Miscibility Studies of Bismesogen CBnCB Forming Nematic Twist-Bend Phase with Cyanobiphenyls nCB
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Compound CB7CB
3.2. Miscibility Studies
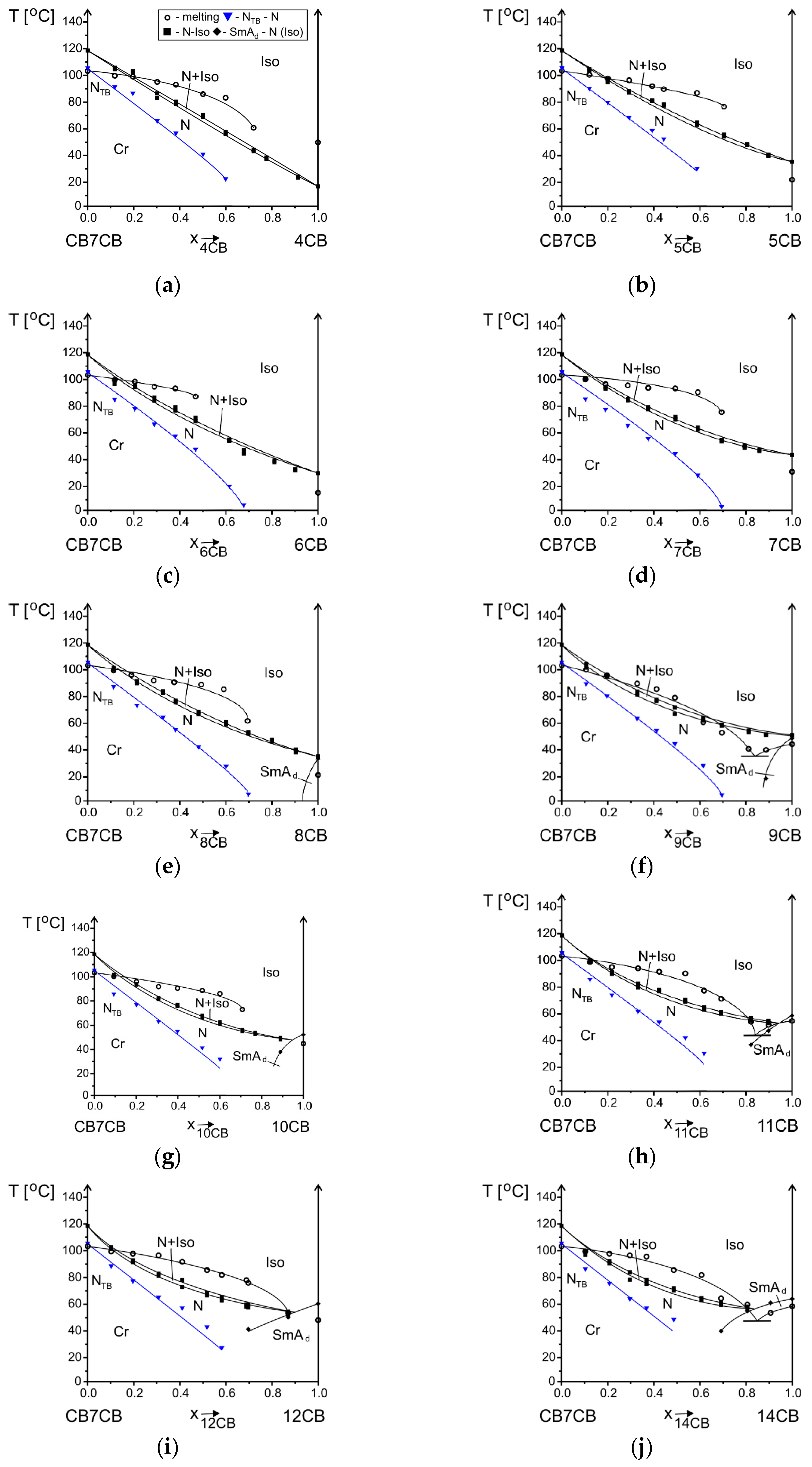
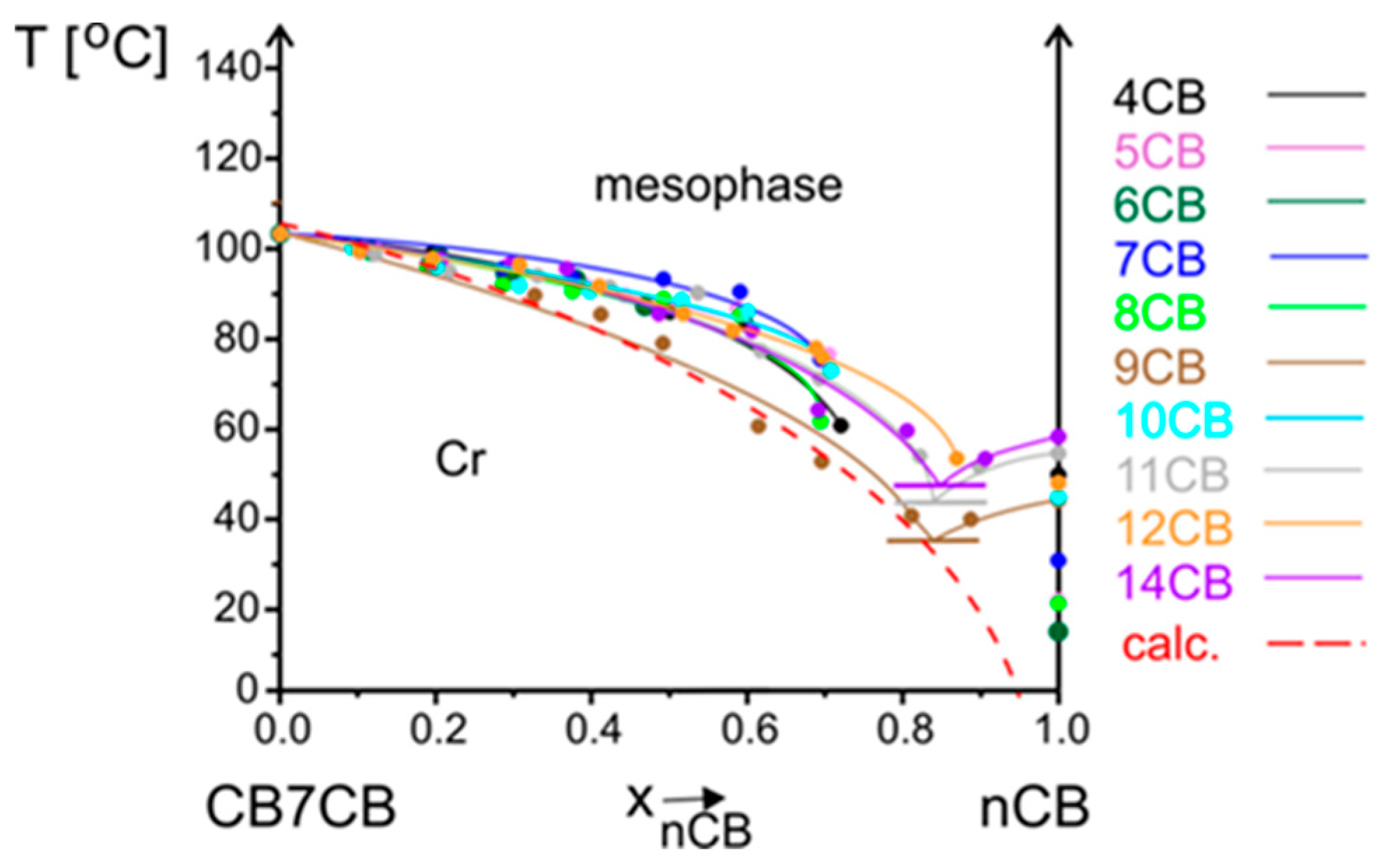
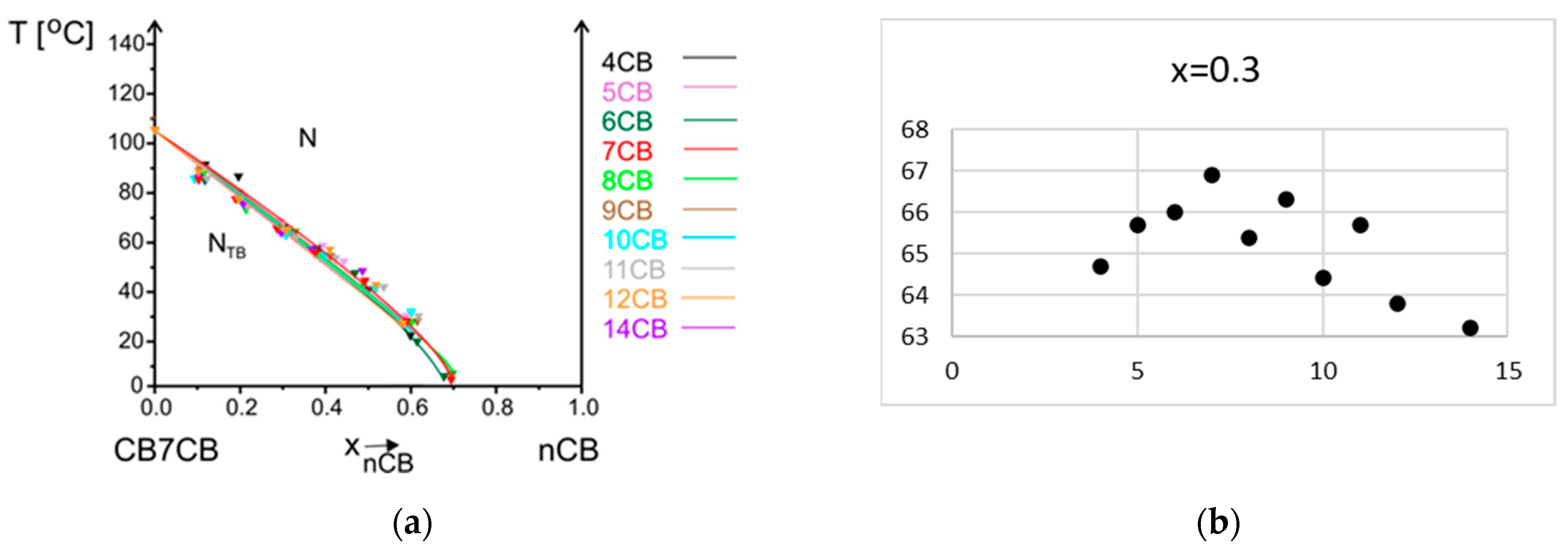
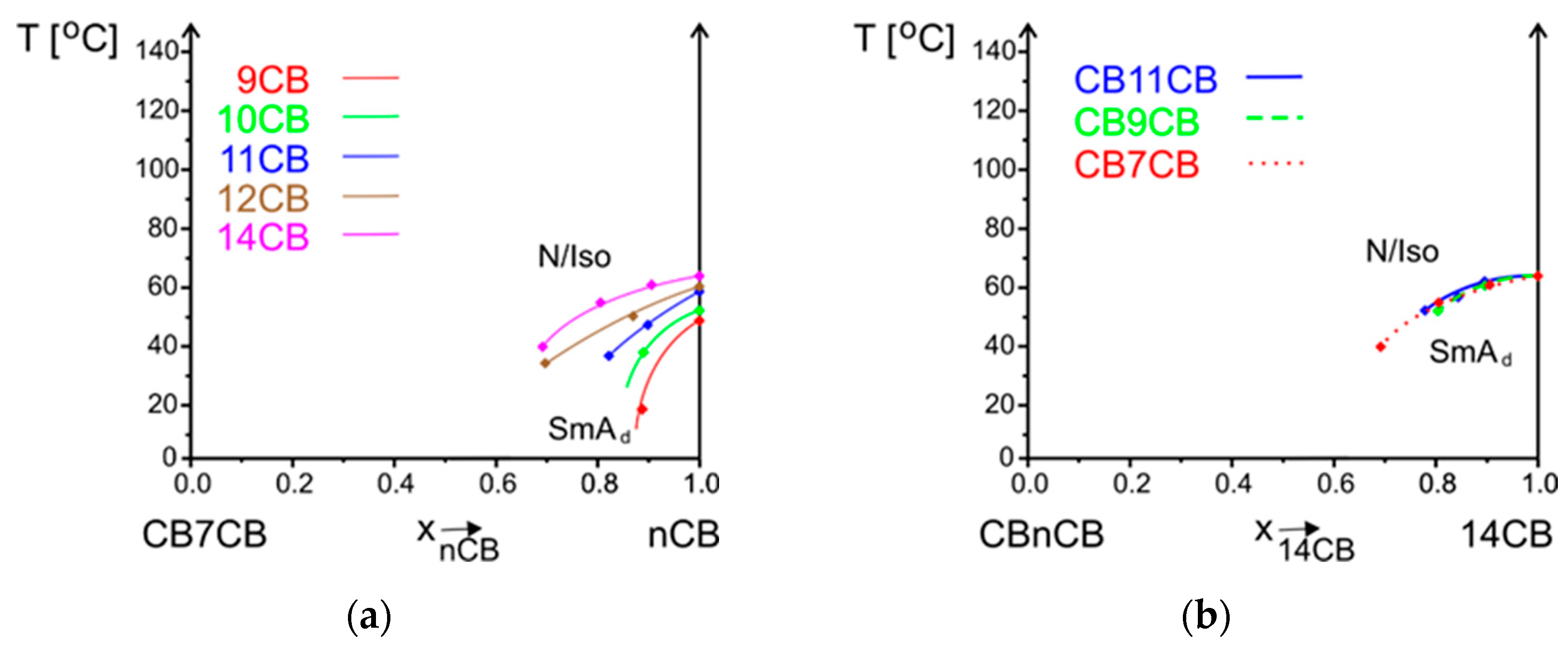
3.2.1. Influence of Alkyl Chain Length of nCB on Phase Situation
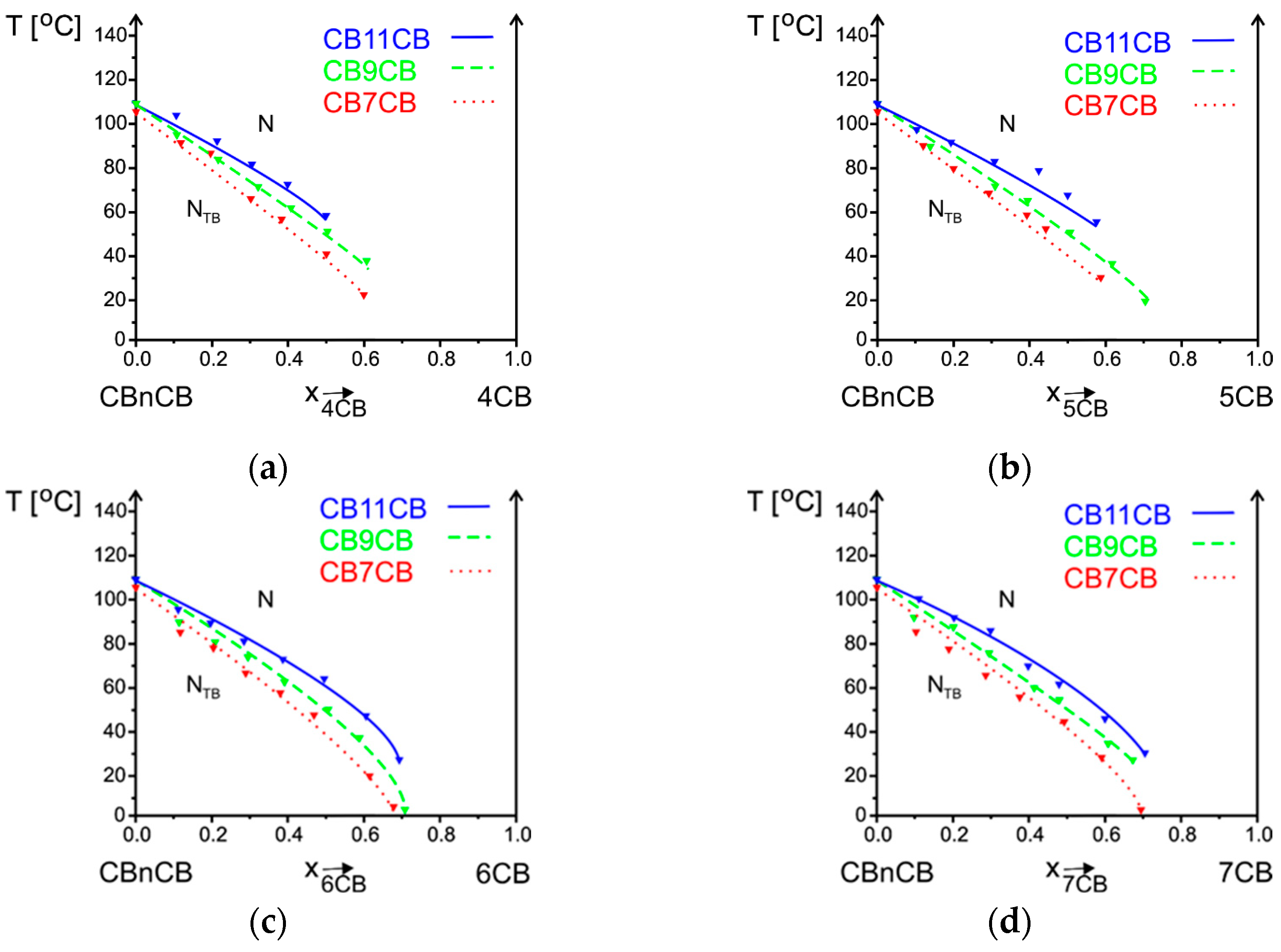
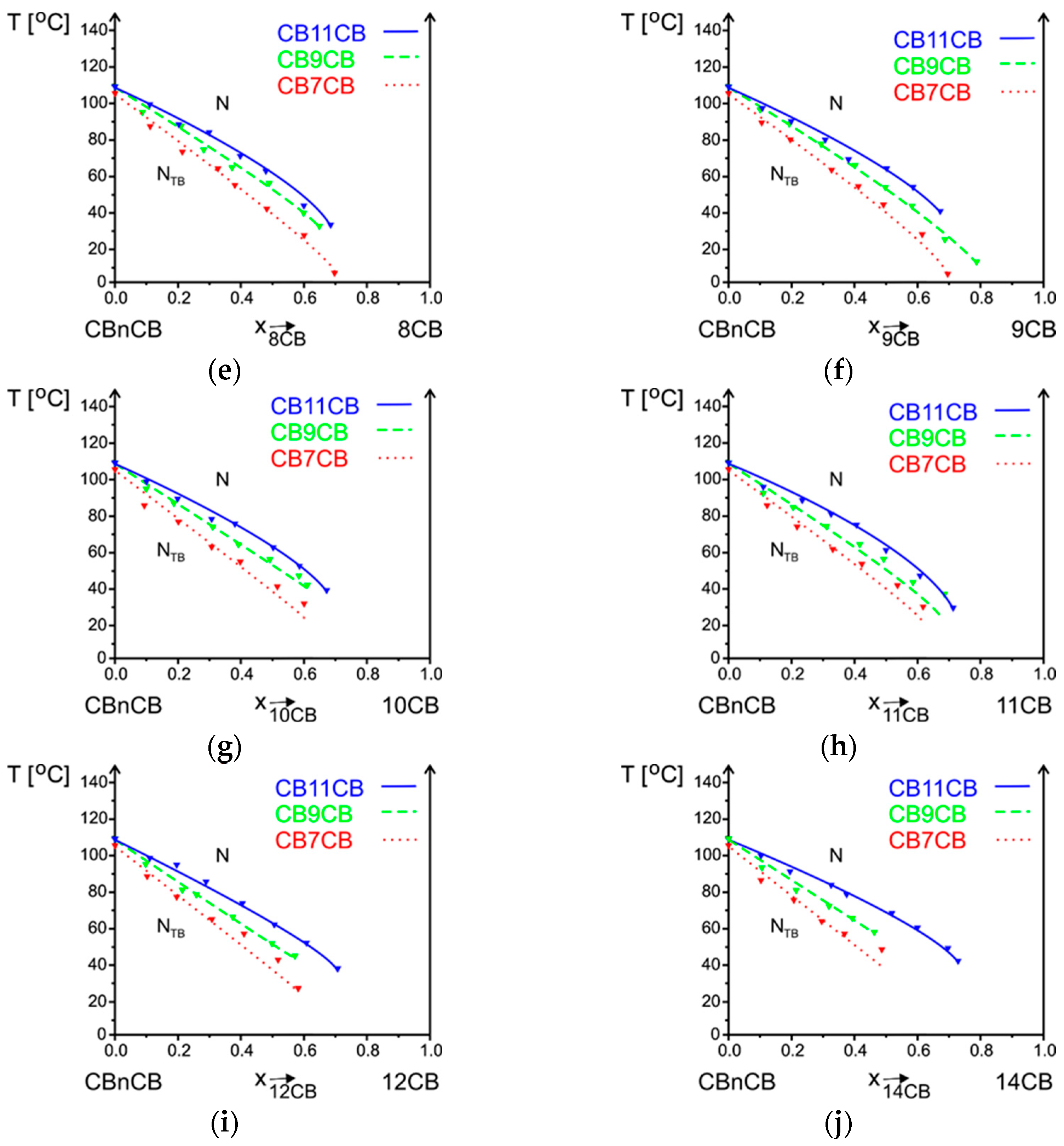
3.2.2. Influence of Alkyl Chain Length of CBnCB on Phase Situation
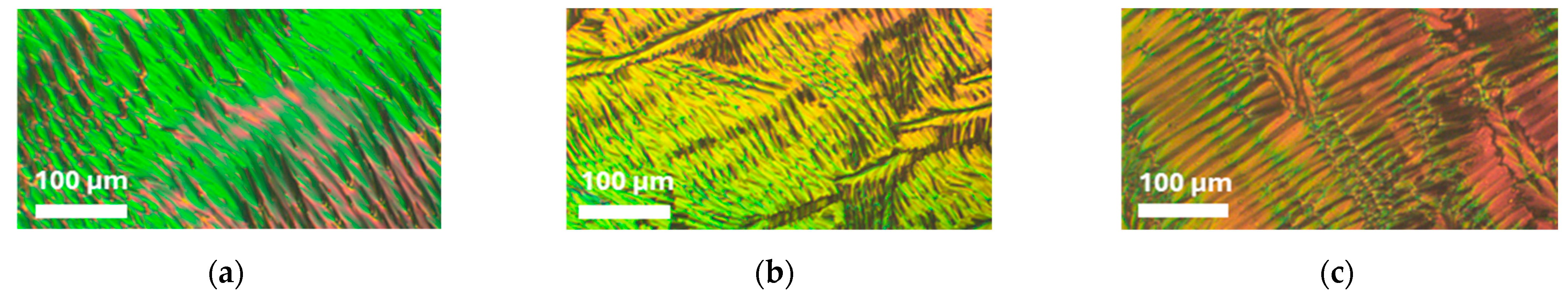
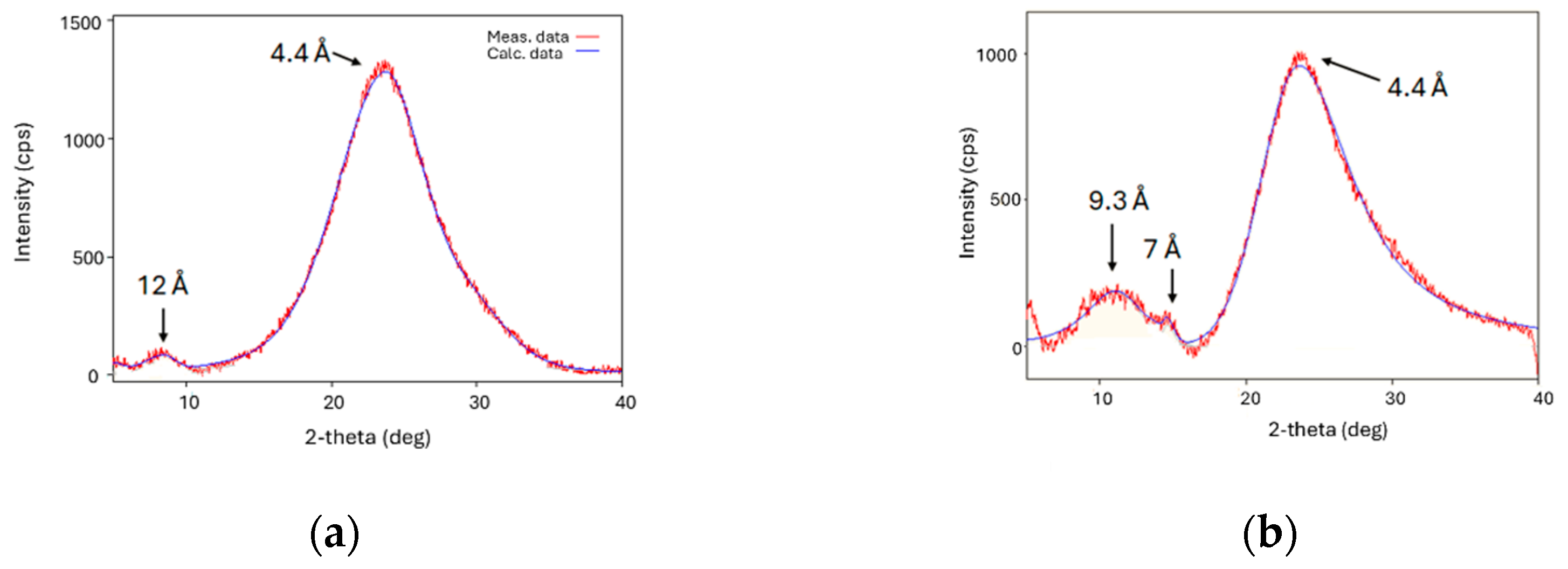
3.3. XRD Studies of Mixtures CB7CB with 9CB and 4CB
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Czupryński, K.; Przedmojski, J.; Dąbrowski, R.; Brodzik, M. Reinvestigation of mesomorphic properties of 4,4′-dialkylbiphenyl. New orthogonal phases. Mol. Cryst. Liq. Cryst. 1997, 299, 503–508. [Google Scholar] [CrossRef]
- Bubnov, A.; Tykarska, M.; Hamplová, V.; Kurp, K. Tuning the phase diagrams: The miscibility studies of multilactate liquid crystalline compounds. Phase Transit. 2016, 89, 885–893. [Google Scholar] [CrossRef]
- Kurp, K.; Tykarska, M. Liquid-crystalline properties of laterally fluorinated alkyl 4″-alkylterphenyl-4-yl carbonates and their mixtures. Liq. Cryst. 2016, 43, 1359–1364. [Google Scholar] [CrossRef]
- Tykarska, M.; Skrzypek, K. The comparison of the ability for induction of antiferroelectric phase of cyanoterminated compounds by means of miscibility studies. Phase Transit. 2007, 80, 203–207. [Google Scholar] [CrossRef]
