Superhydrophobic Anticorrosive Phosphonate–Siloxane Films Formed on Zinc with Different Surface Morphology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Anticorrosion Superhydrophobic Films on the Zinc Surface
- “Smooth” surface, oxidized in air (Type 1);
- Textured surface by chemical etching (Type 2);
- Textured surface by laser (Types 3 and 4).
2.3. Microstructural and Chemical Analysis
2.4. Study of the Protective and Hydrophobic Properties of Films
3. Results and Discussion
3.1. Morphology of Zinc Surface
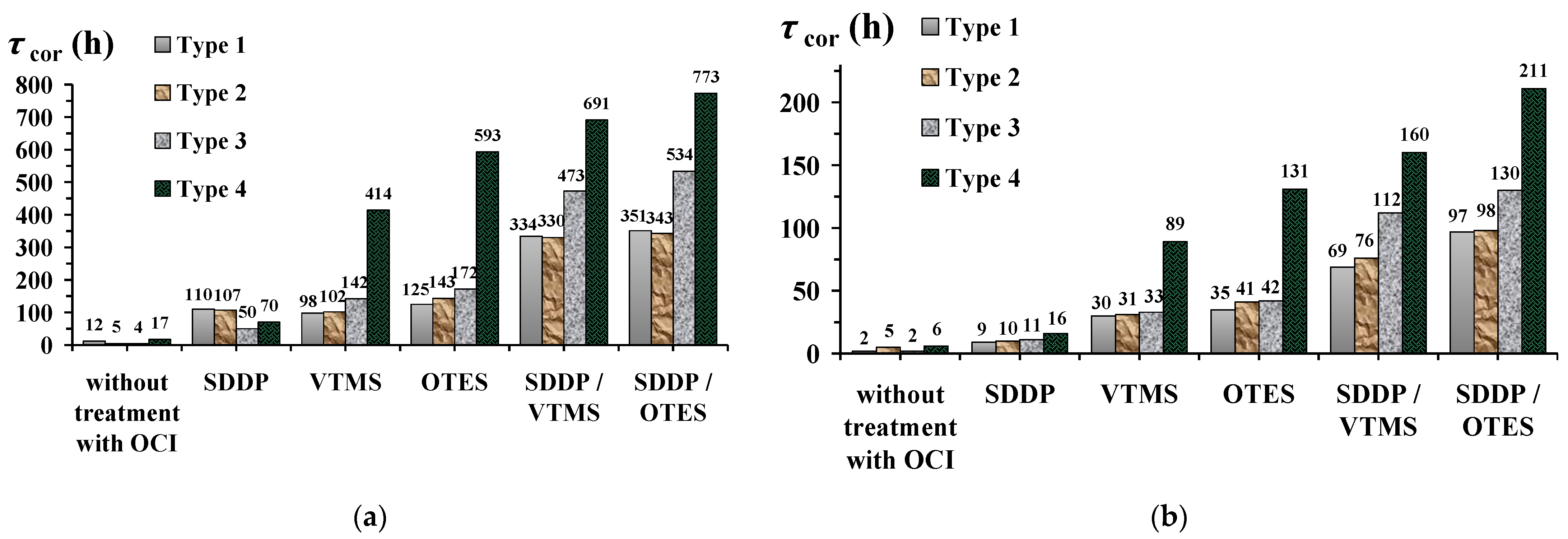
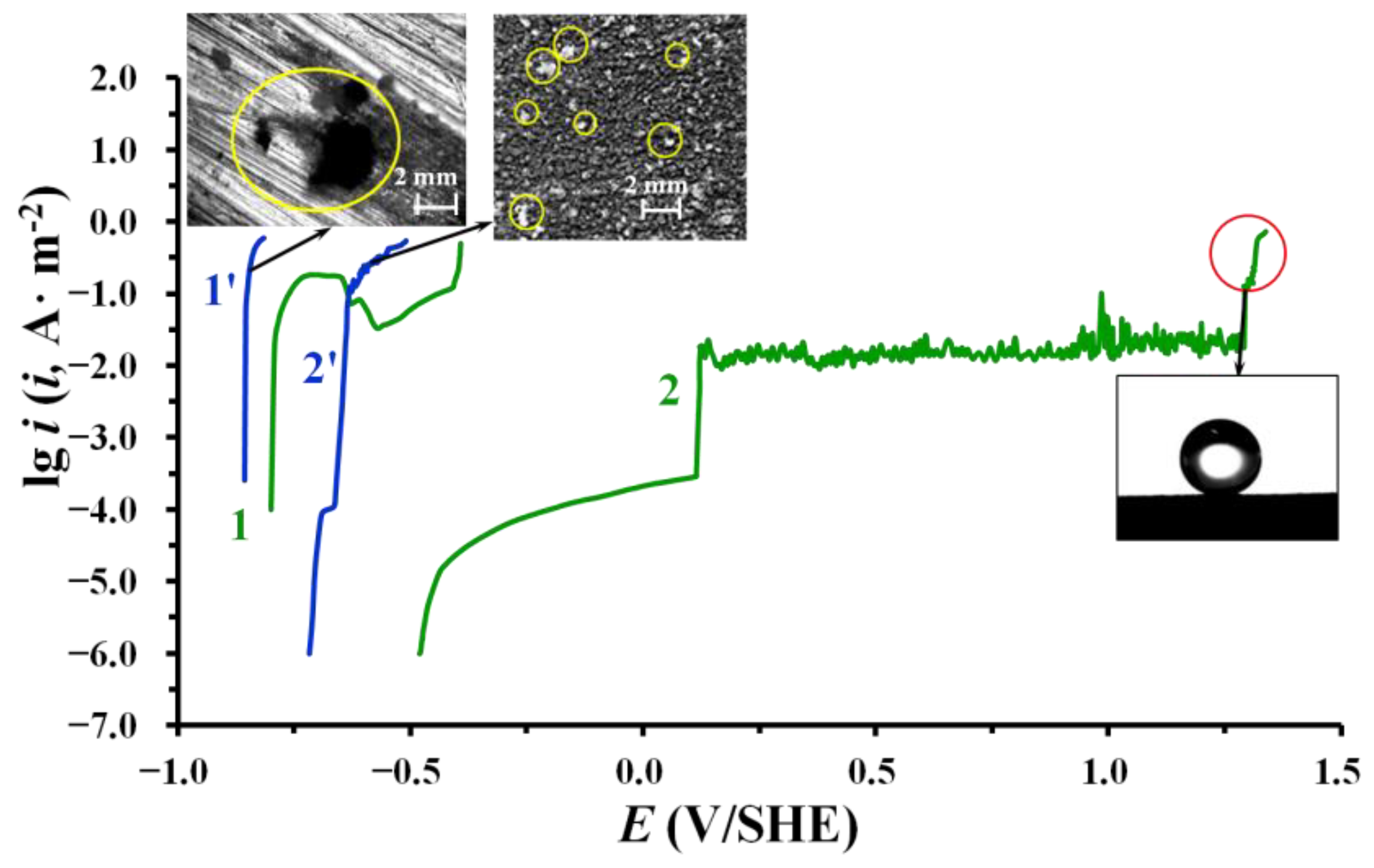
3.2. Hydrophobic and Protective Properties of Phosphonate–Siloxane Films on Zinc
3.3. XPS Studies of the Chemical Composition of Phosphonate-Siloxane Films on Zinc
4. Conclusions
- Chemical etching or laser treatment at different scanning speeds allowed one obtain zinc surfaces that differ significantly in morphology, roughness, and composition of the surface oxide layer.
- Differences in the wettability of the zinc surface with phosphonate–siloxane films depend in combination on its morphology and hydrophobicity of the OCIs used. All studied methods of surface texturing enhance the hydrophobic properties of thin phosphonate–siloxane films. However, superhydrophobic films are obtained either by layer-by-layer passivation of SDDP with a more hydrophobic silane (OTES) or on the surface with fractal morphology obtained by laser treatment at the low scanning speed.
- Hydrophobization or even superhydrophobization of zinc with phosphonate–siloxane films does not always ensure its high corrosion resistance in corrosive atmospheres. Phosphonate–siloxane films obtained on textured zinc surfaces by etching and laser at the high scanning speed are characterized by similar wetting characteristics, despite a significant difference in protective properties.
- Preliminary laser treatment of the zinc surface is more effective than chemical etching to enhance the anticorrosive properties of the resulting thin films.
- According to XPS studies, the mechanism of adsorption of SDDP and OTES on zinc surfaces with different morphology is the same. The thin phosphonate–siloxane film obtained by layer-by-layer technique consists of layers of chemisorbed zinc phosphonate, siloxane, ZnO and Zn(OH)2. The fraction of phosphonate and siloxane components in the protective film increases with roughness of the zinc surface. The thickest and least defective phosphonate–siloxane film, strongly bonded to the surface, is formed on the surface with fractal morphology enriched with ZnO. The combination of the barrier properties of a polysiloxane matrix with a strong chemical bond of phosphonic groups with the zinc surface prevents desorption of OCIs and provides high protective properties of this film during long-term corrosion tests in corrosive atmospheres of high humidity and salt spray.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Symbols | |||
| logP | logarithm of the distribution coefficient of the substance in the water–octanol system (for neutral molecules) | τcor | time to the appearance of the first corrosion damage |
| V | laser scanning speed | time of appearance of the first corrosion damage on the zinc surface without passivation | |
| γ | coefficient of corrosion retardation | time of appearance of the first corrosion damage on the zinc surface after passivation with OCIs | |
| Z | degree of protection | Θc | water contact angle |
| Rz | height of profile irregularities at ten points | Ecor | corrosion potential |
| Ra | the mean arithmetic deviation of the profile | pitting potential on samples without treatment with OCIs | |
| S | the average step of the local projections of the profile within the base length | pitting potential on samples with preliminary passivation with OCIs | |
| Abbreviations | |||
| OCI | organic corrosion inhibitor | SAM | self-assembled monolayer |
| TAS | trialkoxysilane | CnPA | alkylphosphonic acid |
| VTMS | vinyltrimethoxysilane | SDDP | sodium dodecylphosphonate |
| OTES | n-octyltriethoxysilane | ||
References
- Kuznetsov, Y.I.; Redkina, G.V. Thin protective coatings on metals formed by organic corrosion inhibitors in neutral media. Coatings 2022, 12, 149. [Google Scholar] [CrossRef]
- Kuznetsov, Y.I. Organic corrosion inhibitors: Where are we now? A review. Part IV. Passivation and the role of mono—And diphosphonates. Int. J. Corros. Scale Inhib. 2017, 6, 384–427. [Google Scholar] [CrossRef]
- Lisichkin, G.V.; Fadeev, A.Y.; Serdan, A.A.; Nesterenko, P.N.; Mingalev, P.G.; Furman, D.B. The Chemistry of Grafted Surface Compounds; Fizmatlit: Moscow, Russia, 2003. [Google Scholar]
- Aramaki, K.; Shimura, T. Self-assembled monolayers of carboxylate ions on passivated iron for preventing passive film breakdown. Corros. Sci. 2004, 46, 313–328. [Google Scholar] [CrossRef]
- Kuznetsov, Y.I. Organic corrosion inhibitors: Where are we now? A review. Part II. Passivation and the role of chemical structure of carboxylates. Int. J. Corros. Scale Inhib. 2016, 5, 282–318. [Google Scholar] [CrossRef]
- Ferreira, M.G.S.; Duarte, R.G.; Montemor, M.F.; Simoes, A.M.P. Silanes and rare earth salts as chromate replacers for pre-treatments on galvanized steel. Electrochim. Acta 2004, 49, 2927–2935. [Google Scholar] [CrossRef]
- Hansal, W.E.G.; Hansal, S.; Pflzler, M.; Kornherr, A.; Zifferer, G.; Nauer, G.E. Investigation of polysiloxane coatings as corrosion inhibitors of zinc surfaces. Surf. Coat. Tehnol. 2006, 200, 3056–3063. [Google Scholar] [CrossRef]
- Mingalyov, P.G.; Lisichkin, G.V. Chemical modification of oxide surfaces with organophosphorus (V) acids and their esters. Russ. Chem. Rev. 2006, 75, 541–557. [Google Scholar] [CrossRef]
- Hotchkiss, P.J.; Malicki, M.; Giordano, A.J.; Armstrong, N.R.; Marder, S.R. Characterization of phosphonic acid binding to zinc oxide. J. Mater. Chem. 2011, 21, 3107–3112. [Google Scholar] [CrossRef]
- Hoque, E.; DeRose, J.A.; Hoffmann, P.; Bhushan, B.; Mathieu, H.J. Alkylperfluorosilane self-assembled monolayers on aluminum: A comparison with alkylphosphonate self-assembled monolayers. J. Phys. Chem. 2007, 111, 3956–3962. [Google Scholar] [CrossRef]
- Felhősi, I.; Telegdi, J.; Pálinkas, G.; Kálmán, E. Kinetics of self-assembled layer formation on iron. Electrochim. Acta 2002, 47, 2335–2340. [Google Scholar] [CrossRef]
- Kuznetsov, Y.I.; Chirkunov, A.A.; Gorbachev, A.S.; Andreeva, N.P. Passivation of mild steel by sodium octylphosphonate in neutral aqueous solution. Int. J. Corros. Scale Inhib. 2017, 6, 318–332. [Google Scholar] [CrossRef]
- Milošev, I.; Metikoš-Huković, M.; Petrović, Ž. Influence of preparation methods on the properties of self-assembled films of octadecylphosphonate on Nitinol: XPS and EIS studies. Mater. Sci. Eng. C 2012, 32, 2604–2616. [Google Scholar] [CrossRef]
- Attavar, S.; Diwekar, M.; Linford, M.R.; Davis, M.A.; Blair, S. Passivation of aluminum with alkyl phosphonic acids for biochip applications. Appl. Surf. Sci. 2010, 256, 7146–7150. [Google Scholar] [CrossRef]
- Quiñones, R.; Raman, A.; Luliucci, R.J. Investigation of phosphonic acid surface modifications on zinc oxide nanoparticles under ambient conditions. Thin Solid Films 2014, 565, 155–164. [Google Scholar] [CrossRef]
- Perkins, C.L. Molecular anchors for self-assembled monolayers on ZnO: A direct comparison of the thiol and phosphonic acid moieties. J. Phys. Chem. C 2009, 113, 18276–18286. [Google Scholar] [CrossRef]
- Pilbáth, A.; Nyikos, L.; Bertóti, I.; Kálmán, E. Zinc corrosion protection with 1,5-diphosphono-pentane. Corros. Sci. 2008, 50, 3314–3321. [Google Scholar] [CrossRef]
- Pilbáth, A.; Felhősi, I.; Tolnai, G.; Kálmán, E. Application of self-assembly for replacing chromate in corrosion protection of zinc. J. Solid State Electrochem. 2006, 10, 721–729. [Google Scholar] [CrossRef]
- Sinapi, F.; Forget, L.; Delhalle, J.; Mekhalif, Z. Formation and characterization of thin films of H(CH2)xPO(OH)2 on polycrystalline zinc substrates. Surf. Interface Anal. 2002, 34, 148–154. [Google Scholar] [CrossRef]
- GN 2.1.5.1315-03; Maximum permissible concentrations (MPC) of chemicals in water of water objects of drinking and cultural-domestic water use. Publishing House of Standards: Moscow, Russia, 2003. (In Russian)
- RoHS 2002/95/EC Restriction of Hazardous Substances Directive, Official Journal of the European Union, L 37/19, 13.2.2003. Available online: http://data.europa.eu/eli/dir/2002/95/2011-09-10 (accessed on 31 July 2022).
- Kuznetsov, Y.I.; Red’kina, G.V.; Andreeva, N.P. Adsorption from neutral solutions of sodium alkyl phosphonates on zinc and its passivation. Russ. J. Phys. Chem. A 2018, 92, 2548–2555. [Google Scholar] [CrossRef]
- Redkina, G.V.; Kuznetsov, Y.I.; Andreeva, N.P.; Arkhipushkin, I.A.; Kazansky, L.P. Features of zinc passivation by sodium dodecylphosphonate in a neutral aqueous solution. Corros. Sci. 2020, 168, 108554. [Google Scholar] [CrossRef]
- Yuan, W.; van Ooij, W.J. Characterization of organofunctional silane films on zinc substrates. J. Colloid Interface Sci. 1997, 185, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Montemor, M.F.; Simões, A.M.; Ferreira, M.G.S.; Williams, B.; Edwards, H. The corrosion performance of organosilane based pre-treatments for coatings on galvanized steel. Prog. Org. Coat. 2000, 38, 17–26. [Google Scholar] [CrossRef]
- Petrunin, M.A.; Gladkikh, N.A.; Maleeva, M.A.; Maksaeva, L.B.; Yurasova, T.A. The use of organosilanes to inhibit metal corrosion. A review. Int. J. Corros. Scale Inhib. 2019, 8, 882–907. [Google Scholar] [CrossRef]
- Petrunin, M.A.; Maksaeva, L.B.; Gladkikh, N.A.; Narkevich, E.N.; Yurasova, T.A.; Rybkin, A.A.; Terekhova, E.V.; Kotenev, V.A.; Kablov, E.N.; Tsivadze, A.Y. The effect of vinyl-siloxane nanolayers on the corrosion behavior of zinc. Prot. Met. Phys. Chem. Surf. 2018, 5, 795–803. [Google Scholar] [CrossRef]
- Aramaki, K. Protection of zinc from corrosion by coverage with a hydrated cerium (III) oxide layer and ultrathin polymer films of a carboxylate self-assembled monolayer modified with alkyltriethoxysilanes. Corros. Sci. 2007, 49, 1963–1980. [Google Scholar] [CrossRef]
- Gladkikh, N.; Makarychev, Y.; Chirkunov, A.; Shapagin, A.; Petrunin, M.; Maksaeva, L.; Maleeva, M.; Yurasova, T.; Marshakov, A. Formation of polymer-like anticorrosive films based on organosilanes with benzotriazole, carboxylic and phosphonic acids. Protection of copper and steel against atmospheric corrosion. Prog. Org. Coat. 2020, 141, 105544. [Google Scholar] [CrossRef]
- Khramov, A.N.; Balbyshev, V.N.; Kasten, L.S.; Mantz, R.A. Sol-gel coatings with phosphonate functionalities for surface modification of magnesium alloys. Thin Solid Films 2006, 514, 174–181. [Google Scholar] [CrossRef]
- Dalmoro, V.; dos Santos, J.H.Z.; Azambuja, D.S. Corrosion behavior of AA2024-T3 alloy treated with phosphonate-containing TEOS. J. Solid State Electrochem. 2012, 16, 403–414. [Google Scholar] [CrossRef]
- Dalmoro, V.; dos Santos, J.H.Z.; Armelin, E.; Alemán, C.; Azambuja, D.S. Phosphonic acid/silica-based films: A potential treatment for corrosion protection. Corros. Sci. 2012, 60, 173–180. [Google Scholar] [CrossRef]
- Sheffer, M.; Groysman, A.; Starosvetsky, D.; Savchenko, N.; Mandler, D. Anion embedded sol-gel films on Al for corrosion protection. Corros. Sci. 2004, 46, 2975–2985. [Google Scholar] [CrossRef]
- Red’kina, G.V.; Sergienko, A.S.; Kuznetsov, Y.I. Two-stage passivation of zinc by solutions of sodium dodecylphosphonate and trialkoxysilanes. Prot. Met. Phys. Chem. Surf. 2021, 57, 1352–1360. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Emelyanenko, A.M. Hydrophobic materials and coatings: Principles of design, properties and applications. Russ. Chem. Rev. 2008, 77, 583–600. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, L.; Qian, H.; Li, X. Superhydrophobic surfaces for corrosion protection: A review of recent progresses and future directions. J. Coat. Technol. Res. 2016, 13, 11–29. [Google Scholar] [CrossRef] [Green Version]
- Celia, E.; Darmanin, T.; de Givenchy, E.T.; Amigoni, S.; Guittard, F. Recent advances in designing superhydrophobic surfaces. J. Colloid Interface Sci. 2013, 402, 1–18. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Lei, J.; He, J.; Lv, R.; Li, N.; Pan, F. A facile approach to fabricate superhydrophobic Zn surface and its effect on corrosion resistance. Corros. Sci. 2014, 85, 174–182. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, D.; Qiu, R.; Hou, B. Super-hydrophobic film prepared on zinc as corrosion barrier. Corros. Sci. 2011, 53, 2080–2086. [Google Scholar] [CrossRef]
- Qian, B.; Shen, Z. Fabrication of superhydrophobic surfaces by dislocation-selective chemical etching on aluminum, copper, and zinc substrates. Langmuir 2005, 21, 9007–9009. [Google Scholar] [CrossRef]
- Polyakov, N.A.; Botryakova, I.G.; Glukhov, V.G.; Red’kina, G.V.; Kuznetsov, Y.I. Formation and anticorrosion properties of superhydrophobic zinc coatings on steel. Chem. Eng. J. 2021, 421, 127775. [Google Scholar] [CrossRef]
- Zavestovskaya, I.N. Laser nanostructuring of materials surface. Quantum Electron. 2010, 40, 942–954. [Google Scholar] [CrossRef]
- Ma, L.; Wang, L.; Li, C.; Guo, J.; Shrotriya, P.; Deng, C.; Zhao, J. Hybrid nanosecond laser processing and heat treatment for rapid preparation of super-hydrophobic copper surface. Metals 2019, 9, 668. [Google Scholar] [CrossRef] [Green Version]
- Wei, D.; Wang, J.; Wang, H.; Liu, Y.; Li, S.; Li, D. Anti-corrosion behavior of superwetting structured surfaces on Mg-9Al-1Zn magnesium alloy. Appl. Surf. Sci. 2019, 483, 1017–1026. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Emelyanenko, A.M.; Modestov, A.D.; Domantovsky, A.G.; Emelyanenko, K.A. Synergistic effect of superhydrophobicity and oxidized layers on corrosion resistance of aluminum alloy surface textured by nanosecond laser treatment. ACS Appl. Mat. Interfaces 2015, 7, 19500–19508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redkina, G.V.; Sergienko, A.S.; Kuznetsov, Y.I. Hydrophobic and anticorrosion properties of thin phosphonate-siloxane films formed on a laser textured zinc surface. Int. J. Corros. Scale Inhib. 2020, 9, 1550–1563. [Google Scholar] [CrossRef]
- Sergienko, A.S.; Redkina, G.V.; Rozhkov, A.S.; Kuznetsov, Y.I. Corrosion inhibition of galvanized steel by thin superhydrophobic phosphonate-siloxane films. Int. J. Corros. Scale Inhib. 2022, 11, 322–338. [Google Scholar] [CrossRef]
- ISO 1302:2002; Geometrical Product Specifications (GPS)—Indication of Surface Texture in Technical Product Documentation. ISO: Geneva, Switzerland, 2002.
- Shirley, D.A. High-resolution X-ray photoemission spectrum of the valence bands of gold. Phys. Rev. 1972, 5, 4709–4713. [Google Scholar] [CrossRef] [Green Version]
- Mohai, M. XPS MultiQuant: Multimodel XPS quantification software. Surf. Interface Anal. 2004, 36, 828–832. [Google Scholar] [CrossRef]
- Scofield, J.H. Hartree-Slater subshell photoionization cross-sections at 1254 and 1487 eV. J. Electron. Spectrosc. Relat. Phenom. 1976, 8, 129–137. [Google Scholar] [CrossRef]
- ISO 4536:1985; Metallic and Non-Organigs on Metallic Substrates—Saline Droplets Corrosion Test. ISO: Geneva, Switzerland, 1985.
- ISO 9227:2017; Corrosion Tests in Artificial Atmospheres—Salt Spray Tests. ISO: Geneva, Switzerland, 2017.
- Yang, Y.; Yang, J.; Liang, C.; Wang, H. Ultra-broadband enhanced absorption of metal surfaces structured by femtosecond laser pulses. Opt. Express 2008, 16, 1125–11265. [Google Scholar] [CrossRef]
- Vorobyev, A.Y.; Guo, C. Multifunctional surfaces produced by femtosecond laser pulses. J. Appl. Phys. 2015, 117, 033103. [Google Scholar] [CrossRef] [Green Version]
- Hansch, C.; Leo, A. Substituent Constants for Correlation Analysis in Chemistry and Biology; Wiley: New York, NY, USA, 1979; p. 339. [Google Scholar]
- Onda, T.; Shibuichi, S.; Satoh, N.; Tsujii, K. Super-Water-Repellent Fractal Surfaces. Langmuir 1996, 12, 2125–2127. [Google Scholar] [CrossRef]
- Deroubaix, G.; Marcus, P. X-ray photoelectron spectroscopy analysis of copper and zinc oxides and sulfides. Surf. Interface Anal. 1992, 18, 39–46. [Google Scholar] [CrossRef]
- Dake, L.S.; Baer, D.R.; Zachara, J.M. Auger parameter measurements of zinc compounds relevant to zinc transport in the environment. Surf. Interface Anal. 1989, 14, 71–75. [Google Scholar] [CrossRef]
- Castle, J.E.; Epler, D. Chemical shifts in photo-exited Auger spectra. Proc. R. Soc. Lond. A 1974, 339, 49–72. [Google Scholar] [CrossRef]
- Palanivel, V.; Zhu, D.; van Ooij, W.J. Nanoparticle-filled silane films as chromate replacements for aluminum alloys. Prog. Org. Coat. 2003, 47, 384–392. [Google Scholar] [CrossRef]
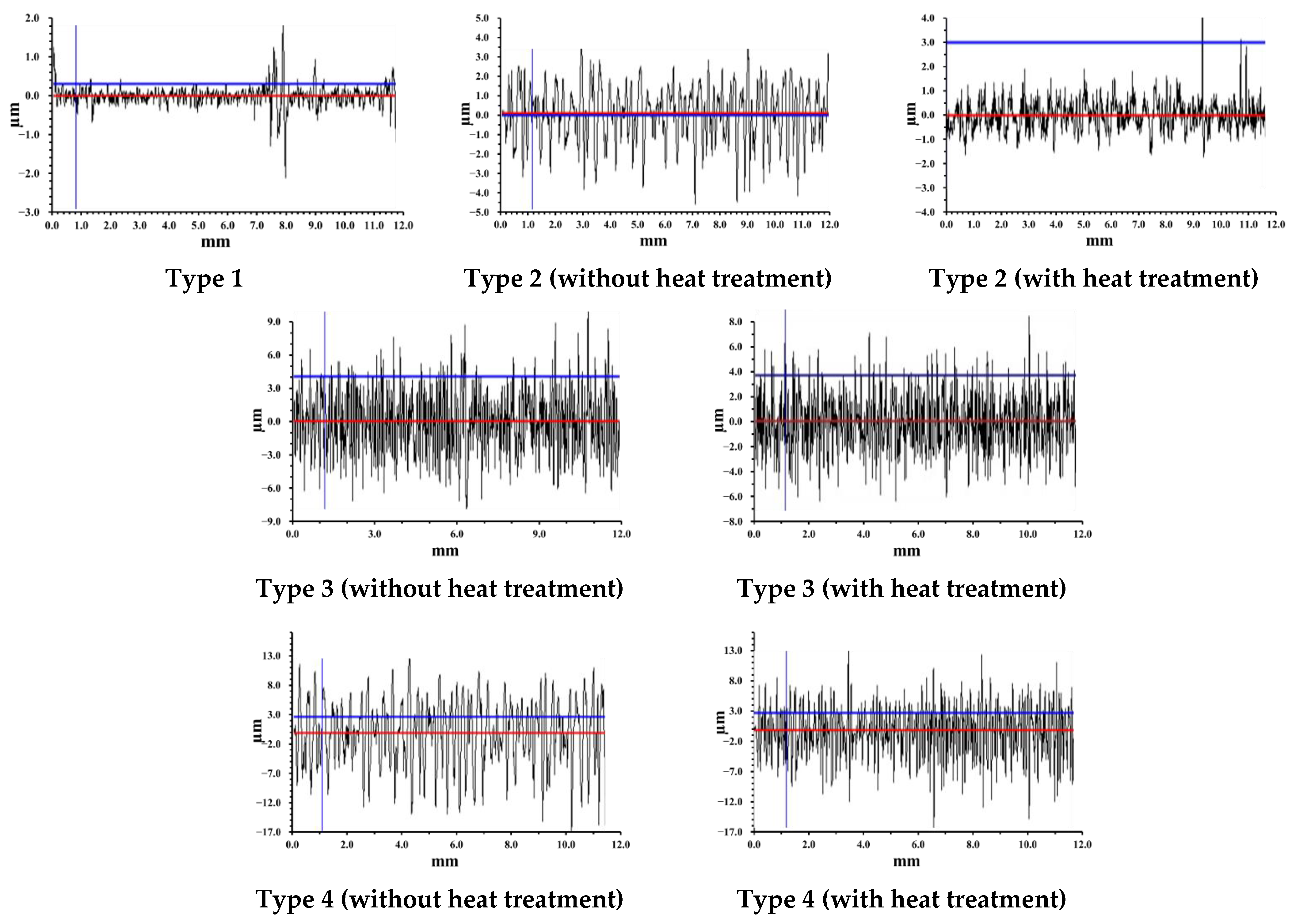
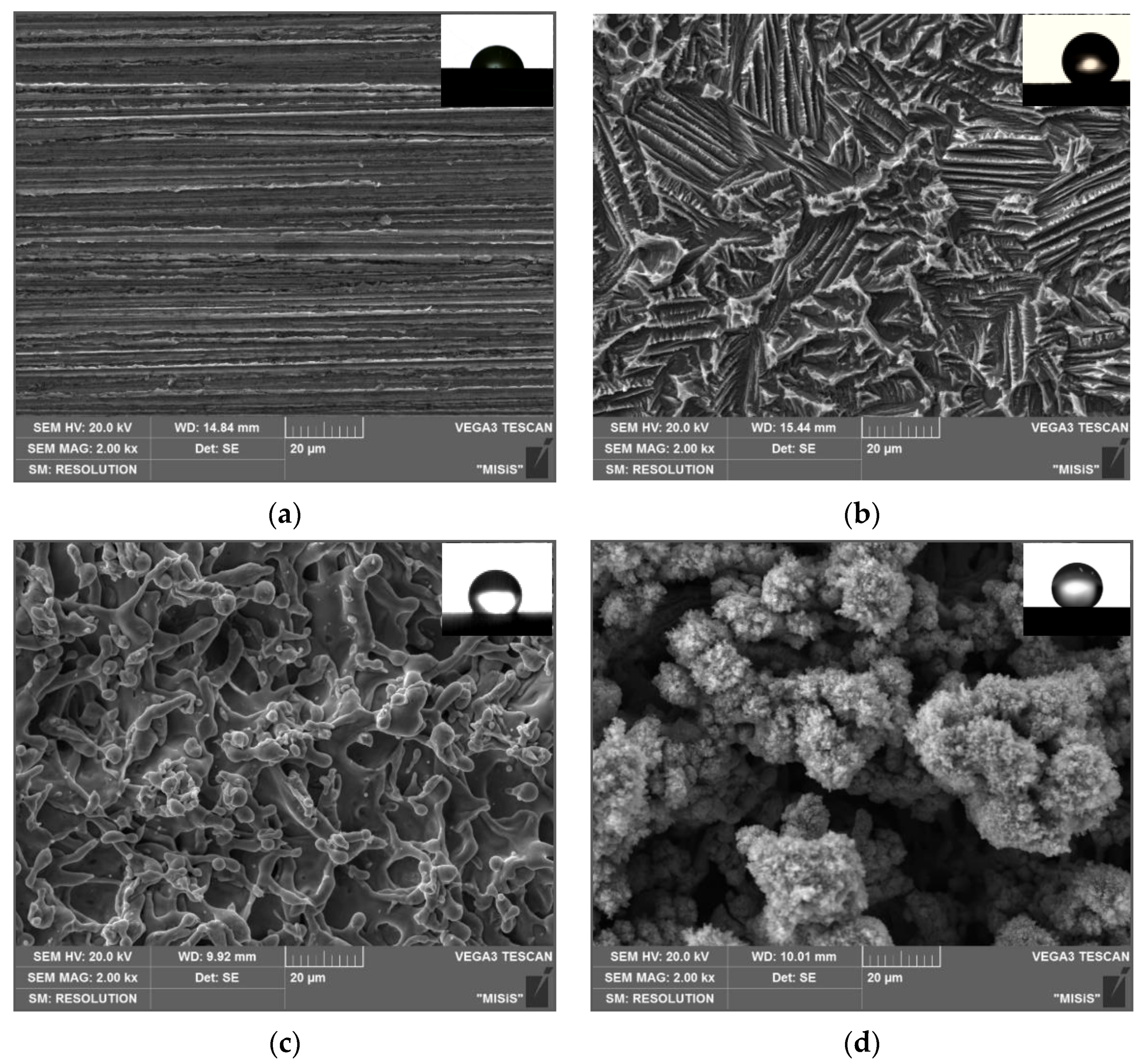

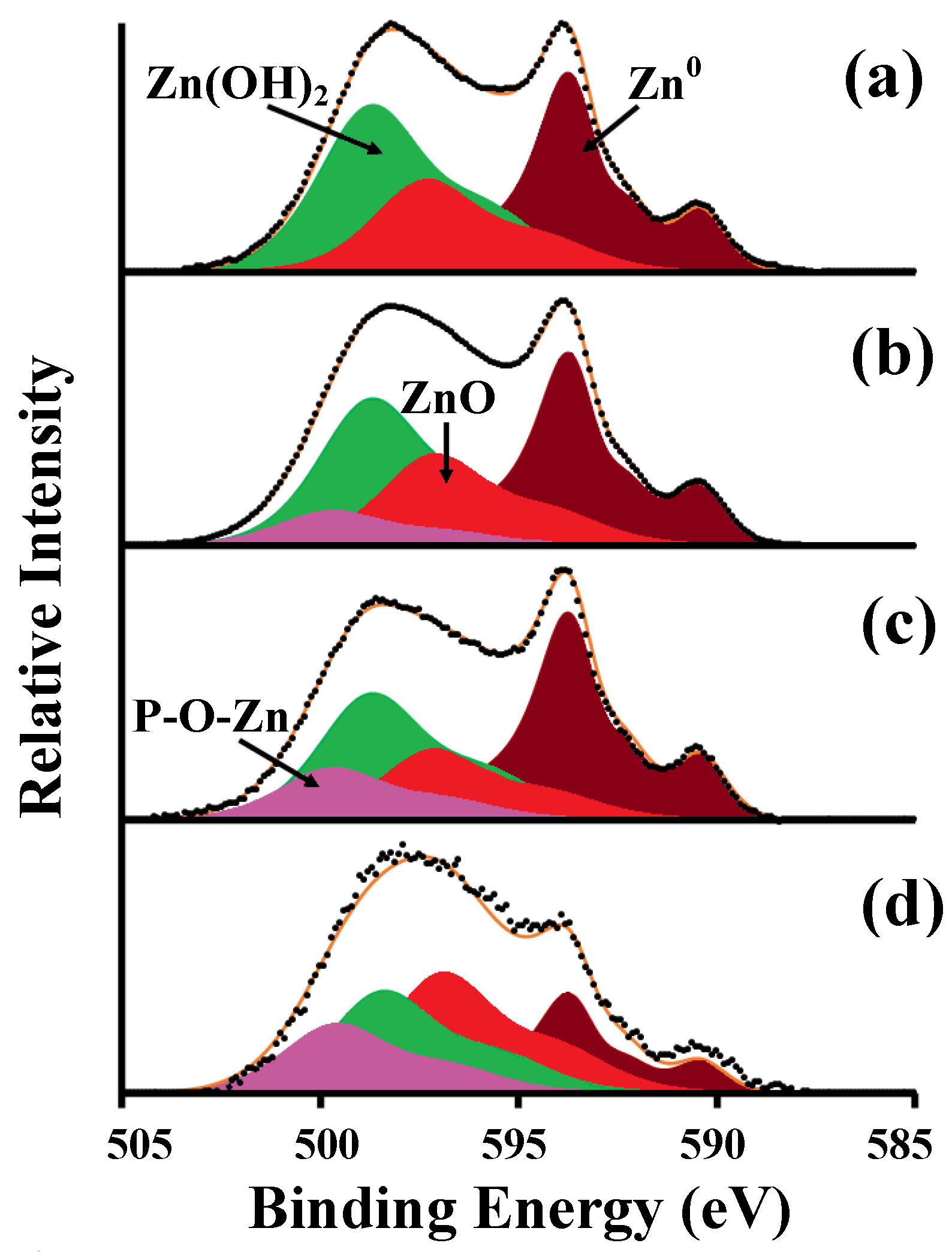
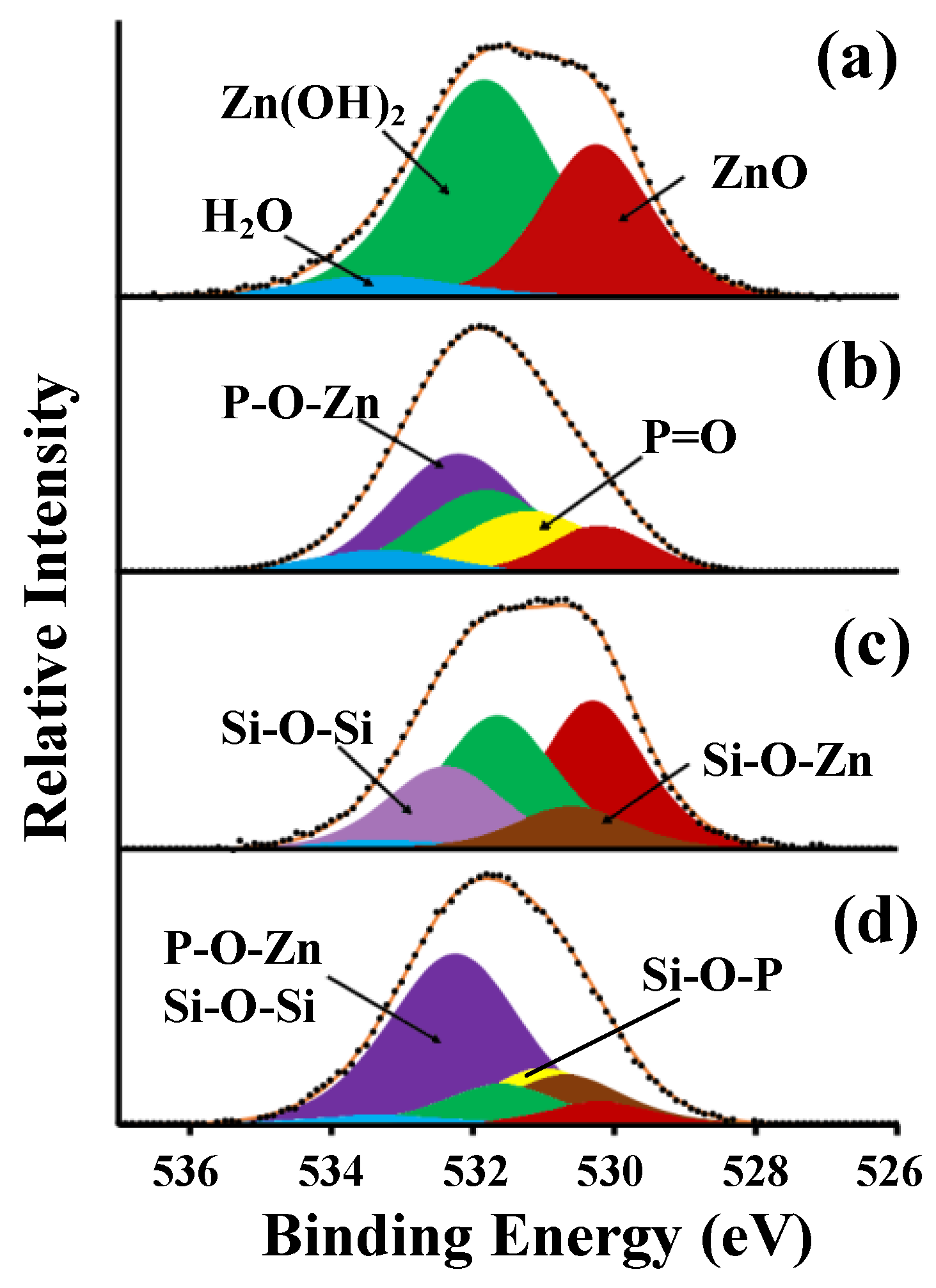

| Surface Types | Rz, µm | Ra, µm | S, µm | Roughness Class | |
|---|---|---|---|---|---|
| Type 1 | 1.70 | 0.09 | 4.26 | 3 | |
| Type 2 | without heat treatment | 9.66 | 0.30 | 23.60 | 6 |
| with heat treatment | 7.67 | 0.21 | 21.32 | 6 | |
| Type 3 | without heat treatment | 16.40 | 1.58 | 7.98 | 7 |
| with heat treatment | 15.85 | 1.38 | 5.99 | 7 | |
| Type 4 | without heat treatment | 33.45 | 5.80 | 9.10 | 9 |
| with heat treatment | 32.32 | 4.43 | 7.69 | 9 | |
| Surface Types | Element, wt.% | ||
|---|---|---|---|
| O | Zn | ||
| Type 1 | 1.09 | 98.91 | |
| Type 2 | 1.06 | 98.94 | |
| Type 3 | without heat treatment | 2.06 | 97.94 |
| with heat treatment | 1.08 | 98.92 | |
| Type 4 | without heat treatment | 11.14 | 88.86 |
| with heat treatment | 10.49 | 89.51 | |
| Composition of the Inhibiting Solution, mM | Θc, ° | |||
|---|---|---|---|---|
| Surface Types | ||||
| Type 1 | Type 2 | Type 3 | Type 4 | |
| without treatment with OCI | 72 ± 3 | 125 ± 1 | 120 ± 3 | 155 ± 1 |
| 2.5 SDDP | 95 ± 2 | 129 ± 2 | 134 ± 2 | 138 ± 3 |
| 10.0 VTMS | 91 ± 3 | 134 ± 2 | 135 ± 3 | 140 ± 3 |
| 10.0 OTES | 89 ± 2 | 149 ± 1 | 148 ± 2 | 159 ± 2 |
| 2.5 SDDP///10.0 VTMS | 102 ± 3 | 145 ± 2 | 142 ± 2 | 160 ± 2 |
| 2.5 SDDP///10.0 OTES | 124 ± 2 | 155 ± 2 | 159 ± 1 | 165 ± 3 |
| Composition of the Inhibiting Solution, mM | γ///Z, % | |||
|---|---|---|---|---|
| Surface Types | ||||
| Type 1 | Type 2 | Type 3 | Type 4 | |
| In a heat and moisture chamber | ||||
| Without treatment with OCI | – | – | – | – |
| 2.5 SDDP | 9.2///89.1 | 8.9///88.8 | 4.2///76.0 | 5.8///82.9 |
| 10.0 VTMS | 8.2///87.8 | 8.5///88.2 | 11.8///91.5 | 34.5///97.1 |
| 10.0 OTES | 10.4///90.4 | 11.9///91.6 | 14.3///93.0 | 49.4///98.0 |
| 2.5 SDDP///10.0 VTMS | 27.8///96.4 | 27.5///96.4 | 39.4///97.5 | 57.6///98.3 |
| 2.5 SDDP///10.0 OTES | 29.3///96.6 | 28.5///96.5 | 44.5///97.8 | 64.4///98.4 |
| In a salt spray chamber | ||||
| Without treatment with OCI | – | – | – | – |
| 2.5 SDDP | 4.5///77.8 | 5.0///80.0 | 5.5///81.8 | 8.0///87.5 |
| 10.0 VTMS | 15.0///93.3 | 15.5///93.5 | 16.5///93.9 | 44.5///97.8 |
| 10.0 OTES | 17.5///94.3 | 20.5///95.1 | 21.0///95.2 | 65.5///98.5 |
| 2.5 SDDP///10.0 VTMS | 34.5///97.1 | 38.0///97.4 | 56.0///98.2 | 80.0///98.8 |
| 2.5 SDDP///10.0 OTES | 48.5///97.9 | 49.0///98.0 | 65.0///98.5 | 105.5///99.1 |
| Surface Types | Element Concentrations (%) | ||
|---|---|---|---|
| O1s | Zn2p | ||
| ZnO | Zn(OH)2 | ||
| Type 1 | 24.00 | 39.50 | 36.50 |
| Type 2 | 16.50 | 42.70 | 40.80 |
| Type 3 | 24.50 | 37.80 | 37.70 |
| Type 4 | 33.30 | 24.00 | 42.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Redkina, G.V.; Sergienko, A.S.; Kuznetsov, Y.I.; Grafov, O.Y. Superhydrophobic Anticorrosive Phosphonate–Siloxane Films Formed on Zinc with Different Surface Morphology. Materials 2022, 15, 5360. https://doi.org/10.3390/ma15155360
Redkina GV, Sergienko AS, Kuznetsov YI, Grafov OY. Superhydrophobic Anticorrosive Phosphonate–Siloxane Films Formed on Zinc with Different Surface Morphology. Materials. 2022; 15(15):5360. https://doi.org/10.3390/ma15155360
Chicago/Turabian StyleRedkina, Galina V., Alexandra S. Sergienko, Yurii I. Kuznetsov, and Oleg Yu. Grafov. 2022. "Superhydrophobic Anticorrosive Phosphonate–Siloxane Films Formed on Zinc with Different Surface Morphology" Materials 15, no. 15: 5360. https://doi.org/10.3390/ma15155360
APA StyleRedkina, G. V., Sergienko, A. S., Kuznetsov, Y. I., & Grafov, O. Y. (2022). Superhydrophobic Anticorrosive Phosphonate–Siloxane Films Formed on Zinc with Different Surface Morphology. Materials, 15(15), 5360. https://doi.org/10.3390/ma15155360






