1. Introduction
Yearly, 3,331,000 tons of municipal solid waste are generated in Flanders. Around 880,000 tons are incinerated to gain energy and reduce the waste volume with 90% (Waste-to-Energy) [
1,
2]. The incineration results in bottom ash (BA), boiler ash and air pollution control residues. BA is mostly disposed of as landfill. The high waste volume, cost of landfill and lack of disposal sites force the industry to recycle the BA as secondary raw material [
3]. This would also be more in agreement with European Directive 2008/98/EC [
4], as it saves valuable natural resources and gives waste a financial value [
5]. To obtain an end-of-waste status
cf. ED 2008/98/EC [
4], several criteria need to be fulfilled. The material should serve a purpose. The technical requirements and legislation of this purpose should be met. There needs to be a market for it and there should be no negative environmental effects [
4]. In this context, companies involved in municipal solid waste incineration (MSWI) intend to recover the BA as aggregates for concrete. The successful production of so-called concrete Lego bricks is seen as the minimal aim. These are prefabricated unreinforced blocks which are used to build modular partition walls at industrial and rural sites. For several years now, the waste management company Indaver nv in Belgium has been trying to tune the properties of BA at its incineration plant to meet this goal. In the past, this already resulted in the publication of papers on the proper characterization and treatment of the Belgian BA (e.g., Vandecasteele
et al. [
6], Van Gerven
et al. [
7]).
According to literature, BA from Belgium [
8,
9], the Netherlands [
10], France [
11], Germany [
5], Italy [
3,
12], Spain [
2] and South-Korea [
13] show quite some similarities in composition. They usually consist of bottle glass (around 15%, composed of Na
2O, CaO, CaCO
3 and SiO
2 [
2]), as well as metals (ferrous and non-ferrous, such as Al, Cu and Zn), ceramics (brick, plaster and mortar) and organic residues (bone fragments, charcoal, plant fibers and polymers). The latter elements have only a small contribution to the total mass (each with an amount of about 2%) [
5,
10]. Evidently, the variety in constituting materials implies a heterogeneous chemical composition of the BA: 30%–70% silica, 10%–15% aluminum oxide, 20% sodium oxide and 10%–15% calcium oxide. A smaller yet still important fraction of (heavy) metals is also present [
5,
10]. BA is lighter than gravel (2210 kg/m
3 vs. 2470 kg/m
3). The water absorption is 4–10 times the value of gravel (4%–10%). Both characteristics are due to a high porosity [
3,
11]. BA is highly angular and has a weaker abrasion strength than limestone [
14]. BA concrete is usually characterized by a lower compressive strength, a lower Young’s modulus, a lower workability and a higher porosity than concrete with limestone as aggregate. Nevertheless, even without modifications and with full replacement of the aggregates by BA, a concrete strength class of C20/25 can be assured [
15].
In the fresh state, extensive aggregate replacement by BA may have a negative effect on the concrete workability. This is mainly due to the higher water absorption of the BA. However, when using rather high water-to-cement (W/C) ratios, workability may not be a real issue. According to Pera
et al., a W/C ratio of 0.60–0.65 is ideal to achieve both an acceptable workability and a sufficient mechanical performance [
11]. Moreover, the use of a superplasticizer (SP) can easily improve the workability if needed [
16].
In hardened state, most damage phenomena are induced by the expansive reaction of metallic Al in an alkaline environment, which increases the porosity and reduces the strength. This effect exists for all hydroxide-forming elements present in the BA [
5,
11]. Alkali-silica reaction (ASR) is considered not detrimental because of the higher porosity of the BA concrete. A literature survey on other expected damage phenomena (e.g., freeze-thaw, acid attack,
etc.) provides little or no information on the performance of BA concrete.
Literature on the leaching behavior of concrete containing BA from MSWI is rather scarce. Since the material is immobilized within the cementitious matrix of the concrete, leaching values are normally lower than when the individual grains are in direct contact with the leaching solution. Regarding the leaching of (heavy) metals, Sorlini and Ginés found values that meet the national criteria (Italy for Sorlini and Spain for Ginés) [
17,
18].
In the past, tests were mainly performed in view of a partial replacement of traditional aggregates by BA [
10,
11,
16,
17] and they included variations in cement type [
17], W/C ratio [
8,
12], workability and compaction [
8,
19]. The concrete quality can also be improved by optimizing the BA properties using opto-mechanical glass separation [
11], reactive washing with NaOH (=Lye treatment) [
11], vitrification [
12] and sintering [
17].
