The Preparation and Chemical Structure Analysis of Novel POSS-Based Porous Materials
Abstract
:1. Introduction
2. Experimental Part
2.1. Materials
2.1.1. The Synthesis of OPS
2.1.2. The Preparation of the Porous Materials
2.2. Characterization
3. Results and Discussion
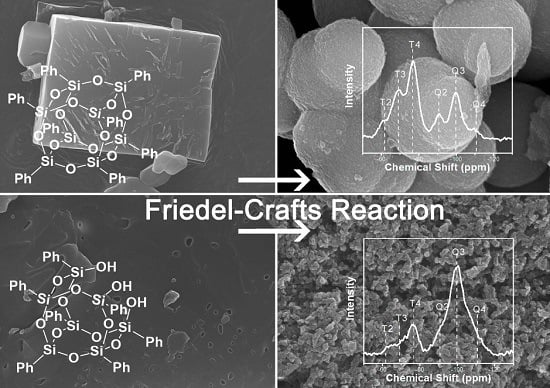
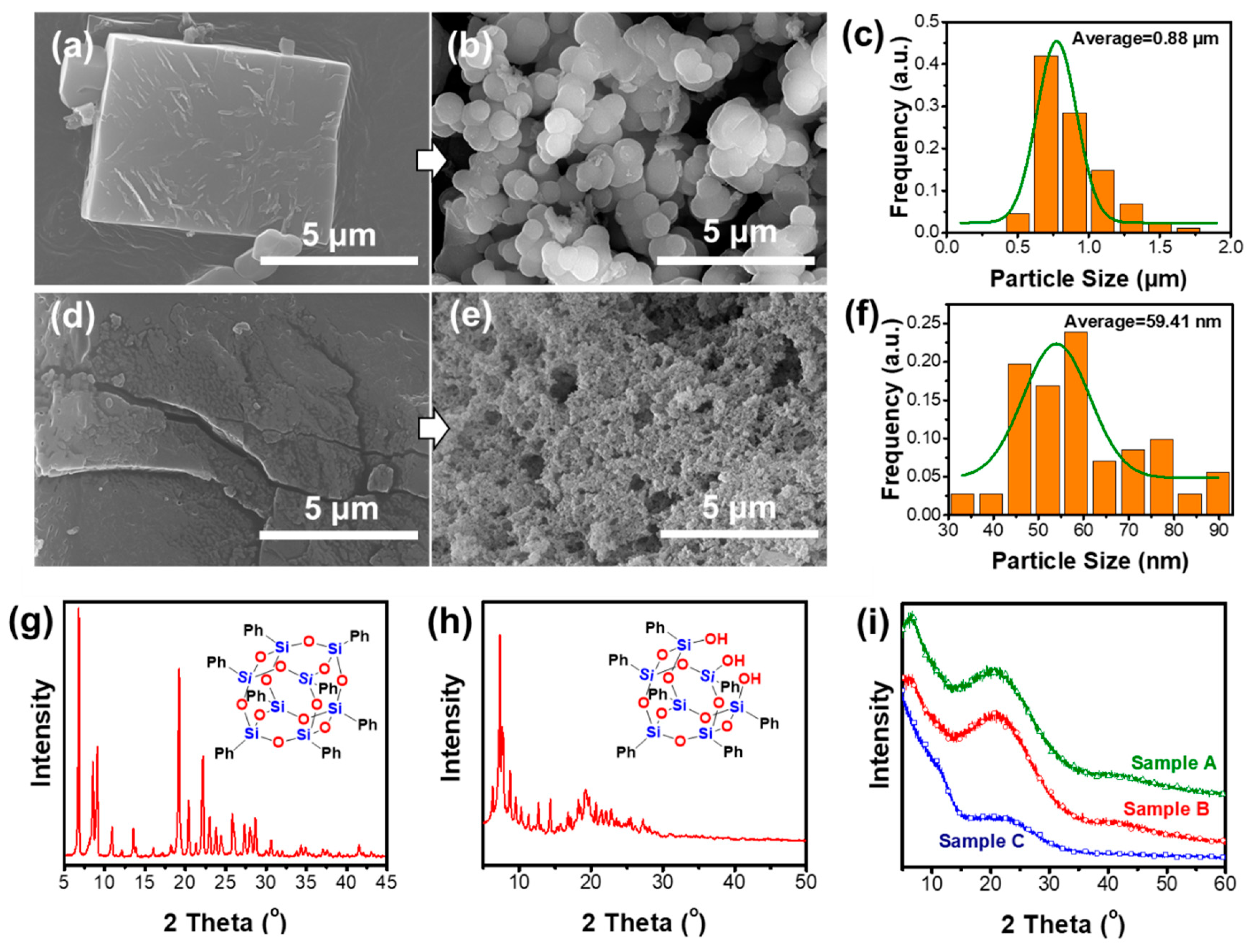
3.1. The Preparation and Morphology Control of the Porous Materials
3.2. The Chemical Structure of the Porous Materials
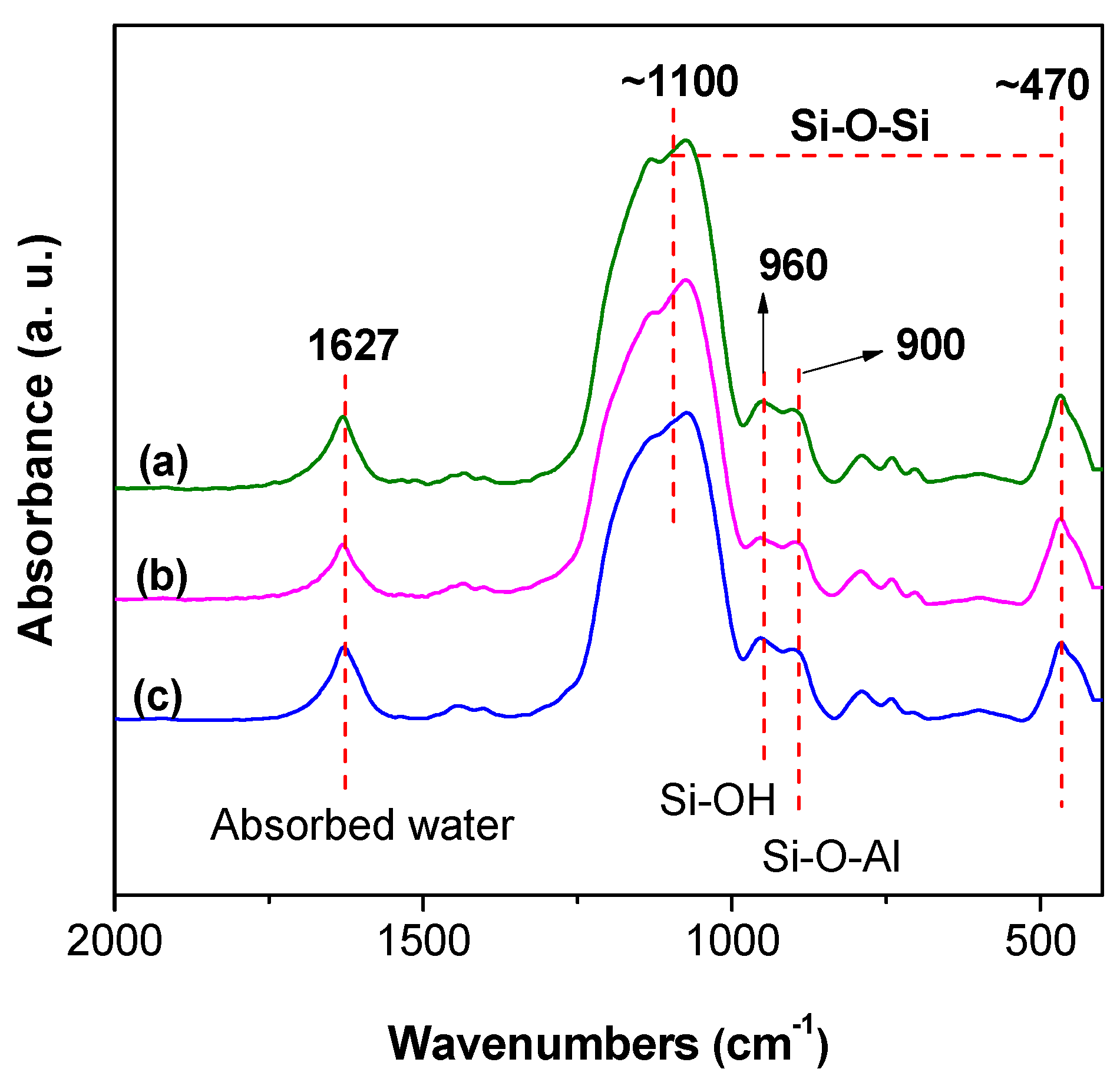
3.2.1. FTIR
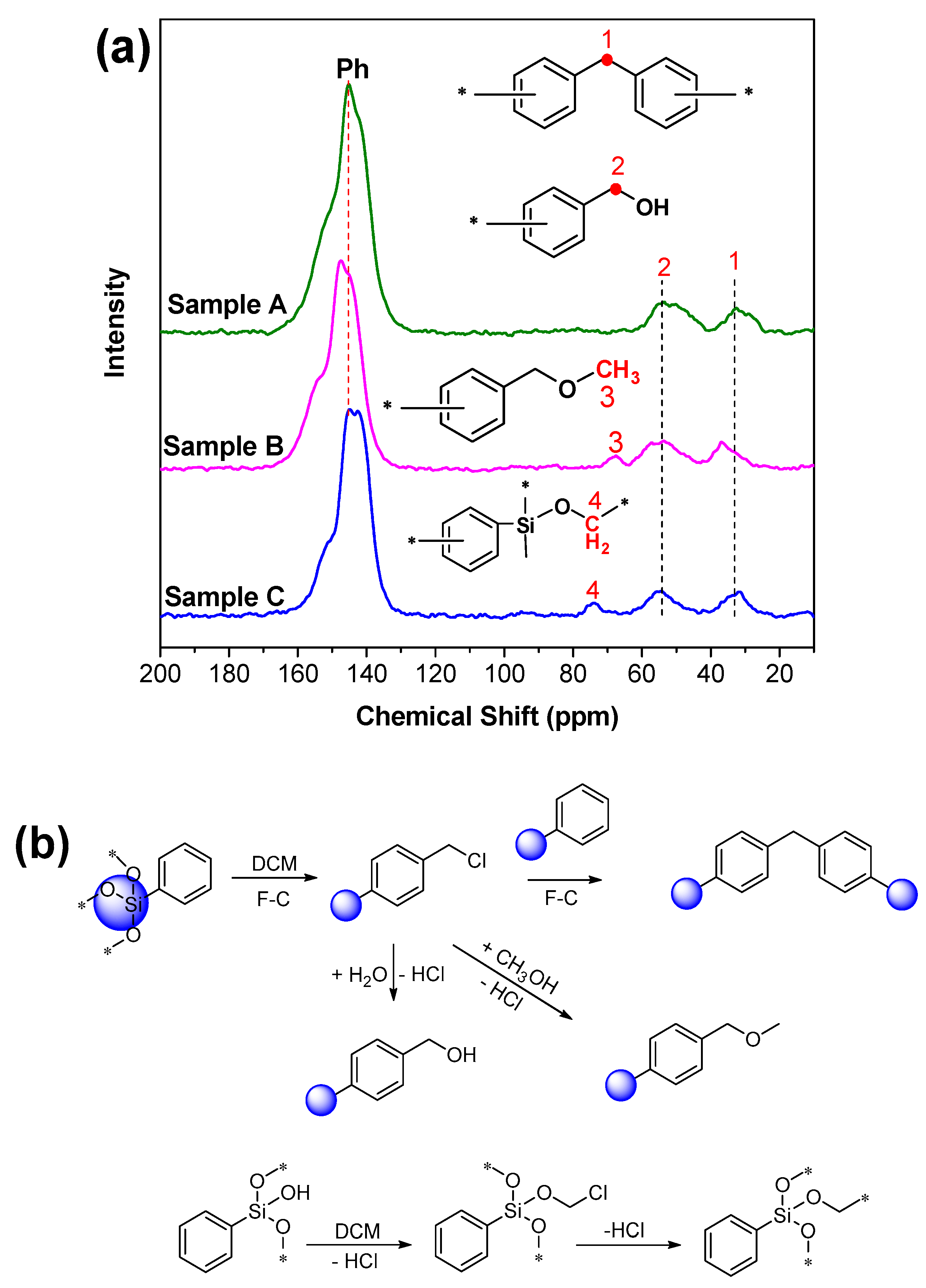
3.2.2. NMR
3.2.3. XPS
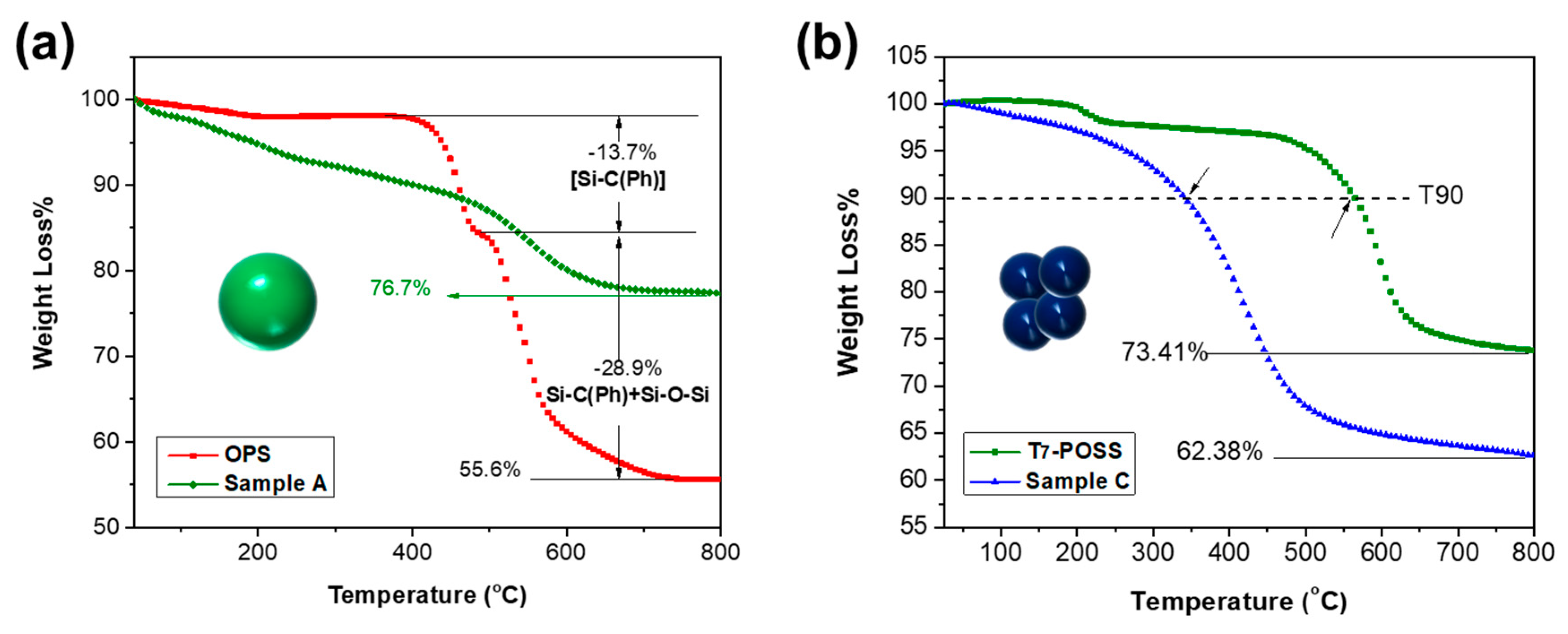
3.3. Thermal Properties (TGA)
3.4. Further Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hasegawa, G.; Kanamori, K.; Ishizuka, N.; Nakanishi, K. New Monolithic Capillary Columns with Well-Defined Macropores Based on Poly(styrene-co-divinylbenzene). ACS Appl. Mater. Interfaces 2012, 4, 2343–2347. [Google Scholar] [CrossRef] [PubMed]
