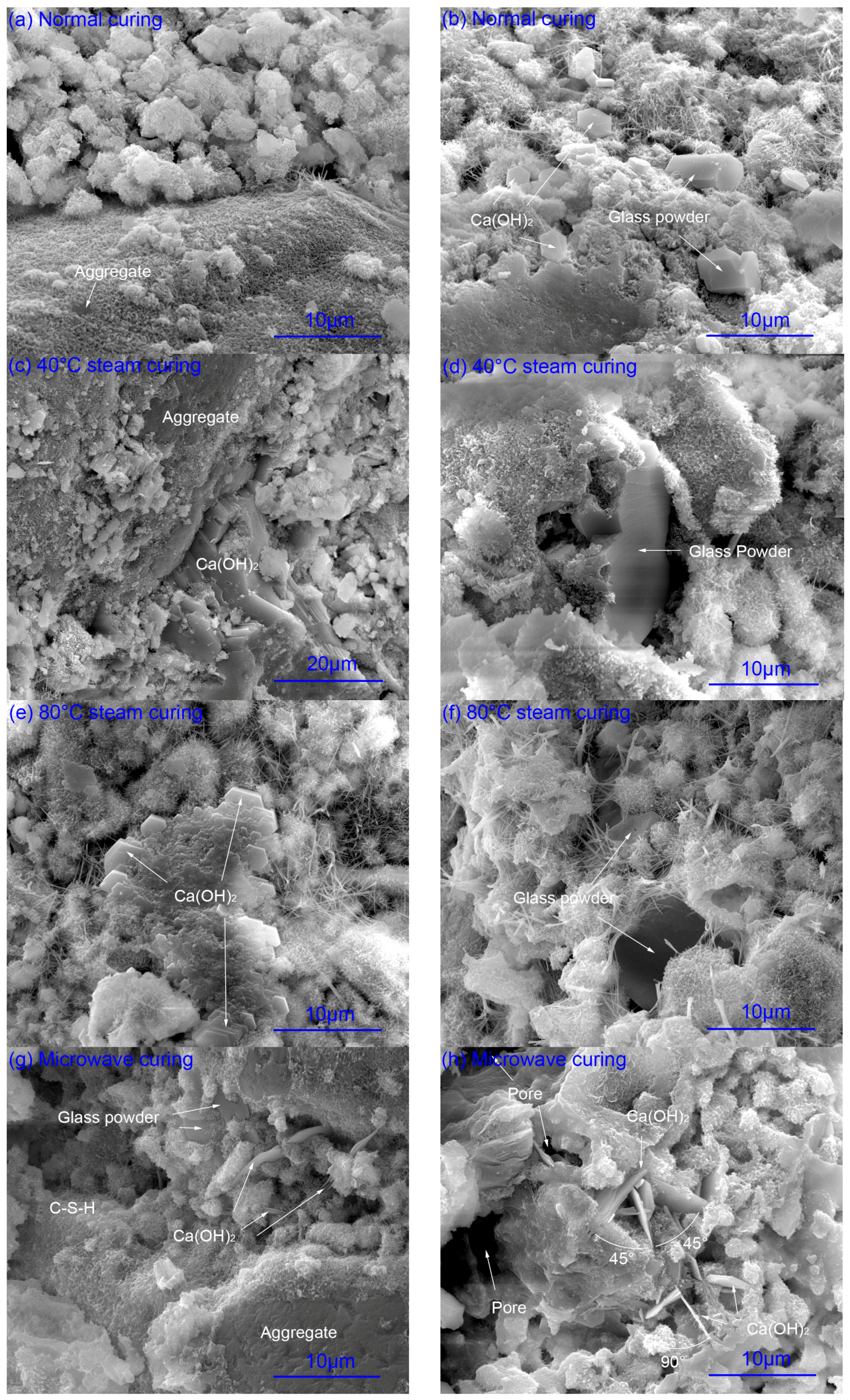
2.2. SEM Microanalysis
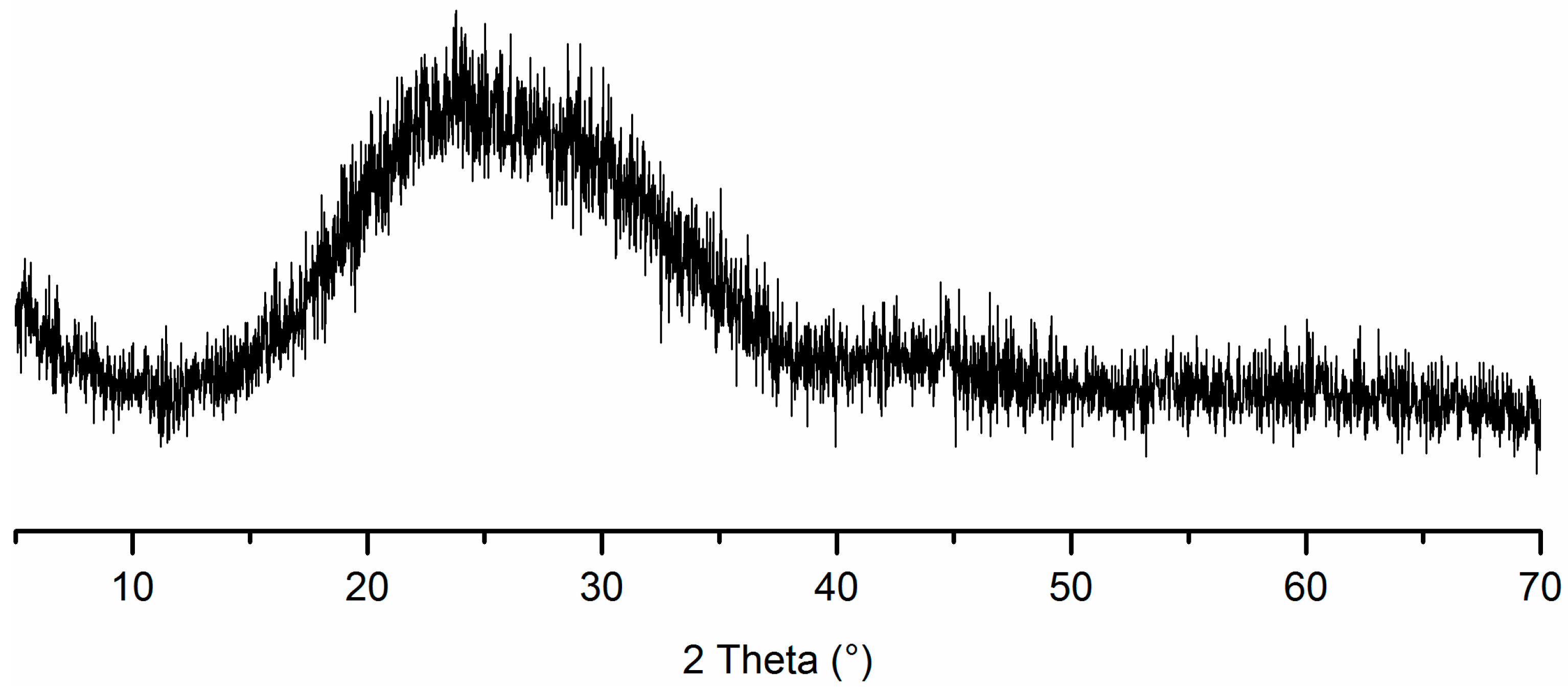
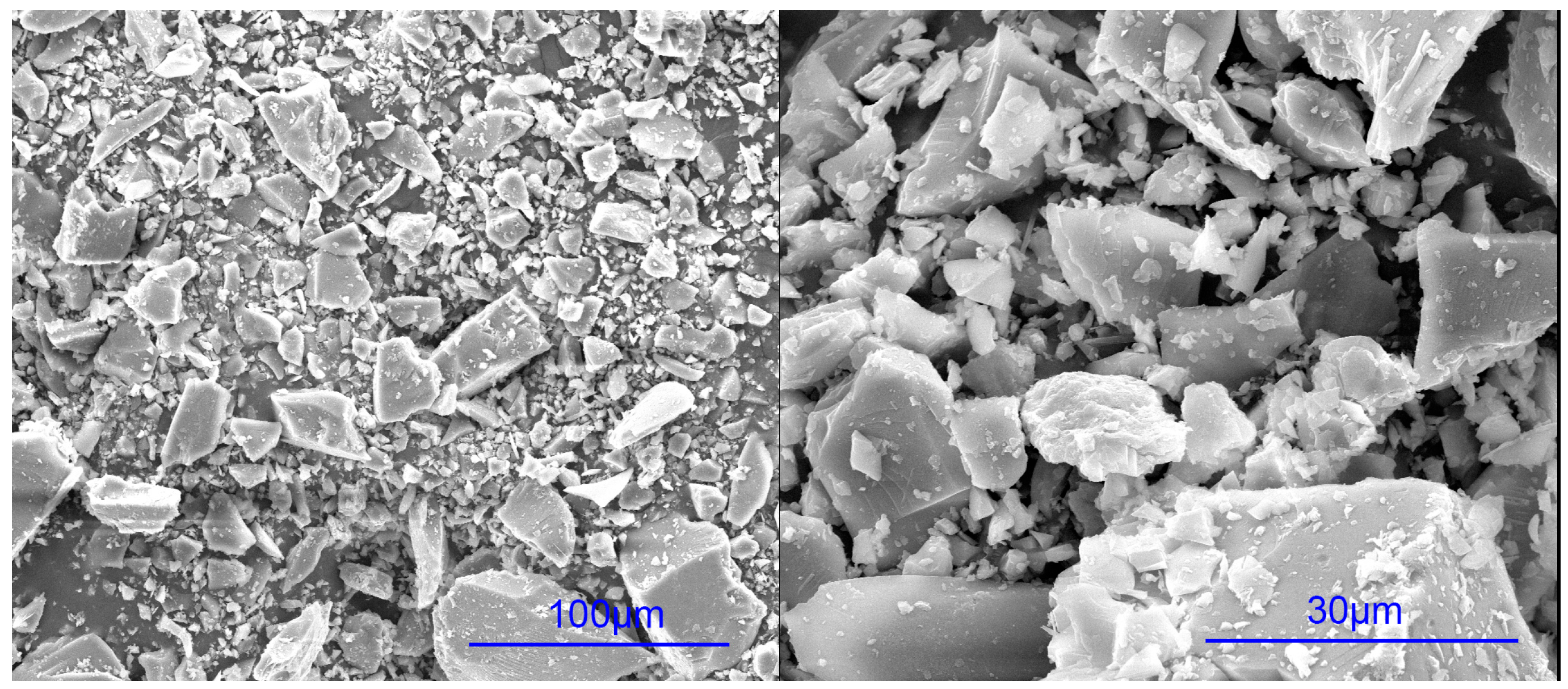
2.2.1. Hydration of Glass Powder
2.2.2. Calcium Hydroxide
2.2.3. Pore Structure
2.2.4. Element Composition
3. Discussion
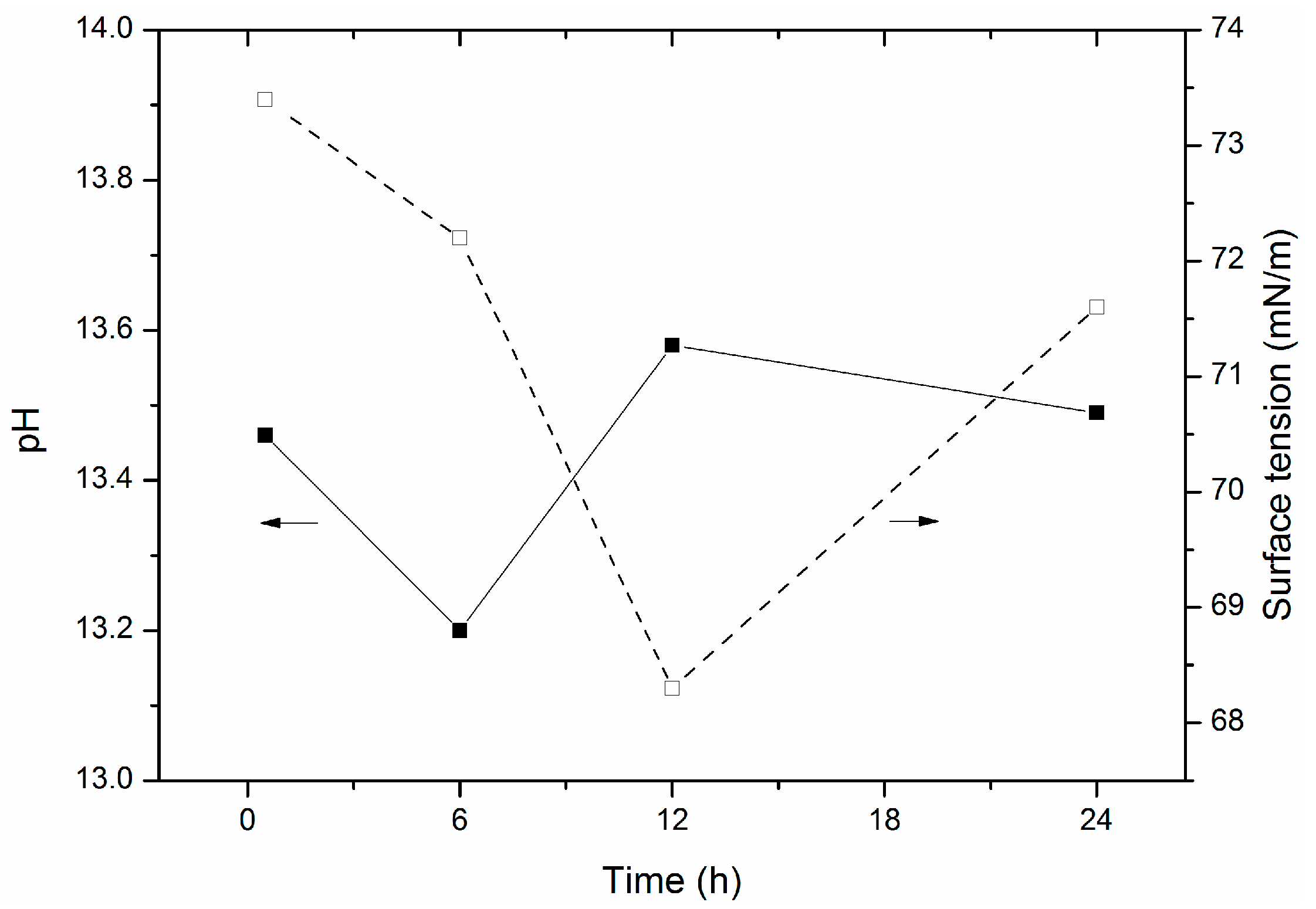
4. Materials and Methods
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Verbeck, G.J.; Helmuth, R.H. Structures and Physical Properties of Cement Paste. In Proceedings of the 5th International Symposium on the Chemistry of Cement, Tokyo, Japan, 7–11 October 1968; pp. 1–32.
- Buttress, A.; Jones, A.; Kingman, S. Microwave processing of cement and concrete materials–towards an industrial reality? Cem. Concr. Res. 2015, 68, 112–123. [Google Scholar] [CrossRef]