3. Materials and Methods
3.1. Concrete Constituents
The inert fraction of concrete traditionally consists of sand and aggregates. For the aggregates, it is often useful to use both a fine and a coarse fraction to achieve a better packing. In this research, the aim was a full replacement of the traditional fine and coarse aggregates by BA processed at the MSWI plant of Indaver nv. In terms of phase composition, this consisted of glass remains, a stony fraction (ceramics, porcelain, bricks, etc.), amorphous slags and some ferrous (e.g., steel) and non-ferrous (e.g., Al, Cu, Pb, Zn) metals that cannot be removed in the treatment installation. The glass remains (bottle glass, grains of glass with devitrification products) normally represent the largest fraction. The proportioning with the other fractions is somewhat seasonally bound.
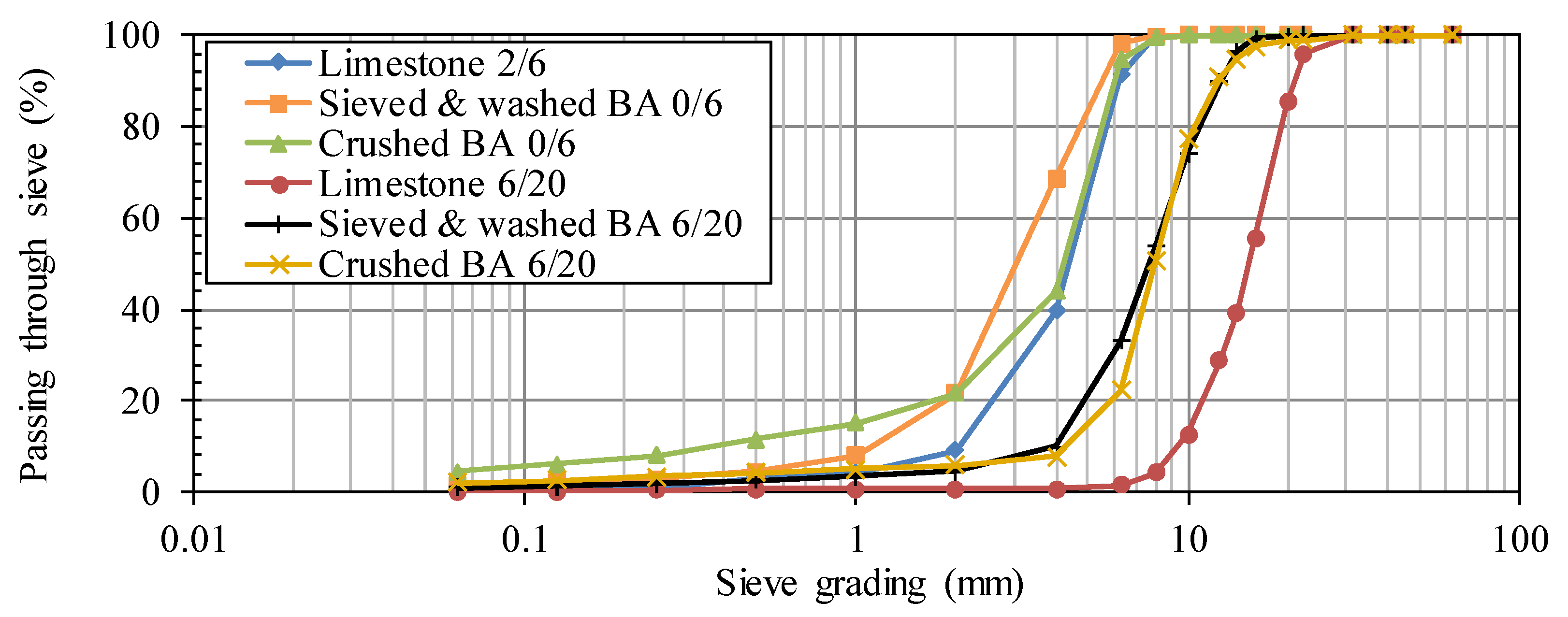
Different types of fine and coarse BA aggregates were considered,
i.e., sieved and aged BA 0/6 and 2/6, crushed BA 0/6, sieved and washed BA 0/6 and 6/20, and crushed BA 6/20. The crushed and sieved and washed fractions originated from the same 6/50 fraction which was obtained after an initial processing stage at Indaver nv. The other fractions were the result of a natural aging process of a 2/6 batch. More details on how the 6/50 fraction and the aged 2/6 batch were obtained can be found in Vandecasteele
et al. [
6]. The crushing, sieving, washing and aging techniques that were applied afterwards to obtain the 0/6, 2/6 and 6/20 fractions were similar to the ones mentioned in that previous research. Crushing was done by means of a commercial mobile cone crusher that allows for setting the required maximum aggregate size. A mobile installation was also used for sieving the material to the desired sieve aperture. The material to be sieved was usually wet due to its outdoor unsheltered storage. No additional water was added during sieving. Washing operations were done in most cases with process water that resulted from drying the filter cake at the Indaver incineration plant. Logically, this process water contains fines that adhere somewhat to the BA. The aging process consisted of piling the BA on 5–10 m high heaps in open air on a paved floor at the incineration plant with the collection of percolating rainwater, exposing them to wind and rain.
The BA-based aggregates should replace the traditional crushed limestone 2/6 and 6/20 which are commonly used for concrete manufacturing in Belgium. The remaining portion of the inert fraction, i.e., the sand, was a natural river sand 0/4.
Two different cement types were used for concrete manufacturing, either a traditional Portland cement CEM I 52.5 N MF or a low alkali (LA) high sulfate resistant CEM I 52.5 N HES LA HSR. The total cement content for each concrete composition amounted to 350 kg/m3.
The mixing water consisted of tap water at a temperature of 20 °C. The amount of water used for the concrete varied with the applied W/C ratio: 0.65, 0.60 or 0.55. When lowering the water content substantially, the use of a SP was often imperative. Initially, polycarboxylic ether-based MasterGlenium 51 con. 35% (dry matter mass percentage: 35%, density at 20 °C: 1100 kg/m3) was used for this purpose. Later on, a switch was made to polycarboxylic ether-based MasterGlenium ACE 30 con. 30% SPL (dry matter mass percentage: 35%, density at 20 °C: 1070 kg/m3) to reduce the delay in setting time.
3.2. Physical and Chemical Characterization of the Aggregates
3.2.1. Water Absorption over 24 h and Mass Density
The water absorption over 24 h (WA24) of all studied aggregates was measured in accordance with NBN EN 1097-6 using a calibrated pycnometer. The apparent mass density ρa (kg/m3) and the relative mass density after oven drying ρrd (kg/m3) were determined in accordance with the same standard.
3.2.2. Water Absorption as a Function of Time
To evaluate the water absorption as a function of time, a hydrostatic weighing technique similar to the one proposed by Garcia-Gonzalez
et al. and Tegguer was applied [
20,
21]. Per aggregate type, a sample of at least 1000 g was inserted in a cylindrical strainer. This strainer was hung from a balance and submerged in a basin filled with demineralized water. The weight increase was monitored over a time period of 10 days.
3.2.3. Particle Size Distribution
The particle size distributions of the aggregates and sand were determined cf. NBN EN 933-1. The sieving column consisted of standardized sieves with following apertures: 63, 45, 40, 31.5, 22.4, 20, 16, 14, 12.5, 10, 8, 6.3, 4, 2, 1, 0.5, 0.25, 0.125 and 0.063 mm. The cumulative percentage of material passing through each sieve was calculated to plot the particle size distributions.
3.2.4. Aggregate Crushing Value
The strength of an aggregate can be characterized by its crushing value. In this research, this parameter—also known as the static compressive strength—was determined in accordance with Belgian standard NBN B11-205, for both the BA and the limestone aggregates. A cylindrical metal mold with an inner diameter of 150 mm was filled with a dried aggregate sample of a certain particle size range. After adding the sample, the mold was fitted with a no-friction plunger which was then loaded to 400 kN in 4 min. Before unloading again, this maximum load onto the sample was maintained for 2 min. Finally, the crushed fraction of the aggregate <2 mm was separated from the sample by means of a sieving operation. The mass ratio of the sample without the crushed fraction over the initial sample before loading counts as the static compressive strength of the aggregate. Normally, this test is to be conducted on coarse aggregate fractions only (minimum aggregate diameter: 6 mm). Nevertheless, the static compressive strengths of the fine BA and limestone aggregates were determined in the same manner. Per type of sample, the test was repeated three times.
3.2.5. Chemical Composition
The chemical properties of the BA were determined using Inductively Coupled Plasma (ICP), Ion Chromatography (IC) and Flow Injection Mercury System (FIMS) techniques available at the laboratories of Indaver nv.
3.3. Concrete Mix Design and Production
Once the total cement content (=350 kg/m3) and the W/C ratio (0.65, 0.60 or 0.55) were set for the concrete compositions under investigation, the required amounts of sand, fine and coarse aggregates were determined. This was done by approximating the optimal particle size distribution curve of Fuller using the least-of-squares method. This approach normally results in a minimal spacing between the different particles of the inert fraction.
In the initial research stage only the fine aggregates were replaced by BA. In the next stage, the same was done for the coarse aggregates. As such, the individual effects of each BA could be studied unambiguously. In the final stage, both the fine and coarse limestone aggregates were replaced.
Concrete was produced in accordance with NBN EN 12390-2. First, the fine and coarse aggregates, the sand and the cement, were mixed for 1 min. Then, the water was added whereupon mixing continued for 2 min. After this step, the workability of the concrete was evaluated by means of the slump and flow test (NBN EN 12350-2, NBN EN 12350-5) and the required amount of SP was determined. This amount was added while mixing for another 2 min. The SP dosing step was repeated until the proper slump and flow class were obtained. Finally, the test samples were cast and optimally cured in a climate chamber at 20 ± 2 °C and 95% ± 5% relative humidity (RH).
3.4. Strength Performance
The compressive strength was determined in accordance with NBN EN 12390-2. This was done after seven days, 28 days and 56 days of optimal curing. Per testing age three cubic specimens (
n = 3) with a 150 mm side were subjected to the test. The characteristic value of the compressive strength f
ck at 28 days was obtained in correspondence with EN 1990
cf. Equation (1).
where x is the mean value of the compressive strength of n samples (N/mm
2); k
n is a factor equal to 3.37 for
n = 3 and a variation coefficient V
X unknown from prior knowledge; and s is the standard deviation on the individual values of the compressive strength of n samples. With the characteristic compressive strength known, the proper compressive strength class as specified in NBN EN 206-1 can be assigned.
3.5. Susceptibility to Expansion
The susceptibility to expansion was evaluated by monitoring the height of the cubic samples intended for the compressive strength tests as a function of time (after 1, 7, 28 and 56 days) and relative to the their original height just after casting (=150 mm). Note that this is only a rudimentary assessment approach to get a first idea on the expansive behavior. The change in length of only one cube side reflects the total expansion only to a certain extent since the initial early age expansion in other directions is hindered by the cubic mold. Moreover, the duration of dihydrogen gas generation after reaction with the metallic Al and the setting time of the concrete also govern the amount of expansion as well. Further investigation on these influencing parameters is certainly still necessary in the future.
3.6. Porosity
The porosity was determined using a vacuum saturation technique followed by hydrostatic weighing
cf. NBN B 05-201. Since the amount of water absorbed during the vacuum saturation is mainly limited to the pores in contact with the outer sample surface, the resulting porosity only reflects the open/permeable porosity. Per concrete mixture, the test was performed on nine cylindrical samples (diameter: 100 mm, height: 50 mm) taken from cubes (side: 150 mm). After 28 days of optimal curing, the samples were weighed and dried at a temperature of 40 ± 5 °C until constant mass (Δm after 24 h < 0.1%). Next, the samples were placed in a normalized vacuum tank. A vacuum with a residual pressure of 2.7 kPa was applied for 2.5 h. While keeping this vacuum condition, water was introduced at a rate of 50 mm/h until complete immersion of the samples. Subsequently, the air pressure was restored and the samples were kept under water for 24 h. Afterwards, they were weighed hydrostatically as well as above water. After the weighing, they were dried at 105 ± 5 °C until constant mass (Δm after 24 h < 0.1%) whereupon the whole procedure was repeated. Then, the open/permeable porosity φ was calculated for the two pre-drying temperatures (Equation (2)).
where m
d is the oven dry mass after pre-drying at 40 °C or 105 °C (g); m
s is the water saturated mass after vacuum saturation (g); and m
l is the mass under water after vacuum saturation (g).
3.7. Long-Term Behavior
3.7.1. Susceptibility to Leaching
After 28 days of optimal curing, six cubes (side: 100 mm) were conserved in a closed tank filled with demineralized water. Conform CMA/2/II/A, the volume of the reagents should be two to five times the sample volume. Conform NEN 7345, this is four times the sample volume. In this research, the latter volume ratio was chosen. A water sample was taken after 30 days of immersion and examined using ICP, IC and FIMS.
3.7.2. Resistance to ASR
The susceptibility to ASR was evaluated using the modified Oberholster test
cf. the Walloon technical guideline STM D424 ST6. This method was chosen because, in contrast with the original Oberholster test or South-African NBRI (National Building Research Institute) accelerated test method, it allows for a test directly on the concrete mixtures under investigation instead of on mortar bars containing the aggregates to be studied after an initial processing (crushing and sieving). Moreover, the modified Oberholster test holds the advantage of being a rather fast assessment technique (exposure period: 20 days) in comparison with some of the other methods designed for concrete (exposure periods of up to 12 months
cf. RILEM TC 106-AAR [
22]). Per mixture, six cylinders (diameter: 50 mm, height: 150 mm) taken from cubes (side: 150 mm) were tested. After 28 days of optimal curing the samples were immersed for 24 h in water at 80 °C. Afterwards, the samples were stored in a 40 g NaOH per L water solution at 80 °C. The expansion was continuously monitored on dial gauges in contact with the upper surface of the cylinders. Normally, an expansion exceeding 0.1% implies a potential ASR sensitivity.
3.7.3. Resistance to Acid Attack
Six cylinders (diameter: 113 mm, height: 150 mm) were drilled and cut from six cubes (side: 150 mm). After 28 days, the cylinders were exposed to 15 cycles involving intermittent exposure to acid. One cycle started by keeping the samples 24 h in a dry environment (minimal 60% ± 5% RH) and continued with 24 h of immersion in an acid solution (30 g acetic acid and 30 g lactic acid per liter), all taking place at a temperature of 20 ± 2 °C and 60% ± 5% RH. After each drying period, the mass of the samples was determined. After the last cycle, the mass was determined once again. The damage was evaluated based on the observed mass decrease and visual appearance.
3.7.4. Resistance to Freeze-Thaw Attack
Four cubes (side: 150 mm), optimally cured for 23 days, were subjected to a freeze-thaw test in compliance with NBN B15-231, which in turn refers to NBN B 05-203. The samples were submerged in demineralized water at a temperature of 20 ± 2 °C until constant mass (Δm 24 h < 0.05%) cf. NBN B 15-215. After saturation was observed, the samples were placed in a laboratory freezing chamber and were subjected to 14 freeze-thaw cycles which consisted of: (i) cooling down till 0 °C in 1–2 h; (ii) progressive freezing from 0 °C to −15 °C in 5 h (at a mean rate of 3 ± 0.5 °C/h and always in the range of 2–4 °C/h); (iii) maintaining a constant freezing temperature of −15 ± 2 °C for 10–11 h; (iv) defrosting in water; (v) keeping the samples in water until the end of the cycle at 24 h.
Two non-destructive test methods were used to evaluate the freeze-thaw resistance: a visual check-up and ultrasonic pulse velocity measurements using a commercial device. The visual damage assessment was done in accordance with NBN B 27-009/A1.
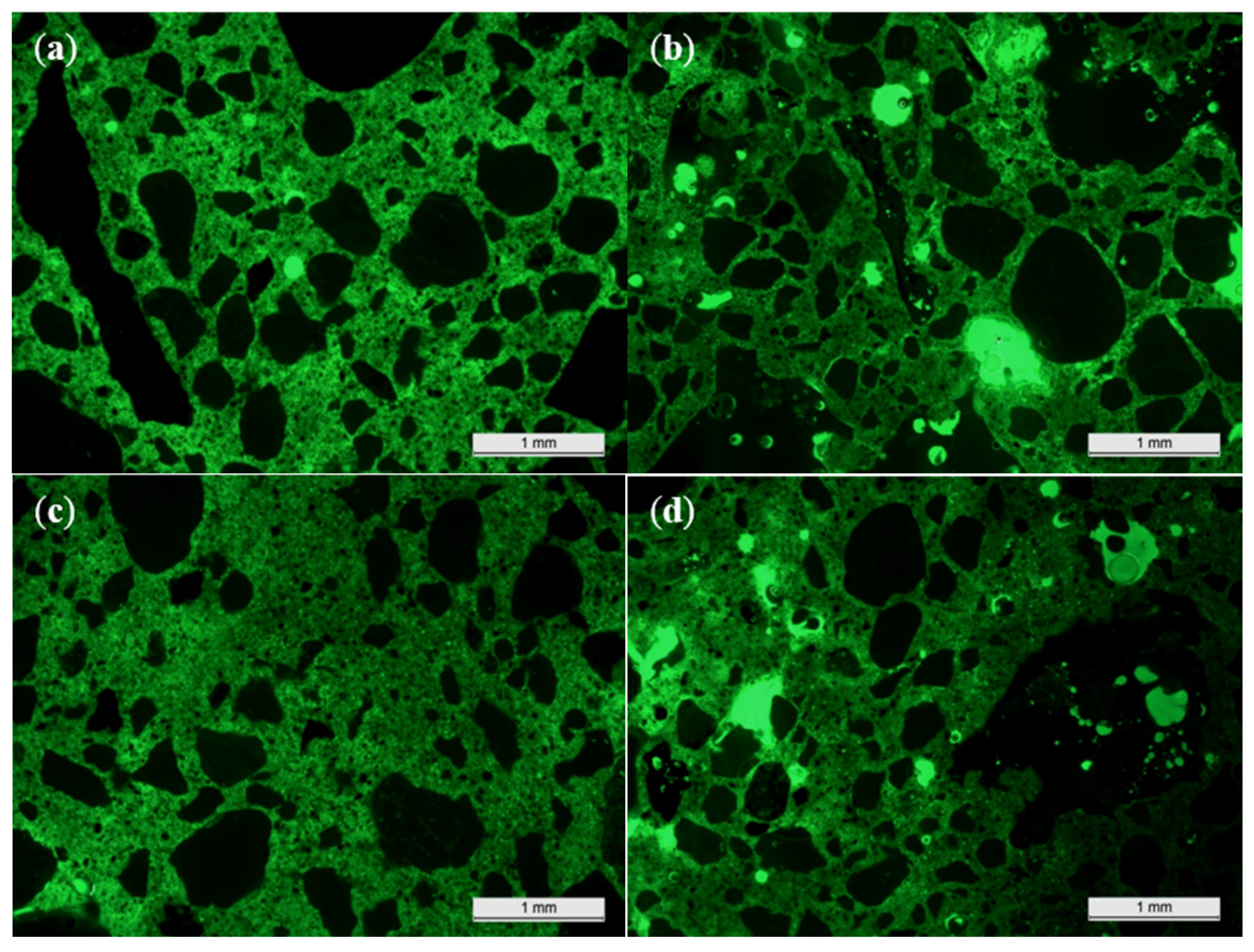
3.8. Microscopic Characterization on Thin Sections
To explain the porosity, a microscopic investigation on thin sections was necessary. These thin sections were prepared as follows: 45 × 30 × 15 mm3 prisms were cut from the concrete and 45 × 30 mm2 faces were glued onto glass slides with a thickness of 2.9 mm. Then, the samples were cut and polished until the height of the concrete specimens and the glass equaled 10.1 mm. In a next step, the samples were impregnated under vacuum with a fluorescent epoxy. After impregnation, the excess epoxy was ground away and an object glass was glued onto the polished surface. In a final step, the glass slides were cut off and the concrete samples on the object glasses were polished until thin sections with a 25 µm thickness were obtained. A cover glass was glued onto them for protection. All thin sections were examined with a Leica DM LP polarizing microscope. Images were taken with a Leica DFC295 camera in fluorescent light mode.
3.9. Optimization of the Bottom Ash
3.9.1. Pre-Wetting
