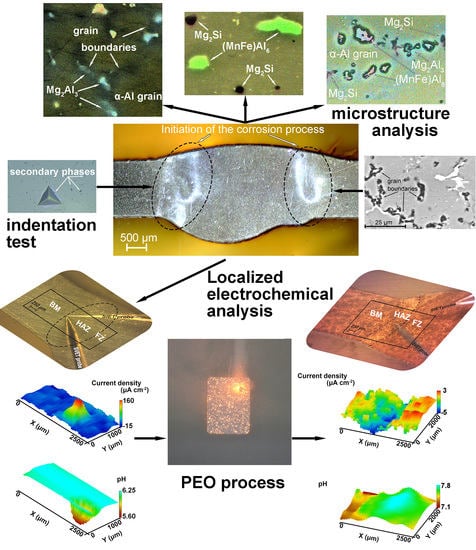
Effect of Microstructure on the Corrosion Resistance of TIG Welded 1579 Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Electrochemical Measurements
2.3. Microstructure Characterization
2.4. Microhardness Test
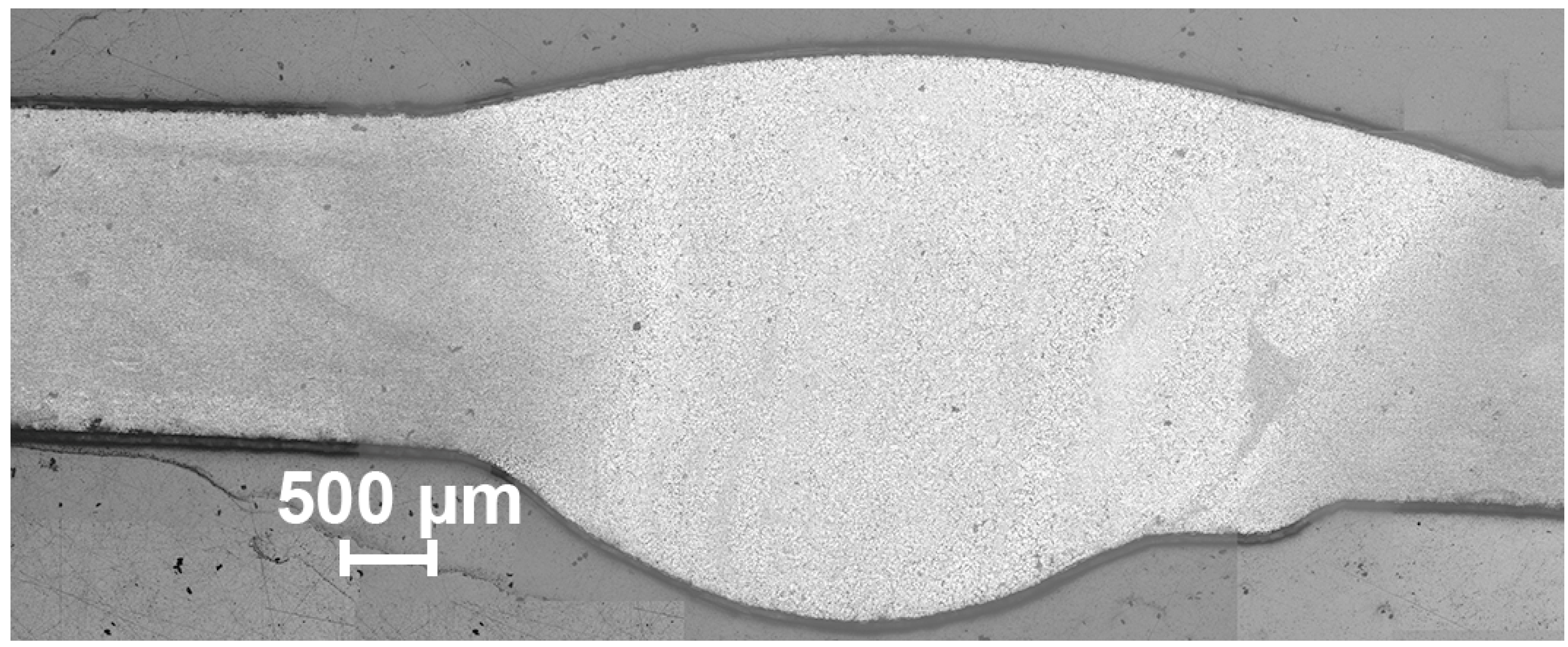
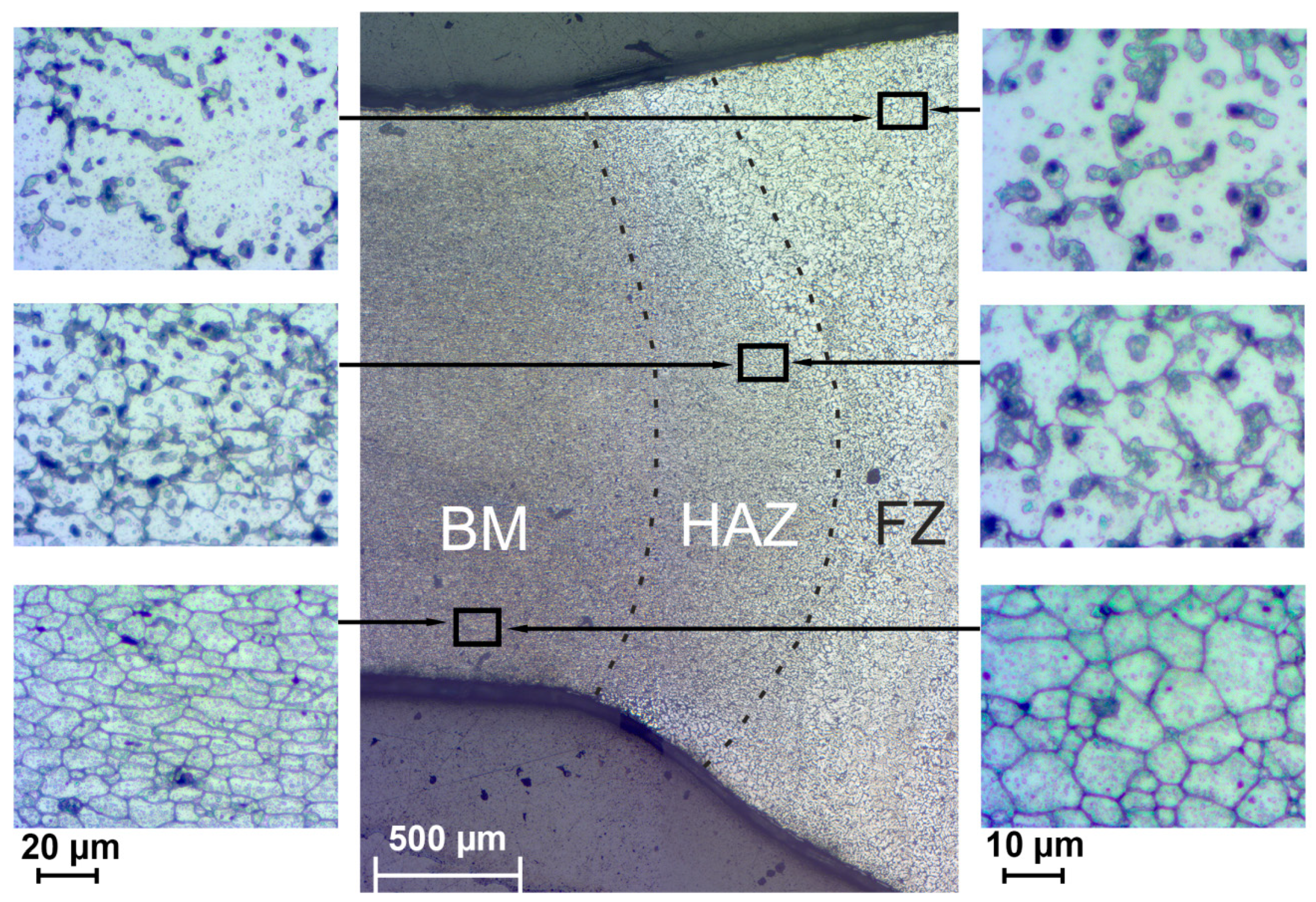
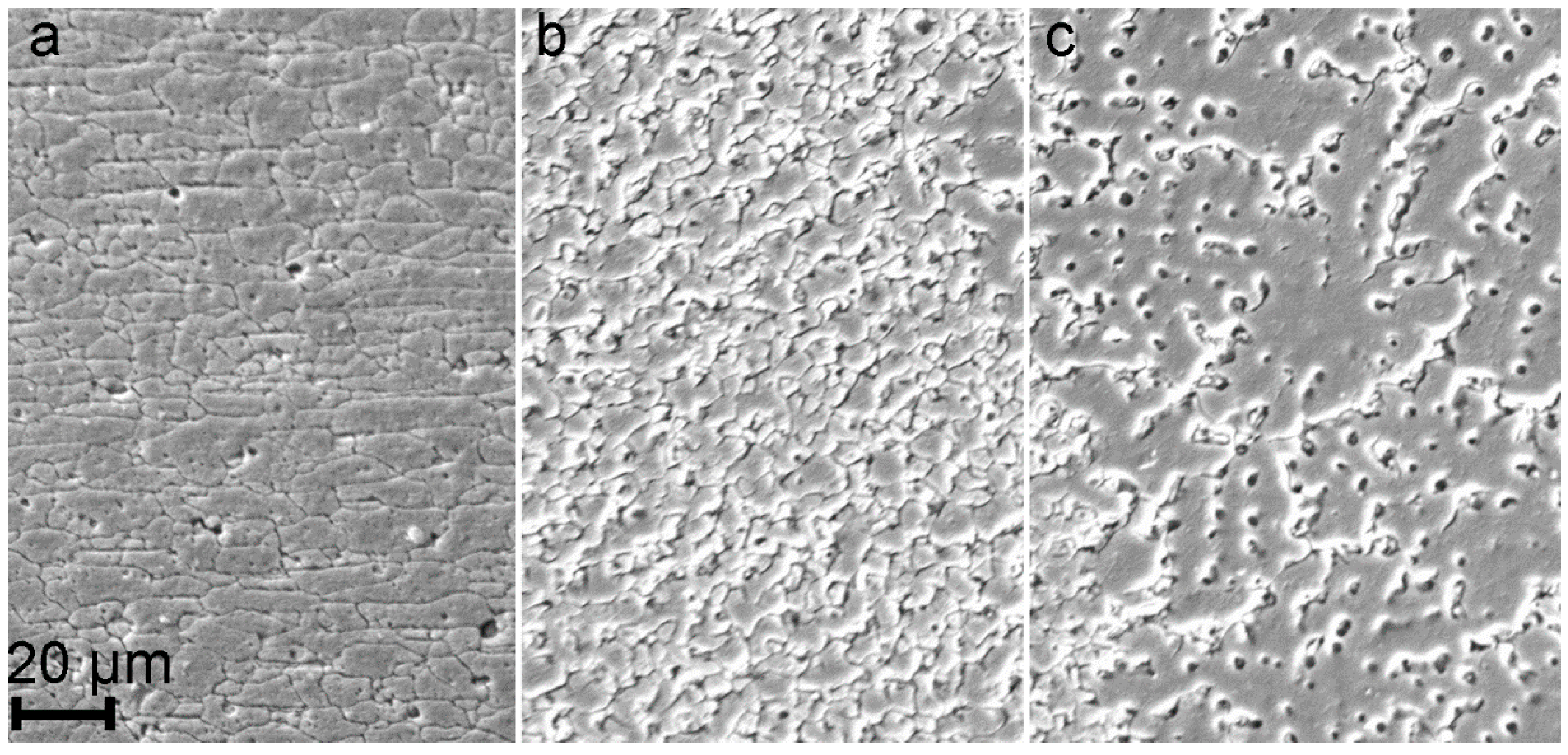
3. Results and Discussion
4. Conclusions
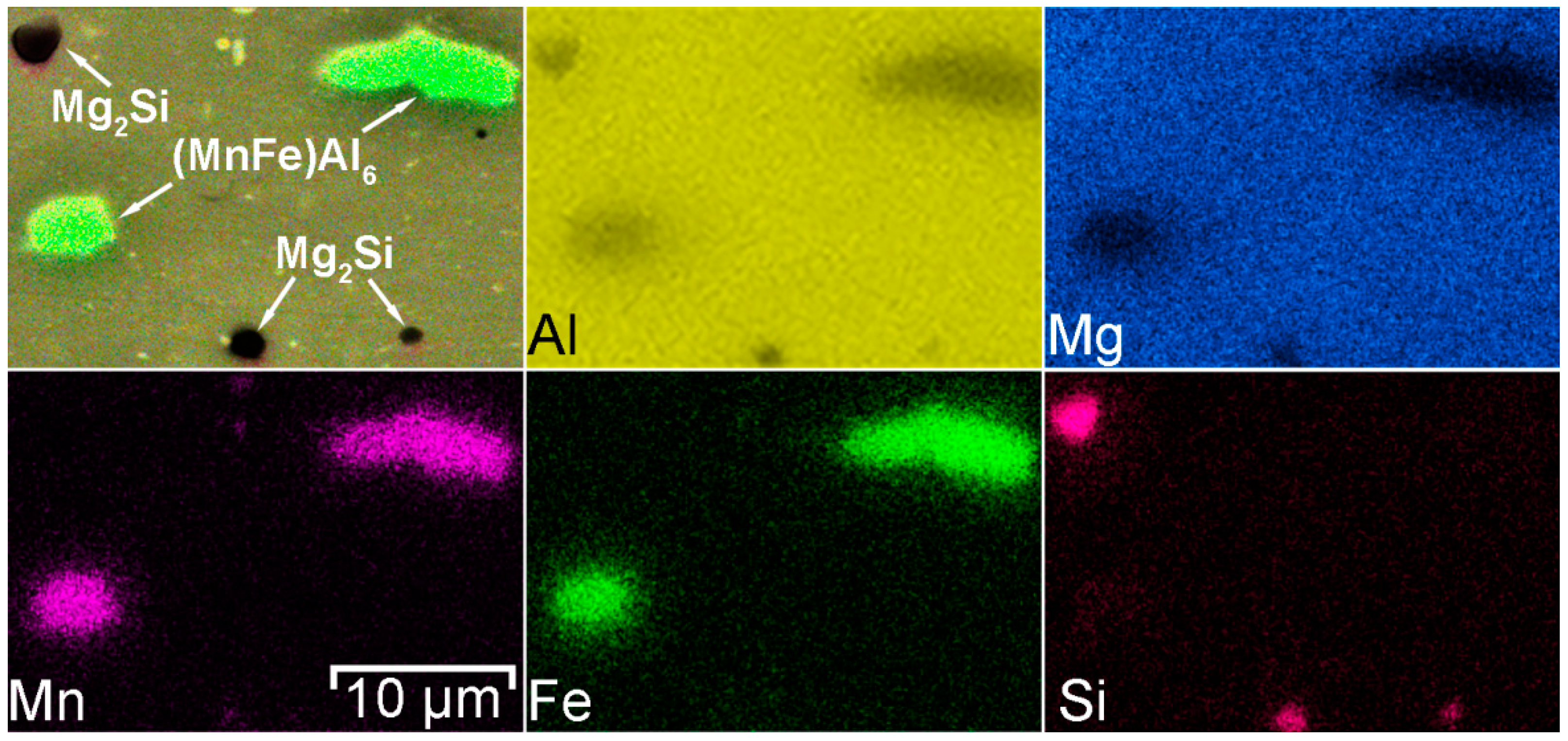
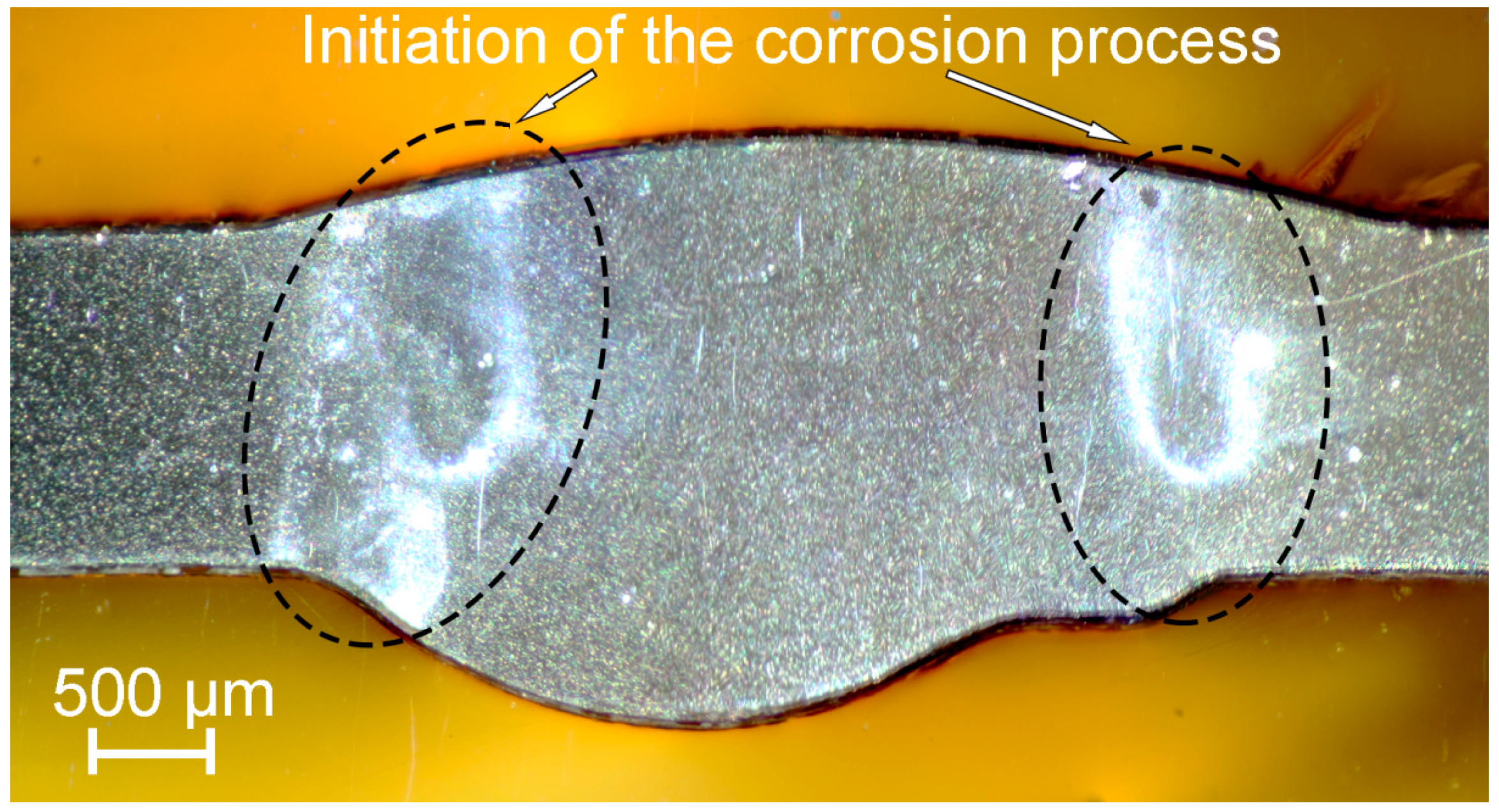
- It has been established that HAZ is more sensitive to localized corrosion as compared to the fusion zone and base material. The higher electrochemical activity of HAZ is