A Facile Synthesis and Characterization of Highly Crystalline Submicro-Sized BiFeO3
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Characterization
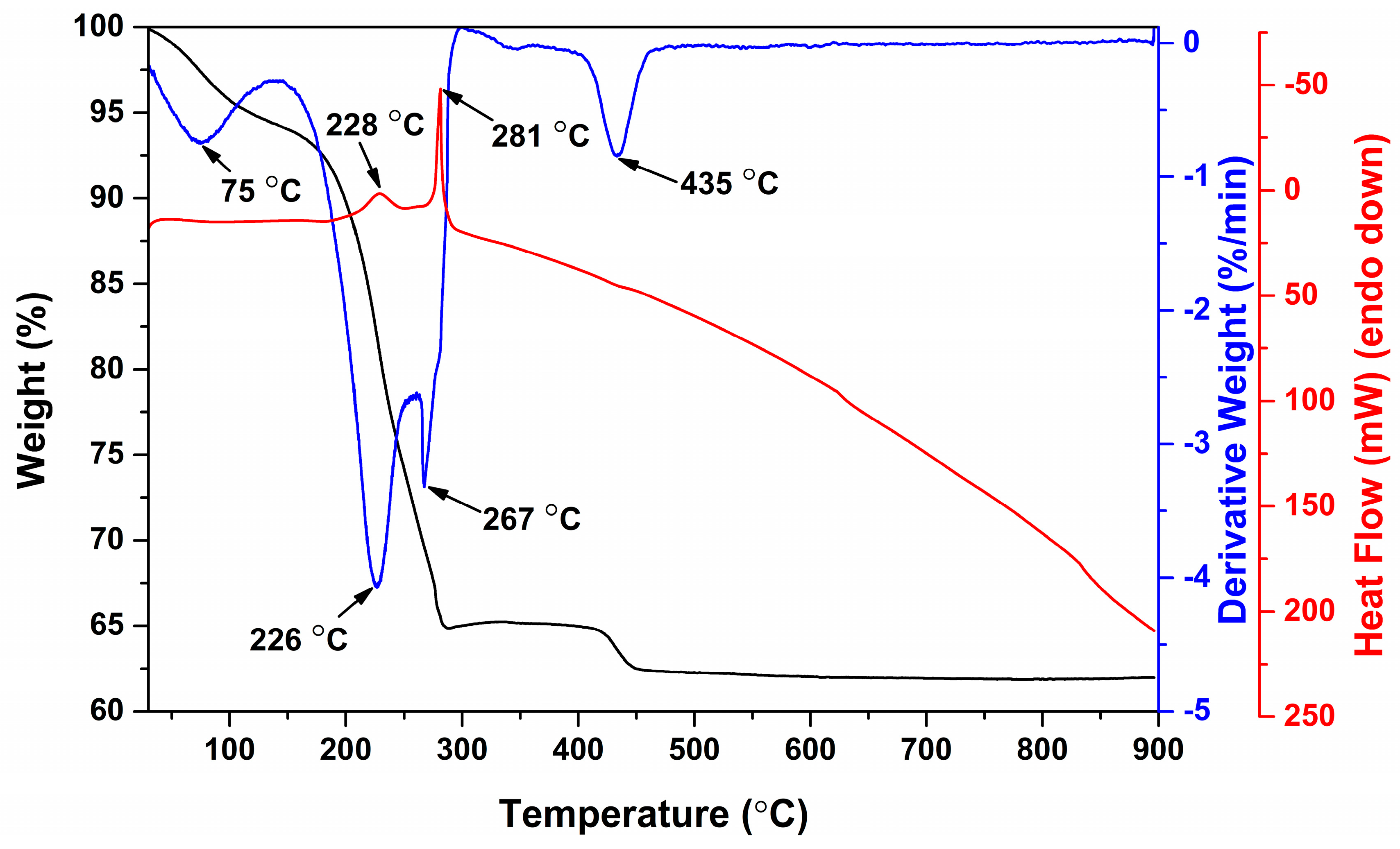
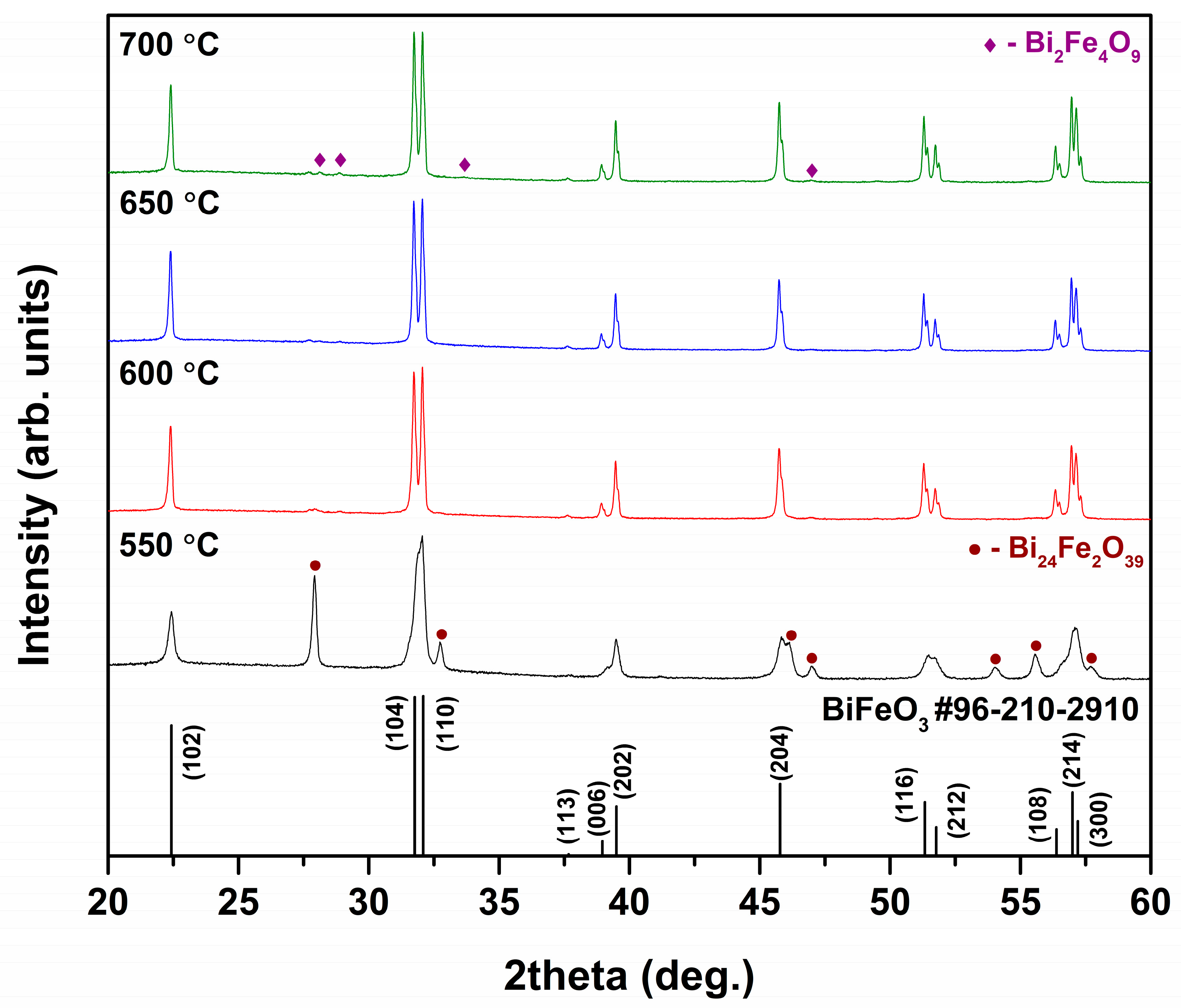
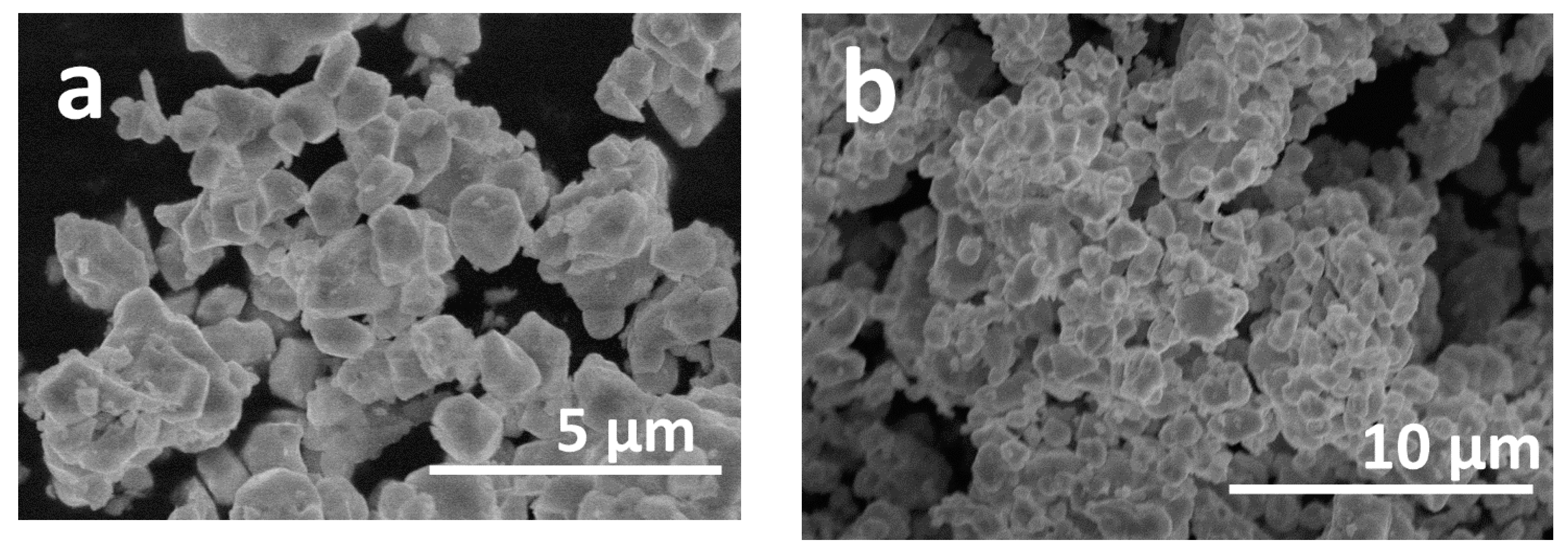
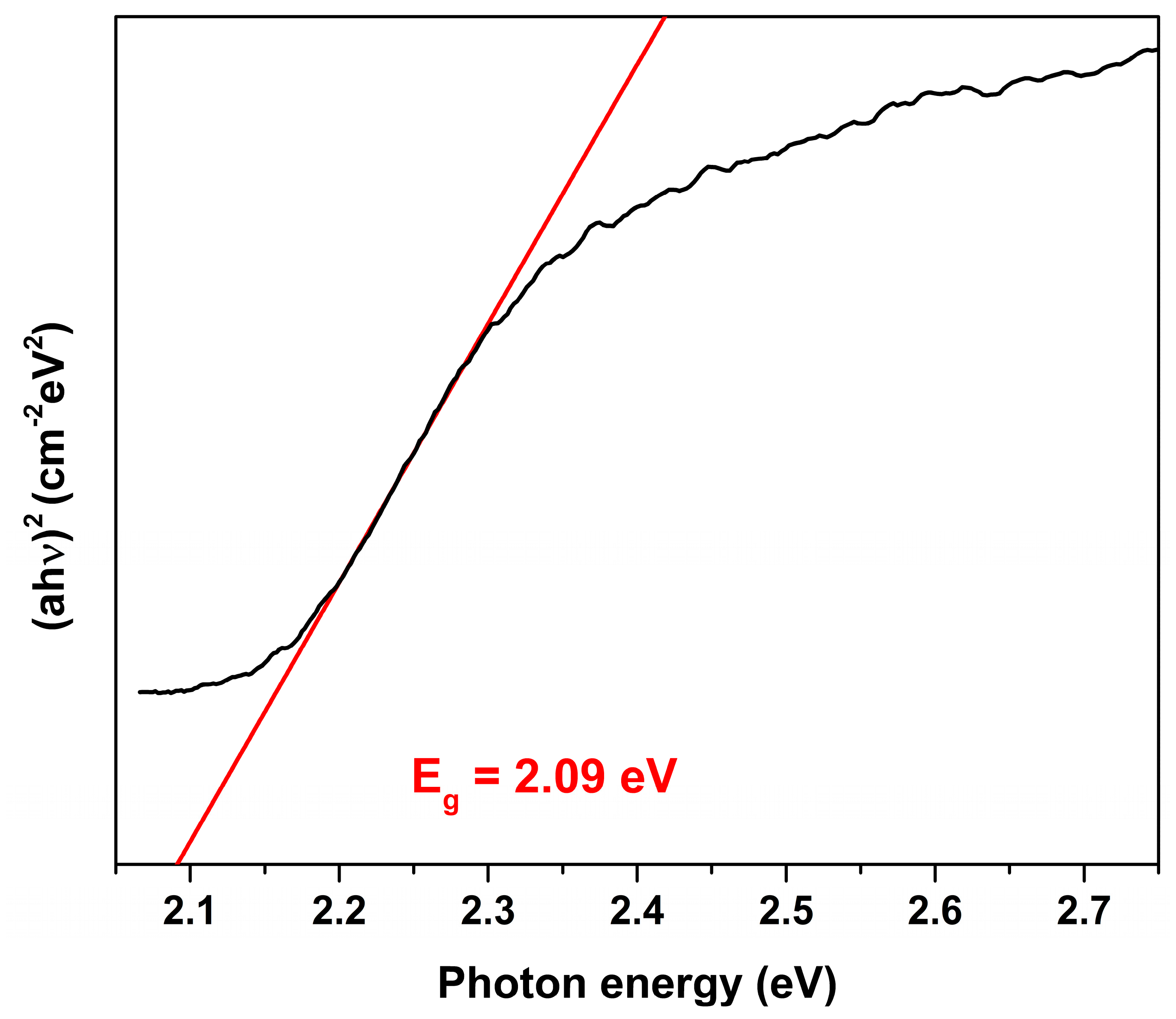
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, J.G.; Fan, Z.; Xiao, D.Q.; Zhu, J.G.; Wang, J. Multiferroic bismuth ferrite-based materials for multifunctional applications: Ceramic bulks, thin films and nanostructures. Prog. Mater. Sci. 2016, 84, 335–402. [Google Scholar] [CrossRef] [Green Version]
- Molak, A.; Mahato, D.K.; Szeremeta, A.Z. Synthesis and characterization of electrical features of bismuth manganite and bismuth ferrite: Effects of doping in cationic and anionic sublattice: Materials for applications. Prog. Cryst. Growth Ch. 2018, 64, 1–22. [Google Scholar] [CrossRef]
- Kubel, F.; Schmid, H. Structure of a ferroelectric and ferroelastic monodomain crystal of the perovskite BiFeO3. Acta Crystallogr. B 1990, 46, 698–702. [Google Scholar] [CrossRef] [Green Version]
- Catalan, G.; Scott, J.F. Physics and Applications of Bismuth Ferrite. Adv. Mater. 2009, 21, 2463–2485. [Google Scholar] [CrossRef]
- Lam, S.-M.; Sin, J.-C.; Mohamed, A.R. A newly emerging visible light-responsive BiFeO3 perovskite for photocatalytic applications: A mini review. Mater. Res. Bull. 2017, 90, 15–30. [Google Scholar] [CrossRef]