- Yong, Y.-C.; Dong, X.-C.; Chan-Park, M.B.; Song, H.; Chen, P. Macroporous and Monolithic Anode Based on Polyaniline Hybridized Three-Dimensional Graphene for High-Performance Microbial Fuel Cells. ACS Nano 2012, 6, 2394–2400. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Pernites, R.B.; Nitta, N.; Isaacson, M.; Sinsabaugh, S.L.; Wong, M.S.; Biswal, S.L. Freestanding Macroporous Silicon and Pyrolyzed Polyacrylonitrile As a Composite Anode for Lithium Ion Batteries. Chem. Mater. 2012, 24, 2998–3003. [Google Scholar] [CrossRef]
- Cho, C.-Y.; Moon, J.H. Hierarchical Twin-Scale Inverse Opal TiO2 Electrodes for Dye-Sensitized Solar Cells. Langmuir 2012, 28, 9372–9377. [Google Scholar] [CrossRef] [PubMed]
- Tétreault, N.; Arsenault, É.; Heiniger, L.-P.; Soheilnia, N.; Brillet, J.; Moehl, T.; Zakeeruddin, S.; Ozin, G.A.; Grätzel, M. High-Efficiency Dye-Sensitized Solar Cell with Three-Dimensional Photoanode. Nano Lett. 2011, 11, 4579–4584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Sumboja, A.; Khoo, E.; Yan, C.; Lee, P.S. Cryogel Synthesis of Hierarchical Interconnected Macro-/Mesoporous Co3O4 with Superb Electrochemical Energy Storage. J. Phys. Chem. C 2012, 116, 4930–4935. [Google Scholar] [CrossRef]