- Makul, N.; Rattanadecho, P.; Agrawal, D.K. Applications of microwave energy in cement and concrete—A review. Renew. Sustain. Energy Rev. 2014, 37, 715–733. [Google Scholar] [CrossRef]
- Mangat, P.; Grigoriadis, K.; Abubakri, S. Microwave curing parameters of in-situ concrete repairs. Constr. Build. Mater. 2016, 112, 856–866. [Google Scholar] [CrossRef]
- Leung, C.K.; Pheeraphan, T. Very high early strength of microwave cured concrete. Cem. Concr. Res. 1995, 25, 136–146. [Google Scholar] [CrossRef]
- Bella, G.D.; Lai, S.; Pinna, M. Beschleunigte erhärtung von beton durch mikrowellen. Betonw. Fert. Tech. (Bft) 1993, 60, 12. [Google Scholar]
- Bella, G.D.; Lai, S.; Pinna, M. Microwaves for the hyperaccelerated curing of concretes [beschleunigte erhartung von beton durch mikrowellen]. Betonw. Fertig. Tech./Concr. Precast. Plant. Technol. 1994, 60, 87–93. [Google Scholar]
- Kong, Y.; Wang, P.; Liu, S.; Gao, Z. Hydration and microstructure of cement-based materials under microwave curing. Constr. Build. Mater. 2016, 114, 831–838. [Google Scholar] [CrossRef]
- Oriol, M.; Pera, J. Pozzolanic activity of metakaolin under microwave treatment. Cem. Concr. Res. 1995, 25, 265–270. [Google Scholar] [CrossRef]
- Zhao, Z.; Song, W.; Dong, B.; Liu, Y.; Long, Y. Effect of microwave radiation activation coal gangue to portland cement system properties. Mater. Rev. 2011, S1. [Google Scholar]
- Makul, N.; Agrawal, D.K. Microwave (2.45 GHz)-assisted rapid sintering of SiO2-rich rice husk ash. Mater. Lett. 2010, 64, 367–370. [Google Scholar] [CrossRef]
- Xi, H. The Effect of Microwave-Activated Lithium Slag on the Coagulation Performance of Sulphoaluminate Cement Concrete; Nanjing University of Science and Technology: Nanjing, China, 2014. [Google Scholar]
- Bai, Y.; Shi, S.; Wang, Y.; Li, H.; Xu, D. Hydration and strength development of high volume fly ash/portland cement blend manufactured with room, thermal and microwave curing methods. In Proceedings of the 33rd Cement and Concrete Science Conference, Portsmouth, UK, 2–3 September 2013.