caused by precipitation of cathodic Fe rich secondary phases (e.g. (MnFe)Al6) along with anodic Mg2Si and Mg2Al3 phases at the recrystallized grain boundaries that stimulate the micro-galvanic corrosion in these areas due to the different corrosion potential;
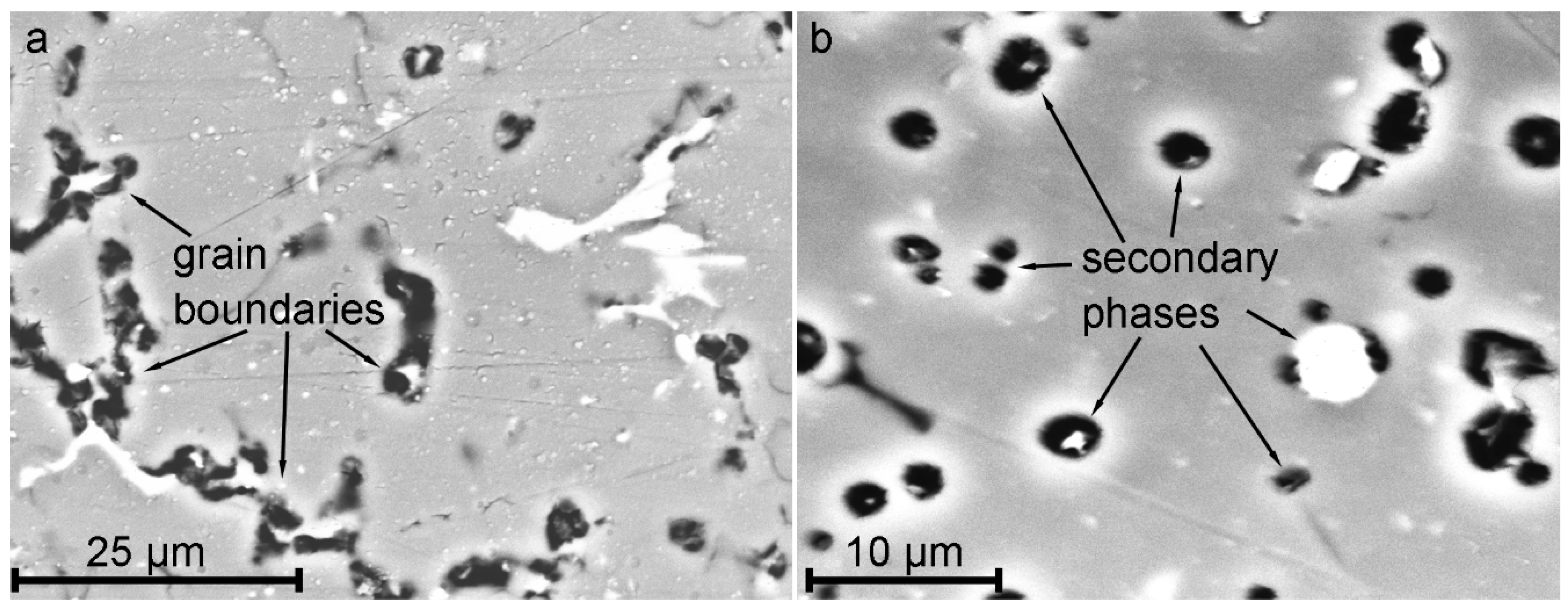
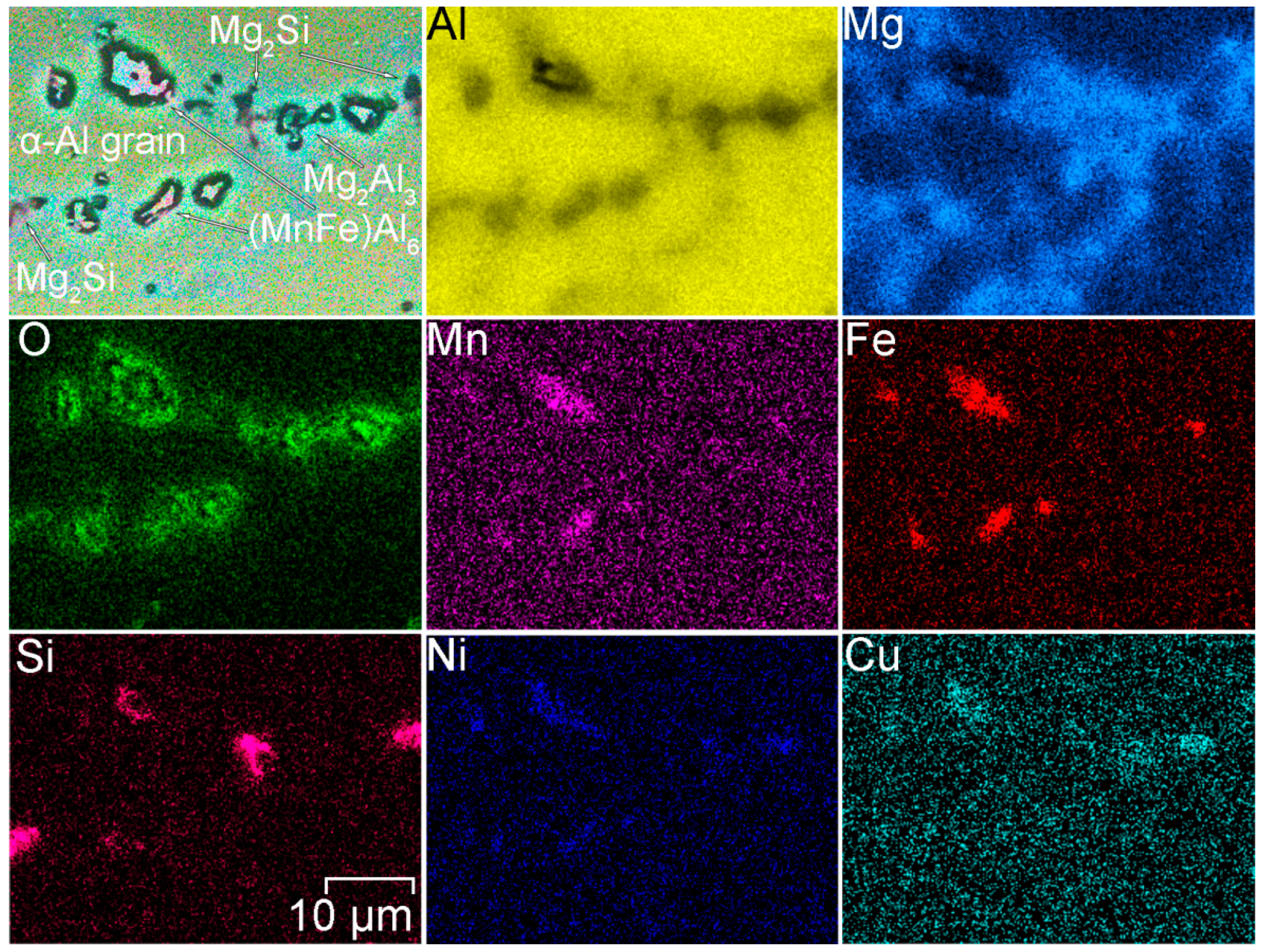
- During heat treatment realized in TIG welding, Mg2Si particles, as well as Mg2Al3, coalesce, while iron-containing phases remain unchanged, which is mainly affected the corrosion resistance of HAZ. The microstructure analysis of the welded joint showed that concentration of the black spots, which are Mg2Al3, Mg2Si phases increased in