Zeolite Adsorption of Chloride from a Synthetic Alkali-Activated Cement Pore Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. X-ray Diffraction (XRD)
2.2.2. Energy- and Wavelength-Dispersive X-ray Spectroscopy (EDS and WDS)
3. Results
3.1. XRD
3.2. EDS and WDS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Osio-Norgaard, J.; Gevaudan, J.P.; Srubar, W.V. A Review of Chloride Transport in Alkali-Activated Cement Paste, Mortar, and Concrete. Constr. Build. Mater. 2018, 186, 191–206. [Google Scholar] [CrossRef]
- Machner, A.; Zajac, M.; Ben Haha, M.; Kjellsen, K.O.; Geiker, M.R.; De Weerdt, K. Chloride-Binding Capacity of Hydrotalcite in Cement Pastes Containing Dolomite and Metakaolin. Cem. Concr. Res. 2018, 107, 163–181. [Google Scholar] [CrossRef]
- Birnin-Yauri, U.; Glasser, F. Friedel?S Salt, Ca2Al(OH)6(Cl,OH)·2H2O: Its Solid Solutions and Their Role in Chloride Binding. Cem. Concr. Res. 1998, 28, 1713–1723. [Google Scholar] [CrossRef]
- Ke, X.; Bernal, S.A.; Provis, J. Assessing the Chloride Binding Capacity of Synthetic Cementitious Phases in Alkali- Activated Slag Simulated Pore Solution. In Proceedings of the 1st International Conference of Construction Materials for Sustainable Future, Zadar, Croatia, 19–21 April 2017. [Google Scholar]
- Vujaković, A.D.; Tomašević-Čanović, M.R.; Daković, A.S.; Dondur, V.T. The Adsorption of Sulphate, Hydrogenchromate and Dihydrogenphosphate Anions on Surfactant-Modified Clinoptilolite. Appl. Clay Sci. 2000, 17, 265–277. [Google Scholar] [CrossRef]
- NING, P.; BART, H.-J.; LI, B.; LU, X.; ZHANG, Y. Phosphate Removal from Wastewater by Model-La(III) Zeolite Adsorbents. J. Environ. Sci. 2008, 20, 670–674. [Google Scholar] [CrossRef]
