Improved Ablation Resistance of Silicone Rubber Composites by Introducing Montmorillonite and Silicon Carbide Whisker
Abstract
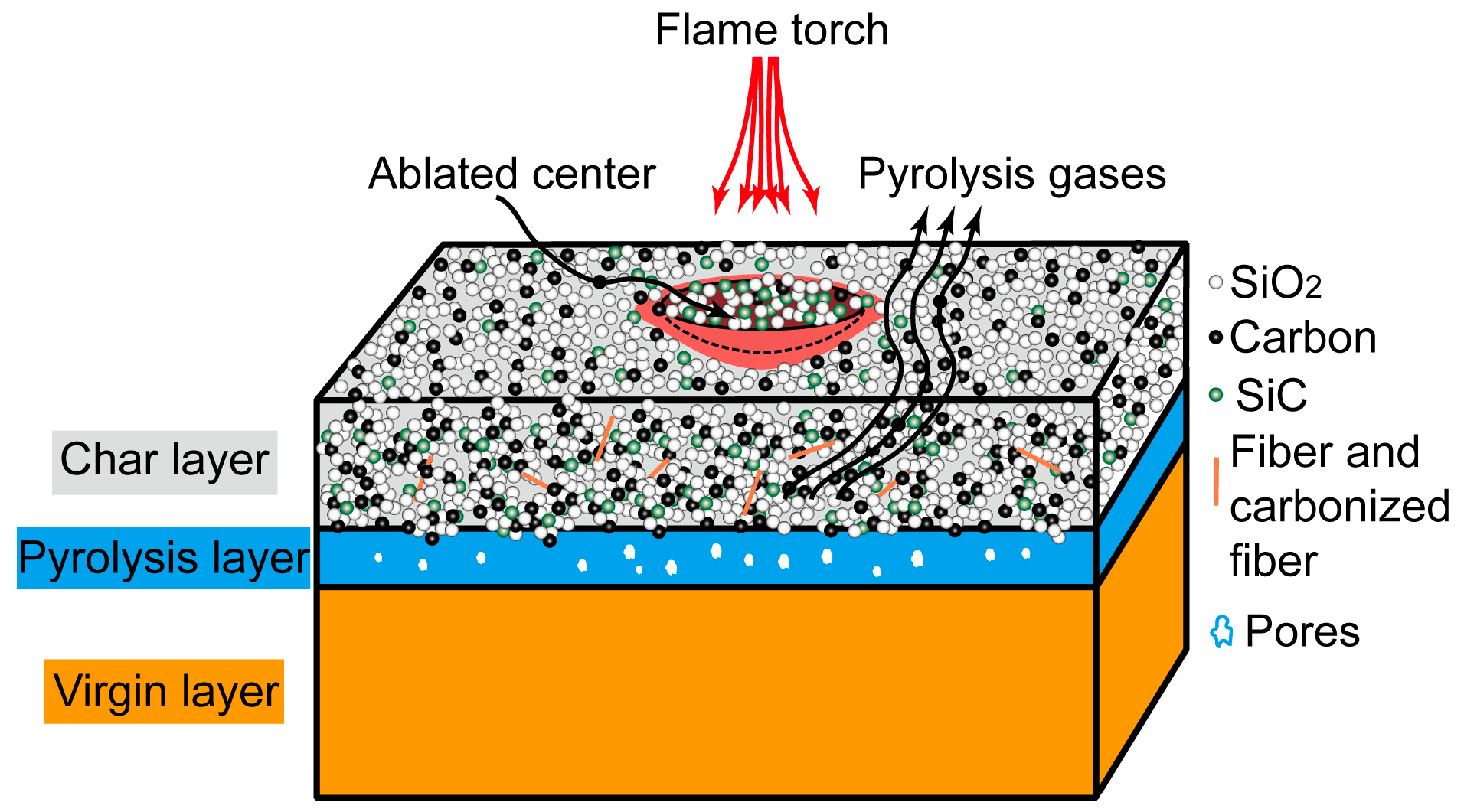
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of MMT/SR Masterbatch
2.3. Fabrication of SR Composites
2.4. Characterization
2.4.1. Ablation Test
2.4.2. X-ray Diffraction (XRD)
2.4.3. Morphology
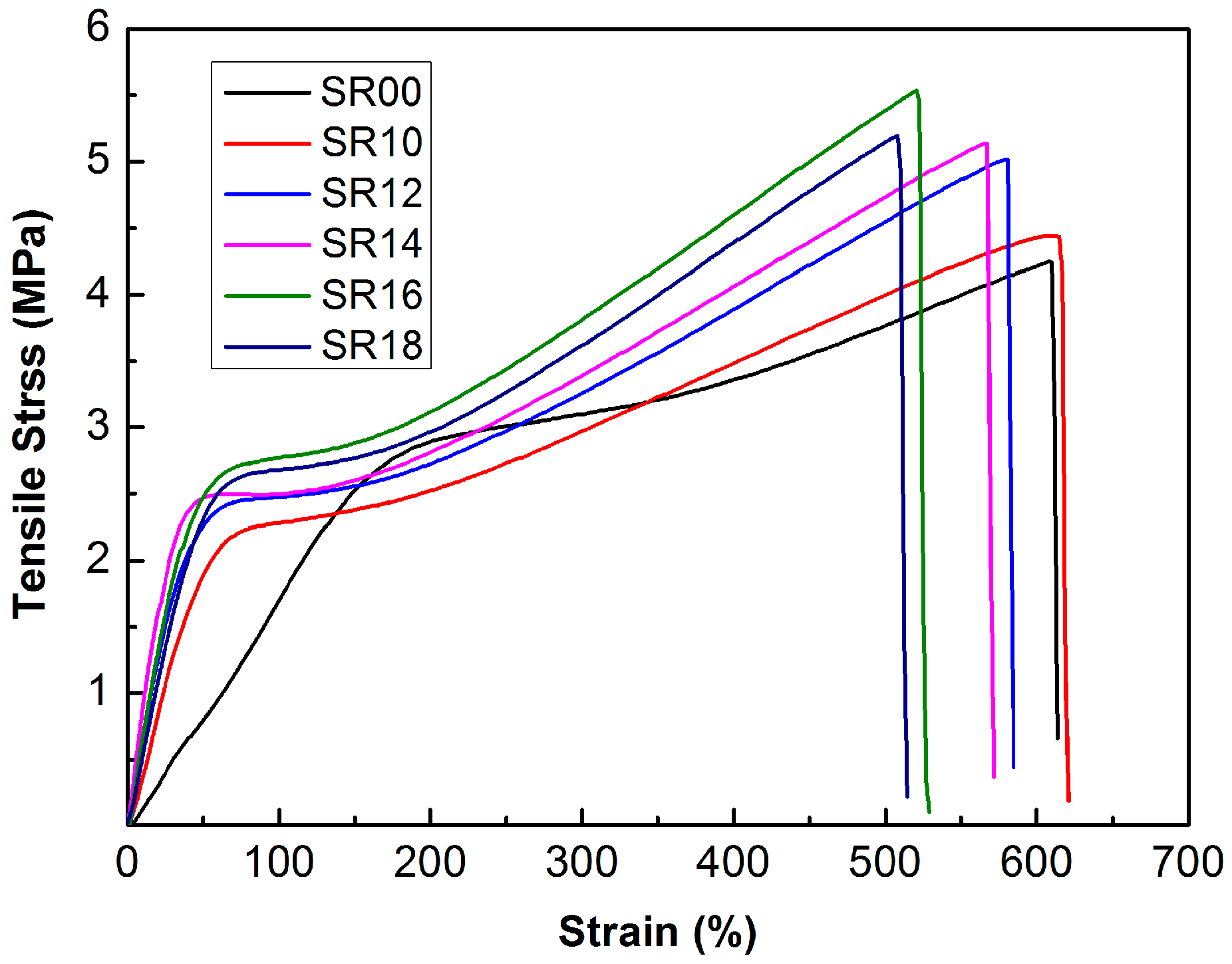
2.4.4. Mechanical and Physical Properties
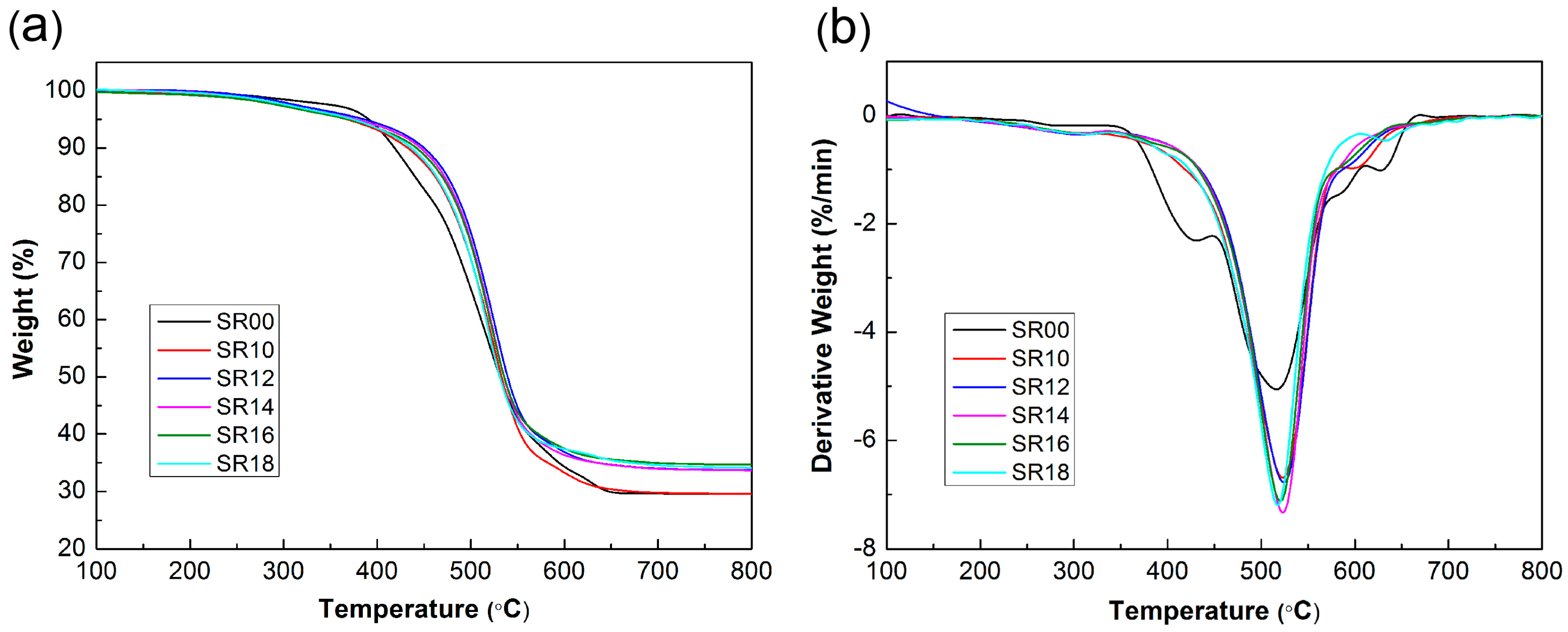
2.4.5. Thermogravimetric Analysis (TGA)
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Koo, J.H.; Natali, M.; Tate, J.; Allcorn, E. Polymer nanocomposites as ablative materials—A comprehensive review. Int. J. Energ. Mater. Chem. Propuls. 2013, 12, 119–162. [Google Scholar] [CrossRef]
- Bian, L.P.; Xiao, J.Y.; Zeng, J.C.; Xing, S.L.; Yin, C.P. Microstructural interpretation of the ablative properties of phenolic–quartz hybrid fabric reinforced phenolic resin composites. Mater. Des. 2014, 62, 424–429. [Google Scholar] [CrossRef]
- Natali, M.; Monti, M.; Puglia, D.; Kenny, J.M.; Torre, L. Ablative properties of carbon black and MWNT/phenolic composites: A comparative study. Compos. Part A Appl. Sci. Manuf. 2012, 43, 174–182. [Google Scholar] [CrossRef]
- Chen, Y.X.; Chen, P.; Hong, C.Q.; Zhang, B.X.; Hui, D. Improved ablation resistance of carbon–phenolic composites by introducing zirconium diboride particles. Compos. Part B Eng. 2013, 47, 320–325. [Google Scholar] [CrossRef]
- Amirsardari, Z.; Mehdinavaz Aghdam, R.; Salavati-Niasari, M.; Shakhesi, S. Enhanced thermal resistance of GO/C/phenolic nanocomposite by introducing ZrB2 nanoparticles. Compos. Part B Eng. 2015, 76, 174–179. [Google Scholar] [CrossRef]