- Makul, N.; Agrawal, D.K. Microstructure and mechanical properties of microwave-assisted heating of pozzolan-portland cement paste at a very early stage. Songklanakarin J. Sci. Technol. 2013, 35, 693–703. [Google Scholar]
- Wu, X.; Dong, J.; Tang, M. Microwave curing technique in concrete manufacture. Cem. Concr. Res. 1987, 17, 205–210. [Google Scholar]
- Leung, C.K.; Pheeraphan, T. Microwave curing of Portland cement concrete: Experimental results and feasibility for practical applications. Constr. Build. Mater. 1995, 9, 67–73. [Google Scholar] [CrossRef]
- Makul, N.; Keangin, P.; Rattanadecho, P.; Chatveera, B.; Agrawal, D.K. Microwave-assisted heating of cementitious materials: Relative dielectric properties, mechanical property, and experimental and numerical heat transfer characteristics. Int. Commun. Heat Mass Transf. 2010, 37, 1096–1105. [Google Scholar] [CrossRef]
- Mehta, P.K.; Monteiro, P.J.M. Concrete: Microstructure, Properties, and Materials; McGraw-Hill: London, UK, 2006. [Google Scholar]
- Ke, Y.; Ortola, S.; Beaucour, A.; Dumontet, H. Identification of microstructural characteristics in lightweight aggregate concretes by micromechanical modelling including the interfacial transition zone (ITZ). Cem. Concr. Res. 2010, 40, 1590–1600. [Google Scholar] [CrossRef]
- Akçaoğlu, T.; Tokyay, M.; Çelik, T. Effect of coarse aggregate size and matrix quality on ITZ and failure behavior of concrete under uniaxial compression. Cem. Concr. Compos. 2004, 26, 633–638. [Google Scholar] [CrossRef]
- Prokopski, G.; Langier, B. Effect of water/cement ratio and silica fume addition on the fracture toughness and morphology of fractured surfaces of gravel concretes. Cem. Concr. Res. 2000, 30, 1427–1433. [Google Scholar] [CrossRef]
- Larbi, J.A. The Cement Paste-Aggregate Interfacial Zone in Concrete; Technical University of Delft: Delft, The Netherlands, 1991. [Google Scholar]
- Van Tuan, N.; Ye, G.; Van Breugel, K.; Copuroglu, O. Hydration and microstructure of ultra high performance concrete incorporating rice husk ash. Cem. Concr. Res. 2011, 41, 1104–1111. [Google Scholar] [CrossRef]