- Zieja, P.; Tykarska, M.; Morawiak, P.; Piecek, W. Influence of the molecular structure of calamitic compounds with trifluoromethoxy terminal group on the induction of the smectic A phase. Part I. Liq. Cryst. 2023, 50, 363–378. [Google Scholar] [CrossRef]
- Tykarska, M.; Kurp, K.; Zieja, P.; Herman, J.; Stulov, S.; Bubnov, A. New quaterphenyls laterally substituted by methyl group and their influence on the self-assembling behaviour of ferroelectric bicomponent mixtures. Liq. Cryst. 2022, 49, 821–835. [Google Scholar] [CrossRef]
- Tykarska, M.; Kurp, K.; Mironov, S.; Rychłowicz, N.; Karcz, J.; Dziaduszek, J.; Kula, P.; Stulov, S.; Bubnov, A. Tuning of self-organizing and electro-optical behaviour for orthoconic ferroelectric liquid crystal by non-chiral dopants. J. Mol. Liq. 2024, 409, 125426. [Google Scholar] [CrossRef]
- Dąbrowski, R.; Czupryński, K. Induced smectic and nematic phases and re-entrant phenomena. In Modern Topics in Liquid Crystals: From Neutron Scattering to Ferroelectricity; Buka, A., Ed.; World Scientific Pub. Co. Inc.: Singapore, 1993; pp. 125–160. [Google Scholar]
- Nakata, M.; Takanishi, Y.; Watanabe, J.; Takezoe, H. Blue phases induced by doping chiral nematic liquid crystals with nonchiral molecules. Phys. Rev. E 2003, 68, 041710. [Google Scholar] [CrossRef]
- Taushanoff, S.; Van Le, K.; Williams, J.; Twieg, R.J.; Sadashiva, B.K.; Takezoe, H.; Jakli, A. Stable amorphous blue phase of bent-core nematic liquid crystals doped with a chiral material. J. Mater. Chem. 2010, 20, 5893–5898. [Google Scholar] [CrossRef]
- Bubnov, A.; Hamplová, V.; Kašpar, M.; Vajda, A.; Stojanović, M.; Obadović, D.Ž.; Éber, N.; Fodor-Csorba, K. Thermal analysis of binary liquid crystalline mixtures. System of bent core and calamitic molecules. J. Therm. Anal. Calorim. 2007, 90, 431–441. [Google Scholar] [CrossRef]
