Crevice Corrosion Behavior of Alloy 690 in High-Temperature Aerated Chloride Solution
Abstract
:1. Introduction
2. Experimental Section
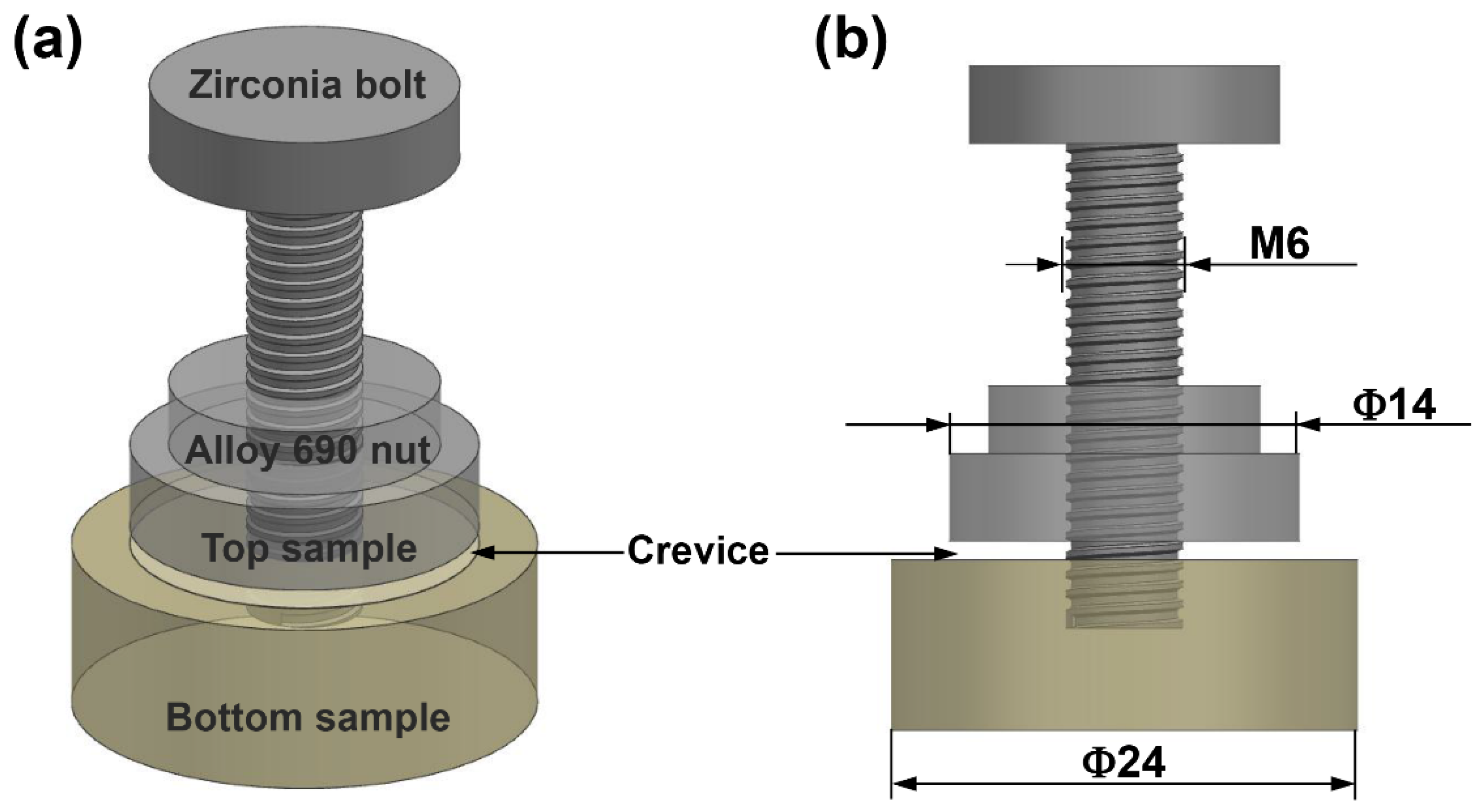
2.1. Materials and Crevice Device
2.2. Crevice Corrosion Immersion Test
2.3. Methodology
3. Results
3.1. Surface Appearance
3.2. XRD Analysis of Oxide Films
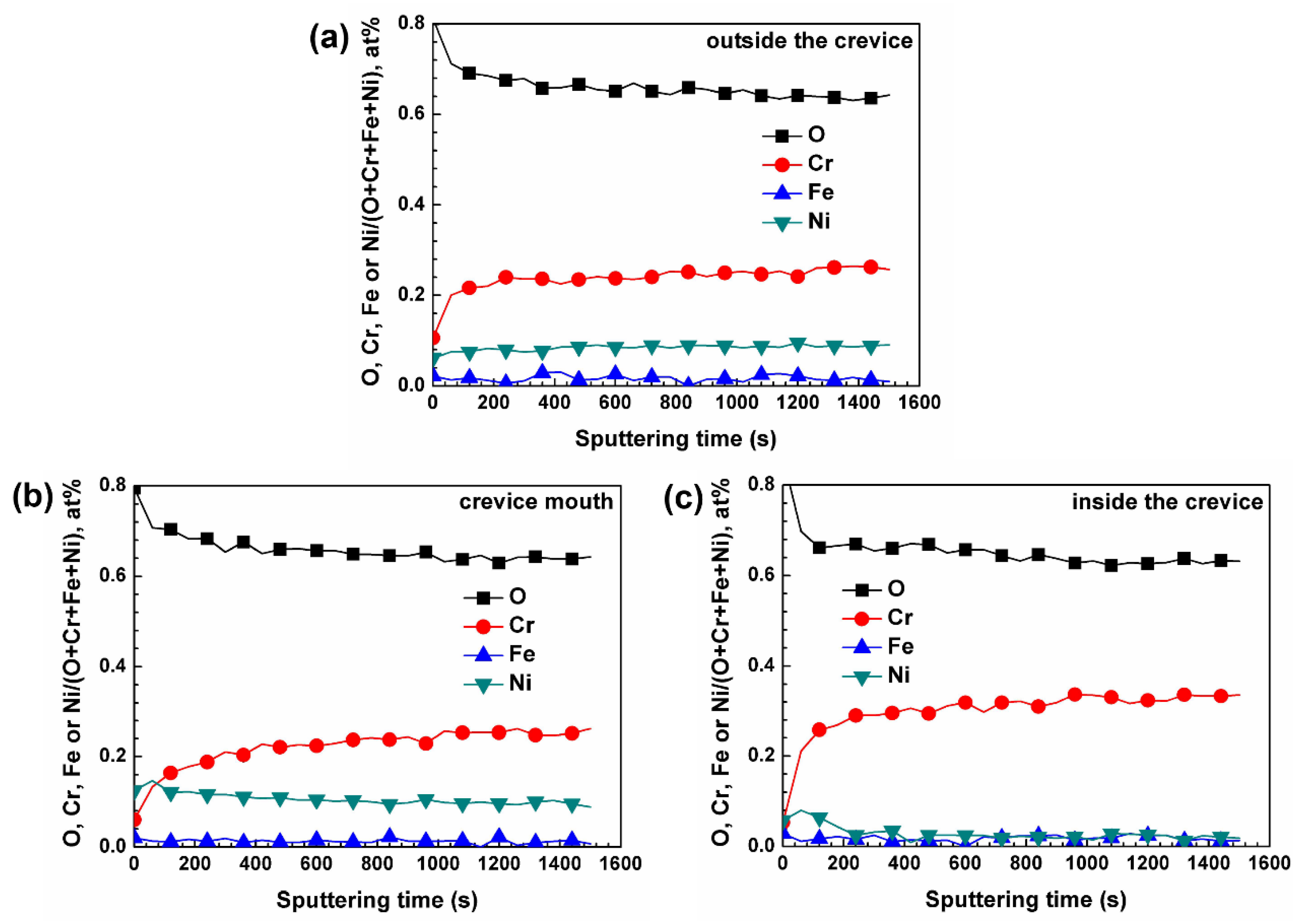
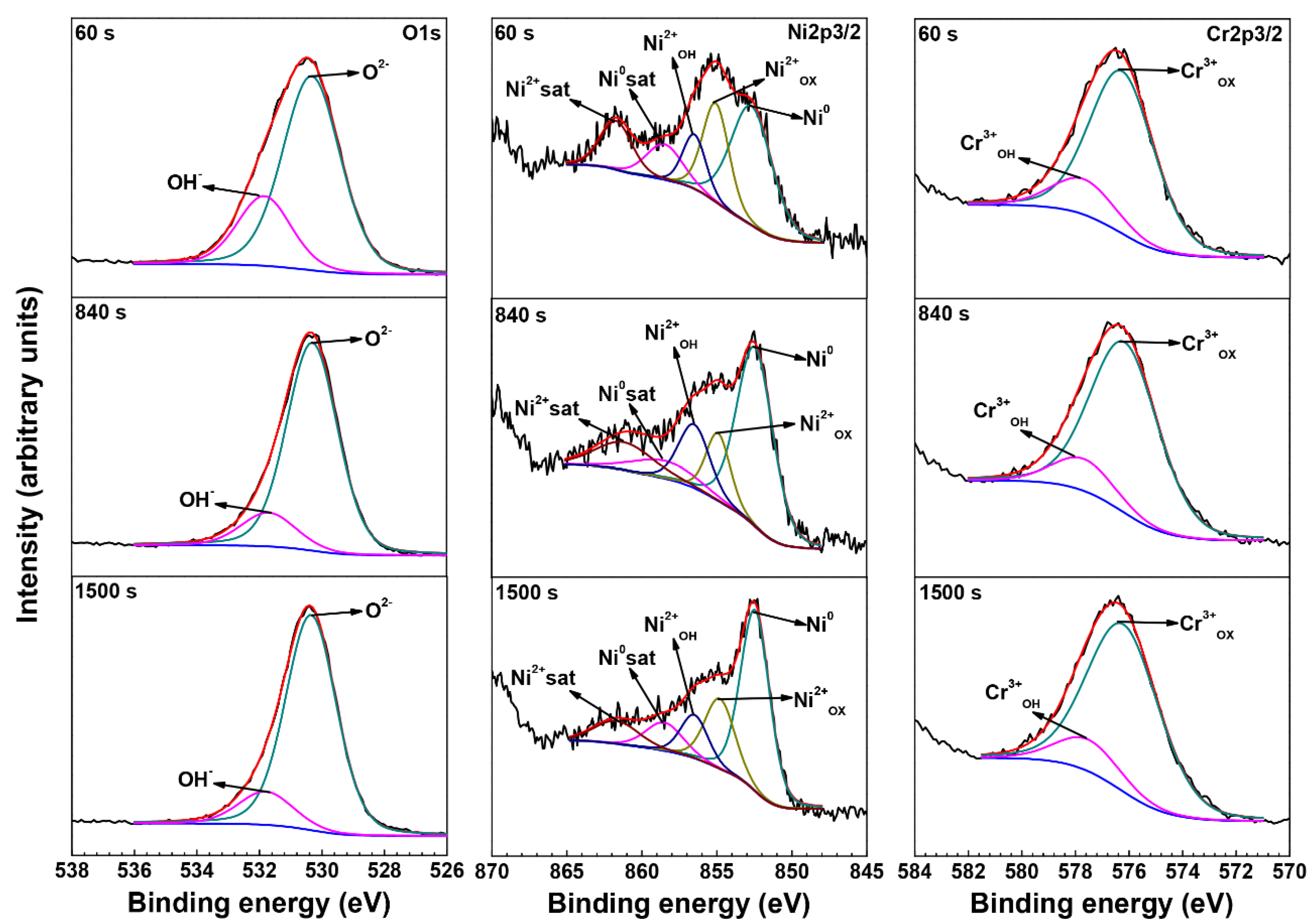
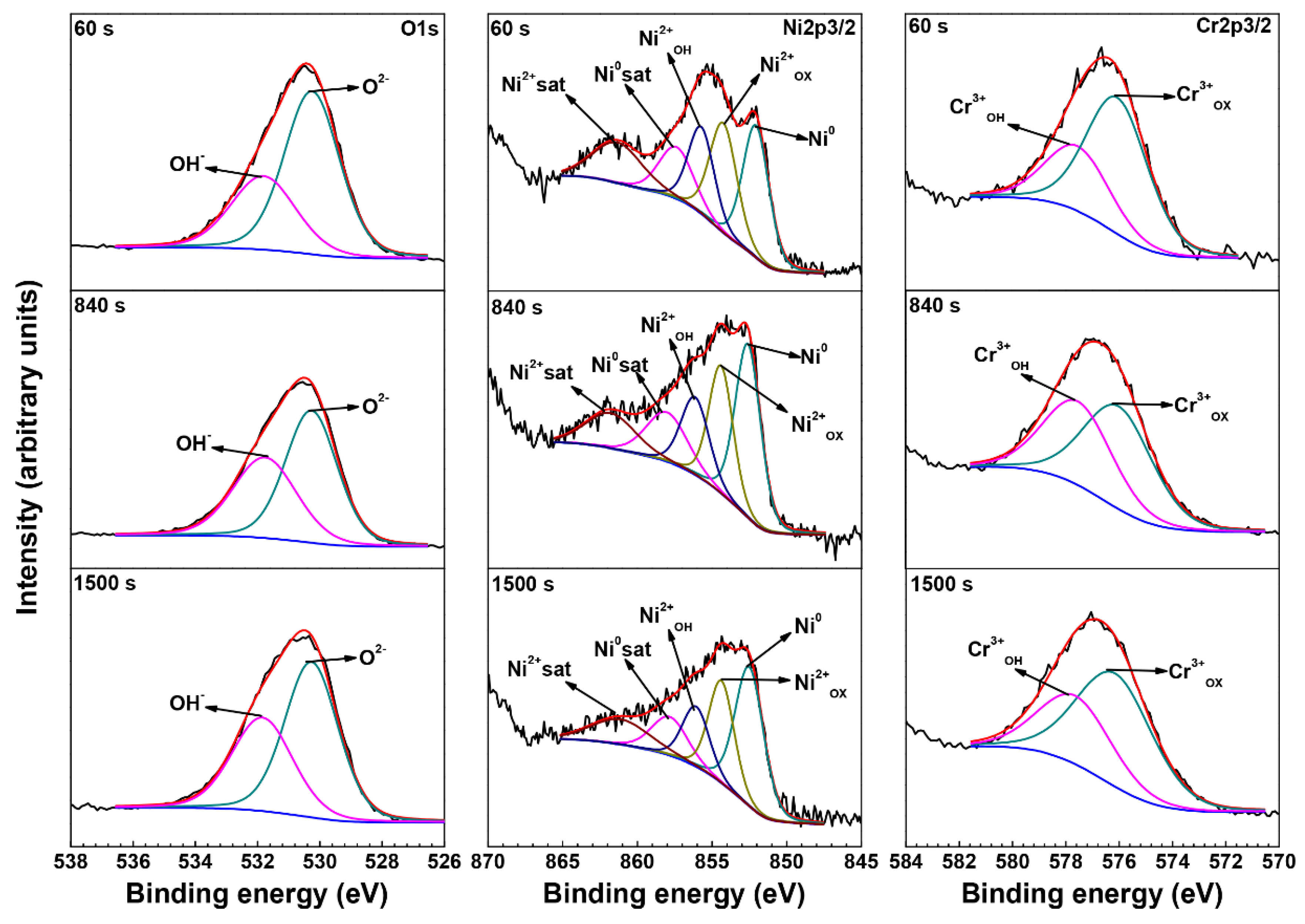
3.3. XPS Analysis of Oxide Films
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zinkle, S.J.; Was, G.S. Materials challenges in nuclear energy. Acta Mater. 2013, 61, 735–758. [Google Scholar] [CrossRef]
- Kuang, W.; Song, M.; Wang, P.; Was, G.S. The oxidation of alloy 690 in simulated pressurized water reactor primary water. Corros. Sci. 2017, 126, 227–237. [Google Scholar] [CrossRef]
- Liao, J.; Wu, X.; Tan, J.; Tang, L.; Qian, H.; Xie, Y. Fretting corrosion fatigue of Alloy 690 in high-temperature pure water. Corros. Sci. 2018, 133, 423–431. [Google Scholar] [CrossRef]
- Kuang, W.; Wu, X.; Han, E.-H. Influence of dissolved oxygen concentration on the oxide film formed on Alloy 690 in high temperature water. Corros. Sci. 2013, 69, 197–204. [Google Scholar] [CrossRef]
- Ning, F.; Wu, X.; Tan, J. Crevice corrosion behavior of Alloy 690 in high-temperature water. J. Nucl. Mater. 2019, 515, 326–337. [Google Scholar] [CrossRef]
- Ning, F.; Tan, J.; Zhang, Z.; Wu, X.; Wang, X.; Han, E.-H.; Ke, W. Effects of thiosulfate and dissolved oxygen on crevice corrosion of Alloy 690 in high-temperature chloride solution. J. Mater. Sci. Technol. 2021, 66, 163–176. [Google Scholar] [CrossRef]