- Menéndez, G.; Bonavetti, V.; Irassar, E. Strength development of ternary blended cement with limestone filler and blast-furnace slag. Cem. Concr. Compos. 2003, 25, 61–67. [Google Scholar] [CrossRef]
- Mirzahosseini, M.; Riding, K.A. Effect of combined glass particles on hydration in cementitious systems. J. Mater. Civ. Eng. 2014, 27, 04014190. [Google Scholar] [CrossRef]
- Serpa, D.; Silva, A.S.; de Brito, J.; Pontes, J.; Soares, D. Asr of mortars containing glass. Constr. Build. Mater. 2013, 47, 489–495. [Google Scholar] [CrossRef]
- Du, H.; Tan, K.H. Waste glass powder as cement replacement in concrete. J. Adv. Concr. Technol. 2014, 12, 468–477. [Google Scholar] [CrossRef]
- Schwarz, N.; Neithalath, N. Influence of a fine glass powder on cement hydration: Comparison to fly ash and modeling the degree of hydration. Cem. Concr. Res. 2008, 38, 429–436. [Google Scholar] [CrossRef]
- Liu, S.; Xie, G.; Wang, S. Effect of curing temperature on hydration properties of waste glass powder in cement-based materials. J. Therm. Anal. Calorim. 2015, 119, 47–55. [Google Scholar] [CrossRef]
- Du, H.; Tan, K.H. Concrete with recycled glass as fine aggregates. ACI Mater. J. 2014, 111, 47–58. [Google Scholar]
- Idir, R.; Cyr, M.; Tagnit-Hamou, A. Use of fine glass as ASR inhibitor in glass aggregate mortars. Constr. Build. Mater. 2010, 24, 1309–1312. [Google Scholar] [CrossRef]
- Liu, S.; Wang, S.; Tang, W.; Hu, N.; Wei, J. Inhibitory effect of waste glass powder on ASR expansion induced by waste glass aggregate. Materials 2015, 8, 6849–6862. [Google Scholar] [CrossRef]
- Du, H.; Tan, K.H. Effect of particle size on alkali–silica reaction in recycled glass mortars. Constr. Build. Mater. 2014, 66, 275–285. [Google Scholar] [CrossRef]
- Du, H.; Tan, K.H. Transport properties of concrete with glass powder as supplementary cementitious material. ACI Mater. J. 2015, 112, 429–439. [Google Scholar] [CrossRef]
