Influence of In-Situ Electrochemical Oxidation on Implant Surface and Colonizing Microorganisms Evaluated by Scanning Electron Microscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Contaminated Implant Surfaces
2.2. Set-up of the Boron-Doped Diamond Electrode
2.3. Decontamination of Implants
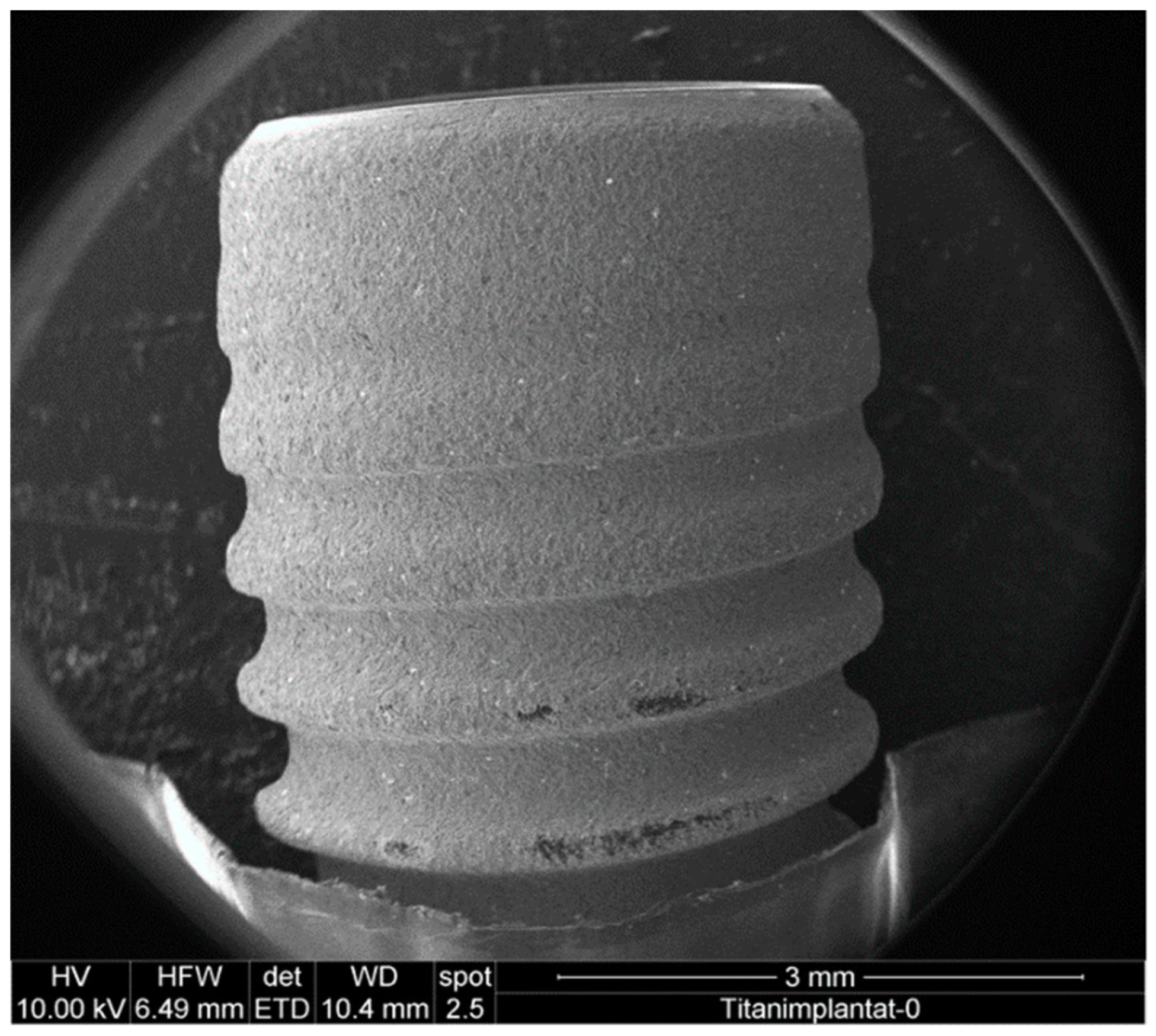
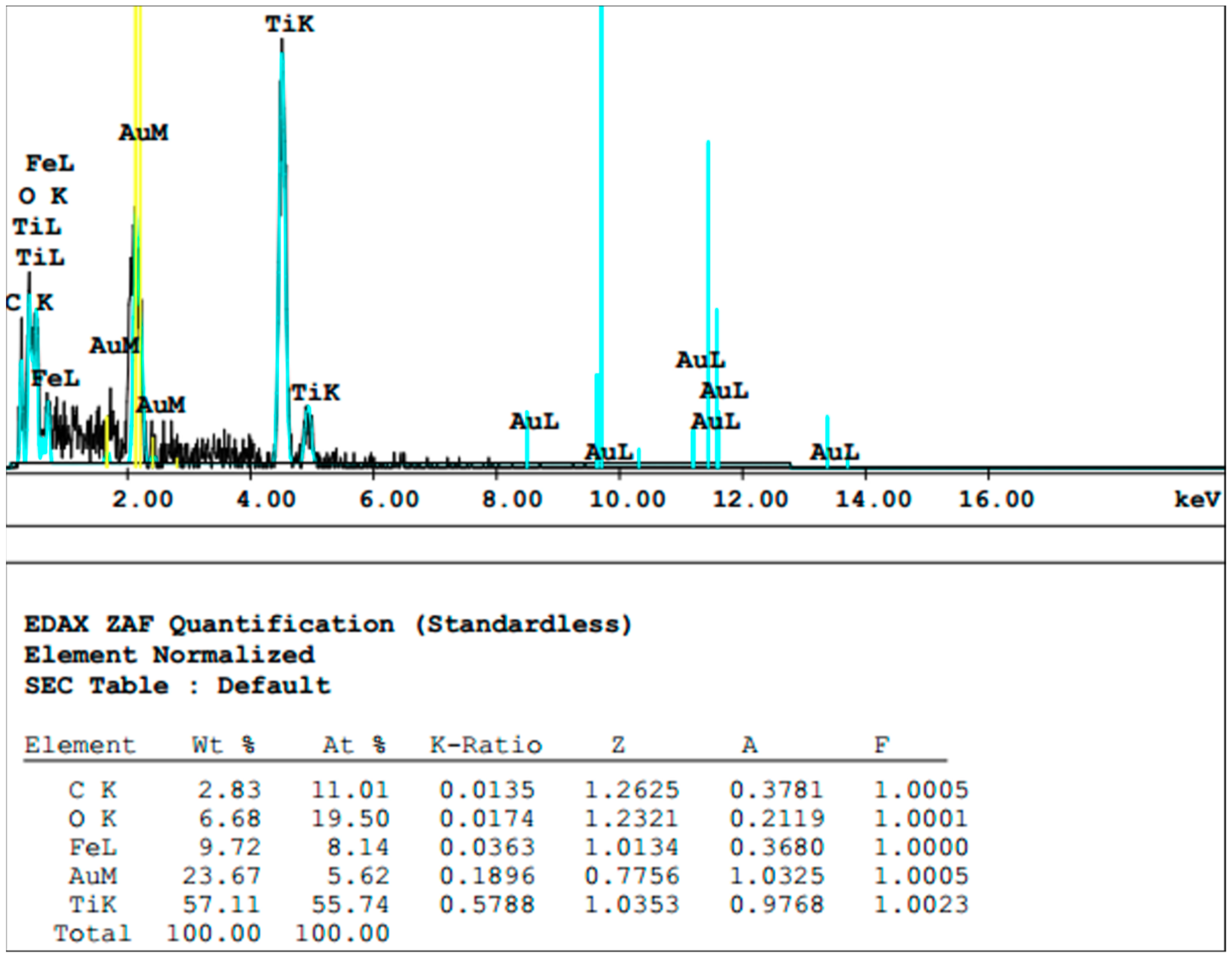
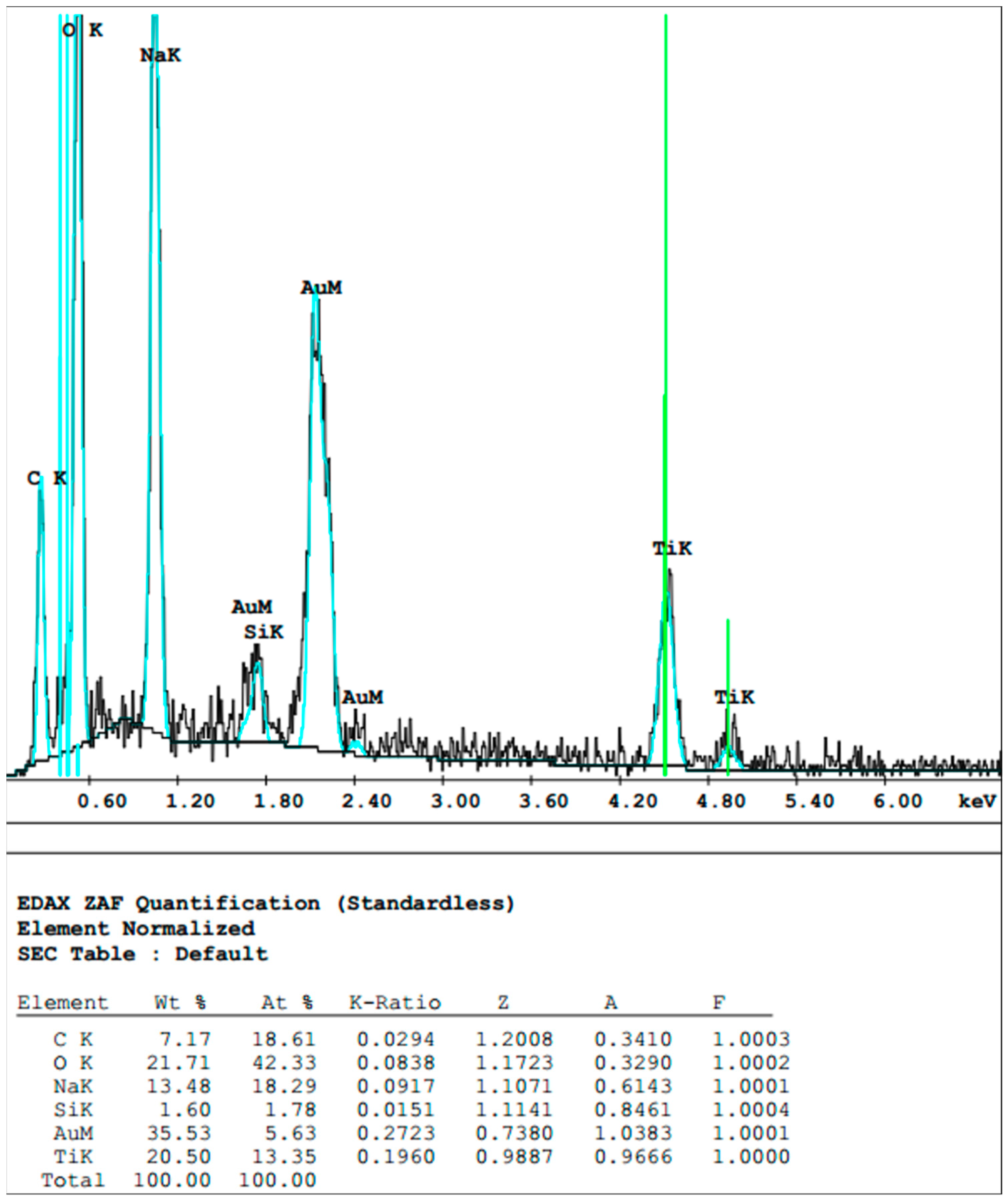
2.4. Sample Preparation for Scanning Electron Microscopy and Energy-Dispersive X-ray Spectroscopy
3. Results
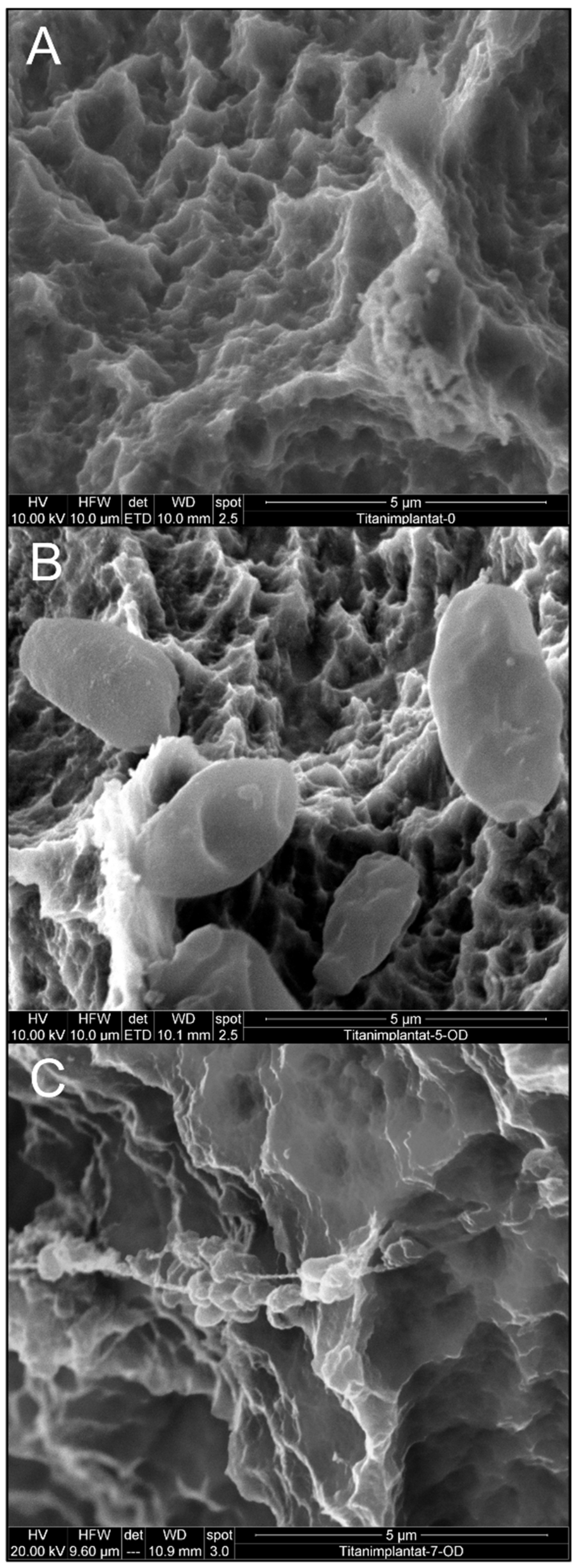
3.1. Effect of Mechanical Debridement by Curettes
3.2. Effect of Air Particle Abrasion
3.3. Effect of BDD Electrode Treatment
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Derks, J.; Tomasi, C. Peri-implant health and disease. A systematic review of current epidemiology. J. Clin. Periodontol. 2015, 42 (Suppl. 16), S158–S171. [Google Scholar] [CrossRef] [PubMed]
- Sirinirund, B.; Garaicoa-Pazmino, C.; Wang, H.L. Effects of Mechanical Instrumentation with Commercially Available Instruments Used in Supportive Peri-implant Therapy: An In Vitro Study. Int. J. Oral Maxillofac. Implants 2019, 34, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Chieruzzi, M.; Pagano, S.; Lombardo, G.; Marinucci, L.; Kenny, J.M.; Torre, L.; Cianetti, S. Effect of nanohydroxyapatite, antibiotic, and mucosal defensive agent on the mechanical and thermal properties of glass ionomer cements for special needs patients. J. Mater. Res. 2018, 33, 638–649. [Google Scholar] [CrossRef]
- Cicciù, M.; Fiorillo, L.; Herford, A.S.; Crimi, S.; Bianchi, A.; D’Amico, C.; Laino, L.; Cervino, G. Bioactive Titanium Surfaces: Interactions of Eukaryotic and Prokaryotic Cells of Nano Devices Applied to Dental Practice. Biomedicines 2019, 7, 12. [Google Scholar] [CrossRef]
- Wang, C.W.; Renvert, S.; Wang, H.L. Nonsurgical treatment of periimplantitis. Implant Dent. 2019, 28, 155–160. [Google Scholar] [CrossRef]
- Koo, K.T.; Khoury, F.; Keeve, P.L.; Schwarz, F.; Ramanauskaite, A.; Sculean, A.; Romanos, G. Implant surface decontamination by surgical treatment of periimplantitis: A literature review. Implant Dent 2019, 28, 173–176. [Google Scholar] [CrossRef]
- Lang, N.P.; Salvi, G.E.; Sculean, A. Nonsurgical therapy for teeth and implants—When and why? Periodontol. 2000 2019, 79, 15–21. [Google Scholar] [CrossRef]
- Berglundh, T.; Wennström, J.L.; Lindhe, J. Long-term outcome of surgical treatment of peri-implantitis. A 2-11-year retrospective study. Clin. Oral Implants Res. 2018, 29, 404–410. [Google Scholar] [CrossRef]
- Carcuac, O.; Derks, J.; Abrahamsson, I.; Wennström, J.L.; Petzold, M.; Berglundh, T. Surgical treatment of peri-implantitis: 3-year results from a randomized controlled clinical trial. J. Clin. Periodontol. 2017, 44, 1294–1303. [Google Scholar] [CrossRef]
- Gershenfeld, L.; Kalos, A.; Whittle, T.; Yeung, S. Randomised clinical trial of the effects of azithromycin use in the treatment of Peri-implantitis. Aust. Dent. J. 2018, 63, 374–381. [Google Scholar] [CrossRef]