- Sun, R.; Feng, S.; Wang, D.; Liu, H. Fluorescence-Tuned Silicone Elastomers for Multicolored Ultraviolet Light-Emitting Diodes: Realizing the Processability of Polyhedral Oligomeric Silsesquioxane-Based Hybrid Porous Polymers. Chem. Mater. 2018, 30, 6370–6376. [Google Scholar] [CrossRef]
- Yin, G.; Zhao, D.; Zhang, L.; Ren, Y.; Ji, S.; Tang, H.; Zhou, Z.; Li, Q. Highly porous 3D PLLA materials composed of nanosheets, fibrous nanosheets, or nanofibrous networks: Preparation and the potential application in oil–water separation. Chem. Eng. J. 2016, 302, 1–11. [Google Scholar] [CrossRef]
- Konishi, J.; Fujita, K.; Oiwa, S.; Nakanishi, K.; Hirao, K. Crystalline ZrO2 Monoliths with Well-Defined Macropores and Mesostructured Skeletons Prepared by Combining the Alkoxy-Derived Sol–Gel Process Accompanied by Phase Separation and the Solvothermal Process. Chem. Mater. 2008, 20, 2165–2173. [Google Scholar] [CrossRef]
- Fuertes, M.C.; Soler-Illia, G.J.A.A. Processing of Macroporous Titania Thin Films: From Multiscale Functional Porosity to Nanocrystalline Macroporous TiO2. Chem. Mater. 2006, 18, 2109–2117. [Google Scholar] [CrossRef]
- Toberer, E.S.; Joshi, A.; Seshadri, R. Template-Free Routes to Macroporous Monoliths of Nickel and Iron Oxides: Toward Porous Metals and Conformally Coated Pore Walls. Chem. Mater. 2005, 17, 2142–2147. [Google Scholar] [CrossRef]
- Tokuyama, H.; Kanehara, A. Novel Synthesis of Macroporous Poly(N-isopropylacrylamide) Hydrogels Using Oil-in-Water Emulsions. Langmuir 2007, 23, 11246–11251. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Hu, J.; Peng, C.; Liu, H.; Hu, Y. Synergic Effects of Imidazolium Ionic Liquids on P123 Mixed Micelles for Inducing Micro/Mesoporous Materials. Langmuir 2012, 28, 2950–2959. [Google Scholar] [CrossRef] [PubMed]
- Minaberry, Y.; Jobbágy, M. Macroporous Bioglass Scaffolds Prepared by Coupling Sol–Gel with Freeze Drying. Chem. Mater. 2011, 23, 2327–2332. [Google Scholar] [CrossRef]