- Badhe, Y.; Balasubramanian, K. Novel hybrid ablative composites of resorcinol formaldehyde as thermal protection systems for re-entry vehicles. RSC Adv. 2014, 4, 28956–28963. [Google Scholar] [CrossRef]
- Hu, Y.; Geng, W.L.; You, H.; Wang, Y.; Loy, D.A. Modification of a phenolic resin with epoxy- and methacrylate-functionalized silica sols to improve the ablation resistance of their glass fiber-reinforced composites. Polymers 2014, 6, 105–113. [Google Scholar] [CrossRef]
- Youren, J.W. Ablation of elastomeric composites for rocket motor insulation. Composites 1971, 2, 180–184. [Google Scholar] [CrossRef]
- Oyumi, Y. Ablation characteristics of silicone insulation. J. Polym. Sci. Polym. Chem. 1998, 36, 233–239. [Google Scholar] [CrossRef]
- Allcorn, E.K.; Natali, M.; Koo, J.H. Ablation performance and characterization of thermoplastic polyurethane elastomer nanocomposites. Compos. Part A Appl. Sci. Manuf. 2013, 45, 109–118. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, S.H.; Liu, W.; An, G.L.; Wu, Z.P.; Wu, D.Z.; Jin, R.G. Nitrile butadiene rubber-based heat-shielding insulations for solid rocket motors: Effect of polyimide fibrous reinforcement on the morphology and properties. High Perform. Polym. 2014, 27, 153–160. [Google Scholar] [CrossRef]
- Ahmed, A.F.; Hoa, S.V. Thermal insulation by heat resistant polymers for solid rocket motor insulation. J. Compos. Mater. 2011, 46, 1549–1559. [Google Scholar] [CrossRef]