To limit the high water absorption during concrete mixing and the resulting workability issues, the BA could be pre-wetted. In total, three pre-wetting techniques were examined. All of them were based on their expected water absorption as a function of time (
Section 3.2.2). The first technique focused on pre-wetting the BA to a level corresponding with the deflection point in the absorption curve. This level is met as soon as the initial high water uptake rate ends. The time needed for this counts as the required immersion time span of the aggregates prior to the concrete mixing. After immersion, a self-made strainer bucket was used to drain the water. Note that this method was only applied for the fine BA. The second and third methods also relate to the water absorption behavior as a function of time. For these two methods, the expected contact time with water was the decisive criterion and not the change in water absorption rate. As the concrete mixing process and specimen casting takes about 6 min after adding the water, it makes sense to pre-wet the BA with the amount of water that is expected to be absorbed within the 6 min timeframe. This can be done in two ways. Either the aggregates can be pre-wetted in the mixer (this second method is commonly used when manufacturing concrete with lightweight aggregates), or the additional amount of water can be added afterwards as a surplus to the mixing water (the third method). Both the fine and coarse BA were treated like this.
3.9.2. Reactive Washing
The applied reactive washing procedure was based upon literature findings [
23] and existing know-how at Indaver nv. To passivate Al inclusions, the BA was washed with 1 M NaOH. The BA was completely immersed in this solution for two weeks. The metallic Al reacts with the NaOH solution and forms non-active Al and H
2. The mixture was stirred from time to time to ensure an optimal interaction between the BA and the solution and to guarantee a proper release of H
2.
To evaluate the effectiveness of this reactive washing, the amount of reactive Al before and after the treatment was determined. Therefore, the BA samples were subjected to a test described in CUR recommendation 116 [
24]. Per sample, the BA was combined with the NaOH solution in a closed system flask. The H
2 being released was captured in a second flask initially full of water. Since a portion of the water is displaced by the H
2, the amount of metallic Al can be calculated from Equation (3) after measuring the initial and final water weight.
where v
H2 is the volume of entrapped H
2 (L); M
Al is the molar mass of Al (27 g/mol); v
0 is the molar volume (22.4 L/mol); and m
Al is the mass of reactive Al (g). Before applying the BA after the reactive washing in concrete, they were first rinsed with water until the pH had decreased sufficiently. For the fine and coarse BA fractions, the pH decreased from 12.8 to 9.0–10.0 and from 12.8 to 8.0, respectively.
3.10. Manufacturing of the Lego Bricks
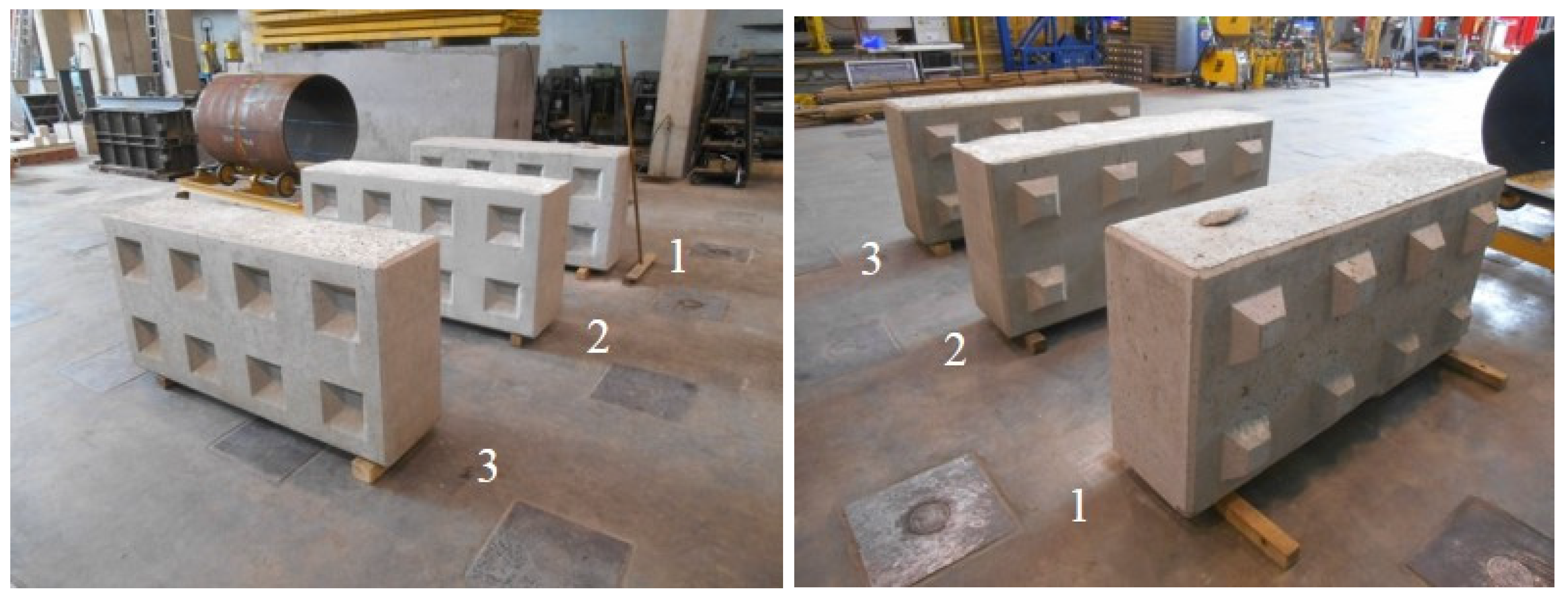
Three 1600 × 800 × 400 mm3 Lego bricks were cast: one made of traditional concrete, one made of the initial BA concrete with full replacement of both the fine and coarse aggregate fractions and one made of the optimized BA concrete, also with full aggregate replacement. The mold for the Lego brick was provided by CB Recycling BV. The concrete was manufactured in a concrete mixer with a total volume capacity of only 200 L. As a consequence, the Lego bricks had to be cast in three subsequent stages. Each layer of concrete was carefully compacted using a vibration needle.
5. Conclusions
It is possible to produce a proper concrete in which both the fine and coarse aggregates have been replaced with BA resulting from MSWI. The concrete seems suitable for casting prefabricated Lego bricks. The minimum required strength class (=C20/25) can be assured even with W/C ratios of no less than 0.65 and without using an expensive SP.
However, a dedicated processing of the BA prior to concrete manufacturing is imperative. Excluding the excess of fines to approximate the particle size distributions of the traditional limestone aggregates is advised. This results in a better packing and a higher strength of the concrete. A crushing operation before the sieving eliminates porous elements in the BA which absorb a lot of water. As such, a better workability of the concrete can be obtained. A controlled pre-wetting of the BA just before concrete mixing in relation to the time-dependent water absorption curve also helps to improve the concrete workability. Given these techniques, it will probably be possible to manufacture a sufficiently workable BA concrete with a design W/C ratio of only 0.60 in the future.