- Scholz, S.; Lercher, J.A. Hierarchically Structured Millimeter-Sized (Organo) Silica Spheres with a Macroporous Shell and a Meso/Microporous Core. Chem. Mater. 2011, 23, 2091–2099. [Google Scholar] [CrossRef]
- Catauro, M.; Tranquillo, E.; Barrino, F.; Blanco, I.; Dal Poggetto, F.; Naviglio, D. Drug Release of Hybrid Materials Containing Fe(II)Citrate Synthesized by Sol-Gel Technique. Materials 2018, 11, 2270. [Google Scholar] [CrossRef] [PubMed]
- Esquena, J.; Nestor, J.; Vílchez, A.; Aramaki, K.; Solans, C. Preparation of Mesoporous/Macroporous Materials in Highly Concentrated Emulsions Based on Cubic Phases by a Single-Step Method. Langmuir 2012, 28, 12334–12340. [Google Scholar] [CrossRef] [PubMed]
- Vílchez, S.; Pérez-Carrillo, L.A.; Miras, J.; Solans, C.; Esquena, J. Oil-in-Alcohol Highly Concentrated Emulsions as Templates for the Preparation of Macroporous Materials. Langmuir 2012, 28, 7614–7621. [Google Scholar] [CrossRef]
- Kido, Y.; Nakanishi, K.; Miyasaka, A.; Kanamori, K. Synthesis of Monolithic Hierarchically Porous Iron-Based Xerogels from Iron(III) Salts via an Epoxide-Mediated Sol–Gel Process. Chem. Mater. 2012, 24, 2071–2077. [Google Scholar] [CrossRef]
- Wu, Q.L.; Subramanian, N.; Rankin, S.E. Hierarchically Porous Titania Thin Film Prepared by Controlled Phase Separation and Surfactant Templating. Langmuir 2011, 27, 9557–9566. [Google Scholar] [CrossRef]
- Blanco, I.; Bottino, F.A.; Cicala, G.; Latteri, A.; Recca, A. Synthesis and characterization of differently substituted phenyl hepta isobutyl-polyhedral oligomeric silsesquioxane/polystyrene nanocomposites. Polym. Compos. 2014, 35, 151–157. [Google Scholar] [CrossRef]
- Blanco, I. The Rediscovery of POSS: A Molecule Rather than a Filler. Polymers 2018, 10, 904. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Babonneau, F.; Bonhomme, C.; Laine, R.M.; Soles, C.L.; Hristov, H.A.; Yee, A.F. Highly Porous Polyhedral Silsesquioxane Polymers. Synthesis and Characterization. J. Am. Chem. Soc. 1998, 120, 8380–8391. [Google Scholar] [CrossRef]
- Wang, D.; Li, L.; Yang, W.; