Inversely 3D-Printed β-TCP Scaffolds for Bone Replacement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Manufacturing
2.2. Sample Characterization
2.2.1. Weight and Dimensions
2.2.2. Surface Roughness
2.2.3. Porosimetry
2.2.4. Mechanical Strength
2.2.5. Microstructure and Elemental Analysis
2.3. Biocompatibility
2.3.1. Live/Dead Assay
2.3.2. Cell Proliferation Assay
2.3.3. Lactate Dehydrogenase (LDH) Assay
2.4. Incubation in Simulated Body Fluid (SBF)
2.5. Statistics
3. Results
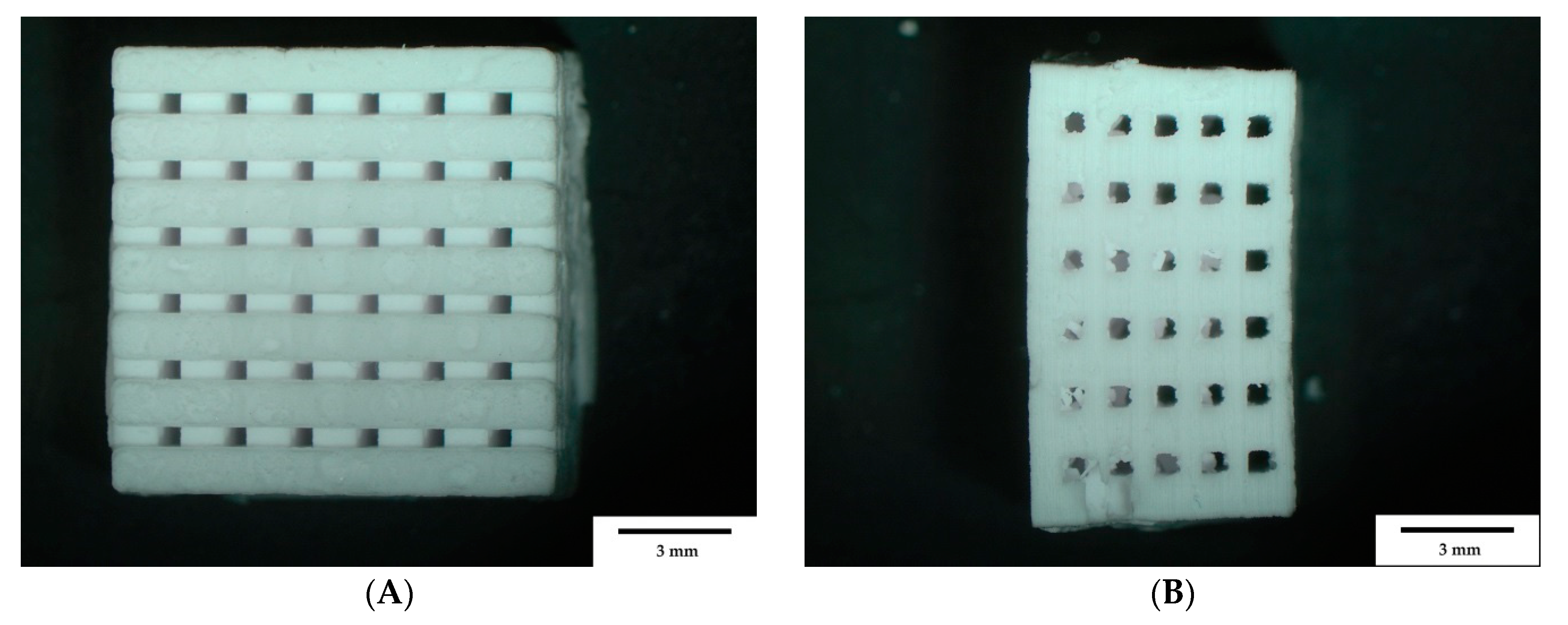
3.1. Sample Characterization
3.1.1. Weight and Dimensions
3.1.2. Surface Roughness
3.1.3. Mechanical Strengths
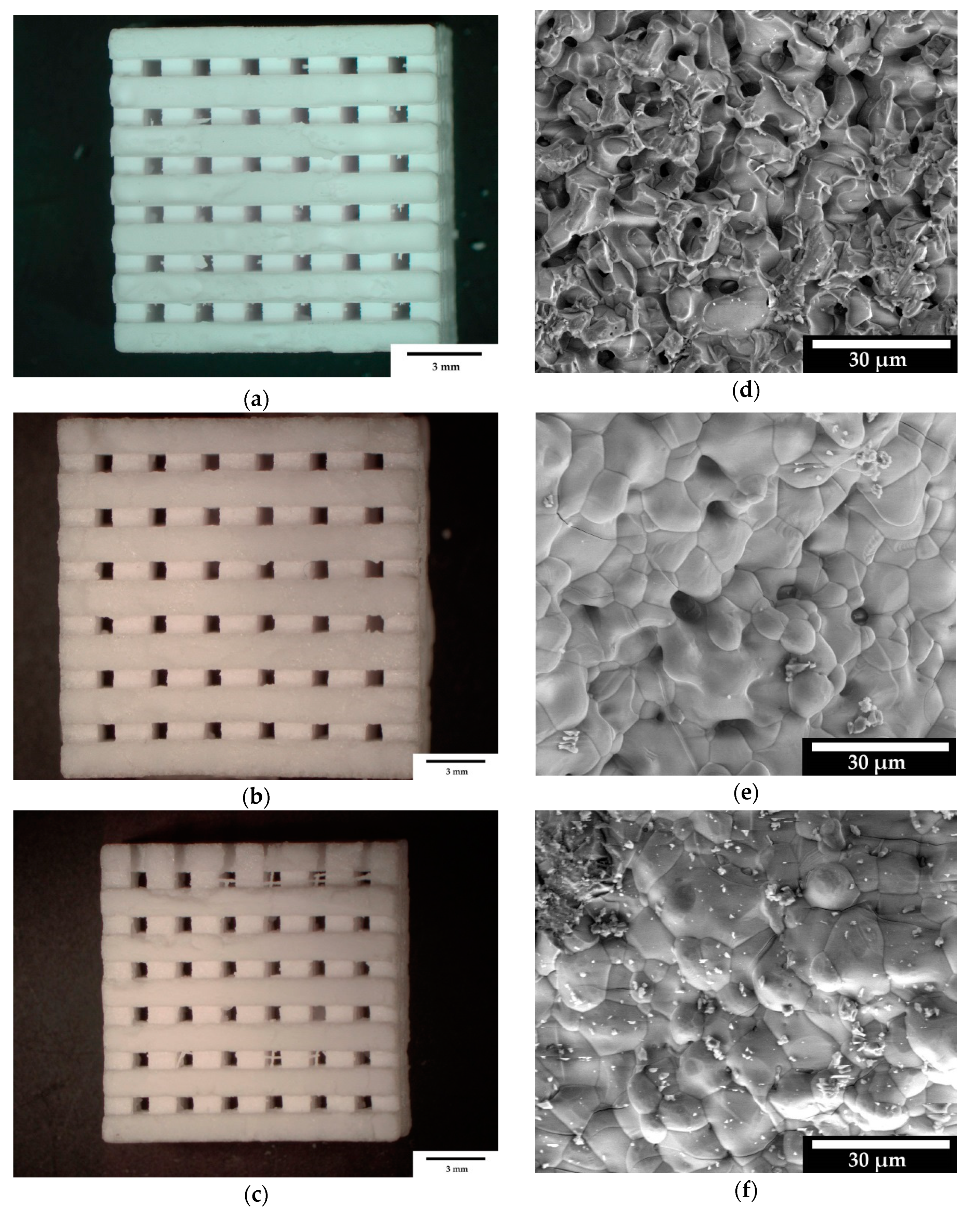
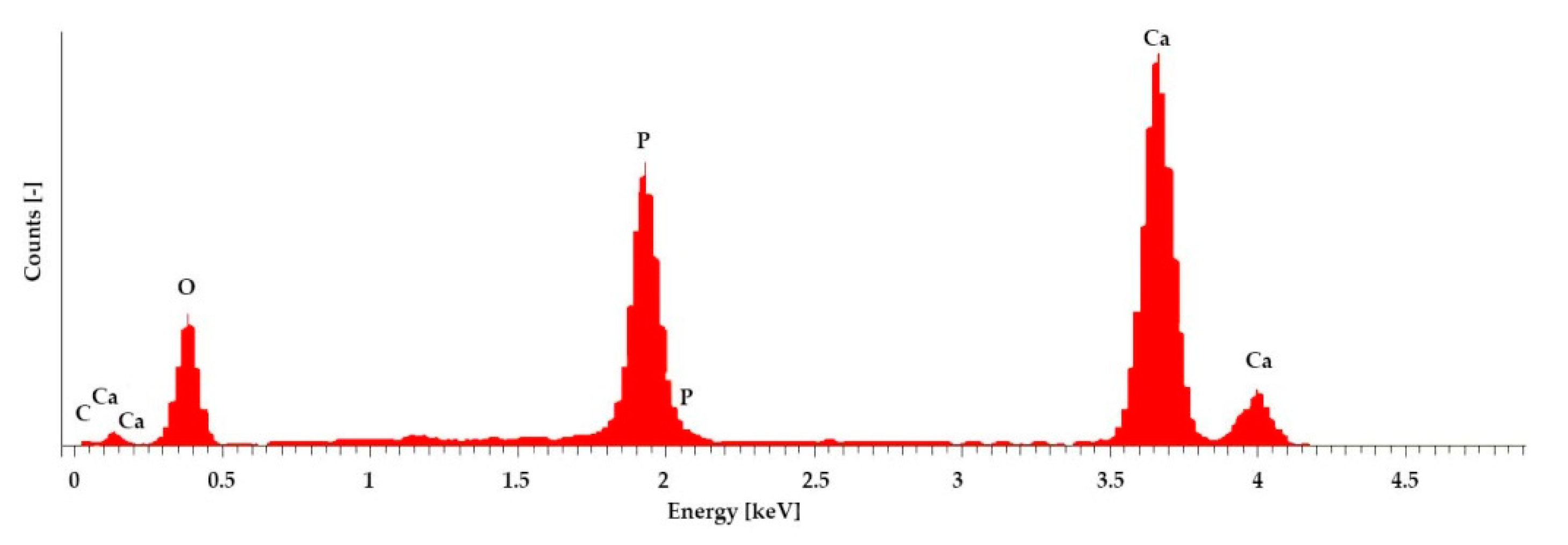
3.1.4. Microstructure and Elemental Analysis
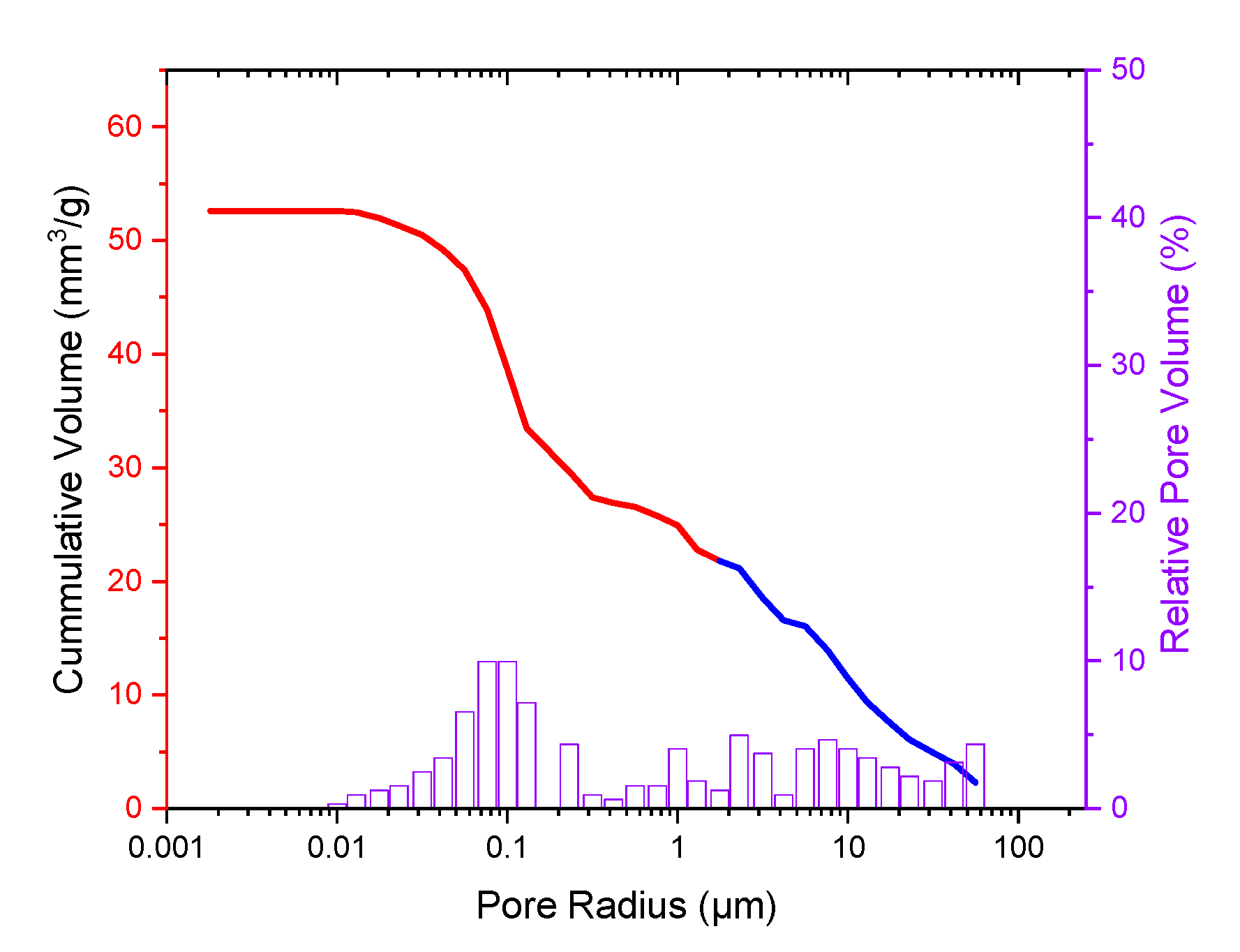
3.1.5. Porosimetry
3.2. Biocompatibility
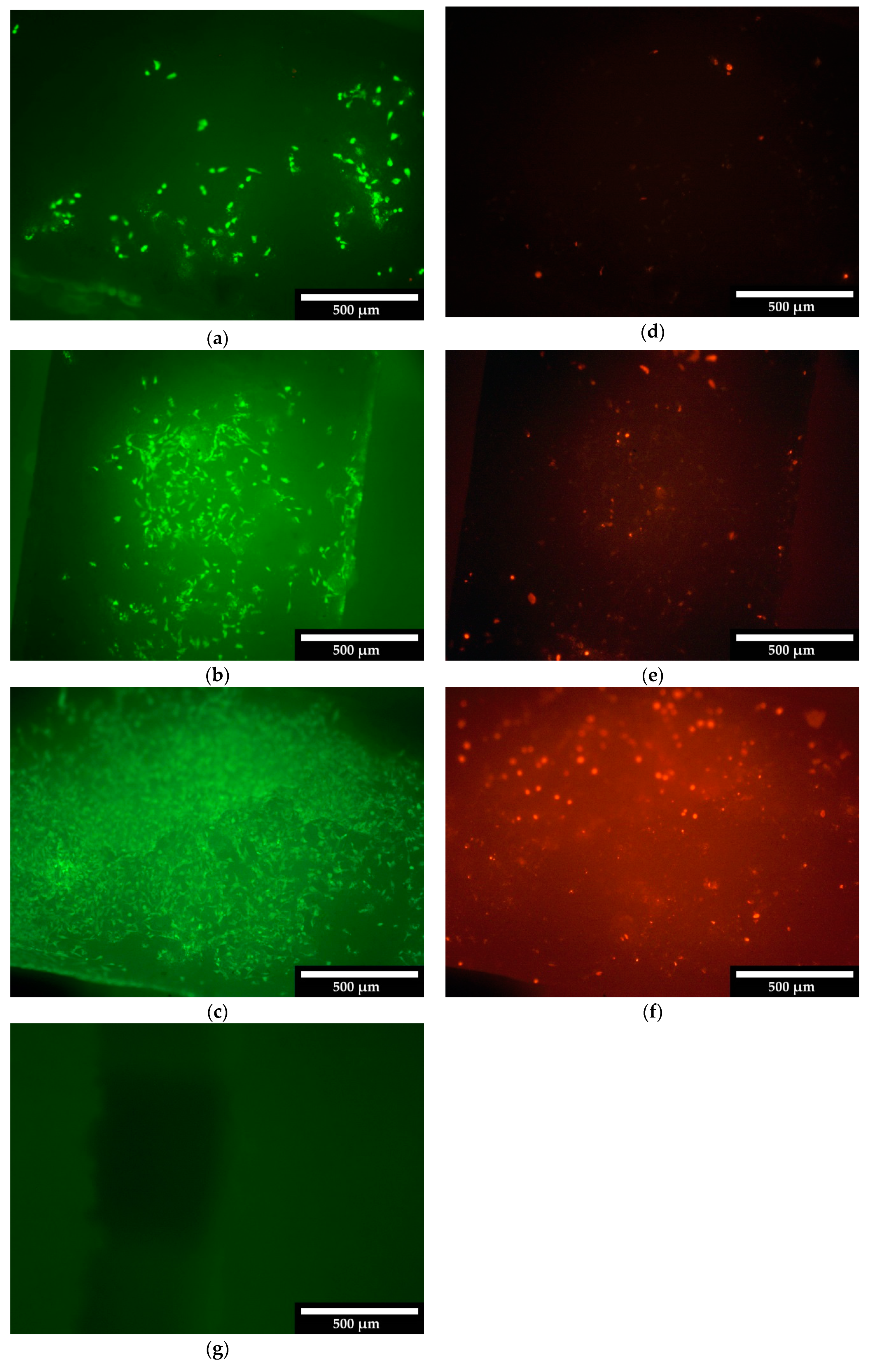
3.2.1. Live/Dead Assay
3.2.2. Cell Proliferation Assay
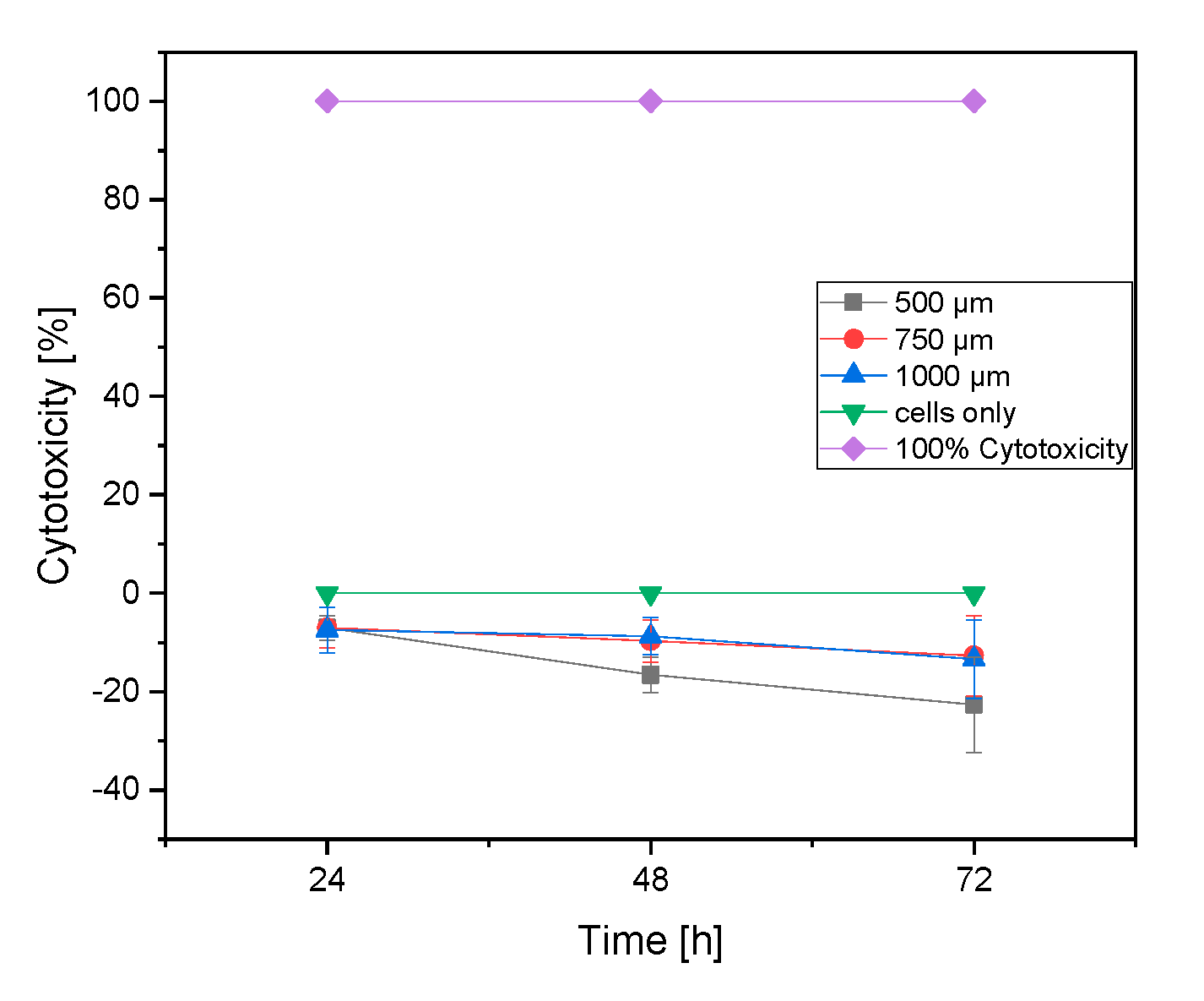
3.2.3. LDH Assay
4. Discussion
4.1. Mechanical Stability
4.2. Sample Characterization via Porosimetry, ESEM and EDX
4.3. Biocompatibility
4.3.1. Live/Dead Assay
4.3.2. Cell Proliferation Assay (WST-I)
4.3.3. LDH Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Destatis, Gesundheit-Fallpauschalenbezogene Krankenhausstatistik (DRG-Statistik) Operationen und Prozeduren der Vollstationären Patientinnen und Patienten in Krankenhäusern (4-Steller); Statistisches Bundesamt (Destatis): Wiesbadem, Germany, 2016; p. 72.
- Salgado, A.J.; Coutinho, O.P.; Reis, R.L. Bone Tissue Engineering: State of the Art and Future Trends. Macromol. Biosci. 2004, 4, 743–765. [Google Scholar] [CrossRef] [Green Version]
- Mohanty, S.; Sanger, K.; Heiskanen, A.; Trifol, J.; Szabo, P.; Dufva, M.; Emnéus, J.; Wolff, A.; Dufva, M. Fabrication of scalable tissue engineering scaffolds with dual-pore microarchitecture by combining 3D printing and particle leaching. Mater. Sci. Eng. C 2016, 61, 180–189. [Google Scholar] [CrossRef] [Green Version]
- Epple, M. Biomaterialien und Biomineralisation; B. G. Teubner: Wiesbaden, Germany, 2003; Volume 1, p. 161. [Google Scholar]
- Du, X.; Fu, S.; Zhu, Y. 3D printing of ceramic-based scaffolds for bone tissue engineering: An overview. J. Mater. Chem. B 2018, 6, 4397–4412. [Google Scholar] [CrossRef]
- Seidenstuecker, M.; Kerr, L.; Bernstein, A.; Mayr, H.O.; Suedkamp, N.P.; Gadow, R.; Krieg, P.; Hernandez Latorre, S.; Thomann, R.; Syrowatka, F.; et al. 3D Powder Printed Bioglass and β-Tricalcium Phosphate Bone Scaffolds. Materials 2018, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.-T.; Lee, M.Y.; Tsai, W.W.; Wang, H.C.; Lu, W.C. Osteogenesis of adipose-derived stem cells on polycaprolactone–β-tricalcium phosphate scaffold fabricated via selective laser sintering and surface coating with collagen type I. J. Tissue Eng. Regen. Med. 2016, 10, E337–E353. [Google Scholar] [CrossRef] [PubMed]
- Vorndran, E.; Geffers, M.; Ewald, A.; Lemm, M.; Nies, B.; Gbureck, U. Ready-to-use injectable calcium phosphate bone cement paste as drug carrier. Acta Biomater. 2013, 9, 9558–9567. [Google Scholar] [CrossRef] [PubMed]
- Crump, S.S. Apparatus and Method for Creating Three-Dimensional Objects; Stratasys Inc.: Eden Prairie, MA, USA, 1989. [Google Scholar]
- Wang, X.; Jiang, M.; Zhou, Z.; Gou, J.; Hui, D. 3D printing of polymer matrix composites: A review and prospective. Compos. Part B Eng. 2017, 110, 442–458. [Google Scholar] [CrossRef]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone tissue engineering using 3D printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Trombetta, R.; Inzana, J.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D printing of calcium phosphate ceramics for bone tissue engineering and drug delivery. Ann. Biomed. Eng. 2017, 45, 23–44. [Google Scholar] [CrossRef]
- Eom, J.-H.; Kim, Y.-W.; Raju, S. Processing and properties of macroporous silicon carbide ceramics: A review. J. Asian Ceram. Soc. 2013, 1, 220–242. [Google Scholar] [CrossRef] [Green Version]
- Bergmann, C.; Lindner, M.; Zhang, W.; Koczur, K.; Kirsten, A.; Telle, R.; Fischer, H. 3D printing of bone substitute implants using calcium phosphate and bioactive glasses. J. Eur. Ceram. Soc. 2010, 30, 2563–2567. [Google Scholar] [CrossRef]
- Moore, R.W.; Graves, S.E.; Bain, G.I. Synthetic bone graft substitutes. ANZ J. Surg. 2001, 71, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Tarafder, S. Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: a review. Acta Biomater. 2012, 8, 1401–1421. [Google Scholar] [CrossRef]
- Klarner, M. 3D-Pulverdruck von Calciumphosphat-Keramiken mit Polymeren und Anorganischen Bindersystemen Polymer and Inorganic 3D-Rapid Prototyping Systems to Build Calciumphospate-Ceramics, in Medizinische Fakultät; Julius-Maximilians-Universität: Würzburg, Germany, 2009. [Google Scholar]
- PromoKine. Live/Dead Cell Staining Kit II-Instruction Manual; PromoCell GmbH: Heidelberg, Germany, 2017. [Google Scholar]
- Jalota, S.; Bhaduri, S.B.; Tas, A.C. Using a synthetic body fluid (SBF) solution of 27 mM HCO3−to make bone substitutes more osteointegrative. Mater. Sci. Eng. C 2008, 28, 129–140. [Google Scholar] [CrossRef]
- Bose, S.; Darsell, J.; Kintner, M.; Hosick, H.; Bandyopadhyay, A. Pore size and pore volume effects on alumina and TCP ceramic scaffolds. Mater. Sci. Eng. C 2002, 23, 479–486. [Google Scholar] [CrossRef]
- Olszta, M.J.; Cheng, X.; Jee, S.S.; Kumar, R.; Kim, Y.-Y.; Kaufman, M.J.; Douglas, E.P.; Gower, L.B. Bone structure and formation: A new perspective. Mater. Sci. Eng. R Rep. 2007, 58, 77–116. [Google Scholar] [CrossRef]
- Vorndran, E.; Kerr, L.; Bernstein, A.; Mayr, H.O.; Suedkamp, N.P.; Gadow, R.; Krieg, P.; Latorre, S.H.; Thomann, R.; Syrowatka, F.; et al. 3D powder printing of beta-tricalcium phosphate ceramics using different strategies. Materials 2008, 11, 13. [Google Scholar]
- Lei, Y.; Rai, B.; Ho, K.H.; Teoh, S.H. In vitro degradation of novel bioactive polycaprolactone-20% tricalcium phosphate composite scaffolds for bone engineering. Mater. Sci. Eng. C 2006, 27, 293–298. [Google Scholar] [CrossRef]
- Jalota, S.; Bhaduri, S.B.; Tas, A.C. In vitro testing of calcium phosphate (HA, TCP, and biphasic HA-TCP) whiskers. J. Biomed. Mater. Res. Part A 2006, 78, 481–490. [Google Scholar] [CrossRef]
- Uchino, T.; Ohtsuki, C.; Kamitakahara, M.; Tanihara, M.; Miyazaki, T. Apatite formation behavior on tricalcium phosphate (TCP) porous body in a simulated body fluid. Key Eng. Mater. 2006, 309, 251–254. [Google Scholar] [CrossRef]
- Duan, Y.R.; Zhang, Z.R.; Wang, C.Y.; Chen, J.Y.; Zhang, X.D. Dynamic study of calcium phosphate formation on porous HA/TCP ceramics. J. Mater. Sci. Mater. Electron. 2005, 16, 795–801. [Google Scholar] [CrossRef] [PubMed]
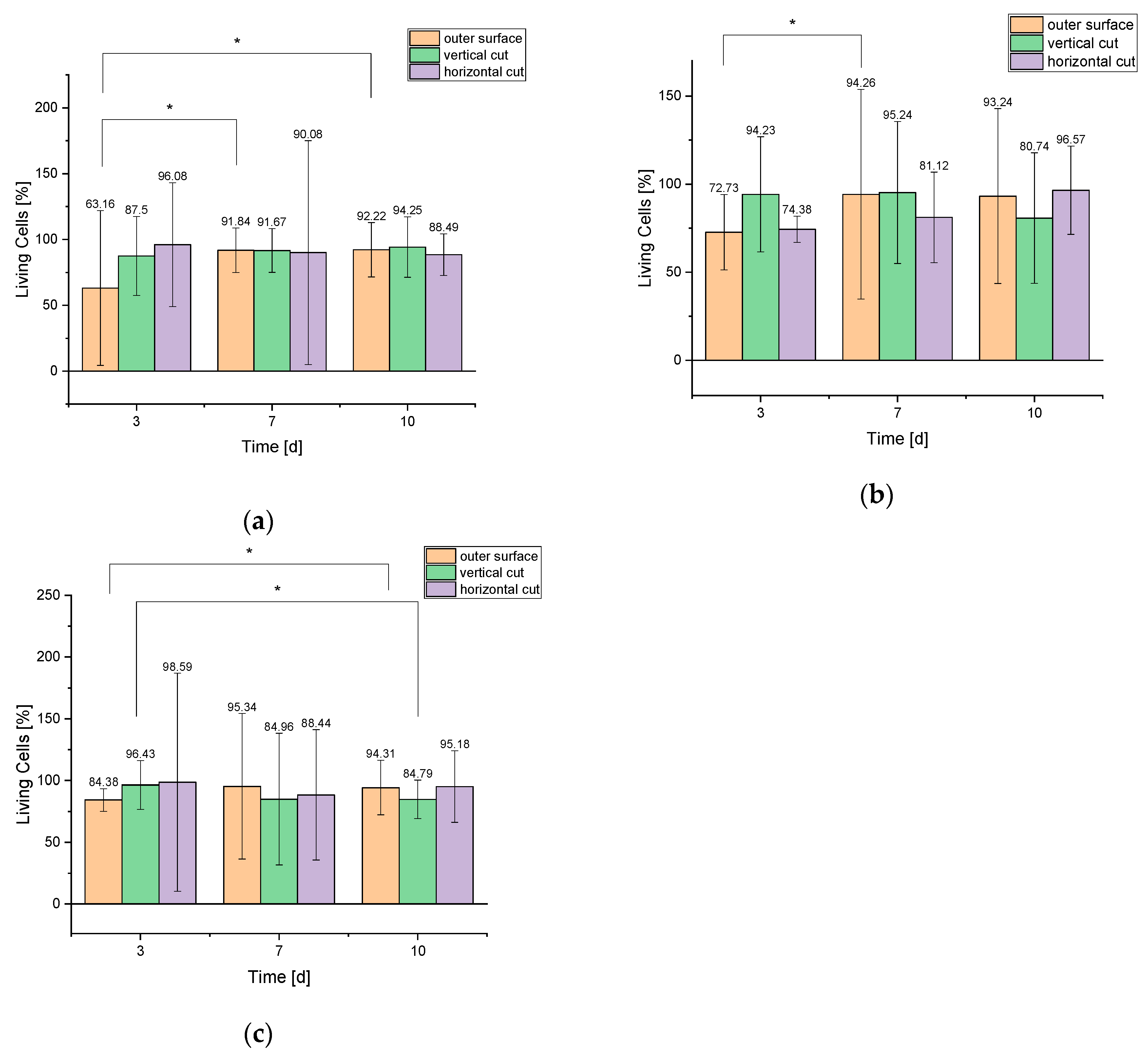

| Chemical Substance | Quantity [g] |
|---|---|
| NaCl | 3.274 |
| NaHCO3 | 1.1134 |
| KCl | 0.187 |
| Na2HPO4 2H2O | 0.089 |
| MgCl2 | 0.071 |
| CaCl2 2H2O | 0.184 |
| Na2SO4 | 0.0355 |
| (CH2OH)3CNH2 | 3.0285 |
| 1M HCl solution | until pH 7.4 |
| Sample | Length (mm) | Width (mm) | Height (mm) | Weight (g) |
|---|---|---|---|---|
| 500 µm | 12.71 ± 0.05 | 12.74 ± 0.14 | 5.59 ± 0.04 | 1.48 ± 0.09 |
| 750 µm | 13.05 ± 0.34 | 13.08 ± 0.2 | 6.43 ± 0.17 | 1.63 ± 0.17 |
| 1000 µm | 14.67 ± 0.53 | 14.65 ± 0.10 | 8.13 ± 0.15 | 2.38 ± 0.12 |
| - | Sa (µm) | ||
|---|---|---|---|
| Material | 500 µm | 750 µm | 1000 µm |
| Mean ± SD | 8.84 ± 0.71 | 7.97 ± 1.54 | 9.61 ± 2.02 |
| 500 µm | 750 µm | 1000 µm | ||||||
|---|---|---|---|---|---|---|---|---|
| Breaking Strength [N] | Fmax [N] | Compressive Strength [MPa] | Breaking Strength [N] | Fmax [N] | Compressive Strength [MPa] | Breaking Strength [N] | Fmax [N] | Compressive Strength [MPa] |
| 543.6 ± 35 | 2754.6 ± 234.9 | 3.4 ± 0.2 | 243.4 ± 34.1 | 1210.6 ± 146.7 | 1.3 ± 0.2 | 117.5 ± 43.4 | 309 ± 89.1 | 0.5 ± 0.18 |
| 500 µm + SBF | 750 µm + SBF | 1000 µm + SBF | ||||||
|---|---|---|---|---|---|---|---|---|
| Breaking Strength [N] | Fmax [N] | Compressive Strength [MPa] | Breaking Strength [N] | Fmax [N] | Compressive Strength [MPa] | Breaking Strength [N] | Fmax [N] | Compressive Strength [MPa] |
| 458.6 ± 40.2 | 2094.8 ± 712.4 | 2.8 ± 0.2 | 155.3 ± 21.3 | 786.4 ± 104.9 | 0.8 ± 0.1 | 64 ± 24 | 150 ± 61 | 0.28 ± 0.1 |
| Elements | Atom [%] |
|---|---|
| C | 7.79 |
| O | 64.83 |
| P | 9.85 |
| Ca | 16.53 |
| - | Day 3 | Day 7 | Day 10 | |||
|---|---|---|---|---|---|---|
| Living | Dead | Living | Dead | Living | Dead | |
| 500 µm | Cells/mm² | |||||
| Outer surface | 36 ± 33 | 21 ± 1 | 180 ± 33 | 16 ± 8 | 308 ± 69 | 26 ± 6 |
| Vertical cut | 35 ± 12 | 5 ± 4 | 121 ± 20 | 11 ± 8 | 164 ± 40 | 10 ± 4 |
| Horizontal cut | 49 ± 24 | 2 ± 1 | 109 ± 115 | 12 ± 13 | 123 ± 22 | 16 ± 11 |
| 750 µm | - | - | - | - | - | - |
| Outer surface | 32 ± 9 | 12 ± 4 | 197 ± 124 | 12 ± 8 | 262 ± 139 | 19 ± 8 |
| Vertical cut | 49 ± 17 | 3 ± 2 | 200 ± 84 | 10 ± 4 | 218 ± 99 | 52 ± 23 |
| Horizontal cut | 119 ± 9 | 2 ± 2 | 189 ± 59 | 44 ± 21 | 225 ± 58 | 8 ± 2 |
| 1000 µm | - | - | - | - | - | - |
| Outer surface | 27 ± 2 | 5 ± 5 | 225 ± 139 | 11 ± 9 | 265 ± 61 | 16 ± 11 |
| Vertical cut | 54 ± 11 | 2 ± 1 | 226 ± 141 | 40 ± 22 | 223 ± 44 | 40 ± 11 |
| Horizontal cut | 70 ± 84 | 1 ± 2 | 176 ± 105 | 23 ± 38 | 158 ± 48 | 8 ± 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seidenstuecker, M.; Lange, S.; Esslinger, S.; Latorre, S.H.; Krastev, R.; Gadow, R.; Mayr, H.O.; Bernstein, A. Inversely 3D-Printed β-TCP Scaffolds for Bone Replacement. Materials 2019, 12, 3417. https://doi.org/10.3390/ma12203417
Seidenstuecker M, Lange S, Esslinger S, Latorre SH, Krastev R, Gadow R, Mayr HO, Bernstein A. Inversely 3D-Printed β-TCP Scaffolds for Bone Replacement. Materials. 2019; 12(20):3417. https://doi.org/10.3390/ma12203417
Chicago/Turabian StyleSeidenstuecker, Michael, Svenja Lange, Steffen Esslinger, Sergio H. Latorre, Rumen Krastev, Rainer Gadow, Hermann O. Mayr, and Anke Bernstein. 2019. "Inversely 3D-Printed β-TCP Scaffolds for Bone Replacement" Materials 12, no. 20: 3417. https://doi.org/10.3390/ma12203417