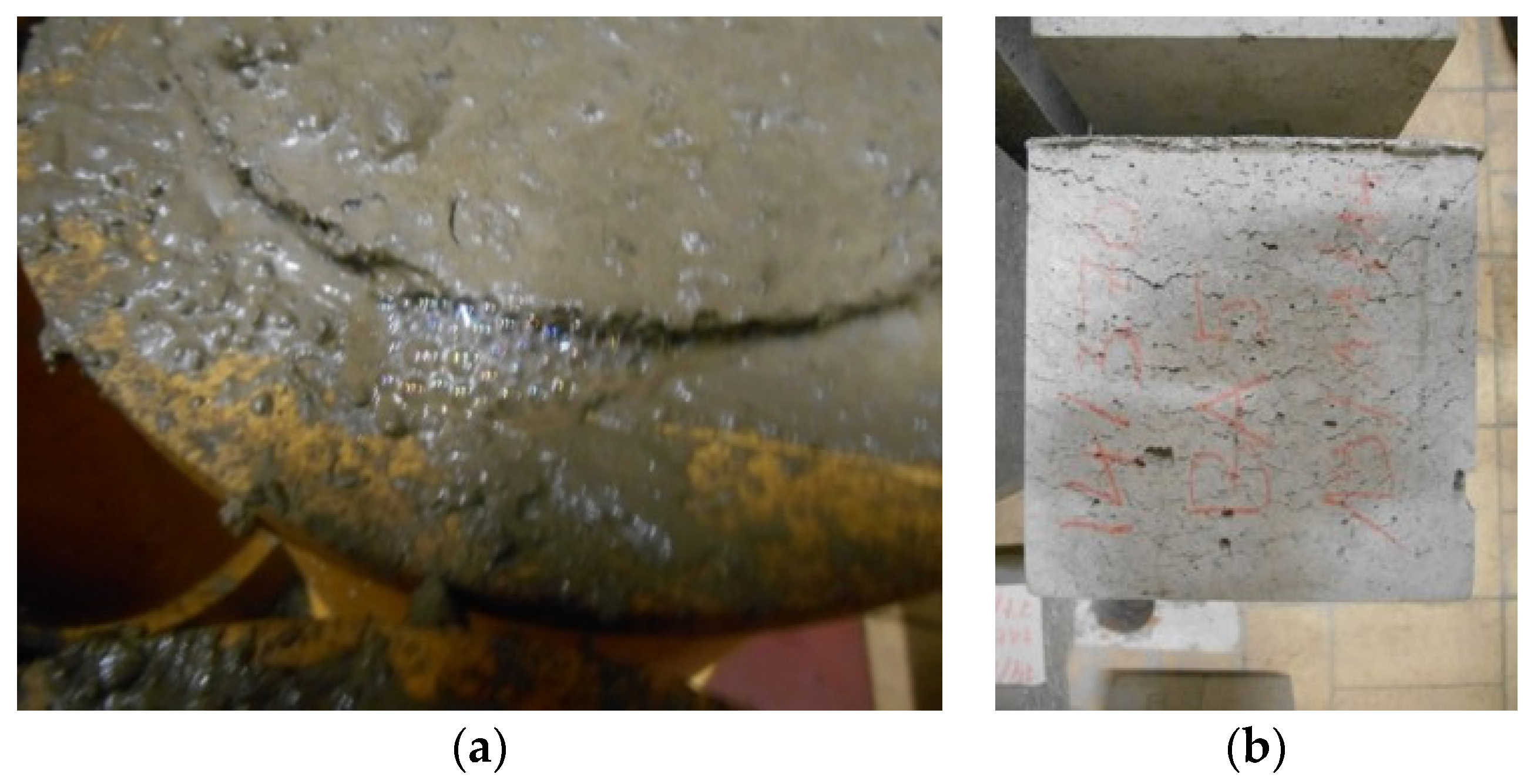
The significant presence of metallic Al in the BA makes the concrete susceptible to the formation of longitudinal voids and expansion. The use of a LA cement can somewhat reduce this problem. A reactive washing of the BA with 1 M NaOH eliminates the problem completely. Still, the latter procedure is not ideal from both an economic and an environmental point of view. Further research on more appropriate reactive washing techniques is still needed.
No problems regarding leaching and freeze-thaw were observed for the BA concrete. On the other hand, the concrete shows a pronounced surface roughening after acetic and lactic acid exposure and does not pass the modified Oberholster test for concrete. The expansion (>0.1%) after the latter test indicates ASR sensitivity. However, further microscopic analysis is needed to see whether ASR is indeed causing the problem. Moreover, the tested BA concrete was not fully optimized yet (e.g., with exclusion of the reactive washing stage). Additional tests on the finally optimized mixture used for Lego brick 3 are, for the moment, still ongoing.