- Ning, F.; Tan, J.; Wu, X. Effects of 405 stainless steel on crevice corrosion behavior of Alloy 690 in high-temperature pure water. J. Mater. Sci. Technol. 2020, 47, 76–87. [Google Scholar] [CrossRef]
- Xia, D.-H.; Sun, Y.-F.; Shen, C.; Chen, X.-Y.; Fan, H.-Q.; Luo, J.-L. A mechanistic study on sulfur-induced passivity degradation on Alloy 800 in simulated alkaline crevice chemistries at temperatures ranging from 21 °C to 300 °C. Corros. Sci. 2015, 100, 504–516. [Google Scholar] [CrossRef]
- Xia, D.; Song, S.; Zhu, R.; Behnamian, Y.; Shen, C.; Wang, J.; Luo, J.; Lu, Y. Klimas, A mechanistic study on thiosulfate-enhanced passivity degradation of Alloy 800 in chloride solutions. Electrochim. Acta 2013, 111, 510–525. [Google Scholar] [CrossRef]
- Xia, D.-H.; Behnamian, Y.; Luo, J.-L. Review—Factors influencing sulfur induced corrosion on the secondary side in pressurized water reactors (PWRs). J. Electrochem. Soc. 2019, 166, C49–C64. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, S.; Xia, D.-H.; Xu, L.; Wang, J.; Song, S.; Fan, H.; Gao, Z.; Zhang, J.; Wu, Z.; et al. Temperature dependence of passivity degradation on UNS N08800 in near neutral crevice chemistries containing thiosulphate. Corros. Sci. 2018, 140, 260–271. [Google Scholar] [CrossRef]
- Shen, C.; Xia, D.-H.; Fan, H.-Q.; Behnamian, Y.; Afacan, A.; Klimas, S.J.; Luo, J.-L. Passivation Degradation of Alloy 800 in Boiling Solution Containing Thiosulphate. Electrochim. Acta 2017, 233, 13–25. [Google Scholar] [CrossRef]
- Ning, F.; Tan, J.; Zhang, Z.; Wang, X.; Wu, X.; Han, E.-H.; Ke, W. Nodular corrosion inside the crevice of Alloy 690 in deaerated high-temperature chloride solution. Corros. Sci. 2021, 185, 109442. [Google Scholar] [CrossRef]
- Abellà, J.; Balachov, I.; Macdonald, D.D.; Millet, P.J. Transport processes in steam generator crevices III. Experimental results. Corros. Sci. 2002, 44, 191–205. [Google Scholar] [CrossRef]
- Staehle, R.W.; Gorman, J.A. Quantitative assessment of submodes of stress corrosion cracking on the secondary side of steam generator tubing in pressurized water reactors: Part I. Corrosion 2003, 59, 931–994. [Google Scholar] [CrossRef]
- Nishimoto, M.; Ogawa, J.; Muto, I.; Sugawara, Y.; Hara, N. Simultaneous visualization of pH and Cl− distributions inside the crevice of stainless steel. Corros. Sci. 2016, 106, 298–302. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Wang, J.; Wu, B.; Guo, X.; Wang, Y.; Chen, D.; Zhang, Y.; Du, K.; Oguzie, E.E.; Ma, X. Unmasking chloride attack on the passive film of metals. Nat. Commun. 2018, 9, 2559. [Google Scholar] [CrossRef]
- Oldfield, J.W.; Sutton, W.H. Crevice Corrosion of Stainless Steels I: A Mathematical Model. Br. Corros. J. 1978, 13, 13–22. [Google Scholar] [CrossRef]
- Force, B.D.; Pickering, H. A clearer view of how crevice corrosion occurs. JOM 1995, 47, 22–27. [Google Scholar] [CrossRef]
- White, S.P.; Weir, G.J.; Laycock, N.J. Calculating chemical concentrations during the initiation of crevice corrosion. Corros. Sci. 2000, 42, 605–629. [Google Scholar] [CrossRef]
- Kennell, G.F.; Evitts, R.W.; Heppner, K.L. A critical crevice solution and IR drop crevice corrosion model. Corros. Sci. 2008, 50, 1716–1725. [Google Scholar] [CrossRef]
- Alavi, A.; Cottis, R.A. The determination of pH, potential and chloride concentration in corroding crevices on 304 stainless steel and 7475 aluminium alloy. Corros. Sci. 1987, 27, 443–451. [Google Scholar] [CrossRef]
- Yeh, C.-P.; Tsai, K.-C.; Huang, J.-Y. Influence of Chloride Concentration on Stress Corrosion Cracking and Crevice Corrosion of Austenitic Stainless Steel in Saline Environments. Materials 2020, 13, 5640. [Google Scholar] [CrossRef] [PubMed]
- Hur, D.H.; Han, J.; Choi, J. Molar Ratio Effect of Sodium to Chloride Ions on the Electrochemical Corrosion of Alloy 600 and SA508 in HCl + NaOH Mixtures. Materials 2020, 13, 1970. [Google Scholar] [CrossRef] [Green Version]
- Shojaei, E.; Moayed, M.H.; Mirjalili, M.; Pahlavan, S. Proposed stability product criterion for open hemispherical metastable pits formed in the crevices of different aspect ratios (l/d) on 316L stainless steel in 3.5% NaCl solution. Corros. Sci. 2021, 184, 109389. [Google Scholar] [CrossRef]
- Eškinja, M.; Moshtaghi, M.; Hönig, S.; Zehethofer, G.; Mori, G. Investigation of the effects of temperature and exposure time on the corrosion behavior of a ferritic steel in CO2 environment using the optimized linear polarization resistance method. Results Mater. 2022, 14, 100282. [Google Scholar] [CrossRef]
- Chen, D.; Wu, X.; Han, E.-H.; Sun, H. Oxidation behavior of 304 stainless steel during crevice corrosion in high-temperature pure water. Corrosion 2015, 71, 1213–1223. [Google Scholar] [CrossRef]
- Chen, D.; Han, E.-H.; Wu, X. Effects of crevice geometry on corrosion behavior of 304 stainless steel during crevice corrosion in high temperature pure water. Corros. Sci. 2016, 111, 518–530. [Google Scholar] [CrossRef]
- Dong, L.; Peng, Q.; Zhang, Z.; Shoji, T.; Han, E.-H.; Ke, W.; Wang, L. Effect of dissolved hydrogen on corrosion of 316NG stainless steel in high temperature water. Nucl. Eng. Des. 2015, 295, 403–414. [Google Scholar] [CrossRef]
- Deng, P.; Peng, Q.; Han, E.-H.; Ke, W.; Sun, C.; Jiao, Z. Effect of irradiation on corrosion of 304 nuclear grade stainless steel in simulated PWR primary water. Corros. Sci. 2017, 127, 91–100. [Google Scholar] [CrossRef]
- Zhong, X.; Han, E.-H.; Wu, X. Corrosion behavior of Alloy 690 in aerated supercritical water. Corros. Sci. 2013, 66, 369–379. [Google Scholar] [CrossRef]
- Mu, P.; Zhang, H.; Lou, T.; Zhou, P.; Shi, H.; Chen, L.; Xu, H. Preparation of chromic oxide green by thermal decomposition of CrOOH with base center orthorhombic structure and trigonal structure. Chin. J. Process Eng. (In Chinese). [CrossRef]
- Machet, A.; Galtayries, A.; Marcus, P.; Combrade, P.; Jolivet, P.; Scott, P. XPS study of oxides formed on nickel-base alloys in high-temperature and high-pressure water. Surf. Interface Anal. 2002, 34, 197–200. [Google Scholar] [CrossRef]
- Machet, A.; Galtayries, A.; Zanna, S.; Klein, L.; Maurice, V.; Jolivet, P.; Foucault, M.; Combrade, P.; Scott, P.; Marcus, P. XPS and STM study of the growth and structure of passive films in high temperature water on a nickel-base alloy. Electrochim. Acta 2004, 49, 3957–3964. [Google Scholar] [CrossRef]
- Marchetti, L.; Miserque, F.; Perrin, S.; Pijolat, M. XPS study of Ni-base alloys oxide films formed in primary conditions of pressurized water reactor. Surf. Interface Anal. 2015, 47, 632–642. [Google Scholar] [CrossRef] [Green Version]
- Fujimoto, S.; Kim, W.-S.; Sato, M.; Son, J.-Y.; Machida, M.; Jung, K.-T.; Tsuchiya, H. Characterization of oxide films formed on Alloy 600 and Alloy 690 in simulated PWR primary water by using hard X-ray photoelectron spectroscopy. J. Solid State Electrochem. 2015, 19, 3521–3531. [Google Scholar] [CrossRef]
- Duan, Z.; Arjmand, F.; Zhang, L.; Meng, F.; Abe, H. Electrochemical and XPS investigation of the corrosion behavior of Alloy 690 at high-temperature water. J. Solid State Electrochem. 2015, 19, 2265–2273. [Google Scholar] [CrossRef]
- Huang, J.; Wu, X.; Han, E.-H. Electrochemical properties and growth mechanism of passive films on Alloy 690 in high-temperature alkaline environments. Corros. Sci. 2010, 52, 3444–3452. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Huang, F.; Zhang, Z.; Wang, J.; Staehle, R.W. Comparison of corrosion resistance of UNS N06690TT and UNS N08800SN in simulated primary water with various concentrations of dissolved oxygen. Corrosion 2014, 70, 598–614. [Google Scholar] [CrossRef]
- Ning, F.; Wang, X.; Yang, Y.; Tan, J.; Zhang, Z.; Jia, D.; Wu, X.; Han, E.-H. Uniform corrosion behavior of FeCrAl alloys in borated and lithiated high temperature water. J. Mater. Sci. Technol. 2021, 70, 136–144. [Google Scholar] [CrossRef]