- Farajpour, T.; Bayat, Y.; Abdollahi, M.; Keshavarz, M.H. Effect of borax on the thermal and mechanical properties of ethylene-propylene-diene terpolymer rubber-based heat insulator. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Guan, Y.; Zhang, L.-X.; Zhang, L.-Q.; Lu, Y.-L. Study on ablative properties and mechanisms of hydrogenated nitrile butadiene rubber (HNBR) composites containing different fillers. Polym. Degrad. Stab. 2011, 96, 808–817. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, W.; Jiang, B.Z.; Guo, Y. Silicone rubber ablative composites improved with zirconium carbide or zirconia. Compos. Part A Appl. Sci. Manuf. 2013, 44, 70–77. [Google Scholar] [CrossRef]
- Arroyo, M.; Lopez-Manchado, M.A.; Herrero, B. Organo-montmorillonite as substitute of carbon black in natural rubber compounds. Polymer 2003, 44, 2447–2453. [Google Scholar] [CrossRef]
- Kim, J.T.; Oh, T.S.; Lee, D.H. Preparation and characteristics of nitrile rubber (NBR) nanocomposites based on organophilic layered clay. Polym. Int. 2003, 52, 1058–1063. [Google Scholar] [CrossRef]
- Bayer, I.S.; Steele, A.; Martorana, P.; Loth, E.; Robinson, S.J.; Stevenson, D. Biolubricant induced phase inversion and superhydrophobicity in rubber-toughened biopolymer/organoclay nanocomposites. Appl. Phys. Lett. 2009, 95, 063702–063703. [Google Scholar] [CrossRef]
- Wu, Y.P.; Huang, H.H.; Zhao, W.; Zhang, H.F.; Wang, Y.Q.; Zhang, L.Q. Flame retardance of montmorillonite/rubber composites. J. Appl. Polym. Sci. 2008, 107, 3318–3324. [Google Scholar] [CrossRef]
- Zhang, H.F.; Wang, Y.Q.; Wu, Y.P.; Zhang, L.Q.; Yang, J. Study on flammability of montmorillonite/styrene-butadiene rubber (SBR) nanocomposites. J. Appl. Polym. Sci. 2005, 97, 844–849. [Google Scholar] [CrossRef]