- Chakraborty, S.; Das, M.K.; Bubnov, A.; Weissflog, W.; Węgłowska, D.; Dąbrowski, R. Induced frustrated twist grain boundary liquid crystalline phases in binary mixtures of achiral hockey stick-shaped and chiral rod-like materials. J. Mater. Chem. C 2019, 7, 10530–10543. [Google Scholar]
- Dozov, I. On the spontaneous symmetry breaking in the mesophases of achiral banana-shaped molecules. Europhys. Lett. 2001, 56, 247–253. [Google Scholar] [CrossRef]
- Cestari, M.; Diez-Berart, S.; Dunmur, D.A.; Ferrarini, A.; de la Fuente, M.R.; Jackson, D.J.B.; López, D.O.; Luckhurst, G.R.; Pérez-Jubindo, M.A.; Richardson, R.M.; et al. Phase behavior and properties of the liquid-crystal dimer 1,7-bis(4-cyanobiphenyl-4-yl)heptane: A twist-bend nematic liquid crystal. Phys. Rev. E 2011, 84, 031704. [Google Scholar]
- Beguin, L.; Emsley, J.W.; Lelli, M.; Lesage, A.; Luckhurst, G.R.; Timimi, B.A.; Zimmermann, H. The Chirality of a Twist–Bend Nematic Phase Identified by NMR Spectroscopy. J. Phys. Chem. B 2012, 116, 7940–7951. [Google Scholar] [PubMed]
- López, D.O.; Sebastian, N.; de la Fuente, M.R.; Martínez-García, J.C.; Salud, J.; Pérez-Jubindo, M.A.; Diez-Berart, S.; Dunmur, D.A.; Luckhurst, G.R. Disentangling molecular motions involved in the glass transition of a twist-bend nematic liquid crystal through dielectric studies. J. Chem. Phys. 2012, 137, 034502. [Google Scholar]
- Jokisaari, J.P.; Luckhurst, G.R.; Timimi, B.A.; Zhu, J.; Zimmermann, H. Twist-bend nematic phase of the liquid crystal dimer CB7CB: Orientational order and conical angle determined by 129Xe and 2H NMR spectroscopy. Liq. Cryst. 2015, 42, 708–721. [Google Scholar]