- Seyfouri, M.M.; Wang, D.Y. Recent progress in bismuth ferrite-based thin films as a promising photovoltaic material. Crit. Rev. Solid State Mat. Sci. 2020. [Google Scholar] [CrossRef]
- Zhou, J.; Jiang, L.; Chen, D.; Liang, J.; Qin, L.; Bai, L.; Sun, X.; Huang, Y. Facile synthesis of Er-doped BiFeO3 nanoparticles for enhanced visible light photocatalytic degradation of tetracycline hydrochloride. J. Sol-Gel Sci. Technol. 2019, 90, 535–546. [Google Scholar] [CrossRef]
- Haruna, A.; Abdulkadir, I.; Idris, S.O. Photocatalytic activity and doping effects of BiFeO3 nanoparticles in model organic dyes. Heliyon 2020, 6, e03237. [Google Scholar] [CrossRef] [Green Version]
- Rabbani, M.; Rahimi, R.; Farajnejad Ghadi, H. Photocatalytic application of BiFeO3 synthesized via a facile microwave-assisted solution combustion method. J. Sol-Gel Sci. Technol. 2018, 87, 340–346. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, S.; Kong, F.; Tong, J.; Ruan, L.; Duan, Q.; Zhou, J.; Zhang, X. α-Fe2O3/BiFeO3 composites as visible-active photocatalysts and their optical response mechanism. J. Phys. Chem. Solids 2020, 141, 109329. [Google Scholar] [CrossRef]
- Ali, S.; Humayun, M.; Pi, W.; Yuan, Y.; Wang, M.; Khan, A.; Yue, P.; Shu, L.; Zheng, Z.; Fu, Q.; et al. Fabrication of BiFeO3-g-C3N4-WO3 Z-scheme heterojunction as highly efficient visible-light photocatalyst for water reduction and 2,4-dichlorophenol degradation: Insight mechanism. J. Hazard. Mater. 2020, 397, 122708. [Google Scholar] [CrossRef]
- Selbach, S.M.; Einarsrud, M.-A.; Grande, T. On the Thermodynamic Stability of BiFeO3. Chem. Mater. 2009, 21, 169–173. [Google Scholar] [CrossRef]
- Bera, S.; Ghosh, S.; Shyamal, S.; Bhattacharya, C.; Basu, R.N. Photocatalytic hydrogen generation using gold decorated BiFeO3 heterostructures as an efficient catalyst under visible light irradiation. Sol. Energy Mater. Sol. Cells 2019, 194, 195–206. [Google Scholar] [CrossRef]
- Wang, X.; Yang, C.; Zhou, D.; Wang, Z.; Jin, M. Chemical co-precipitation synthesis and properties of pure-phase BiFeO3. Chem. Phys. Lett. 2018, 713, 185–188. [Google Scholar] [CrossRef]
- Asefi, N.; Masoudpanah, S.M.; Hasheminiasari, M. Microwave-assisted solution combustion synthesis of BiFeO3 powders. J. Sol-Gel Sci. Technol. 2018, 86, 751–759. [Google Scholar] [CrossRef]
- Volnistem, E.A.; Leonardo, J.M.P.; Silva, V.S.; Silva, D.M.; Dias, G.S.; Cótica, L.F.; Santos, I.A. Tuning the magnetic response of cryo-milled BiFeO3 nanoparticles by controlling crystallite sizes and internal strain. Powder Technology 2019, 347, 215–219. [Google Scholar] [CrossRef]
- Chermahini, M.D.; Safaee, I.; Kazazi, M.; Shahraki, M.M. Enhanced multiferroic properties of sono-synthesized BiFeO3 nanoceramics by co-doping of Sm and Mn elements. Ceram. Int. 2018, 44, 14281–14285. [Google Scholar] [CrossRef]