HAZ and decreased in BM and FZ. SEM-EDX analysis showed the intensive intergranular corrosion in HAZ, where a high concentration of (MnFe)Al6, Mg2Si, Mg2Al3 particles was detected;
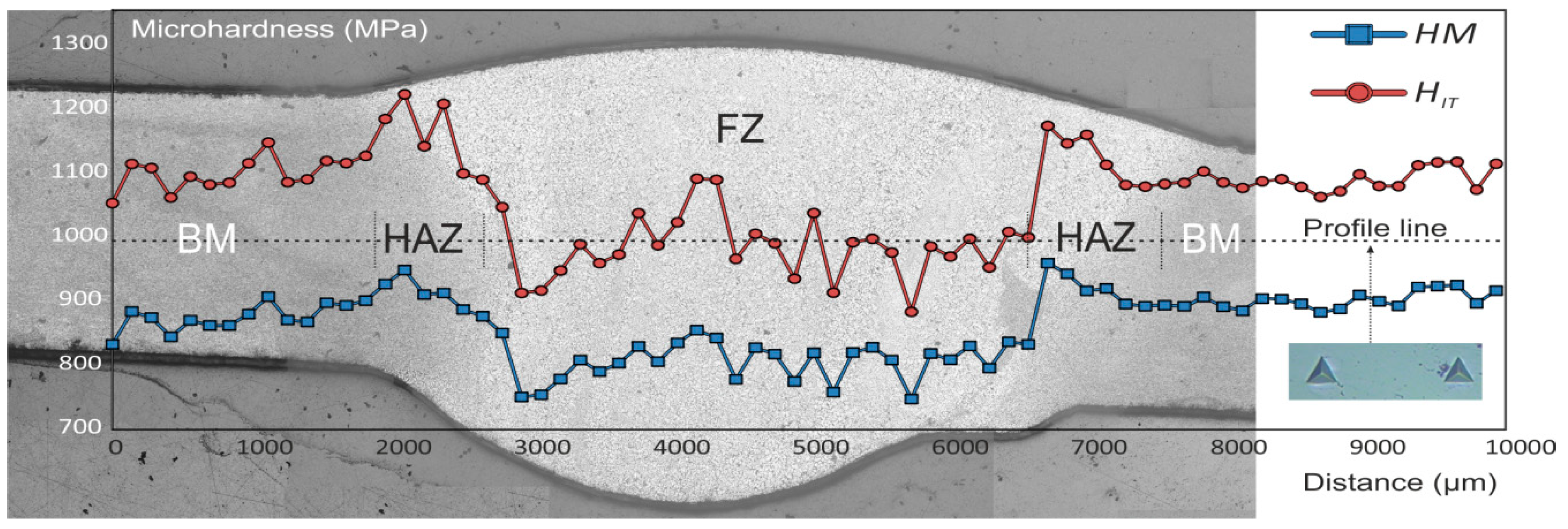
- It has been established the higher microhardness values of HAZ as compared to those of BM and FZ, which is related to the enrichment with hard Mg2Si phase and hard (MnFe)Al6 intermetallic compounds;
- According to SVET and SIET, the coating, obtained using PEO treatment reduces the electrochemical activity of the welded joint and inhibits its corrosion degradation. This method enables one to improve the corrosion properties of the welded material.