- Zhu, C.; Tuchband, M.R.; Young, A.; Shuai, M.; Scarbrough, A.; Walba, D.M.; Maclennan, J.E.; Wang, C.; Hexemer, A.; Clark, N.A. Resonant Carbon K-Edge Soft X-Ray Scattering from Lattice-Free Heliconical Molecular Ordering: Soft Dilative Elasticity of the Twist-Bend Liquid Crystal Phase. Phys. Rev. Lett. 2016, 116, 147803. [Google Scholar]
- Babakhanova, G.; Parsouzi, Z.; Paladugu, S.; Wang, H.; Nastishin, Y.A.; Shiyanovskii, S.V.; Sprunt, S.; Lavrentovich, O.D. Elastic and viscous properties of the nematic dimer CB7CB. Phys. Rev. E 2017, 96, 062704. [Google Scholar]
- Krishnamurthy, K.S.; Kanakala, M.B.; Yelamaggad, C.V.; Kleman, M. Instabilities in the electric Freedericksz state of the twist-bend nematic liquid crystal CB7CB. Soft Matter 2018, 14, 5393. [Google Scholar]
- Paterson, D.A.; Abberley, J.P.; Harrison, W.T.A.; Storey, J.M.D.; Imrie, C.T. Cyanobiphenyl-based liquid crystal dimers and the twist-bend nematic phase. Liq. Cryst. 2017, 44, 127–146. [Google Scholar]
- Imrie, C.T.; Walker, R.; Storey, J.M.D.; Górecka, E.; Pociecha, D. Liquid Crystal Dimers and Smectic Phases from the Intercalated to the Twist-Bend. Crystals 2022, 12, 1245. [Google Scholar] [CrossRef]
- Mandle, R.J. A Ten-Year Perspective on Twist-Bend Nematic Materials. Molecules 2022, 27, 2689. [Google Scholar] [CrossRef] [PubMed]
- Dunmur, D.A. Anatomy of a Discovery: The Twist–Bend Nematic Phase. Crystals 2022, 12, 309. [Google Scholar] [CrossRef]
- Kumar, M.P.; Karcz, J.; Kula, P.; Dhara, S. Dynamics of a sheared twist-bend nematic liquid crystal. Phys. Rev. Mat. 2021, 5, 115605. [Google Scholar] [CrossRef]
- Merkel, K.; Loska, B.; Arakawa, Y.; Mehl, G.H.; Karcz, J.; Kocot, A. How do intermolecular interactions evolve at the Nematic to Twist–Bent phase transition? Int. J. Mol. Sci. 2022, 23, 11018. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Henderson, P.A.; Paterson, B.J.A.; Imrie, C.T. Liquid crystal dimers and the twist-bend nematic phase. The preparation and characterization of the α,ω-bis(4-cyanobiphenyl-4′-yl) alkanedioates. Liq. Cryst. 2014, 41, 471–483. [Google Scholar] [CrossRef]
- Robles-Hernandez, B.; Sebastian, N.; Salud, J.; Diez-Berart, S.; Dunmur, D.A.; Luckhurst, G.R.; López, D.O.; de la Fuente, M.R. Molecular dynamics of a binary mixture of twist-bend nematic liquid crystal dimers studied by dielectric spectroscopy. Phys. Rev. E 2016, 93, 062705. [Google Scholar]
- Paterson, D.A.; Gao, M.; Kim, Y.-K.; Jamali, A.; Finley, K.L.; Robles-Hernandez, B.; Diez-Berart, S.; Salud, J.; de la Fuente, M.R.; Timimi, B.A.; et al. Understanding the twist-bend nematic phase: The characterisation of 1-(4-cyanobiphenyl-40-yloxy)-6-(4-cyanobiphenyl-40-yl)hexane (CB6OCB) and comparison with CB7CB. Soft Matter 2016, 12, 6827. [Google Scholar] [CrossRef]
- Tuchband, M.R.; Shuai, M.; Graber, K.A.; Chen, D.; Radzihovsky, L.; Klittnick, A.; Foley, L.; Scarbrough, A.; Porada, J.H.; Moran, M.; et al. The twist-bend nematic phase of bent mesogenic dimer CB7CB and its mixtures. arXiv 2015, arXiv:1511.07523. [Google Scholar]
- Trbojevic, N.; Read, D.J.; Nagaraj, M. Dielectric properties of liquid crystalline dimer mixtures exhibiting the nematic and twist-bend nematic phases. Phys. Rev. E 2017, 96, 052703. [Google Scholar]