- Yang, L.; Hu, Y.; Lu, H.D.; Song, L. Morphology, thermal, and mechanical properties of flame-retardant silicone rubber/montmorillonite nanocomposites. J. Appl. Polym. Sci. 2006, 99, 3275–3280. [Google Scholar] [CrossRef]
- Iqbal, N.; Sagar, S.; Khan, M.B.; Rafique, H.M. Ablation, thermal stability/transport and mechanical investigations of modified nanokaolinite impregnated acrylonitrile butadiene rubber composites. J. Compos. Mater. 2013, 48, 1221–1231. [Google Scholar] [CrossRef]
- He, J.; Zhang, X.; Li, X.; Yang, R. Effect of OMMT on ablative, thermal and flame retardant properties of ethylene propylene diene rubber (EPDM) composite. Plast. Rubber Compos. 2015, 44, 206–210. [Google Scholar] [CrossRef]
- Shit, S.C.; Shah, P. A review on silicone rubber. Natl. Acad. Sci. Lett. 2013, 36, 355–365. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, Q.Y.; Xu, H.H.; Bei, Y.L.; Feng, S.Y. Preparation and characterization of room temperature vulcanized silicone rubber using α-amine ketoximesilanes as auto-catalyzed cross-linkers. RSC Adv. 2016, 6, 38447–38453. [Google Scholar] [CrossRef]
- Hernández-Ortiz, J.P.; Osswald, T.A. Modeling processing of silicone rubber: Liquid versus hard silicone rubbers. J. Appl. Polym. Sci. 2011, 119, 1864–1871. [Google Scholar] [CrossRef]
- Delebecq, E.; Ganachaud, F. Looking over liquid silicone rubbers: (1) network topology vs chemical formulations. ACS Appl. Mater. Interfaces 2012, 4, 3340–3352. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.-S.; Li, J.; Zhao, X.-S. Improving the thermal and mechanical properties of silicone polymer by incorporating functionalized graphene oxide. J. Mater. Sci. 2013, 48, 5287–5294. [Google Scholar] [CrossRef]
- Deshpande, G.; Rezac, M.E. Kinetic aspects of the thermal degradation of poly(dimethyl siloxane) and poly(dimethyl diphenyl siloxane). Polym. Degrad. Stab. 2002, 76, 17–24. [Google Scholar] [CrossRef]
- Ramseyer, J.A. Elastomeric Composition Containing Silicon Carbide for Use as an Ablative Coating. U.S. Patent 3,623,904, 30 November 1971. [Google Scholar]
- Yu, L.; Zhou, S.T.; Zou, H.W.; Liang, M. Thermal stability and ablation properties study of aluminum silicate ceramic fiber and acicular wollastonite filled silicone rubber composite. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Zhou, C.; Yu, L.; Luo, W.; Chen, Y.; Zou, H.W.; Liang, M. Ablation properties of aluminum silicate ceramic fibers and calcium carbonate filled silicone rubber composites. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Yang, X.; Huang, Q.Z.; Su, Z.A.; Chang, X.; Xue, L.; Zhong, P.; Li, J. Ablative property and mechanism of C/C-ZrB2-ZrC-SiC composites reinforced by SiC networks under plasma flame. Corros. Sci. 2016, 107, 9–20. [Google Scholar]
- Wang, M.C.; Miao, R.; He, J.K.; Xu, X.Q.; Liu, J.C.; Du, H.Y. Silicon carbide whiskers reinforced polymer-based adhesive for joining C/C composites. Mater. Des. 2016, 99, 293–302. [Google Scholar] [CrossRef]
- Wang, M.C.; Liu, J.C.; Du, H.Y.; Guo, A.R.; Tao, X.; Dong, X.; Geng, H.T. A SiC whisker reinforced high-temperature resistant phosphate adhesive for bonding carbon/carbon composites. J. Alloys Compd. 2015, 633, 145–152. [Google Scholar] [CrossRef]
- Chinese Standard. Rubber, Vulcanized or Thermalplastic—Determination of Tensile Stress-Strain Properties; GB/T 528-2009; Standardization Administration of the People’s Republic of China: Beijing, China, 2009.
- Chinese Standard. Rubber, Vulcanized or Thermalplastic—Determination of Indentation Hardness—Part 1: Duromerer Method (Shore Hardness); GB/T 531.1-2008; Standardization Administration of the People’s Republic of China: Beijing, China, 2008.
- Chinese Standard. Rubber, Vulcanized or Thermalplastic—Determination of Density; GB/T 533-2008; Standardization Administration of the People’s Republic of China: Beijing, China, 2008.
- Ambuken, P.V.; Stretz, H.A.; Koo, J.H.; Messman, J.M.; Wong, D. Effect of addition of montmorillonite and carbon nanotubes on a thermoplastic polyurethane: High temperature thermomechanical properties. Polym. Degrad. Stab. 2014, 102, 160–169. [Google Scholar] [CrossRef]
- Monti, M.; Tsampas, S.A.; Fernberg, S.P.; Blomqvist, P.; Cuttica, F.; Fina, A.; Camino, G. Fire reaction of nanoclay-doped PA6 composites reinforced with continuous glass fibers and produced by commingling technique. Polym. Degrad. Stab. 2015, 121, 1–10. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Zhang, J.; Lu, B.-X.; Xin, Z.X.; Kang, C.K.; Kim, J.K. Effect of flame retardants on mechanical properties, flammability and foamability of PP/wood–fiber composites. Compos. Part B Eng. 2012, 43, 150–158. [Google Scholar] [CrossRef]
- Burnside, S.D.; Giannelis, E.P. Synthesis and properties of new poly(dimethylsiloxane) nanocomposites. Chem. Mater. 1995, 7, 1597–1600. [Google Scholar] [CrossRef]
- Natali, M.; Rallini, M.; Puglia, D.; Kenny, J.; Torre, L. Epdm based heat shielding materials for solid rocket motors: A comparative study of different fibrous reinforcements. Polym. Degrad. Stab. 2013, 98, 2131–2139. [Google Scholar] [CrossRef]
- Bahramian, A.R.; Kokabi, M. Ablation mechanism of polymer layered silicate nanocomposite heat shield. J. Hazard. Mater. 2009, 166, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, W.L.; Maahs, H.G. Active-to-passive transition in the oxidation of silicon carbide and silicon nitride in air. J. Am. Ceram. Soc. 1990, 73, 1540–1543. [Google Scholar] [CrossRef]
- Zhang, X.-H.; Hu, P.; Han, J.-C.; Xu, L.; Meng, S.-H. The addition of lanthanum hexaboride to zirconium diboride for improved oxidation resistance. Scr. Mater. 2007, 57, 1036–1039. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, W.; Jiang, B.Z. Ceramization and oxidation behaviors of silicone rubber ablative composite under oxyacetylene flame. Ceram. Int. 2013, 39, 1575–1581. [Google Scholar] [CrossRef]
- Ladacki, M. Silicon carbide in ablative chars. AIAA J. 1966, 4, 1445–1447. [Google Scholar] [CrossRef]
- Srikanth, I.; Daniel, A.; Kumar, S.; Padmavathi, N.; Singh, V.; Ghosal, P.; Kumar, A.; Devi, G.R. Nano silica modified carbon–phenolic composites for enhanced ablation resistance. Scr. Mater. 2010, 63, 200–203. [Google Scholar] [CrossRef]
- Vaia, R.A.; Price, G.; Ruth, P.N.; Nguyen, H.T.; Lichtenhan, J. Polymer/layered silicate nanocomposites as high performance ablative materials. Appl. Clay Sci. 1999, 15, 67–92. [Google Scholar] [CrossRef]
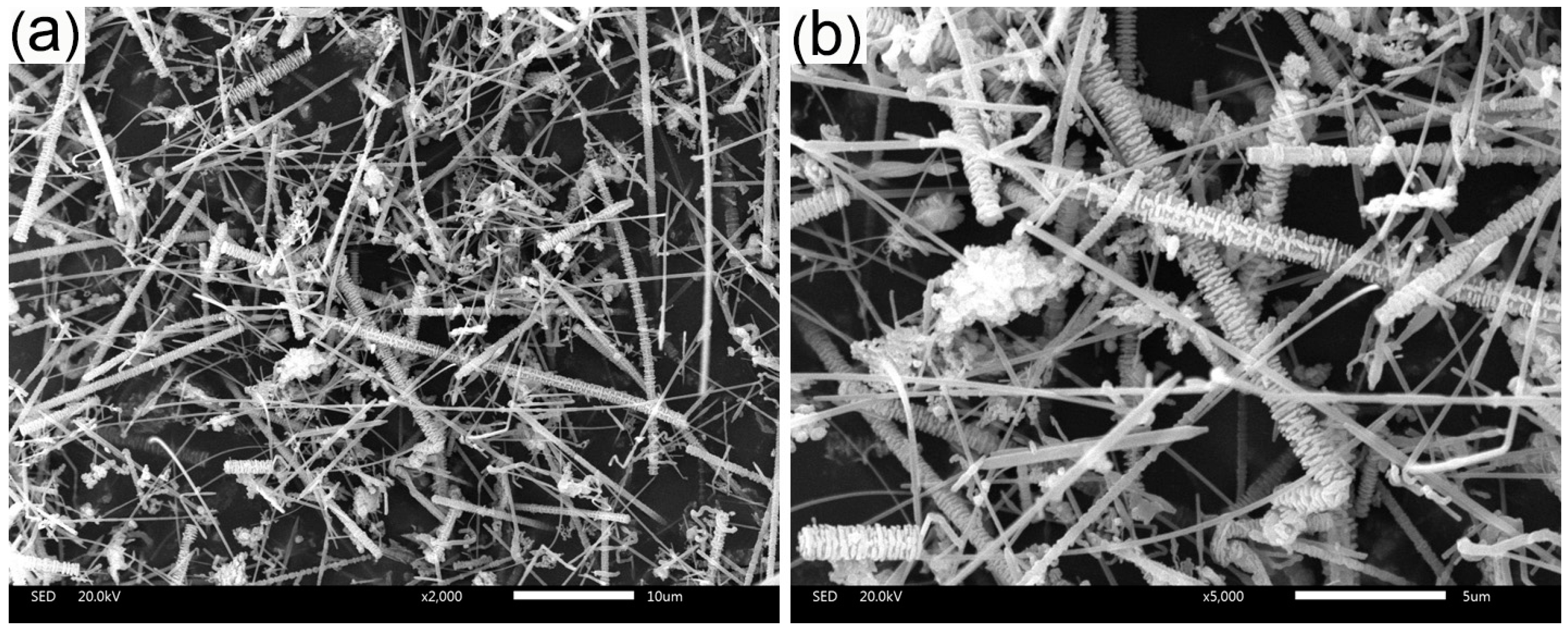

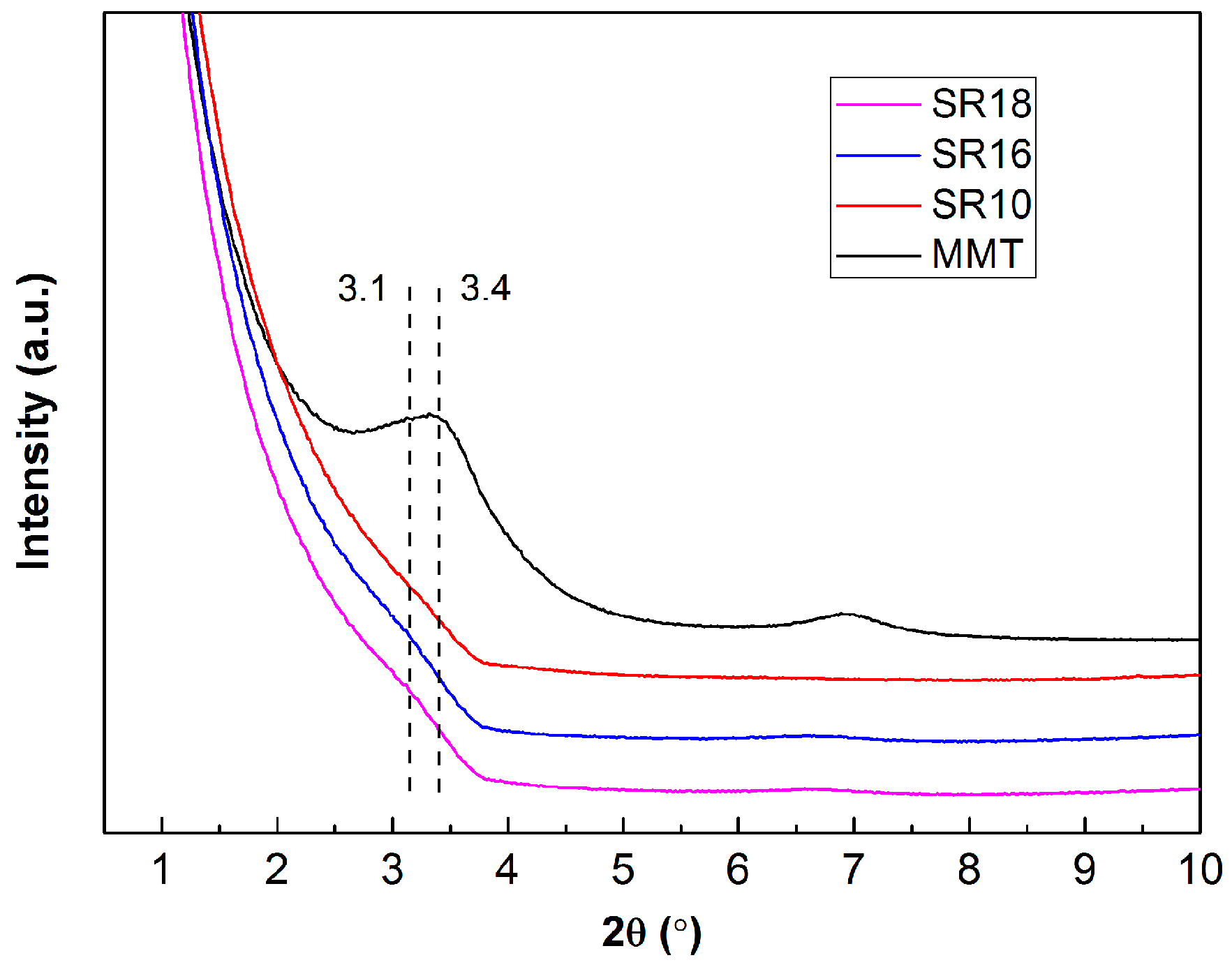
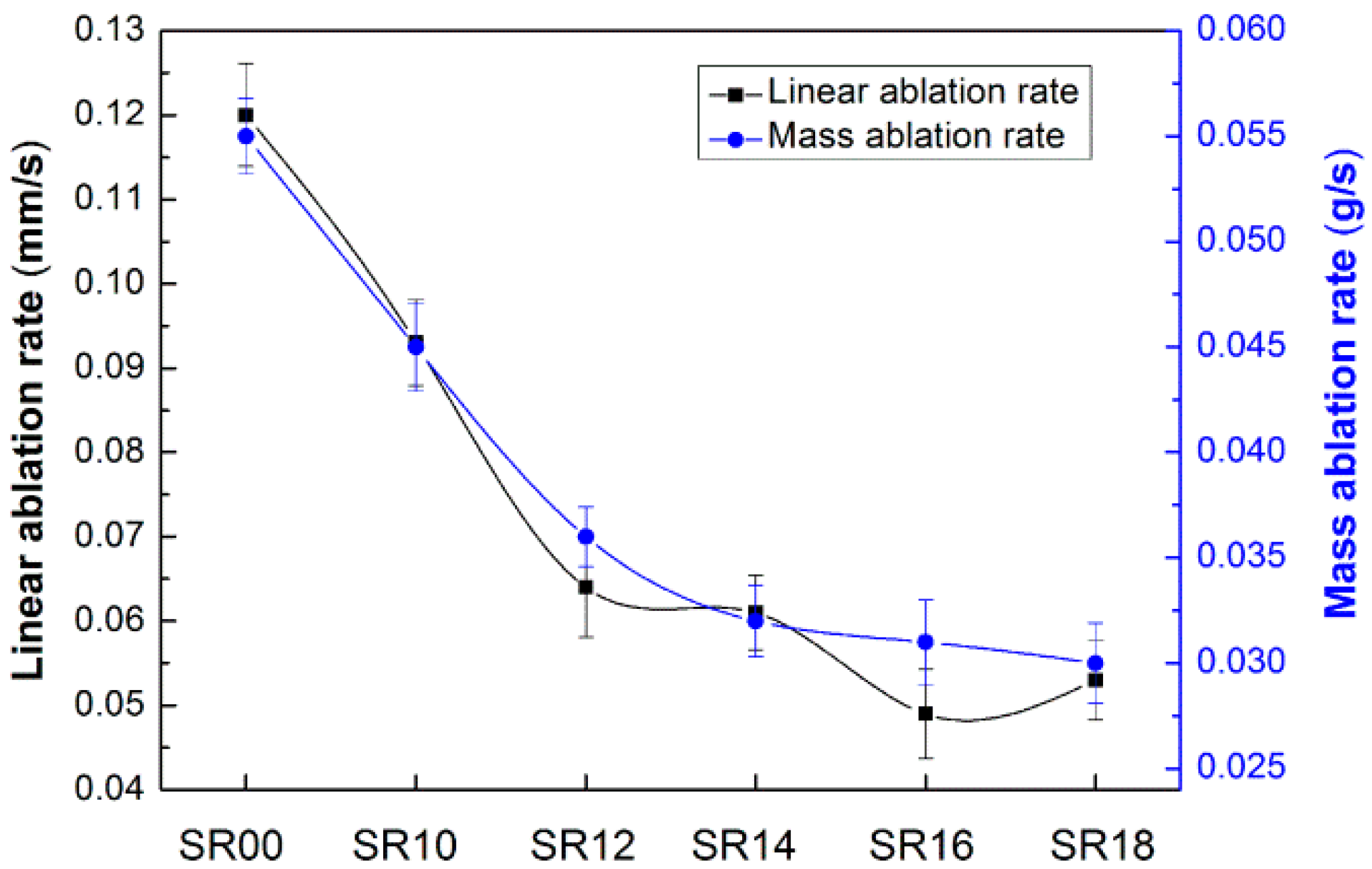

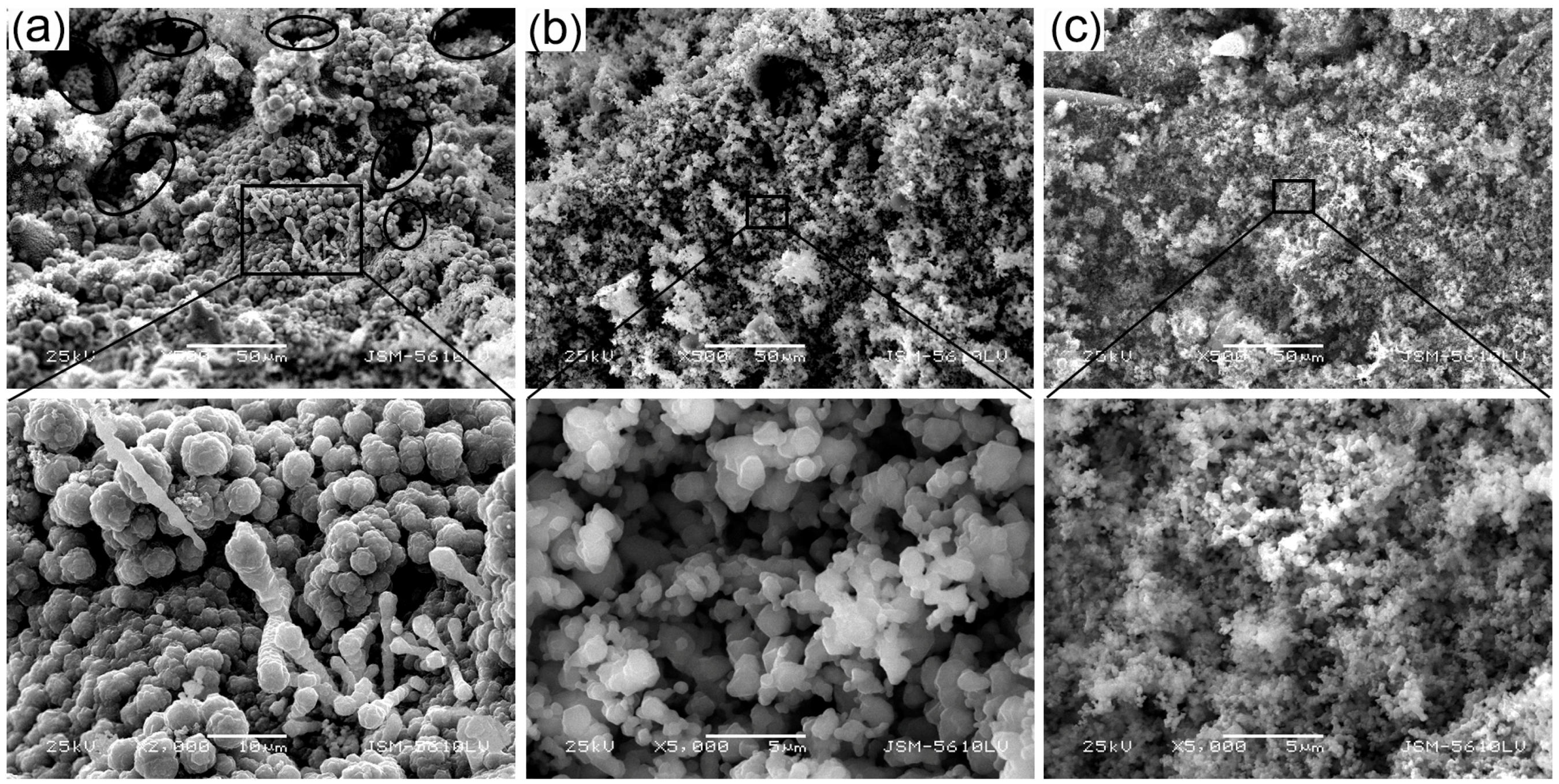
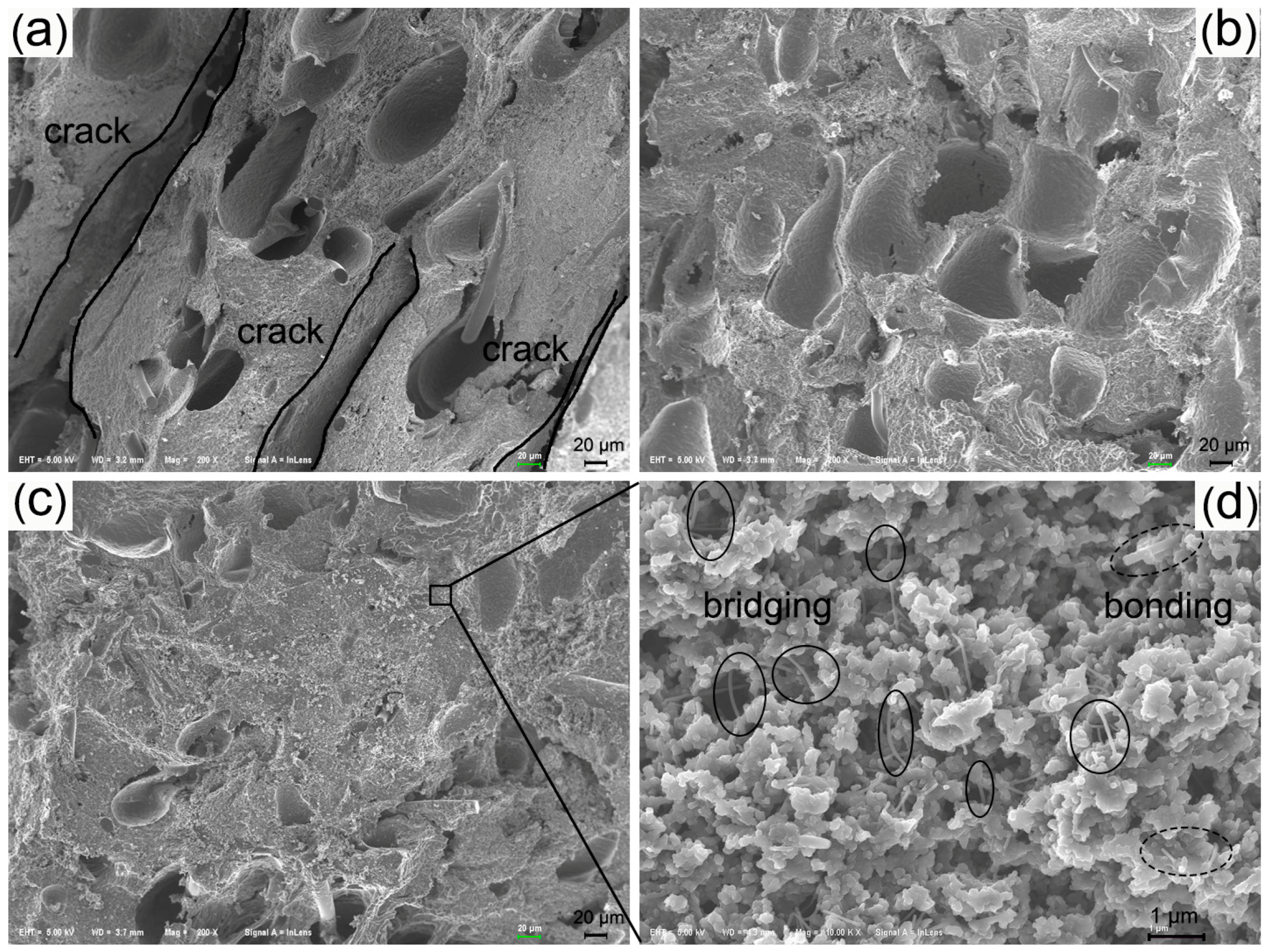
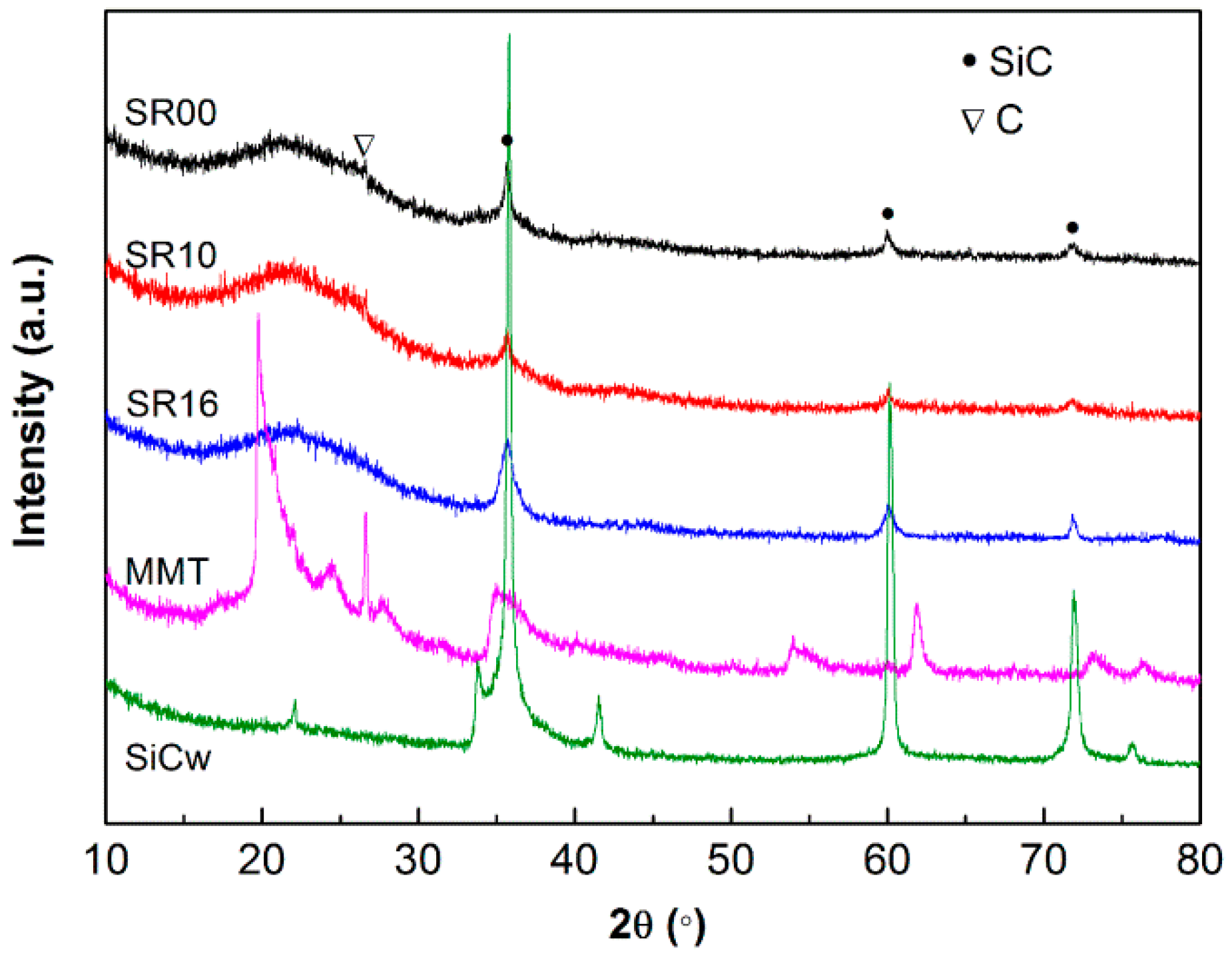
| Sample | Content (phr) a | ||||||
|---|---|---|---|---|---|---|---|
| MVS | MPVS | SiO2 | Kynol | DBPMH | MMT | SiCw | |
| SR00 (control) | 40 | 60 | 30 | 10 | 1.5 | 0 | 0 |