- Kuang, W.; Wu, X.; Han, E.-H.; Rao, J. The mechanism of oxide film formation on Alloy 690 in oxygenated high temperature water. Corros. Sci. 2011, 53, 3853–3860. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Han, E.-H.; Ke, W. Influence of dissolved oxygen on oxide films of Alloy 690TT with different surface status in simulated primary water. Corros. Sci. 2011, 53, 3623–3635. [Google Scholar] [CrossRef]
- Lemire, R.J.; McRae, G.A. The corrosion of Alloy 690 in high-temperature aqueous media–thermodynamic considerations. J. Nucl. Mater. 2001, 294, 141–147. [Google Scholar] [CrossRef]
- Beverskog, B.; Puigdomenech, I. Pourbaix diagrams for the ternary system of iron-chromium-nickel. Corrosion 1999, 55, 1077–1087. [Google Scholar] [CrossRef]
- Sharland, S.M. A review of the theoretical modelling of crevice and pitting corrosion. Corros. Sci. 1987, 27, 289–323. [Google Scholar] [CrossRef]
- Vermilyea, D.A.; Tedmon, C.S., Jr. A Simple Crevice Corrosion Theory. J. Electrochem. Soc. 1970, 117, 437–440. [Google Scholar] [CrossRef]
- Sharland, S.M.; Tasker, P.W. A mathematical model of crevice and pitting corrosion-I. The physical model. Corros. Sci. 1988, 28, 603–620. [Google Scholar] [CrossRef]
- Song, F.M. Predicting the chemistry, corrosion potential and corrosion rate in a crevice formed between substrate steel and a disbonded permeable coating with a mouth. Corros. Sci. 2012, 55, 107–115. [Google Scholar] [CrossRef]
- Tsai, K.-C.; Yeh, C.-P. The Corrosion Susceptibility of 304L Stainless Steel Exposed to Crevice Environments. Materials 2022, 15, 3055. [Google Scholar] [CrossRef]
- Mu, J.; Li, Y.; Wang, X. Crevice corrosion behavior of X70 steel in NaCl solution with different pH. Corros. Sci. 2021, 182, 109310. [Google Scholar] [CrossRef]