- Gevaudan, J.P.; Campbell, K.M.; Kane, T.J.; Shoemaker, R.K.; Srubar, W.V.; Srubar, W.V., III. Mineralization Dynamics of Metakaolin-Based Alkali-Activated Cements. Cem. Concr. Res. 2017, 94, 1–12. [Google Scholar] [CrossRef]
- Jun, Y.; Yoon, S.; Oh, J.E. A Comparison Study for Chloride-Binding Capacity between Alkali-Activated Fly Ash and Slag in the Use of Seawater. Appl. Sci. 2017, 7, 971. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; Van Deventer, J.S.J. Geopolymer Technology: The Current State of the Art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Ruiz-Santaquiteria, C.; Fernández-Jiménez, A.; Skibsted, J.; Palomo, A. Clay Reactivity: Production of Alkali Activated Cements. Appl. Clay Sci. 2013, 73, 11–16. [Google Scholar] [CrossRef]
- Ruiz-Santaquiteria, C.; Skibsted, J.; Fernández-Jiménez, A.; Palomo, A. Alkaline Solution/Binder Ratio as a Determining Factor in the Alkaline Activation of Aluminosilicates. Cem. Concr. Res. 2012, 42, 1242–1251. [Google Scholar] [CrossRef]
- Li, N.; Shi, C.; Wang, Q.; Zhang, Z.; Ou, Z. Composition Design and Performance of Alkali-Activated Cements. Mater. Struct. 2017, 50, 178. [Google Scholar] [CrossRef]
- Uzal, B.; Turanli, L. Blended Cements Containing High Volume of Natural Zeolites: Properties, Hydration and Paste Microstructure. Cem. Concr. Compos. 2012, 34, 101–109. [Google Scholar] [CrossRef]
- Xiaodong, S.; Sheng, Y.; Xuequan, W.; Mingshu, T.; Liji, Y. Immobilization of Stimulated High Level Wastes into AASC Waste Form. Cem. Concr. Res. 1994, 24, 133–138. [Google Scholar] [CrossRef]
- Vigil de la Villa, R.; Fernández, R.; García, R.; Villar-Cociña, E.; Frías, M. Pozzolanic Activity and Alkaline Reactivity of a Mordenite-Rich Tuff. Microporous Mesoporous Mater. 2009, 126, 125–132. [Google Scholar] [CrossRef]
- Hu, M.; Zhu, X.; Long, F. Alkali-Activated Fly Ash-Based Geopolymers with Zeolite or Bentonite as Additives. Cem. Concr. Compos. 2009, 31, 762–768. [Google Scholar] [CrossRef]