- Patianna, G.; Valente, N.A.; D’Addona, A.; Andreana, S. In vitro evaluation of controlled-release 14% doxycycline gel for decontamination of machined and sandblasted acid-etched implants. J. Periodontol. 2018, 89, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.V.; Casati, M.Z.; Casarin, R.C.; Corrêa, M.G.; Cirano, F.R.; Negri, B.M.; Pimentel, S.P. Impact of a triclosan-containing toothpaste during the progression of experimental peri-implant mucositis: Clinical parameters and local pattern of osteo-immunoinflammatory mediators in peri-implant fluid. J. Periodontol. 2018, 89, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Takagi, T.; Aoki, A.; Ichinose, S.; Taniguchi, Y.; Tachikawa, N.; Shinoki, T.; Meinzer, W.; Sculean, A.; Izumi, Y. Effective removal of calcified deposits on microstructured titanium fixture surfaces of dental implants with erbium lasers. J. Periodontol. 2018, 89, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Alagl, A.S.; Madi, M.; Bedi, S.; Al Onaizan, F.; Al-Aql, Z.S. The Effect of Er,Cr:YSGG and Diode Laser Applications on Dental Implant Surfaces Contaminated with Acinetobacter Baumannii and Pseudomonas Aeruginosa. Materials 2019, 12, 2073. [Google Scholar] [CrossRef]
- Bombeccari, G.P.; Guzzi, G.; Gualini, F.; Gualini, S.; Santoro, F.; Spadari, F. Photodynamic therapy to treat periimplantitis. Implant Dent. 2013, 22, 631–638. [Google Scholar] [CrossRef]
- Fraga, R.S.; Antunes, L.A.A.; Fontes, K.B.F.D.C.; Küchler, E.C.; Iorio, N.L.P.P.; Antunes, L.S. Is Antimicrobial Photodynamic Therapy Effective for Microbial Load Reduction in Peri-implantitis Treatment? A Systematic Review and Meta-Analysis. Photochem. Photobiol. 2018, 94, 752–759. [Google Scholar] [CrossRef]
- Ramos, U.D.; Suaid, F.; Wikesjö, U.M.; Susin, C.; Vital, P.C.; de Souza, S.L.S.; Messora, M.R.; Palioto, D.B.; Novaes, A.B., Jr. Microbiologic effect of two topical anti-infective treatments on ligature-induced peri-implantitis: A pilot study in dogs. J. Periodontol. 2018, 89, 995–1002. [Google Scholar] [CrossRef]
- Rupf, S.; Idlibi, A.N.; Marrawi, F.A.; Hannig, M.; Schubert, A.; von Mueller, L.; Spitzer, W.; Holtmann, H.; Lehmann, A.; Rueppell, A.; et al. Removing biofilms from microstructured titanium ex vivo: A novel approach using atmospheric plasma technology. PLoS ONE 2011, 6, e25893. [Google Scholar] [CrossRef]
- Idlibi, A.N.; Al-Marrawi, F.; Hannig, M.; Lehmann, A.; Rueppell, A.; Schindler, A.; Jentsch, H.; Rupf, S. Destruction of oral biofilms formed in situ on machined titanium (Ti) surfaces by cold atmospheric plasma. Biofouling 2013, 29, 369–379. [Google Scholar] [CrossRef]
- Wei, M.C.T.; Tran, C.; Meredith, N.; Walsh, L.J. Effectiveness of implant surface debridement using particle beams at differing air pressures. Clin. Exp. Dent. Res. 2017, 3, 148–153. [Google Scholar] [CrossRef]
- Lee, S.T.; Subu, M.G.; Kwon, T.G. Emphysema following air-powder abrasive treatment for peri-implantitis. Maxillofac. Plast. Reconstr. Surg. 2018, 40, 12. [Google Scholar] [CrossRef] [PubMed]
- Rosen, P.S.; Qari, M.; Froum, S.J.; Dibart, S.; Chou, L.L. A Pilot Study on the Efficacy of a Treatment Algorithm to Detoxify Dental Implant Surfaces Affected by Peri-implantitis. Int. J. Periodontics Restor. Dent. 2018, 38, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Tastepe, C.S.; Lin, X.; Werner, A.; Donnet, M.; Wismeijer, D.; Liu, Y. Cleaning effect of osteoconductive powder abrasive treatment on explanted human implants and biofilm-coated titanium discs. Clin. Exp. Dent. Res. 2018, 4, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Sinjab, K.; Garaicoa-Pazmino, C.; Wang, H.L. Decision Making for Management of Periimplant Diseases. Implant Dent. 2018, 27, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Parlar, A.; Bosshardt, D.D.; Cetiner, D.; Schafroth, D.; Ünsal, B.; Haytac, C.; Lang, N.P. Effects of decontamination and implant surface characteristics on re-osseointegration following treatment of peri-implantitis. Clin. Oral Implants Res. 2009, 20, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Louropoulou, A.; Slot, D.E.; van der Weijden, F. The effect of mechanical instruments on contaminated titanium dental implant surfaces: A systematic review. Clin. Oral Implants Res. 2014, 25, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Larsen, O.I.; Enersen, M.; Kristoffersen, A.K.; Wennerberg, A.; Bunæs, D.F.; Lie, S.A.; Leknes, K.N. Antimicrobial Effects of Three Different Treatment Modalities on Dental Implant Surfaces. J. Oral Implantol. 2017, 43, 429–436. [Google Scholar] [CrossRef]
- Schneider, S.; Rudolph, M.; Bause, V.; Terfort, A. Electrochemical removal of biofilms from titanium dental implant surfaces. Bioelectrochemistry 2018, 121, 84–94. [Google Scholar] [CrossRef]
- Kraft, A. Doped diamond: A compact review on a new, versatile electrode material. Int. J. Electrochem. Sci. 2007, 2, 355–385. [Google Scholar]
- Canizares, P.; Paz, R.; Saez, C.; Rodrigo, M.A. Electrochemical oxidation of alcohols and carboxylic acids with diamond anodes - A comparison with other advanced oxidation processes. Electrochim. Acta 2008, 53, 2144–2153. [Google Scholar] [CrossRef]
- Schorr, B.; Ghanem, H.; Rosiwal, S.; Geißdörfer, W.; Burkovski, A. Elimination of bacterial contaminations by treatment of water with boron-doped diamond electrodes. World J. Microbiol. Biotechnol. 2019, 35, 48. [Google Scholar] [CrossRef] [PubMed]
- Böhm, A.-L.; Koch, M.; Rosiwal, S.; Burkovski, A.; Karl, M.; Grobecker-Karl, T. Electrochemical disinfection of experimentally infected teeth by boron-doped diamond electrode treatment. J. Clin. Med. 2019, 8, 2037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koch, M.; Göltz, M.; Xiangjun, M.; Karl, M.; Rosiwal, S.; Burkovski, A. Diamond-coated electrodes optimize the electrochemical disinfection of dental implants affected by periimplantitis. in preparation.
- Diesendorf, N.; Köhler, S.; Geißdörfer, W.; Grobecker-Karl, T.; Karl, M.; Burkovski, A. Characterisation of Roseomonas mucosa isolated from the root canal of an infected tooth. BMC Res. Notes 2017, 10, 212. [Google Scholar] [CrossRef] [PubMed]
- Burkovski, A.; Karl, M. Lack of evidence for the necessity of root canal obturation. Quintessence Int. 2019, 50, 22–28. [Google Scholar] [CrossRef]
- Schwarz, F.; Becker, K.; Rahn, S.; Hegewald, A.; Pfeffer, K.; Henrich, B. Real-time PCR analysis of fungal organisms and bacterial species at peri-implantitis sites. Int. J. Implant Dent. 2015, 1, 9. [Google Scholar] [CrossRef] [Green Version]
- Flanagan, D. Enterococcus faecalis and Dental Implants. J. Oral Implantol. 2017, 43, 8–11. [Google Scholar] [CrossRef]
- Lemmer, O.; Cremer, R.; Breidt, D.; Frank, M. CVD-Diamant-Dünnschichten nach dem Hot-Filament-Verfahren. In Moderne Beschichtungsverfahren, 2nd ed.; Bach, F.-W., Möhwald, K., Laarmann, A., Wenz, T., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2004; pp. 95–109. [Google Scholar]
- Daubert, D.M.; Weinstein, B.F. Biofilm as a risk factor in implant treatment. Periodontol. 2000 2019, 81, 29–40. [Google Scholar] [CrossRef]
- Bareiß, J.C.; Hackl, G.; Popovska, N.; Rosiwal, S.M.; Singer, R.F. CVD diamond coating of steel on a CVD-TiBN interlayer. Surf. Coat. Technol. 2006, 201, 718–723. [Google Scholar] [CrossRef]
- Misba, L.; Zaidi, S.; Khan, A.U. A comparison of antibacterial and antibiofilm efficacy of phenothiazinium dyes between Gram positive and Gram negative bacterial biofilm. Photodiagn. Photodyn. Ther. 2017, 18, 24–33. [Google Scholar] [CrossRef]
- Cervino, G.; Fiorillo, L.; Iannello, G.; Santonocito, D.; Risitano, G.; Cicciù, M. Sandblasted and Acid Etched Titanium Dental Implant Surfaces Systematic Review and Confocal Microscopy Evaluation. Materials 2019, 12, 1763. [Google Scholar] [CrossRef] [Green Version]
- Bramanti, E.; Matacena, G.; Cecchetti, F.; Arcuri, C.; Cicciù, M. Oral health-related quality of life in partially edentulous patients before and after implant therapy: A 2-year longitudinal study. Oral Implantol. 2013, 6, 37–42. [Google Scholar] [CrossRef]
- Zissis, A.J.; Polyzois, G.L.; Yannikakis, S.A.; Harrison, A. Roughness of denture materials: A comparative study. Int. J. Prosthodont. 2000, 13, 136–140. [Google Scholar] [PubMed]
- Ramel, C.F.; Lüssi, A.; Özcan, M.; Jung, R.E.; Hämmerle, C.H.; Thoma, D.S. Surface roughness of dental implants and treatment time using six different implantoplasty procedures. Clin. Oral Implants Res. 2016, 27, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Madi, M.; Htet, M.; Zakaria, O.; Alagl, A.; Kasugai, S. Re-osseointegration of Dental Implants After Periimplantitis Treatments: A Systematic Review. Implant Dent. 2018, 27, 101–110. [Google Scholar] [CrossRef]



| Oxidation Product | Electrochemical Reaction | E Red (V) |
|---|---|---|
| OH• | HO• + H+ + e− → H2O | 2.80 |
| O• | O• + 2H+ + 2e− → H2O | 2.42 |
| O3 | O3 + 2H+ + 2e− → O2 + H2O | 2.08 |
| H2O2 | H2O2 + 2H+ + 2e− → 2H2O | 1.76 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Göltz, M.; Koch, M.; Detsch, R.; Karl, M.; Burkovski, A.; Rosiwal, S. Influence of In-Situ Electrochemical Oxidation on Implant Surface and Colonizing Microorganisms Evaluated by Scanning Electron Microscopy. Materials 2019, 12, 3977. https://doi.org/10.3390/ma12233977
Göltz M, Koch M, Detsch R, Karl M, Burkovski A, Rosiwal S. Influence of In-Situ Electrochemical Oxidation on Implant Surface and Colonizing Microorganisms Evaluated by Scanning Electron Microscopy. Materials. 2019; 12(23):3977. https://doi.org/10.3390/ma12233977
Chicago/Turabian StyleGöltz, Maximilian, Maximilian Koch, Rainer Detsch, Matthias Karl, Andreas Burkovski, and Stefan Rosiwal. 2019. "Influence of In-Situ Electrochemical Oxidation on Implant Surface and Colonizing Microorganisms Evaluated by Scanning Electron Microscopy" Materials 12, no. 23: 3977. https://doi.org/10.3390/ma12233977