- Zavvou, E.E.; Ramou, E.; Ahmed, Z.; Welch, C.; Mehl, G.H.; Vanakaras, A.G.; Karahaliou, P.K. Dipole-dipole correlations in the nematic phases of symmetric cyanobiphenyl dimers and their binary mixtures with 5CB. Soft Matter 2023, 19, 9224. [Google Scholar] [CrossRef] [PubMed]
- Oswald, P. Surface-field-induced heliconical instability in the cholesteric phase of a mixture of a flexible dimer (CB7CB) and a rodlike molecule (8CB). Phys. Rev. E 2022, 105, 024704. [Google Scholar] [CrossRef] [PubMed]
- Aouini, A.; Nobili, M.; Chauveau, E.; Dieudonné-George, P.; Damême, G.; Stoenescu, D.; Dozov, I.; Blanc, C. Chemical-Physical Characterisation of a Binary Mixture of a Twist Bend Nematic Liquid Crystal with a Smectogen. Crystals 2020, 10, 1110. [Google Scholar] [CrossRef]
- Klucznik, B.; Tykarska, M. Miscibility studies of bimesogen CB9CB with members of homologous series nCB. Phase Transit. 2024; submitted. [Google Scholar]
- Gray, G.W.; Lydon, J.E. New type of smectic mesophase? Nature 1974, 252, 221–222. [Google Scholar] [CrossRef]
- Barnes, P.J.; Douglass, A.G.; Heeks, S.K.; Luckhurst, G.R. An enhanced odd-even effect of liquid crystal dimers Orientational order in the α,ω-bis(4′-cyanobiphenyl-4-yl)alkanes. Liq. Cryst. 1993, 13, 603–613. [Google Scholar] [CrossRef]
- Robles-Hernandez, B.; Sebastian, N.; de la Fuente, M.R.; López, D.O.; Diez-Berart, S.; Salud, J.; Blanca Ros, M.; Dunmur, D.A.; Luckhurst, G.R.; Timimi, B.A. Twist, tilt, and orientational order at the nematic to twist-bend nematic phase transition of 1″,9″-bis(4-cyanobiphenyl-4′-yl) nonane: A dielectric, 2H NMR, and calorimetric study. Phys. Rev. E 2015, 92, 062505. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, C.S.P.; Losada-Perez, P.; Glorieux, C.; Kohlmeier, A.; Tamba, M.G.; Mehl, G.H. Nematic-nematic phase transition in the liquid crystal dimer CBC9CB and its mixtures with 5CB: A high-resolution adiabatic scanning calorimetric study. Phys. Rev. E 2011, 84, 041707. [Google Scholar] [CrossRef]
- Mandle, R.J.; Davis, E.J.; Archbold, C.T.; Cowling, S.J.; Goodby, J.W. Microscopy studies of the nematic NTB phase of 1,11-di-(1″-cyanobiphenyl-4-yl)undecane. J. Mater. Chem. C 2014, 2, 556–566. [Google Scholar]
- Gray, G.W.; Harrison, K.J.; Nash, J.A. New family of nematic liquid crystals for displays. Electron. Lett. 1973, 9, 130–131. [Google Scholar] [CrossRef]
- Urban, S.; Massalska-Arodź, M.; Würflinger, A.; Dąbrowski, R. Pressure-temperature phase diagrams of smectogenic 4′-alkyl-4-cyanobiphenyl (9CB, 10CB, 11CB and 12CB). Liq. Cryst. 2003, 30, 313–318. [Google Scholar] [CrossRef]
- Urban, S.; Würflinger, A.; Gestblom, B.; Dąbrowski, R.; Przedmojski, J. DTA, X-ray and dielectric relaxation studies of 14CB at atmospheric and elevated pressures. Liq. Cryst. 2003, 30, 305–311. [Google Scholar] [CrossRef]
- Krzyżewska, K.; Jaroch, T.; Maranda-Niedbała, A.; Pociecha, D.; Górecka, E.; Ahmed, Z.; Welch, C.; Mehl, G.H.; Proń, A.; Nowakowski, R. Supramolecular organization of liquid-crystal dimers-bis-cyanobiphenyl alkanes on HOPG by scanning tunneling microscopy. Nanoscale 2018, 10, 16201–16210. [Google Scholar] [CrossRef] [PubMed]
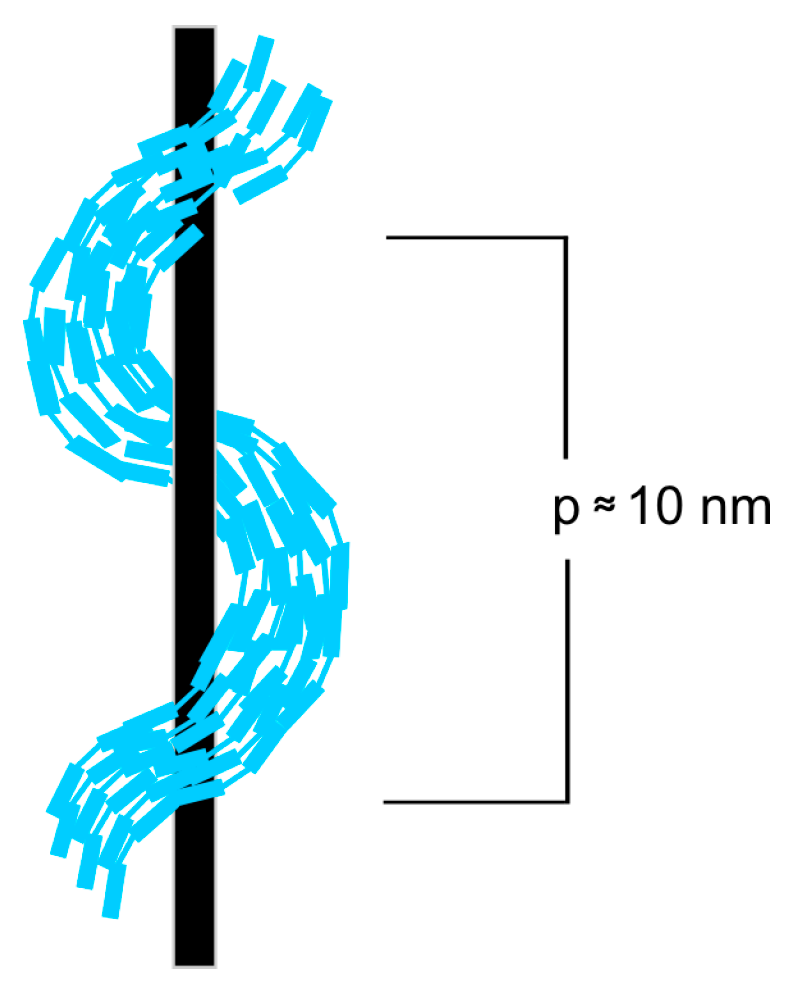
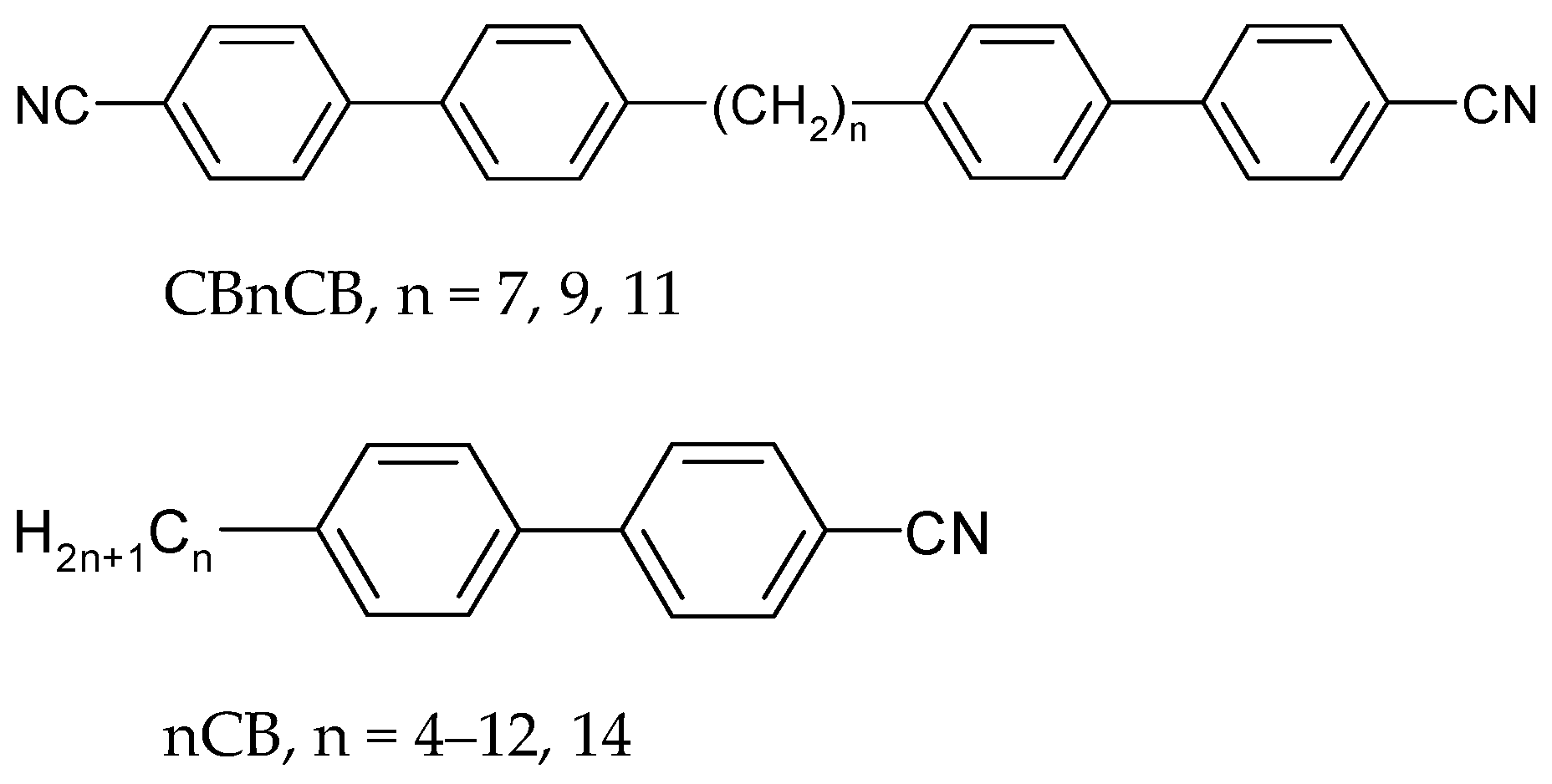
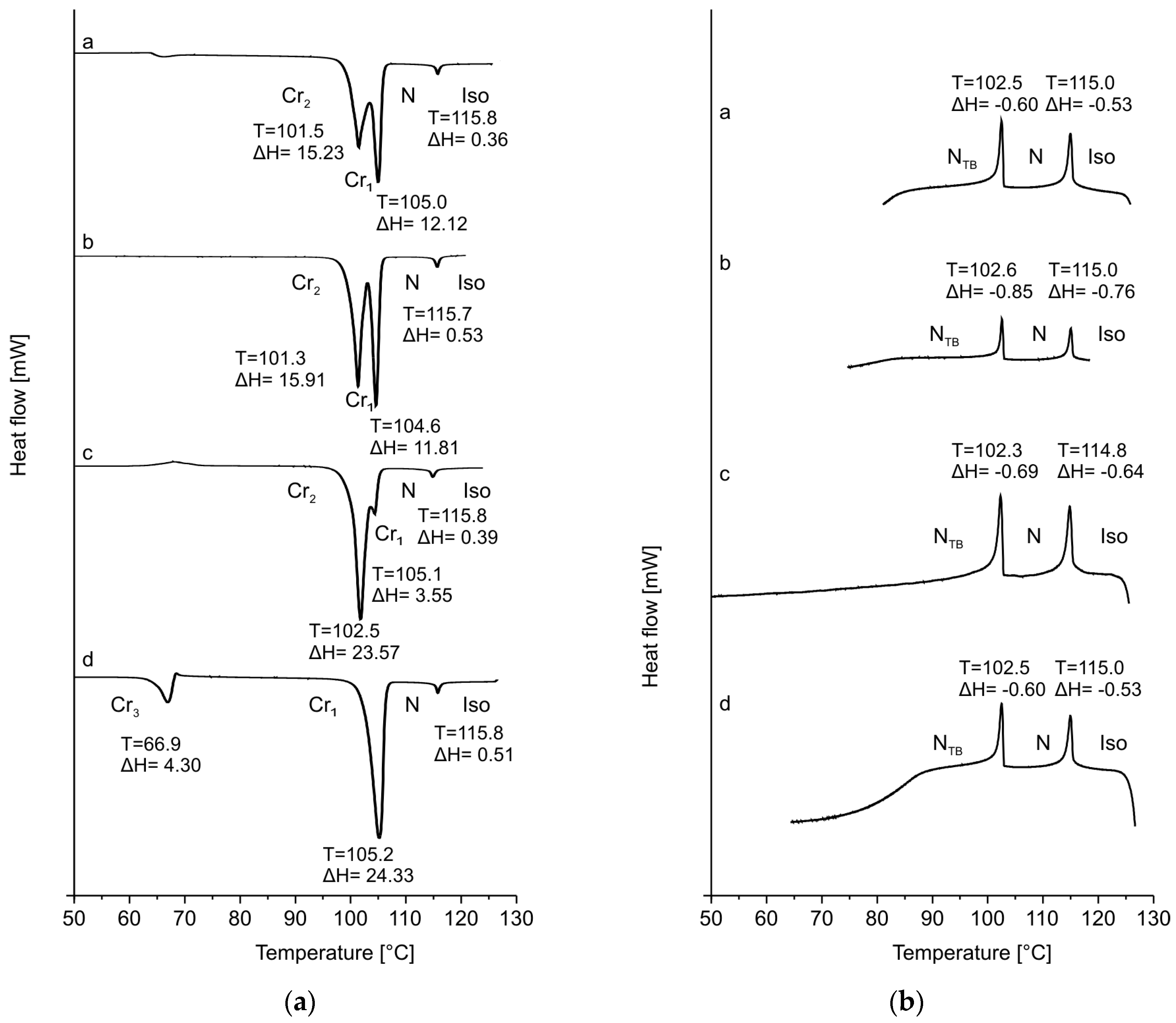
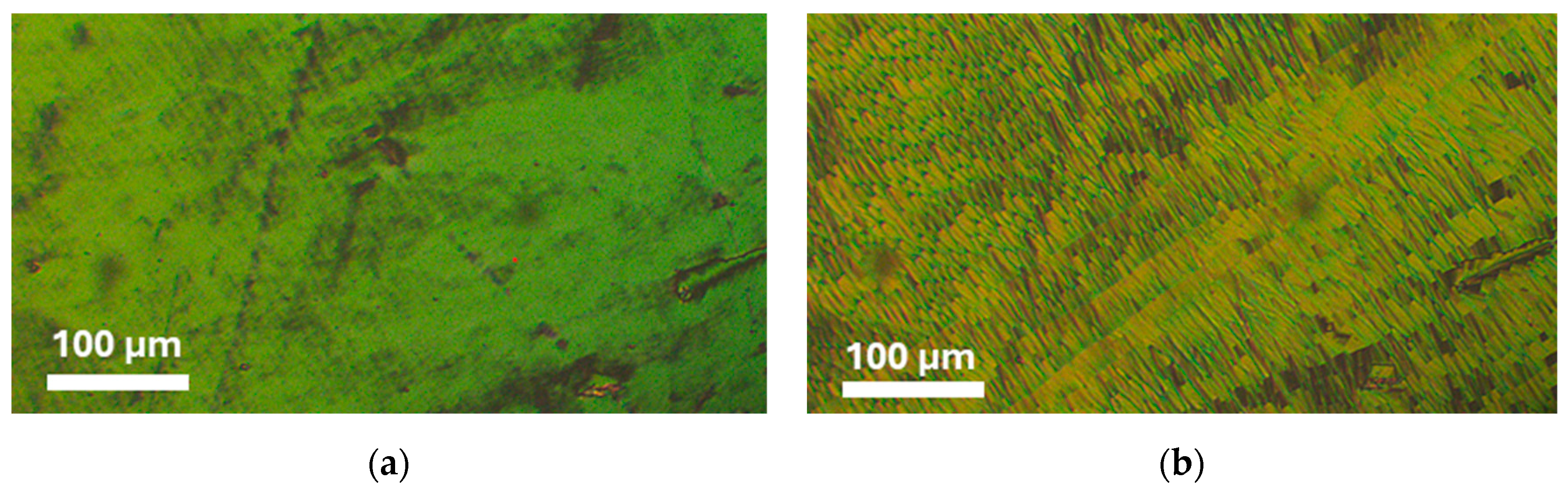

| Acronym | Cr | NTB | SmAd | N | Iso | Refs. | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| CB7CB | * | 102.9 1 | * | 104.4 | - | - | * | 117.2 | * | [14,37] |