- Rodríguez, E.D.; Bernal, S.A.; Provis, J.L.; Gehman, J.D.; Monzó, J.M.; Payá, J.; Borrachero, M.V. Geopolymers Based on Spent Catalyst Residue from a Fluid Catalytic Cracking (FCC) Process. Fuel 2013, 109, 493–502. [Google Scholar] [CrossRef]
- Cornejo, M.H.; Elsen, J.; Togra, B.; Baykara, H.; Soriano, G.; Paredes, C. Effect of Calcium Hydroxide and Water to Solid Ratio on Compressive Strength of Mordenite-Based Geopolymer and the Evaluation of Its Thermal Transmission Property. In Materials Genetics to Structures; ASME: Pittsburgh, Pennsylvania, 2018; Volume 12, p. V012T11A022. [Google Scholar] [CrossRef]
- Lynch, J.L.V.; Baykara, H.; Cornejo, M.; Soriano, G.; Ulloa, N.A. Preparation, Characterization, and Determination of Mechanical and Thermal Stability of Natural Zeolite-Based Foamed Geopolymers. Constr. Build. Mater. 2018, 172, 448–456. [Google Scholar] [CrossRef]
- Rożek, P.; Król, M.; Mozgawa, W. Geopolymer-Zeolite Composites: A Review. J. Clean. Prod. 2019, 230, 557–579. [Google Scholar] [CrossRef]
- Ke, X.; Bernal, S.A.; Provis, J.L. Uptake of Chloride and Carbonate by Mg-Al and Ca-Al Layered Double Hydroxides in Simulated Pore Solutions of Alkali-Activated Slag Cement. Cem. Concr. Res. 2017, 100, 1–13. [Google Scholar] [CrossRef]
- Mundra, S.; Criado, M.; Bernal, S.A.; Provis, J.L. Chloride-Induced Corrosion of Steel Rebars in Simulated Pore Solutions of Alkali-Activated Concretes. Cem. Concr. Res. 2017, 100, 385–397. [Google Scholar] [CrossRef]
- Ke, X.; Provis, J.L.; Bernal, S.A. Structural Ordering of Aged and Hydrothermally Cured Metakaolin Based Potassium Geopolymers. In Rilem Bookseries; Springer: Dordrecht, The Netherlands, 2018; Volume 16, pp. 232–237. [Google Scholar] [CrossRef]