- Jawed, I.; Skalny, J. Alkalies in cement: A review: II. Effects of alkalies on hydration and performance of Portland cement. Cem. Concr. Res. 1978, 8, 37–51. [Google Scholar] [CrossRef]
- Ramachandran, V.; Beaudoin, J.; Sarkar, S.; Xu, A. Physico-chemical and microstructural investigations of the effect of NaOH on the hydration of 3CaO·SiO2. Cemento 1993, 90, 73–73. [Google Scholar]
- Redden, R.; Neithalath, N. Microstructure, strength, and moisture stability of alkali activated glass powder-based binders. Cem. Concr. Compos. 2014, 45, 46–56. [Google Scholar] [CrossRef]
- Yuan, R. Cementitious Materials; Wuhan University of Technology Press: Wuhan, China, 1996. [Google Scholar]
- Shi, C. Corrosion of glasses and expansion mechanism of concrete containing waste glasses as aggregates. J. Mater. Civ. Eng. 2009, 21, 529–534. [Google Scholar] [CrossRef]
- Glasser, L.D.; Kataoka, N. The chemistry of ‘alkali-aggregate’reaction. Cem. Concr. Res. 1981, 11, 1–9. [Google Scholar] [CrossRef]
- Shayan, A.; Xu, A. Performance of glass powder as a pozzolanic material in concrete: A field trial on concrete slabs. Cem. Concr. Res. 2006, 36, 457–468. [Google Scholar] [CrossRef]
- Tang, M.-S.; Xu, Z.-Z.; Han, S.-F. Alkali reactivity of glass aggregate. Durab. Build. Mater. 1987, 4, 377–385. [Google Scholar]
- Saccani, A.; Bignozzi, M.C. ASR expansion behavior of recycled glass fine aggregates in concrete. Cem. Concr. Res. 2010, 40, 531–536. [Google Scholar] [CrossRef]
- Shi, C.; Wu, Y.; Riefler, C.; Wang, H. Characteristics and pozzolanic reactivity of glass powders. Cem. Concr. Res. 2005, 35, 987–993. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Crumbie, A.K.; Laugesen, P. The interfacial transition zone (ITZ) between cement paste and aggregate in concrete. Interface Sci. 2004, 12, 411–421. [Google Scholar] [CrossRef]
- Sun, G.W.; Sun, W.; Zhang, Y.S.; Liu, Z.Y. Numerical calculation and influencing factors of the volume fraction of interfacial transition zone in concrete. Sci. China 2012, 55, 1515–1522. [Google Scholar] [CrossRef]