- Wu, H.; Zhu, X. Microstructures, magnetic, and dielectric properties of Ba-doped BiFeO3 nanoparticles synthesized via molten salt route. Journal of the American Ceramic Society 2019, 102, 4698–4709. [Google Scholar] [CrossRef]
- Gumiel, C.; Jardiel, T.; Bernardo, M.S.; Villanueva, P.G.; Urdiroz, U.; Cebollada, F.; Aragó, C.; Caballero, A.C.; Peiteado, M. Combination of structural and microstructural effects in the multiferroic response of Nd and Ti co-doped BiFeO3 bulk ceramics. Ceram. Int. 2019, 45, 5276–5283. [Google Scholar] [CrossRef]
- Yang, T.; Wei, J.; Lv, Z.; Guo, Y.; Xu, Z. Ion dopants tuning the interband electronic structure for huge saturated ferroelectric polarization in bismuth ferrite films. J. Sol-Gel Sci. Technol. 2018, 88, 618–627. [Google Scholar] [CrossRef]
- Queralto, A.; Frohnhoven, R.; Mathur, S.; Gomez, A. Intrinsic piezoelectric characterization of BiFeO3 nanofibers and its implications for energy harvesting. Appl. Surf. Sci. 2020, 509, 8. [Google Scholar] [CrossRef]
- Rani, B.J.; Ravi, G.; Yuvakkumar, R.; Thambidurai, M. Perovskite BiFeO3 nanocatalysts for electrochemical water oxidation. J. Sol-Gel Sci. Technol. 2019, 91, 247–254. [Google Scholar] [CrossRef]
- Yang, X.; Zeng, R.; Ren, Z.; Wu, Y.; Chen, X.; Li, M.; Chen, J.; Zhao, R.; Zhou, D.; Liao, Z.; et al. Single-Crystal BiFeO3 Nanoplates with Robust Antiferromagnetism. ACS Appl. Mater. Interfaces 2018, 10, 5785–5792. [Google Scholar] [CrossRef]
- Carranza-Celis, D.; Cardona-Rodríguez, A.; Narváez, J.; Moscoso-Londono, O.; Muraca, D.; Knobel, M.; Ornelas-Soto, N.; Reiber, A.; Ramírez, J.G. Control of Multiferroic properties in BiFeO3 nanoparticles. Sci. Rep. 2019, 9, 3182. [Google Scholar] [CrossRef]
- Rouhani, Z.; Karimi-Sabet, J.; Mehdipourghazi, M.; Hadi, A.; Dastbaz, A. Response surface optimization of hydrothermal synthesis of Bismuth ferrite nanoparticles under supercritical water conditions: Application for photocatalytic degradation of Tetracycline. Environ. Nanotechnol. Monit. Manag. 2019, 11, 100198. [Google Scholar] [CrossRef]
- Wang, T.; Song, S.H.; Xu, T.; Wang, M. Maltose-assisted sol–gel synthesis, structural, magnetic and optical properties of multiferroic BiFeO3 nanopowders. J. Sol-Gel Sci. Technol. 2016, 80, 675–682. [Google Scholar] [CrossRef]
- Wu, H.; Xue, P.; Lu, Y.; Zhu, X. Microstructural, optical and magnetic characterizations of BiFeO3 multiferroic nanoparticles synthesized via a sol-gel process. J. Alloys Compd. 2018, 731, 471–477. [Google Scholar] [CrossRef]
- Asefi, N.; Masoudpanah, S.M.; Hasheminiasari, M. Photocatalytic performances of BiFeO3 powders synthesized by solution combustion method: The role of mixed fuels. Mater. Chem. Phys. 2019, 228, 168–174. [Google Scholar] [CrossRef]
- Pujar, P.; Gupta, D.; Mandal, S. High-performance low voltage operation of indium zinc tin oxide thin film transistors using chemically derived sodium β-alumina dielectric. J. Mater. Sci. Mater. Electron. 2019, 30, 9097–9105. [Google Scholar] [CrossRef]
- Park, J.S.; Yoo, Y.J.; Hwang, J.S.; Kang, J.-H.; Lee, B.W.; Lee, Y.P. Enhanced ferromagnetic properties in Ho and Ni co-doped BiFeO3 ceramics. J. Appl. Phys. 2014, 115, 013904. [Google Scholar] [CrossRef]
- Layek, S.; Verma, H.C.; Garg, A. Enhancement in magnetic properties of Ba-doped BiFeO3 ceramics by mechanical activation. J. Alloys Compd. 2015, 651, 294–301. [Google Scholar] [CrossRef]
- Madolappa, S.; Anupama, A.V.; Jaschin, P.W.; Varma, K.B.R.; Sahoo, B. Magnetic and ferroelectric characteristics of Gd3+and Ti4+ co-doped BiFeO3 ceramics. Bull. Mater. Sci. 2016, 39, 593–601. [Google Scholar] [CrossRef] [Green Version]
- Suresh, P.; Srinath, S. Effect of synthesis route on the multiferroic properties of BiFeO3: A comparative study between solid state and sol-gel methods. J. Alloys Compd. 2015, 649, 843–850. [Google Scholar] [CrossRef]
- Lebeugle, D.; Colson, D.; Forget, A.; Viret, M.; Bonville, P.; Marucco, J.F.; Fusil, S. Room-temperature coexistence of large electric polarization and magnetic order in BiFeO3 single crystals. Phys. Rev. B 2007, 76, 8. [Google Scholar] [CrossRef] [Green Version]
- Huang, F.; Wang, Z.; Lu, X.; Zhang, J.; Min, K.; Lin, W.; Ti, R.; Xu, T.; He, J.; Yue, C.; et al. Peculiar magnetism of BiFeO3 nanoparticles with size approaching the period of the spiral spin structure. Sci. Rep. 2013, 3, 2907. [Google Scholar] [CrossRef] [Green Version]
- Papaefthymiou, G.C.; Viescas, A.J.; Le Breton, J.-M.; Chiron, H.; Juraszek, J.; Park, T.-J.; Wong, S.S. Magnetic and Mössbauer characterization of the magnetic properties of single-crystalline sub-micron sized Bi2Fe4O9 cubes. Curr. Appl. Phys. 2015, 15, 417–422. [Google Scholar] [CrossRef] [Green Version]
- Sobolev, A.; Presniakov, I.; Rusakov, V.; Belik, A.; Matsnev, M.; Gorchakov, D.; Glazkova, I. Mössbauer investigations of hyperfine interactions features of 57Fe nuclei in BiFeO3 ferrite. AIP Conf. Proc. 2014, 1622, 104–108. [Google Scholar] [CrossRef]
- Kubelka, P. New Contributions to the Optics of Intensely Light-Scattering Materials. Part I. J. Opt. Soc. Am. 1948, 38, 448–457. [Google Scholar] [CrossRef]
- Papadas, I.T.; Subrahmanyam, K.S.; Kanatzidis, M.G.; Armatas, G.S. Templated assembly of BiFeO3 nanocrystals into 3D mesoporous networks for catalytic applications. Nanoscale 2015, 7, 5737–5743. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Niu, F.; Qin, L.; Wang, S.; Zhang, N.; Huang, Y. Defective BiFeO3 with surface oxygen vacancies: Facile synthesis and mechanism insight into photocatalytic performance. Sol. Energy Mater. Sol. Cells 2017, 171, 24–32. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, P.; Shi, T.; Shao, X. Nd-Cr co-doped BiFeO3 thin films for photovoltaic devices with enhanced photovoltaic performance. Thin Solid Films 2020, 698, 137852. [Google Scholar] [CrossRef]

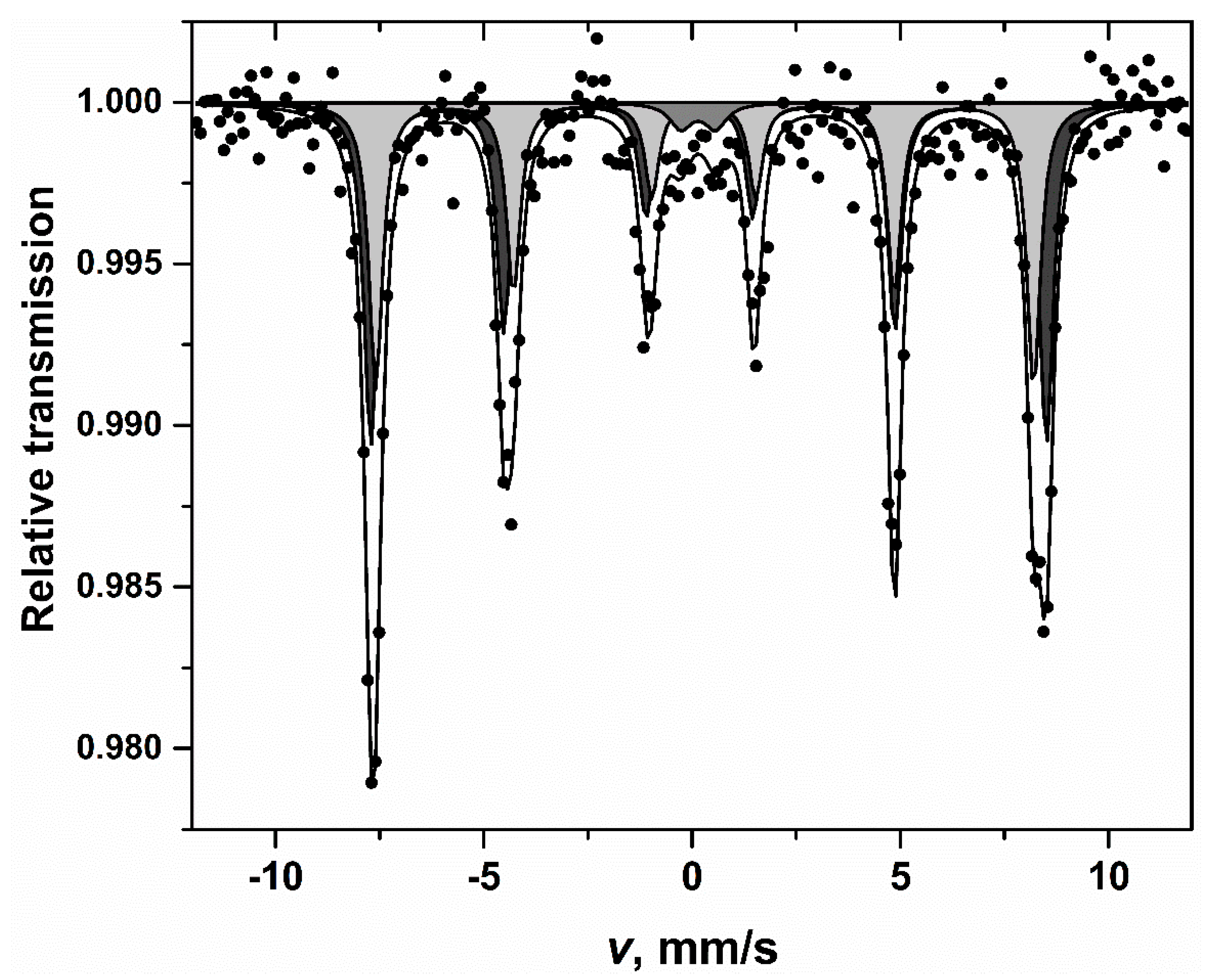
| S, % | Γ, mm/s | δ, mm/s | 2ε(Δ), mm/s | B, T |
|---|---|---|---|---|
| 53 ± 6 | 0.4 ± 0.03 | 0.39 ± 0.01 | 0.22 ± 0.02 | 50.28 ± 0.08 |
| 42 ± 6 | 0.4 ± 0.04 | 0.40 ± 0.01 | 0.02 ± 0.02 | 48.97 ± 0.10 |
| 5 ± 2 | 0.6 ± 0.2 | 0.25 ± 0.09 | 0.83 ± 0.16 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karoblis, D.; Griesiute, D.; Mazeika, K.; Baltrunas, D.; Karpinsky, D.V.; Lukowiak, A.; Gluchowski, P.; Raudonis, R.; Katelnikovas, A.; Zarkov, A.; et al. A Facile Synthesis and Characterization of Highly Crystalline Submicro-Sized BiFeO3. Materials 2020, 13, 3035. https://doi.org/10.3390/ma13133035
Karoblis D, Griesiute D, Mazeika K, Baltrunas D, Karpinsky DV, Lukowiak A, Gluchowski P, Raudonis R, Katelnikovas A, Zarkov A, et al. A Facile Synthesis and Characterization of Highly Crystalline Submicro-Sized BiFeO3. Materials. 2020; 13(13):3035. https://doi.org/10.3390/ma13133035
Chicago/Turabian StyleKaroblis, Dovydas, Diana Griesiute, Kestutis Mazeika, Dalis Baltrunas, Dmitry V. Karpinsky, Anna Lukowiak, Pawel Gluchowski, Rimantas Raudonis, Arturas Katelnikovas, Aleksej Zarkov, and et al. 2020. "A Facile Synthesis and Characterization of Highly Crystalline Submicro-Sized BiFeO3" Materials 13, no. 13: 3035. https://doi.org/10.3390/ma13133035