- Lloyd, R.R.; Provis, J.L.; van Deventer, J.S.J. Pore Solution Composition and Alkali Diffusion in Inorganic Polymer Cement. Cem. Concr. Res. 2010, 40, 1386–1392. [Google Scholar] [CrossRef]
- Hermassi, M.; Valderrama, C.; Moreno, N.; Font, O.; Querol, X.; Batis, N.; Cortina, J.L. Powdered Ca-Activated Zeolite for Phosphate Removal from Treated Waste-Water. J. Chem. Technol. Biotechnol. 2016, 91, 1962–1971. [Google Scholar] [CrossRef]
- Castaldi, P.; Santona, L.; Enzo, S.; Melis, P. Sorption Processes and XRD Analysis of a Natural Zeolite Exchanged with Pb 2+, Cd 2+ and Zn 2+ Cations. J. Hazard. Mater. 2008, 156, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Jha, B.; Singh, D.N. Fly Ash Zeolites; Springer: Singapore, 2016; Volume 78. [Google Scholar] [CrossRef]
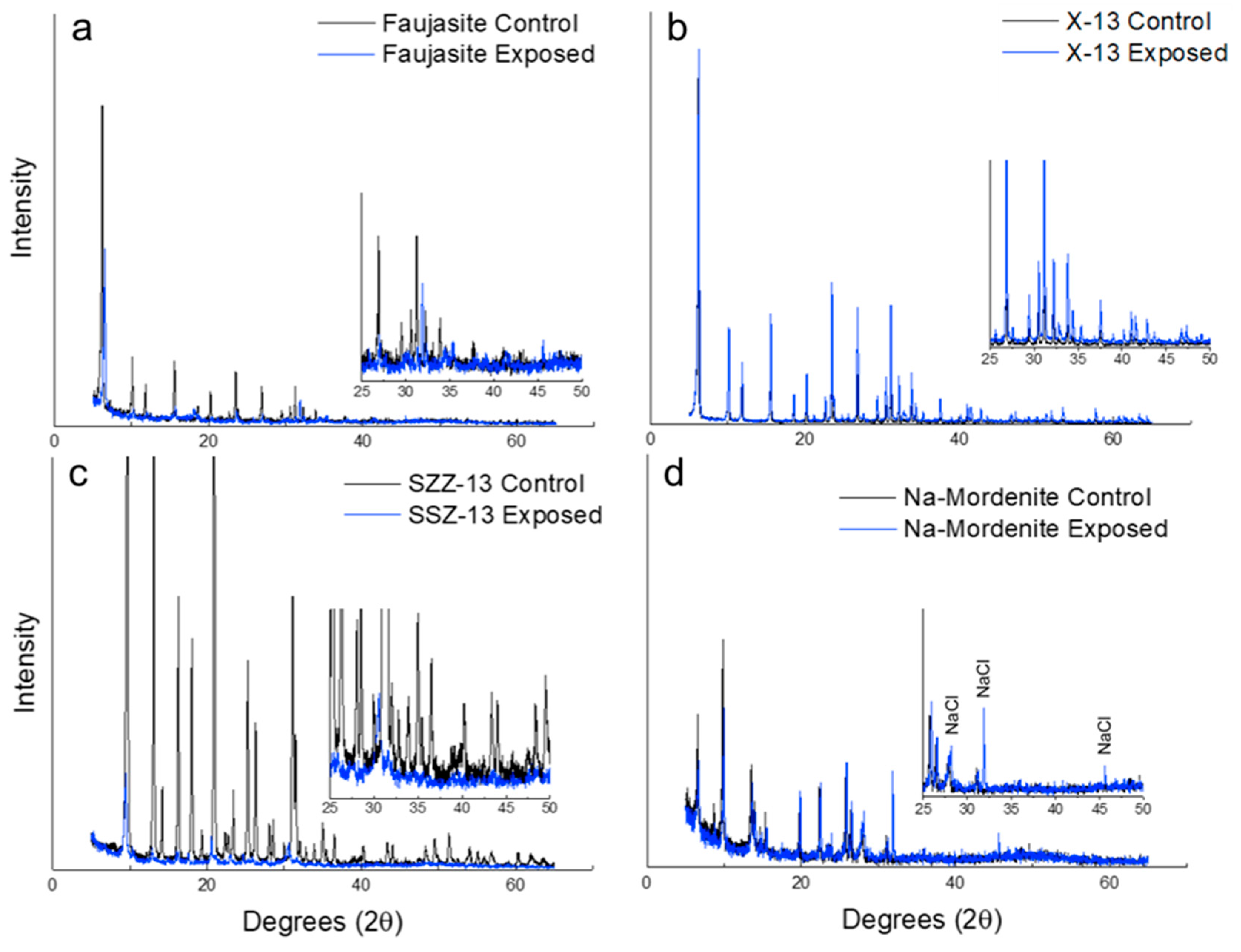
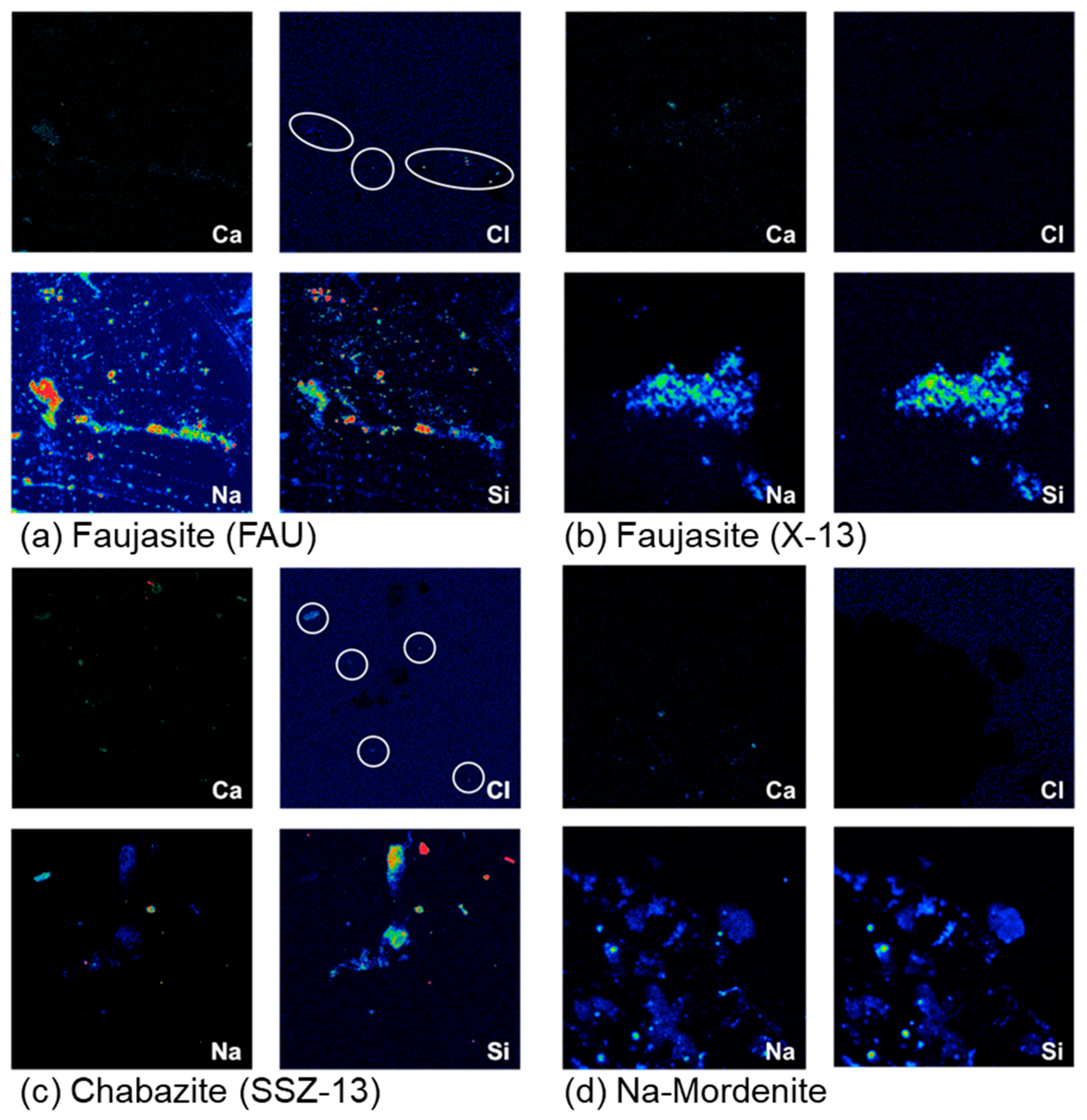
| Sample | Zeolite | XRD Peak Shifts or Broadening | XRD NaCl Presence | EDS Chlorine Presence | WDS Chlorine Presence |
|---|---|---|---|---|---|
| FAU | Faujasite | Yes | No | Yes | Yes |
| X-13 | Faujasite | No | No | No | No |
| SSZ-13 | Chabazite | Yes | No | Yes | Yes |
| Na-Mordenite | Mordenite | No | Yes | No | No |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osio-Norgaard, J.; Srubar, W.V., III. Zeolite Adsorption of Chloride from a Synthetic Alkali-Activated Cement Pore Solution. Materials 2019, 12, 2019. https://doi.org/10.3390/ma12122019
Osio-Norgaard J, Srubar WV III. Zeolite Adsorption of Chloride from a Synthetic Alkali-Activated Cement Pore Solution. Materials. 2019; 12(12):2019. https://doi.org/10.3390/ma12122019
Chicago/Turabian StyleOsio-Norgaard, Jorge, and Wil V. Srubar, III. 2019. "Zeolite Adsorption of Chloride from a Synthetic Alkali-Activated Cement Pore Solution" Materials 12, no. 12: 2019. https://doi.org/10.3390/ma12122019