Author Contributions
Funding
Conflicts of Interest
References
- Moreto, J.A.; Marino, C.E.B.; Bose Filho, W.W.; Rocha, L.A.; Fernandes, J.C.S. SVET, SKP and EIS study of the corrosion behaviour of high strength Al and Al-Li alloys used in aircraft fabrication. Corros. Sci. 2014, 84, 30–41. [Google Scholar] [CrossRef]
- Grilli, R.; Baker, M.A.; Castle, J.E.; Dunn, B.; Watts, J.F. Localized corrosion of a 2219 aluminium alloy exposed to a 3.5% NaCl solution. Corros. Sci. 2010, 52, 2855–2866. [Google Scholar] [CrossRef] [Green Version]
- Lv, J.; Hongyun, L.; Liang, T. Investigation of microstructure and corrosion behavior of burnished aluminum alloy by TEM, EWF, XPS and EIS techniques. Mater. Res. Bull. 2016, 83, 148–154. [Google Scholar] [CrossRef]
- Lee, D.; Kim, B.; Lee, S.; Baek, S.M.; Kim, J.C.; Son, H.T.; Lee, J.G.; Lee, K.S.; Park, S.S. Enhanced corrosion resistance of Mg-Sn-Zn-Al alloy by Y microalloying. Scr. Mater. 2019, 163, 125–129. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, K.; Frankel, G.S. Intermetallic Phases in Aluminum Alloys and Their Roles in Localized Corrosion. J. Electrochem. Soc. 2018, 165, C807–C820. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Z. On the electrochemical dealloying of Al-based alloys in a NaCl aqueous solution. Phys. Chem. Chem. Phys. 2010, 12, 1453–1472. [Google Scholar] [CrossRef]
- Palcut, M.; Ďuriška, L.; Špoták, M.; Vrbovský, M.; Gerhátová, Ž.; Černičková, I.; Janovec, J. Electrochemical Corrosion of Al-Pd Alloys in HCl and NaOH Solutions. J. Min. Metall. Sect. B-Metall. 2017, 53, 333–340. [Google Scholar] [CrossRef]
- Li, J.; Dang, J. A Summary of Corrosion Properties of Al-Rich Solid Solution and Secondary Phase Particles in Al Alloys. Metals (Basel) 2017, 7, 84. [Google Scholar] [CrossRef]
- Chaturvedi, M.C. Welding and Joining of Aerospace Materials; Woodhead Pub: Cambridge, UK, 2012; ISBN 9781845695323. [Google Scholar]
- Queiroz, F.M.; Donatus, U.; Prada Ramirez, O.M.; de Sousa Araujo, J.V.; Gonçalves de Viveiros, B.V.; Lamaka, S.; Zheludkevich, M.; Masoumi, M.; Vivier, V.; Costa, I.; et al. Effect of unequal levels of deformation and fragmentation on the electrochemical response of friction stir welded AA2024-T3 alloy. Electrochim. Acta 2019, 313, 271–281. [Google Scholar] [CrossRef]
- Lomolino, S.; Tovo, R.; Dos Santos, J. On the fatigue behaviour and design curves of friction stir butt-welded Al alloys. Int. J. Fatigue 2005, 27, 305–316. [Google Scholar] [CrossRef]
- Dursun, T.; Soutis, C. Recent developments in advanced aircraft aluminium alloys. Mater. Des. 2014, 56, 862–871. [Google Scholar] [CrossRef]
- Chen, Q.; Lin, S.; Yang, C.; Fan, C.; Ge, H. Grain fragmentation in ultrasonic-assisted TIG weld of pure aluminum. Ultrason. Sonochem. 2017, 39, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, G.; Wu, A.; Zhao, Y.; Li, Q.; Liu, X.; Meng, D.; Song, J.; Zhang, Z. Study on the inconsistency in mechanical properties of 2219 aluminium alloy TIG-welded joints. J. Alloy. Compd. 2019, 777, 1044–1053. [Google Scholar] [CrossRef]
- Lin, Y.T.; Wang, M.C.; Zhang, Y.; He, Y.Z.; Wang, D.P. Investigation of microstructure evolution after post-weld heat treatment and cryogenic fracture toughness of the weld metal of AA2219 VPTIG joints. Mater. Des. 2017, 113, 54–59. [Google Scholar] [CrossRef]
- Li, H.; Zou, J.; Yao, J.; Peng, H. The effect of TIG welding techniques on microstructure, properties and porosity of the welded joint of 2219 aluminum alloy. J. Alloys Compd. 2017, 727, 531–539. [Google Scholar] [CrossRef]
- Niu, L.Q.; Li, X.Y.; Zhang, L.; Liang, X.B.; Li, M. Correlation between microstructure and mechanical properties of 2219-T8 aluminum alloy joints by VPTIG welding. Acta Met. Sin. (Engl. Lett.) 2017, 30, 438–446. [Google Scholar] [CrossRef]
- Proton, V.; Alexis, J.; Andrieu, E.; Blanc, C.; Delfosse, J.; Lacroix, L.; Odemer, G. Influence of Post-Welding Heat Treatment on the Corrosion Behavior of a 2050-T3 Aluminum-Copper-Lithium Alloy Friction Stir Welding Joint. J. Electrochem. Soc. 2011, 158, C139. [Google Scholar] [CrossRef]
- Proton, V.; Alexis, J.; Andrieu, E.; Delfosse, J.; Lafont, M.C.; Blanc, C. Characterisation and understanding of the corrosion behaviour of the nugget in a 2050 aluminium alloy Friction Stir Welding joint. Corros. Sci. 2013, 73, 130–142. [Google Scholar] [CrossRef] [Green Version]
- Gnedenkov, A.; Sinebryukhov, S.; Mashtalyar, D.; Vyaliy, I.; Egorkin, V.; Gnedenkov, S. Corrosion of the Welded Aluminium Alloy in 0.5 M NaCl Solution. Part 1: Specificity of Development. Materials (Basel) 2018, 11, 2053. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Vyaliy, I.E.; Egorkin, V.S.; Gnedenkov, S.V. Corrosion of the welded aluminium alloy in 0.5 M NaCl solution. Part 2: Coating protection. Materials (Basel) 2018, 11, 2177. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Gnedenkov, S.V. Localized corrosion of the Mg alloys with inhibitor-containing coatings: SVET and SIET studies. Corros. Sci. 2016, 102, 269–278. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Gnedenkov, S.V. Inhibitor-Containing Composite Coatings on Mg Alloys: Corrosion Mechanism and Self-Healing Protection. Solid State Phenom. 2016, 245, 89–96. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Gnedenkov, S.V. Features of the magnesium alloys corrosion in the chloride-containing media. Solid State Phenom. 2014, 213, 143–148. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Gnedenkov, S.V. Features of the corrosion processes development at the magnesium alloys surface. Surf. Coat. Technol. 2013, 225, 112–118. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Gnedenkov, S.V. Microscale morphology and properties of the PEO-coating surface. Phys. Procedia 2012, 23, 98–101. [Google Scholar] [CrossRef] [Green Version]
- Mohedano, M.; Serdechnova, M.; Starykevich, M.; Karpushenkov, S.; Bouali, A.C.; Ferreira, M.G.S.; Zheludkevich, M.L. Active protective PEO coatings on AA2024: Role of voltage on in-situ LDH growth. Mater. Des. 2017, 120, 36–46. [Google Scholar] [CrossRef]
- Williams, G.; McMurray, H.N.; Grace, R. Inhibition of magnesium localised corrosion in chloride containing electrolyte. Electrochim. Acta 2010, 55, 7824–7833. [Google Scholar] [CrossRef]
- Kirkland, N.T.; Williams, G.; Birbilis, N. Observations of the galvanostatic dissolution of pure magnesium. Corros. Sci. 2012, 65, 5–9. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Pampliega, A.; Lamaka, S.V.; Taryba, M.G.; Madani, M.; De Strycker, J.; Tourwé, E.; Ferreira, M.G.S.; Terryn, H. Cut-edge corrosion study on painted aluminum rich metallic coated steel by scanning vibrating electrode and micro-potentiometric techniques. Electrochim. Acta 2012, 61, 107–117. [Google Scholar] [CrossRef]
- Yan, M.; Gelling, V.J.; Hinderliter, B.R.; Battocchi, D.; Tallman, D.E.; Bierwagen, G.P. SVET method for characterizing anti-corrosion performance of metal-rich coatings. Corros. Sci. 2010, 52, 2636–2642. [Google Scholar] [CrossRef]
- Falcón, J.M.; Otubo, L.M.; Aoki, I.V. Highly ordered mesoporous silica loaded with dodecylamine for smart anticorrosion coatings. Surf. Coat. Technol. 2016, 303, 319–329. [Google Scholar] [CrossRef]
- Nardeli, J.V.; Snihirova, D.V.; Fugivara, C.S.; Montemor, M.F.; Pinto, E.R.P.; Messaddecq, Y.; Benedetti, A.V. Localised corrosion assessement of crambe-oil-based polyurethane coatings applied on the ASTM 1200 aluminum alloy. Corros. Sci. 2016, 111, 422–435. [Google Scholar] [CrossRef] [Green Version]
- Gnedenkov, S.V.; Sinebryukhov, S.L.; Egorkin, V.S.; Mashtalyar, D.V.; Vyaliy, I.E.; Nadaraia, K.V.; Imshinetskiy, I.M.; Nikitin, A.I.; Subbotin, E.P.; Gnedenkov, A.S. Magnesium fabricated using additive technology: Specificity of corrosion and protection. J. Alloy. Compd. 2019, 151629. [Google Scholar] [CrossRef]
- Němcová, A.; Skeldon, P.; Thompson, G.E.; Morse, S.; Čížek, J.; Pacal, B. Influence of plasma electrolytic oxidation on fatigue performance of AZ61 magnesium alloy. Corros. Sci. 2014, 82, 58–66. [Google Scholar] [CrossRef]
- Gnedenkov, S.V.; Khrisanfova, O.A.; Sinebryukhov, S.L.; Puz, A.V.; Gnedenkov, A.S. Composite protective coatings on nitinol surface. Mater. Manuf. Process. 2008, 23. [Google Scholar] [CrossRef]
- Sinebryukhov, S.L.; Gnedenkov, A.S.; Khrisanfova, O.A.; Gnedenkov, S.V. Influence of plasma electrolytic oxidation on mechanical characteristics of NiTi alloy. Surf. Eng. 2009, 25, 565–569. [Google Scholar] [CrossRef]
- Gnedenkov, S.V.; Sinebryukhov, S.L.; Puz, A.V.; Gnedenkov, A.S.; Vyaliy, I.E.; Mashtalyar, D.V.; Egorkin, V.S. Plasma electrolytic oxidation coatings on titanium formed with microsecond current pulses. Solid State Phenom. 2014, 213, 149–153. [Google Scholar] [CrossRef]
- Sun, M.; Yerokhin, A.; Bychkova, M.Y.; Shtansky, D.V.; Levashov, E.A.; Matthews, A. Self-healing plasma electrolytic oxidation coatings doped with benzotriazole loaded halloysite nanotubes on AM50 magnesium alloy. Corros. Sci. 2016, 111, 753–769. [Google Scholar] [CrossRef]
- Egorkin, V.S.; Gnedenkov, S.V.; Sinebryukhov, S.L.; Vyaliy, I.E.; Gnedenkov, A.S.; Chizhikov, R.G. Increasing thickness and protective properties of PEO-coatings on aluminum alloy. Surf. Coat. Technol. 2018, 334, 29–42. [Google Scholar] [CrossRef]
- Matykina, E.; Arrabal, R.; Mohedano, M.; Mingo, B.; Gonzalez, J.; Pardo, A.; Merino, M.C. Recent advances in energy efficient PEO processing of aluminium alloys. Trans. Nonferrous Met. Soc. China 2017, 27, 1439–1454. [Google Scholar] [CrossRef]
- Dehnavi, V.; Shoesmith, D.W.; Luan, B.L.; Yari, M.; Liu, X.Y.; Rohani, S. Corrosion properties of plasma electrolytic oxidation coatings on an aluminium alloy-The effect of the PEO process stage. Mater. Chem. Phys. 2015, 161, 49–58. [Google Scholar] [CrossRef]
- Kasalica, B.; Radić-Perić, J.; Perić, M.; Petković-Benazzouz, M.; Belča, I.; Sarvan, M. The mechanism of evolution of microdischarges at the beginning of the PEO process on aluminum. Surf. Coat. Technol. 2016, 298, 24–32. [Google Scholar] [CrossRef]
- Mashtalyar, D.V.; Gnedenkov, S.V.; Sinebryukhov, S.L.; Imshinetskiy, I.M.; Gnedenkov, A.S.; Bouznik, V.M. Composite coatings formed using plasma electrolytic oxidation and fluoroparaffin materials. J. Alloy. Compd. 2018, 767, 353–360. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Gnedenkov, S.V. Protective properties of inhibitor-containing composite coatings on a Mg alloy. Corros. Sci. 2016, 102, 348–354. [Google Scholar] [CrossRef]
- Overview of Mechanical Testing Standards; Randall, N. (Ed.) CSM Instruments Applications Bulletin: Peseux, Switzerland, 2002; p. 3. [Google Scholar]
- Peng, Z.; Li, J.; Sang, F.; Chen, Y.; Zhang, X.; Zheng, Z.; Pan, Q. Structures and tensile properties of Sc-containing 1445 Al-Li alloy sheet. J. Alloy. Compd. 2018, 747, 471–483. [Google Scholar] [CrossRef]
- Seidman, D.N.; Marquis, E.A.; Dunand, D.C. Precipitation strengthening at ambient and elevated temperatures of heat-treatable Al(Sc) alloys. Acta Mater. 2002, 50, 4021–4035. [Google Scholar] [CrossRef]
- Novotny, G.M.; Ardell, A.J. Precipitation of Al3Sc in binary Al-Sc alloys. Mater. Sci. Eng. A 2001, 318, 144–154. [Google Scholar] [CrossRef]
- Yin, Z.; Pan, Q.; Zhang, Y.; Jiang, F. Effect of minor Sc and Zr on the microstructure and mechanical properties of Al-Mg based alloys. Mater. Sci. Eng. A 2000, 280, 151–155. [Google Scholar] [CrossRef]
- Ralston, K.D.; Fabijanic, D.; Birbilis, N. Effect of grain size on corrosion of high purity aluminium. Electrochim. Acta 2011, 56, 1729–1736. [Google Scholar] [CrossRef]
- Cavanaugh, M.K.; Birbilis, N.; Buchheit, R.G.; Bovard, F. Investigating localized corrosion susceptibility arising from Sc containing intermetallic Al3Sc in high strength Al-alloys. Scr. Mater. 2007, 56, 995–998. [Google Scholar] [CrossRef]
- Xia, X. Precipitation and recrystallization in Al-Mn AA3104 alloy. Scr. Met. Mater. 1993, 28, 1213–1218. [Google Scholar] [CrossRef]
- Vlach, M.; Stulikova, I.; Smola, B.; Kekule, T.; Kudrnova, H.; Danis, S.; Gemma, R.; Ocenasek, V.; Malek, J.; Tanprayoon, D.; et al. Precipitation in cold-rolled Al-Sc-Zr and Al-Mn-Sc-Zr alloys prepared by powder metallurgy. Mater. Charact. 2013, 86, 59–68. [Google Scholar] [CrossRef]
- Liu, D.; Wang, J.; Li, J. Microstructures evolution and mechanical properties disparity in 2070 Al-Li alloy with minor Sc addition. Trans. Nonferrous Met. Soc. China 2018, 28, 2151–2161. [Google Scholar] [CrossRef]
- Harada, Y.; Dunand, D. Microstructure of Al3Sc with ternary transition-metal additions. Mater. Sci. Eng. A 2002, 329–331, 686–695. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Chen, X.G. Effect of magnesium on dispersoid strengthening of Al-Mn-Mg-Si (3xxx) alloys. Trans. Nonferrous Met. Soc. China 2016, 26, 2793–2799. [Google Scholar] [CrossRef]
- Liu, K.; Nabawy, A.M.; Chen, X.G. Influence of TiB2 nanoparticles on elevated-temperature properties of Al-Mn-Mg 3004 alloy. Trans. Nonferrous Met. Soc. China 2017, 27, 771–778. [Google Scholar] [CrossRef]
- Lucadamo, G.; Yang, N.Y.C.; Marchi, C.S.; Lavernia, E.J. Microstructure characterization in cryomilled Al 5083. Mater. Sci. Eng. A 2006, 430, 230–241. [Google Scholar] [CrossRef]
- Lyndon, J.A.; Gupta, R.K.; Gibson, M.A.; Birbilis, N. Electrochemical behaviour of the β-phase intermetallic (Mg2Al3) as a function of pH as relevant to corrosion of aluminium-magnesium alloys. Corros. Sci. 2013, 70, 290–293. [Google Scholar] [CrossRef]
- Aluminum-Magnesium (5000) Alloys. Available online: http://www.totalmateria.com/Article75.htm (accessed on 1 May 2003).
- Ghali, E. Corrosion Resistance of Aluminum and Magnesium Alloys: Understanding, Performance, and Testing; John Wiley: Hoboken, NJ, USA, 2010; ISBN 9783527330010. [Google Scholar]
- Wong, T.W.; Hadadzadeh, A.; Benoit, M.J.; Wells, M.A. Impact of homogenization heat treatment on the high temperature deformation behavior of cast AZ31B magnesium alloy. J. Mater. Process. Technol. 2018, 254, 238–247. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, P.; Peng, Y.; Du, Y.; Sundman, B.; Long, J.; Xu, T.; Zhang, Z. A thermodynamic description of the Al-Co-Ni system and site occupancy in Co + AlNi3 composite binder phase. J. Alloy. Compd. 2016, 687, 855–866. [Google Scholar] [CrossRef]
- Birbilis, N.; Buchheit, R.G. Electrochemical Characteristics of Intermetallic Phases in Aluminum Alloys. J. Electrochem. Soc. 2005, 152, B140. [Google Scholar] [CrossRef]
- Huang, Z.L.; Wang, K.; Zhang, Z.M.; Li, B.; Xue, H.S.; Yang, D.Z. Effects of Mg content on primary Mg2Si phase in hypereutectic Al-Si alloys. Trans. Nonferrous Met. Soc. China 2015, 25, 3197–3203. [Google Scholar] [CrossRef]
- Linardi, E.; Haddad, R.; Lanzani, L. Stability Analysis of the Mg2Si Phase in AA 6061 Aluminum Alloy. Procedia Mater. Sci. 2012, 1, 550–557. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, B.; Zhou, X.; Wang, J.; Hashimoto, T.; Luo, C.; Sun, Z.; Tang, Z.; Lu, F. Laser welding introduced segregation and its influence on the corrosion behaviour of Al-Cu-Li alloy. Corros. Sci. 2018, 135, 177–191. [Google Scholar] [CrossRef]
- Sinhmar, S.; Dwivedi, D.K. A study on corrosion behavior of friction stir welded and tungsten inert gas welded AA2014 aluminium alloy. Corros. Sci. 2018, 133, 25–35. [Google Scholar] [CrossRef]
- Li, S.; Dong, H.; Shi, L.; Li, P.; Ye, F. Corrosion behavior and mechanical properties of Al-Zn-Mg aluminum alloy weld. Corros. Sci. 2017, 123, 243–255. [Google Scholar] [CrossRef]
- Yan, S.; Chen, H.; Ma, C.; Nie, Y.; Wang, X.; Qin, Q.H. Local corrosion behaviour of hybrid laser-MIG welded Al-Zn-Mg alloy joints. Mater. Des. 2015, 88, 1353–1365. [Google Scholar] [CrossRef]
- Wu, K.; Yuan, X.; Li, T.; Wang, H.; Xu, C.; Luo, J. Effect of ultrasonic vibration on TIG welding–brazing joining of aluminum alloy to steel. J. Mater. Process. Technol. 2019, 266, 230–238. [Google Scholar] [CrossRef]
- Ardeshiri, A.; Sohi, M.H.; Safaei, A. Surface alloying of A2618 aluminum with silicon and iron by TIG process. Surf. Coat. Technol. 2017, 310, 87–92. [Google Scholar] [CrossRef]
- Coy, A.E.; Viejo, F.; Skeldon, P.; Thompson, G.E. Susceptibility of rare-earth-magnesium alloys to micro-galvanic corrosion. Corros. Sci. 2010, 52, 3896–3906. [Google Scholar] [CrossRef]
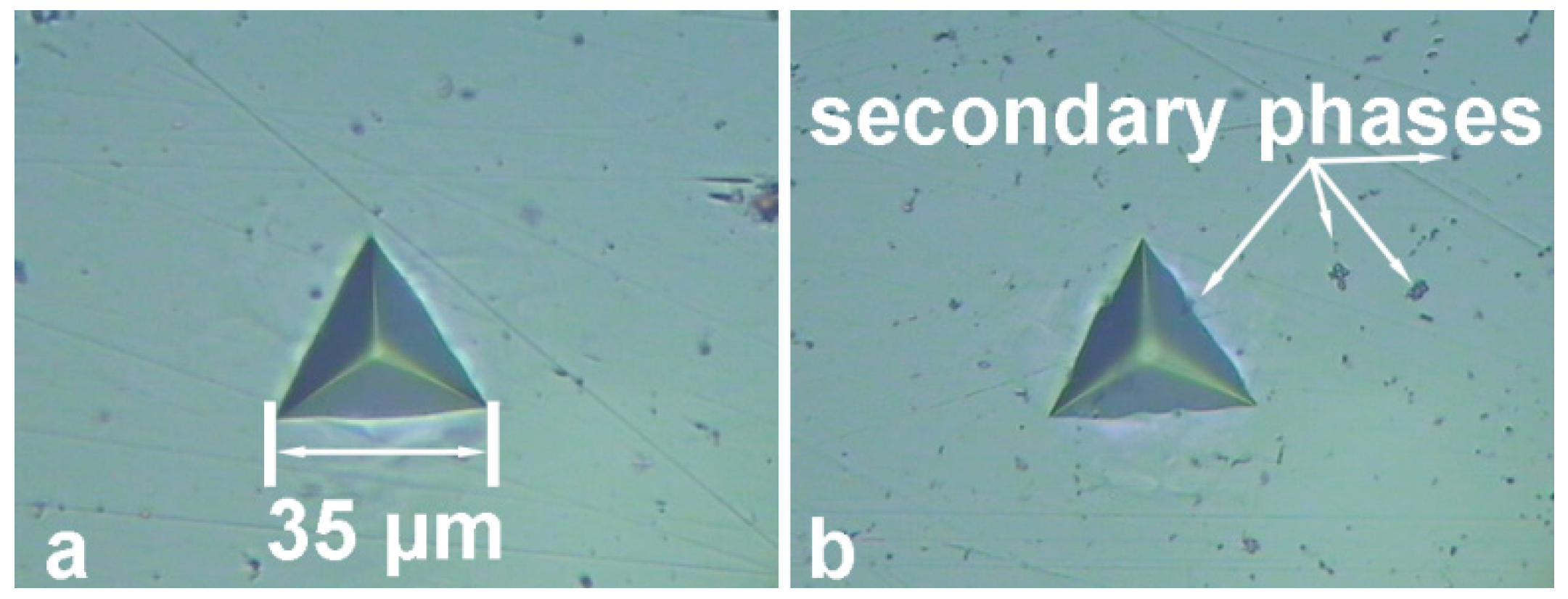
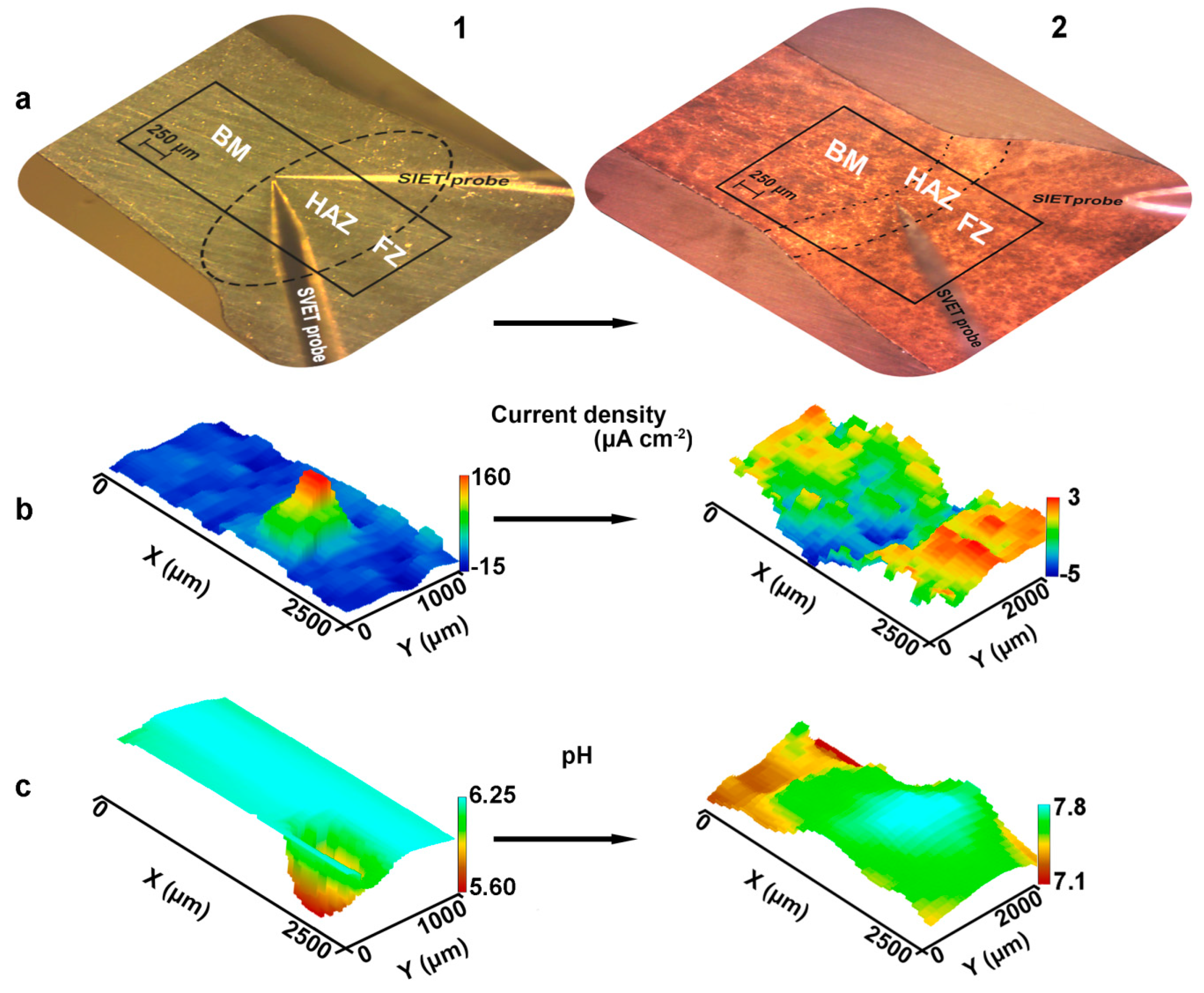
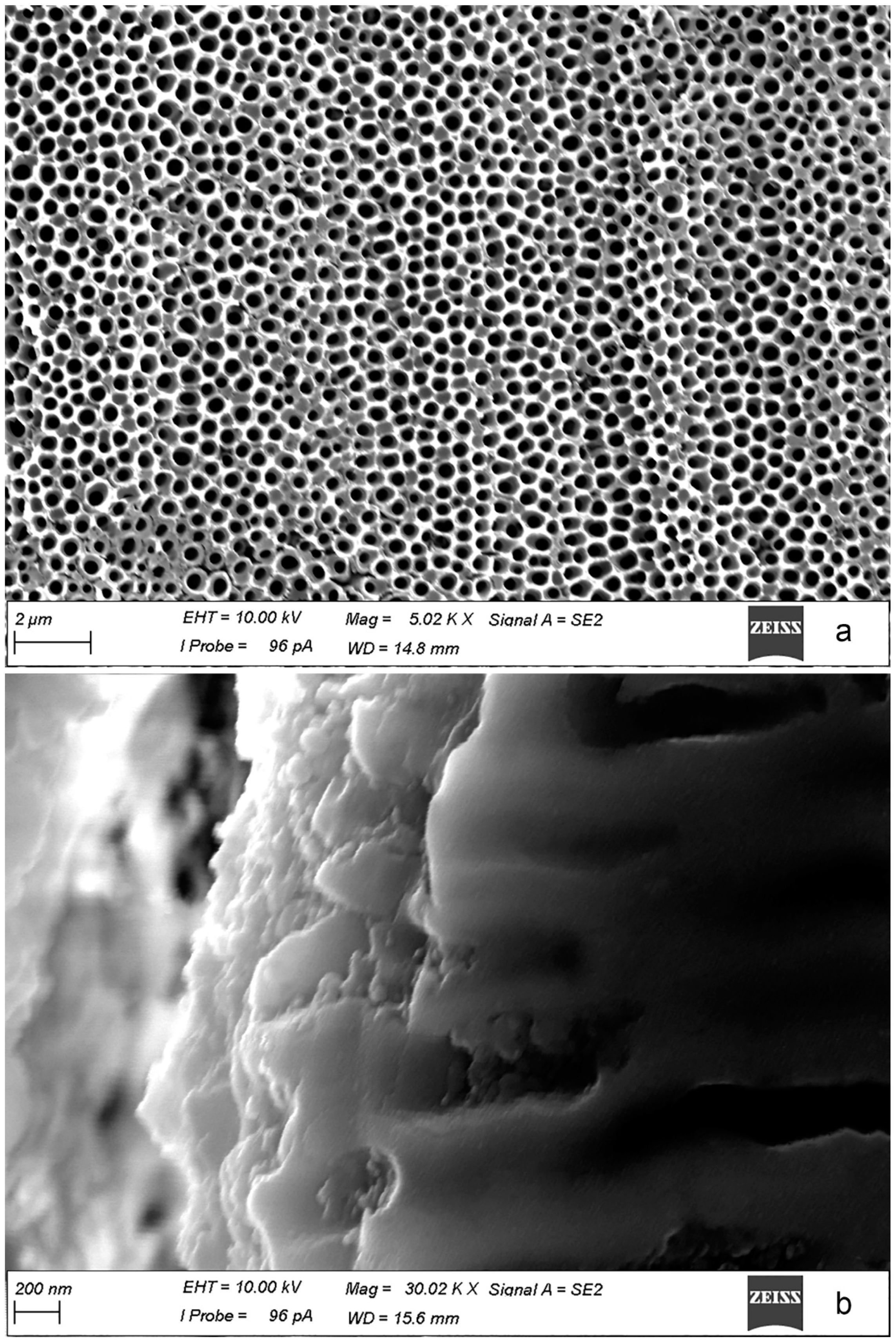
| Element | Al | Mg | Si | Mn | Zn | Cr | Fe | Cu | Sc | Zr | Ni | Ti |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wt.% | balance | 6.78 | 0.51 | 0.30 | 0.62 | 0.17 | 0.15 | 0.14 | 0.13 | 0.13 | 0.10 | 0.02 |
| Phases | Al | Mg2Al3 | Mg2Si | Al3Fe | Al6Mn | Al3Ti | Al3Zr |
|---|---|---|---|---|---|---|---|