| SR10 | 40 | 60 | 30 | 10 | 1.5 | 10 | 0 |
| SR12 | 40 | 60 | 30 | 10 | 1.5 | 10 | 2 |
| SR14 | 40 | 60 | 30 | 10 | 1.5 | 10 | 4 |
| SR16 | 40 | 60 | 30 | 10 | 1.5 | 10 | 6 |
| SR18 | 40 | 60 | 30 | 10 | 1.5 | 10 | 8 |
| Sample | Tensile Strength (MPa) | Elongation at Break (%) | Shore A | Density (g·cm−3) |
|---|---|---|---|---|
| SR00 | 4.23 ± 0.26 | 603 ± 6 | 65.1 | 1.081 |
| SR10 | 4.72 ± 0.35 | 611 ± 5 | 67.5 | 1.114 |
| SR12 | 5.08 ± 0.31 | 575 ± 9 | 72.3 | 1.121 |
| SR14 | 5.20 ± 0.23 | 562 ± 11 | 73.8 | 1.136 |
| SR16 | 5.61 ± 0.31 | 520 ± 7 | 75.2 | 1.143 |
| SR18 | 5.28 ± 0.24 | 502 ± 13 | 75.5 | 1.159 |
| Sample | T0.1 (°C) | Tmax (°C) | R800 (%) |
|---|---|---|---|
| SR00 | 418.5 | 515.0 | 29.58 |
| SR10 | 433.1 | 523.1 | 29.59 |
| SR12 | 449.8 | 524.8 | 33.69 |
| SR14 | 445.6 | 523.1 | 33.69 |
| SR16 | 442.6 | 521.1 | 34.11 |
| SR18 | 436.1 | 518.6 | 34.68 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Wang, F.; Huang, Z.; Dai, J.; Shi, M. Improved Ablation Resistance of Silicone Rubber Composites by Introducing Montmorillonite and Silicon Carbide Whisker. Materials 2016, 9, 723. https://doi.org/10.3390/ma9090723
Zhang G, Wang F, Huang Z, Dai J, Shi M. Improved Ablation Resistance of Silicone Rubber Composites by Introducing Montmorillonite and Silicon Carbide Whisker. Materials. 2016; 9(9):723. https://doi.org/10.3390/ma9090723
Chicago/Turabian StyleZhang, Guangwu, Fuzhong Wang, Zhixiong Huang, Jing Dai, and Minxian Shi. 2016. "Improved Ablation Resistance of Silicone Rubber Composites by Introducing Montmorillonite and Silicon Carbide Whisker" Materials 9, no. 9: 723. https://doi.org/10.3390/ma9090723