| CB9CB | * | 84.9 | * | 107.8 | - | - | * | 123.4 | * | [38,39] |
| CB11CB | * | 105.7 2 | * | 108.7 | - | - | * | 125.4 | * | [40] |
| 4CB | * | 49.5 | - | - | - | - | * | (16.5) | * | [41] |
| 5CB | * | 21.6 | - | - | - | - | * | 39.4 | * | [41] |
| 6CB | * | 14.8 | - | - | - | - | * | 29.5 | * | [41] |
| 7CB | * | 30.5 | - | - | - | - | * | 43.3 | * | [41] |
| 8CB | * | 20.3 | - | - | * | 33.0 | * | 39.7 | * | [41] |
| 9CB | * | 43.9 | - | - | * | 48.4 | * | 50.3 | * | [41] |
| 10CB | * | 44.5 | - | - | * | 51.7 | - | - | * | [42] |
| 11CB | * | 54.3 | - | - | * | 58.3 | - | - | * | [42] |
| 12CB | * | 47.4 | - | - | * | 59.9 | - | - | * | [42] |
| 14CB | * | 58.0 | - | - | * | 63.6 | - | - | * | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tykarska, M.; Klucznik, B.; Dziaduszek, J.; Jóźwiak, S. Miscibility Studies of Bismesogen CBnCB Forming Nematic Twist-Bend Phase with Cyanobiphenyls nCB. Materials 2024, 17, 4256. https://doi.org/10.3390/ma17174256
Tykarska M, Klucznik B, Dziaduszek J, Jóźwiak S. Miscibility Studies of Bismesogen CBnCB Forming Nematic Twist-Bend Phase with Cyanobiphenyls nCB. Materials. 2024; 17(17):4256. https://doi.org/10.3390/ma17174256
Chicago/Turabian StyleTykarska, Marzena, Barbara Klucznik, Jerzy Dziaduszek, and Stanisław Jóźwiak. 2024. "Miscibility Studies of Bismesogen CBnCB Forming Nematic Twist-Bend Phase with Cyanobiphenyls nCB" Materials 17, no. 17: 4256. https://doi.org/10.3390/ma17174256
APA StyleTykarska, M., Klucznik, B., Dziaduszek, J., & Jóźwiak, S. (2024). Miscibility Studies of Bismesogen CBnCB Forming Nematic Twist-Bend Phase with Cyanobiphenyls nCB. Materials, 17(17), 4256. https://doi.org/10.3390/ma17174256