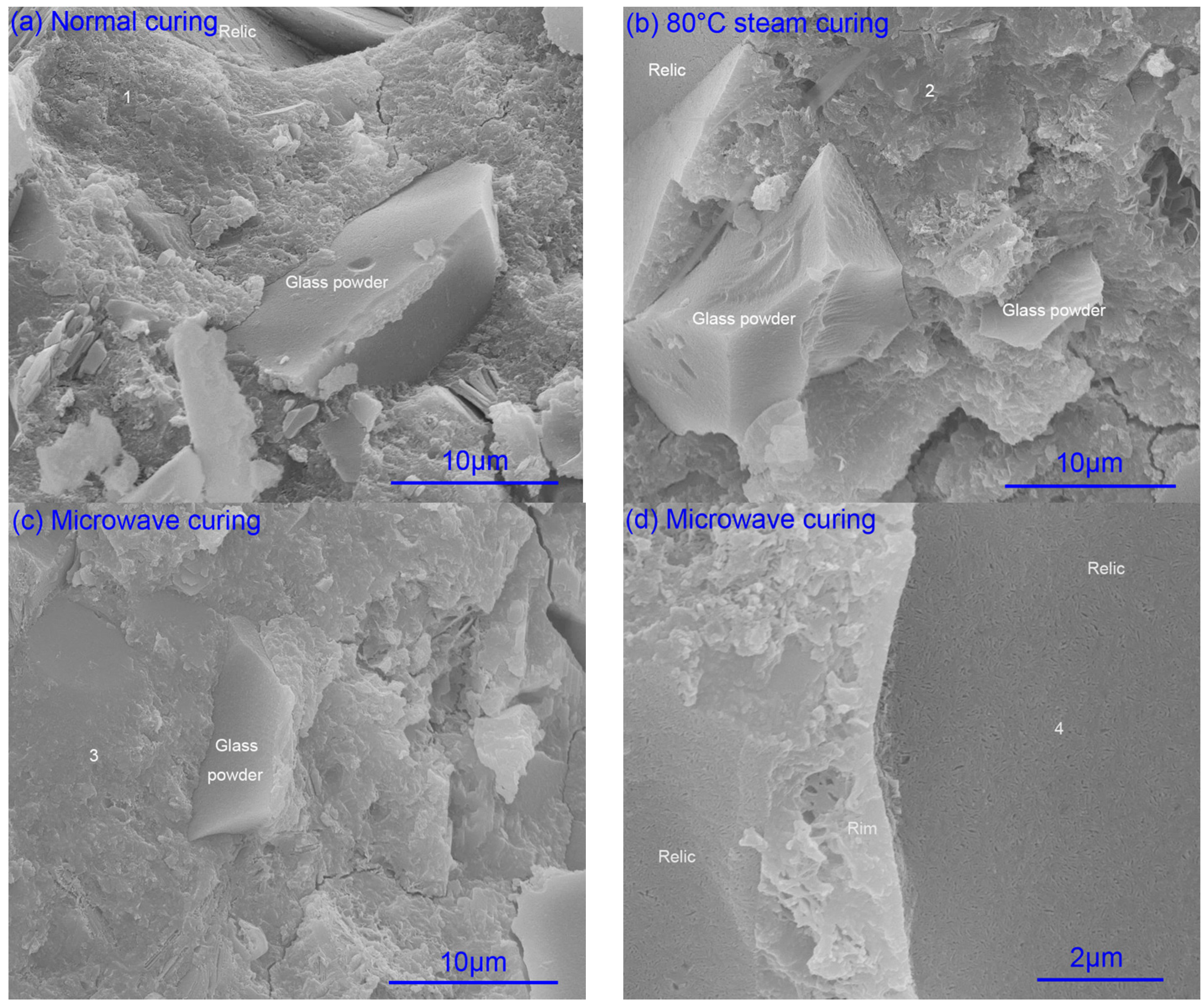
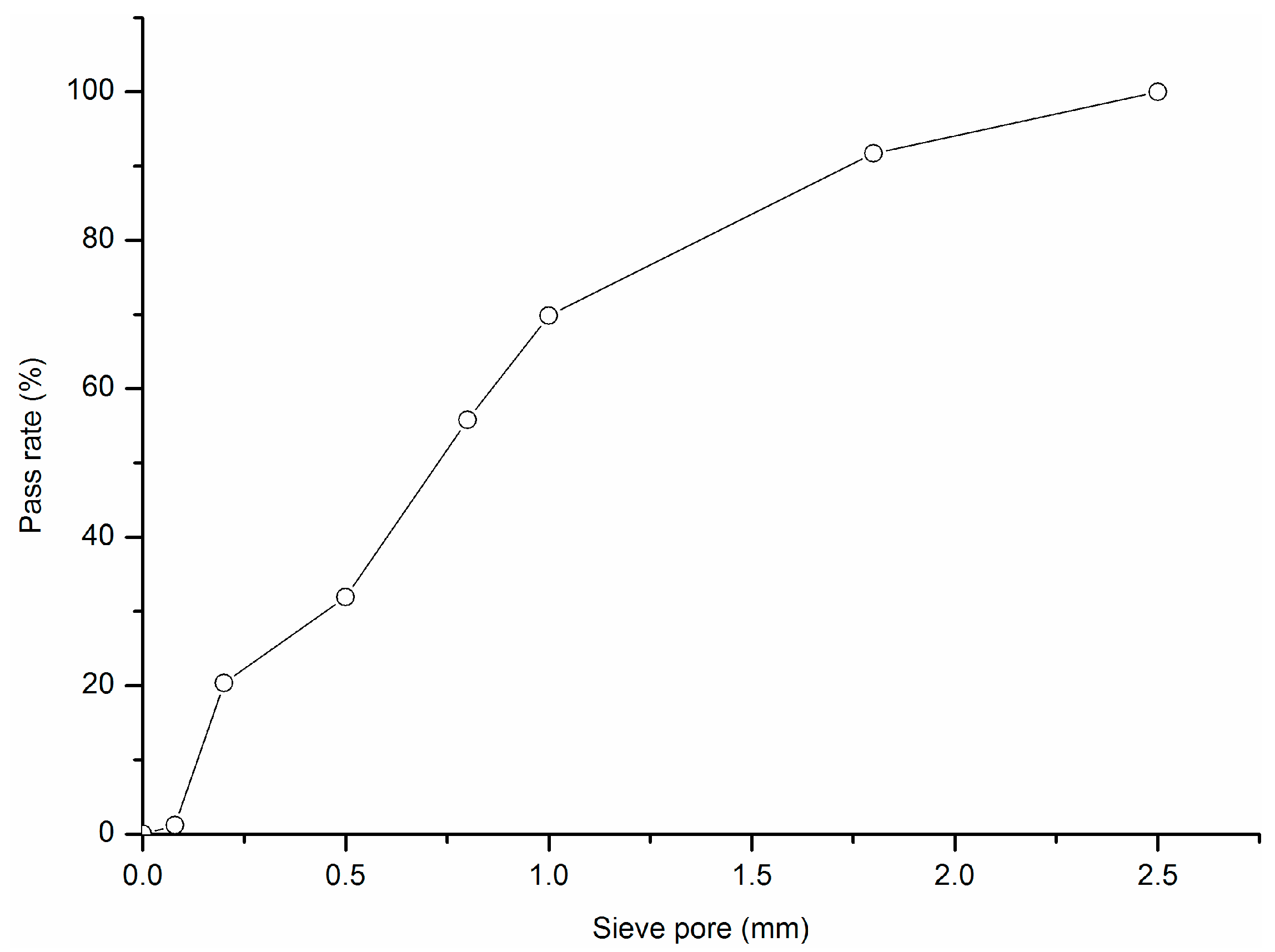
| EDS Spots in Figure 4 | O | Na | Mg | Al | Si | S | K | Ca | Na/Si | Ca/(Si+Na) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 62.75 | 2.29 | 1.67 | 2.76 | 12.87 | 1.62 | 0.99 | 15.05 | 0.18 | 0.99 |
| 2 | 59.73 | 2.82 | 0.66 | 1.72 | 13.62 | 1.66 | 0.8 | 19.01 | 0.21 | 1.16 |
| 3 | 44.38 | 3.09 | 1.36 | 2.14 | 17.12 | 1.99 | 1.29 | 28.62 | 0.18 | 1.42 |
| 4 | 34.05 | 5.07 | 1.49 | 3.26 | 17.22 | 3.54 | 2.1 | 33.27 | 0.29 | 1.49 |
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Na2O | Cl | f-CaO | Loss on Ignition | |
|---|---|---|---|---|---|---|---|---|---|---|
| Cement | 20.81 | 4.92 | 3.41 | 62.65 | 2.38 | 2.65 | 0.67 | 0.012 | 0.81 | 2.01 |
| Glass powder | 71.8 | 1.6 | 0.39 | 10.7 | 0.43 | 0.46 | 13.2 | 0.11 | - | 0.27 |
| Cumulative Percentage (%) | Characteristic Particle Diameter (μm) | Specific Surface Area (m2/kg) | |||||
|---|---|---|---|---|---|---|---|
| <25 μm | <45 μm | <80 μm | D10 | D50 | D90 | ||
| Cement | 66.3 | 89.6 | 98.4 | 2.92 | 17.18 | 47.94 | 347 |
| Glass powder | 42.7 | 63.1 | 81.1 | 1.75 | 33.01 | 110.98 | 270 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, Y.; Wang, P.; Liu, S.; Zhao, G.; Peng, Y. SEM Analysis of the Interfacial Transition Zone between Cement-Glass Powder Paste and Aggregate of Mortar under Microwave Curing. Materials 2016, 9, 733. https://doi.org/10.3390/ma9090733
Kong Y, Wang P, Liu S, Zhao G, Peng Y. SEM Analysis of the Interfacial Transition Zone between Cement-Glass Powder Paste and Aggregate of Mortar under Microwave Curing. Materials. 2016; 9(9):733. https://doi.org/10.3390/ma9090733
Chicago/Turabian StyleKong, Yaning, Peiming Wang, Shuhua Liu, Guorong Zhao, and Yu Peng. 2016. "SEM Analysis of the Interfacial Transition Zone between Cement-Glass Powder Paste and Aggregate of Mortar under Microwave Curing" Materials 9, no. 9: 733. https://doi.org/10.3390/ma9090733