| Corrosion potential, mVSCE | −849 | −1162 | −1536 | −566 | −913 | −799 | −801 |
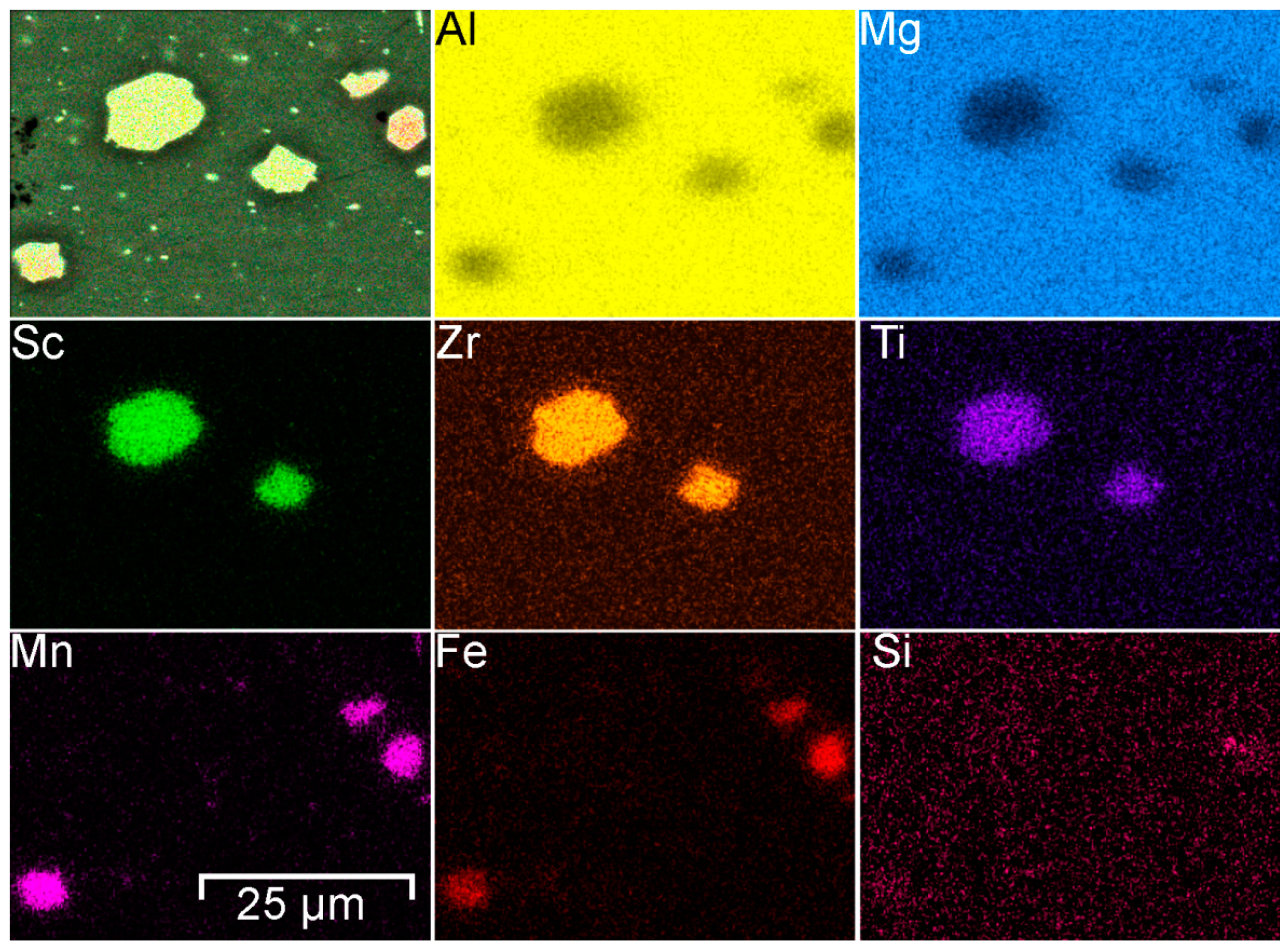
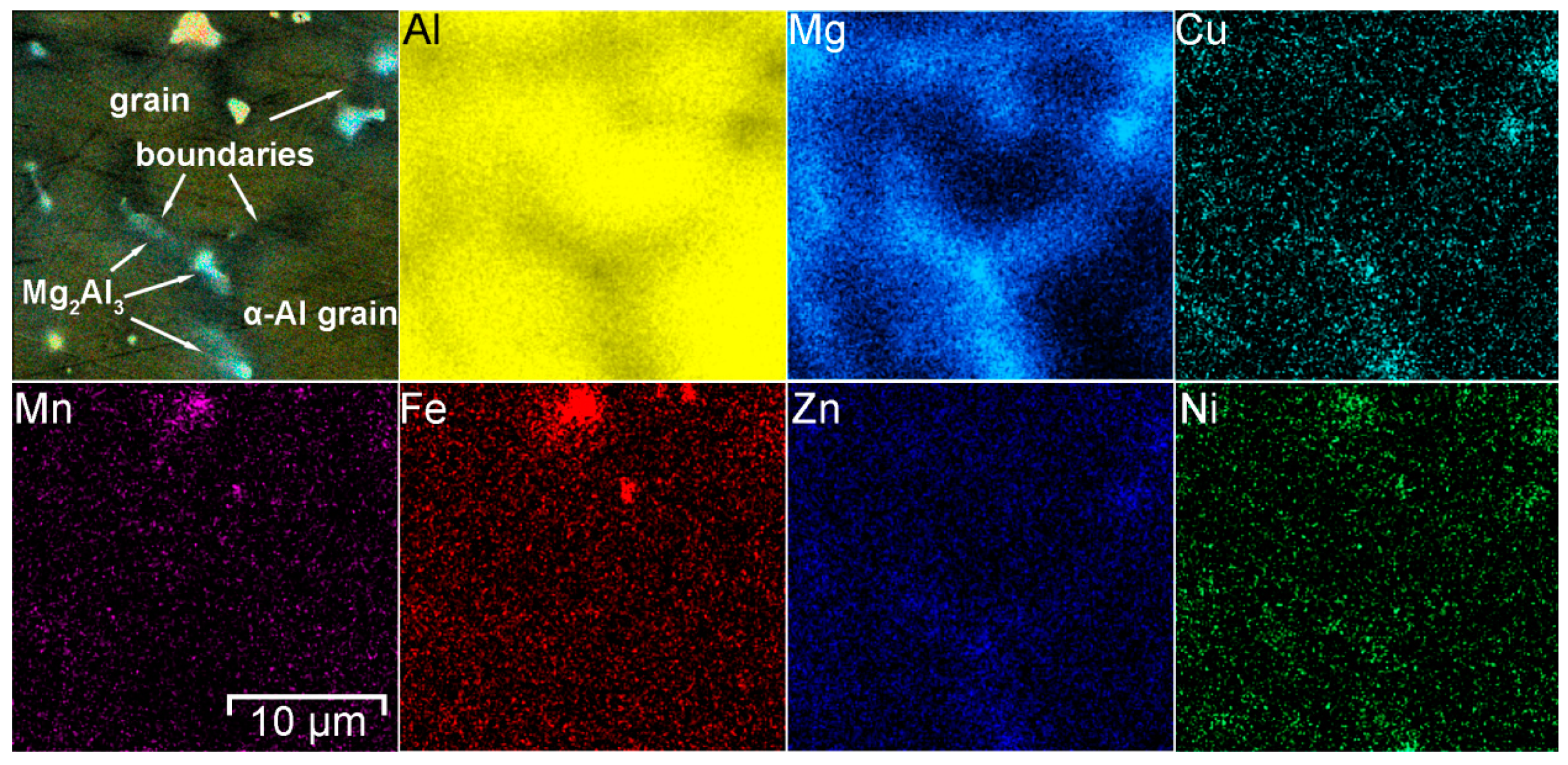
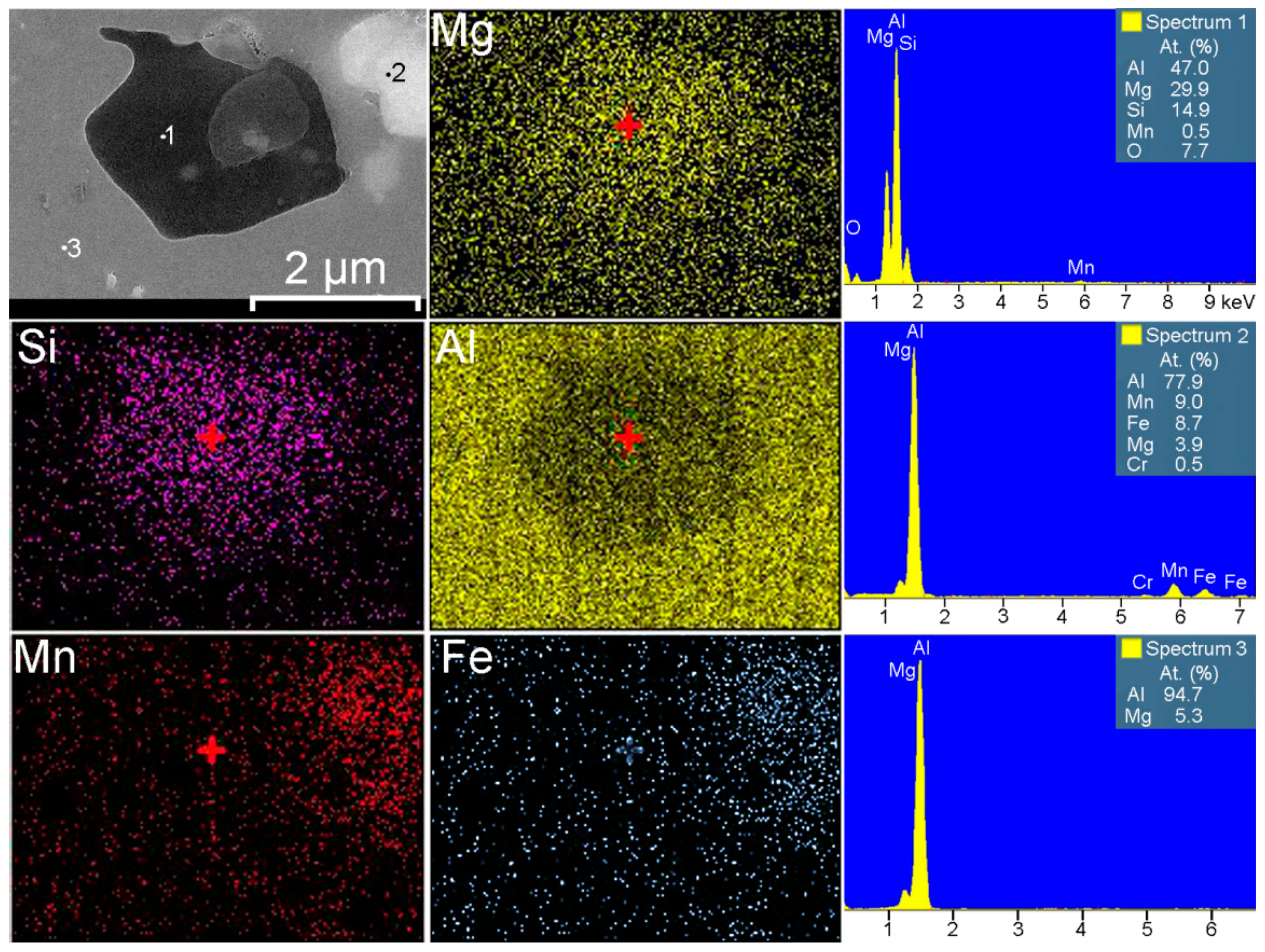
| Phases | α-Al | Mg2Al3 | Mg2Si | (MnFe)Al6 | Al3(ScZr) | |
|---|---|---|---|---|---|---|
| Size, µm | 16 ± 3 | 3 ± 1 | 2 ± 1 | 6 ± 3 | 9 ± 2 | |
| EDX analysis, at.% | Al | 94.7 | 59.2 | 47.0 | 77.9 | 58.1 |
| Mg | 5.3 | 38.9 | 29.9 | 3.9 | – | |
| Si | – | – | 14.9 | – | – | |
| Mn | – | – | 0.5 | 9.0 | – | |
| Fe | – | – | – | 8.7 | – | |
| Sc | – | – | – | – | 18.9 | |
| Zr | – | – | – | – | 18.7 | |
| Ti | – | – | – | – | 4.3 | |
| Cr | – | – | – | 0.5 | – | |
| Zn | – | 1.4 | – | – | – | |
| Cu | – | 0.5 | – | – | – | |
| O | – | – | 7.7 | – | – |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gnedenkov, A.S.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Imshinetskiy, I.M.; Vyaliy, I.E.; Gnedenkov, S.V. Effect of Microstructure on the Corrosion Resistance of TIG Welded 1579 Alloy. Materials 2019, 12, 2615. https://doi.org/10.3390/ma12162615
Gnedenkov AS, Sinebryukhov SL, Mashtalyar DV, Imshinetskiy IM, Vyaliy IE, Gnedenkov SV. Effect of Microstructure on the Corrosion Resistance of TIG Welded 1579 Alloy. Materials. 2019; 12(16):2615. https://doi.org/10.3390/ma12162615
Chicago/Turabian StyleGnedenkov, Andrey S., Sergey L. Sinebryukhov, Dmitry V. Mashtalyar, Igor M. Imshinetskiy, Igor E. Vyaliy, and Sergey V. Gnedenkov. 2019. "Effect of Microstructure on the Corrosion Resistance of TIG Welded 1579 Alloy" Materials 12, no. 16: 2615. https://doi.org/10.3390/ma12162615