- Liu, X.; Wu, X.; Han, E.-H. Influence of Zn injection on characteristics of oxide film on 304 stainless steel in borated and lithiated high temperature water. Corros. Sci. 2011, 53, 3337–3345. [Google Scholar] [CrossRef]



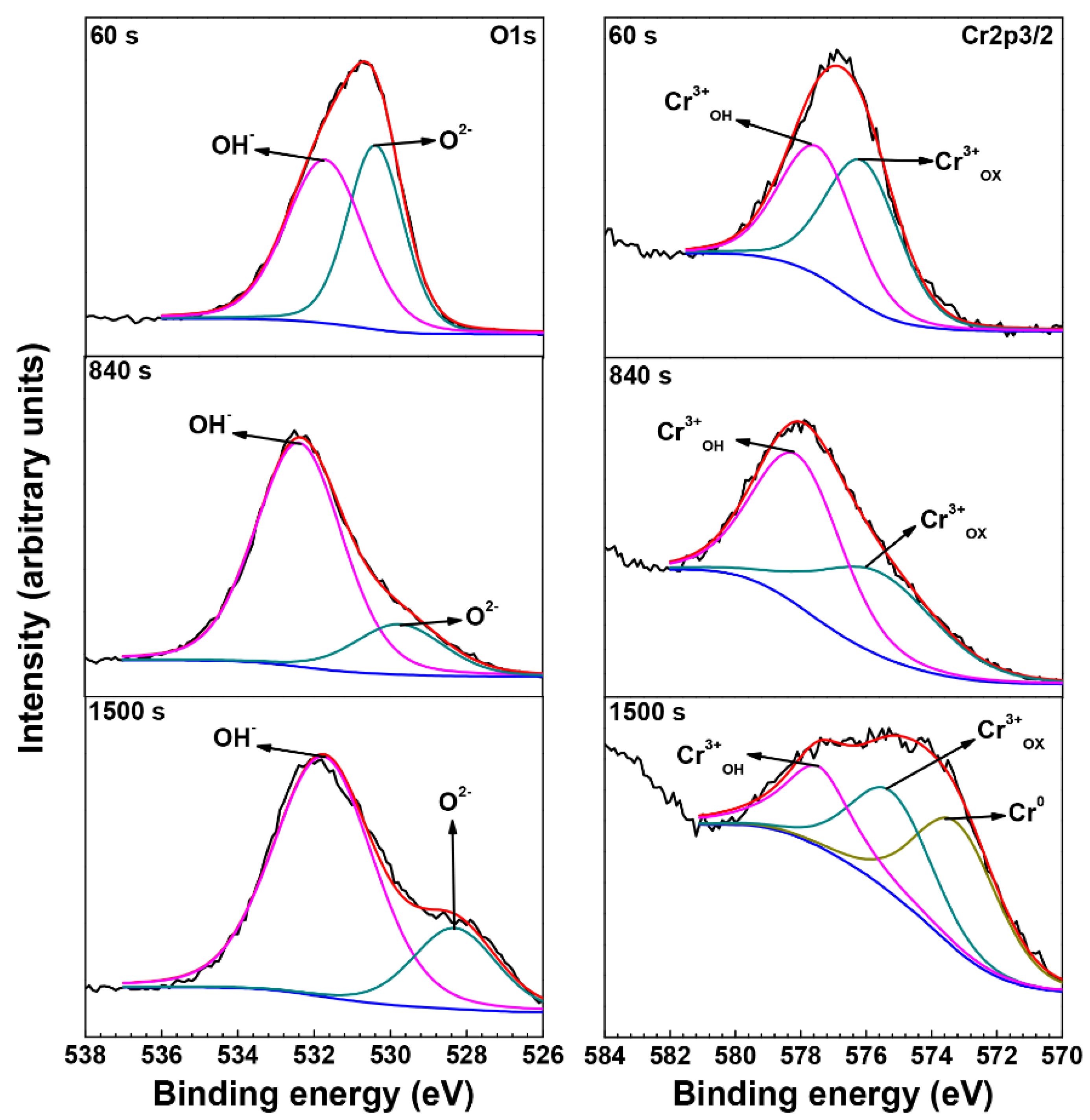
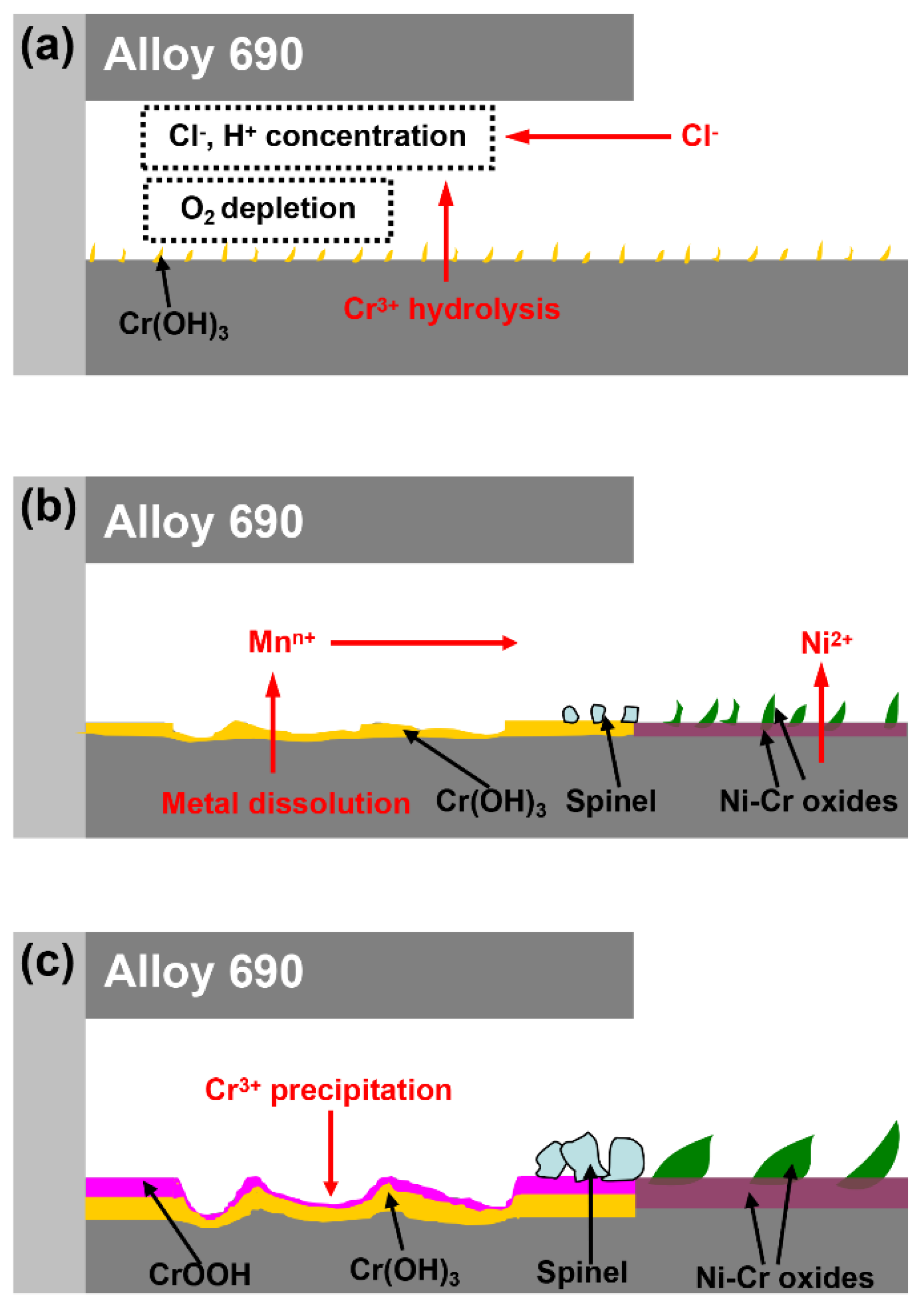
| Material | C | N | S | P | Mn | Ti | Al | Si | Cu | Fe | Cr | Ni |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alloy 690 | 0.03 | 0.013 | 0.001 | 0.007 | 0.293 | 0.202 | 0.202 | 0.292 | 0.01 | 10.61 | 30.04 | 58.3 |
| Element | Species | Binding Energy (eV) |
|---|---|---|
| O | O2− (1s) | 530.3 |
| OH− (1s) | 531.7 | |
| Ni | Ni0 (2p3/2) | 852.7 |
| Ni0 sat (2p3/2) | 858.5 | |
| Ni2+OX (2p3/2) | 854.4 | |
| Ni2+OH (2p3/2) | 856.5 | |
| Ni2+sat (2p3/2) | 861.7 | |
| Cr | Cr0 (2p3/2) | 574.3 |
| Cr3+OX (2p3/2) | 576.1 | |
| Cr3+OH (2p3/2) | 577.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ning, F.; Tan, J.; Zhang, Z.; Wang, X.; Wu, X.; Han, E.-H.; Ke, W. Crevice Corrosion Behavior of Alloy 690 in High-Temperature Aerated Chloride Solution. Materials 2022, 15, 5434. https://doi.org/10.3390/ma15155434
Ning F, Tan J, Zhang Z, Wang X, Wu X, Han E-H, Ke W. Crevice Corrosion Behavior of Alloy 690 in High-Temperature Aerated Chloride Solution. Materials. 2022; 15(15):5434. https://doi.org/10.3390/ma15155434
Chicago/Turabian StyleNing, Fangqiang, Jibo Tan, Ziyu Zhang, Xiang Wang, Xinqiang Wu, En-Hou Han, and Wei Ke. 2022. "Crevice Corrosion Behavior of Alloy 690 in High-Temperature Aerated Chloride Solution" Materials 15, no. 15: 5434. https://doi.org/10.3390/ma15155434






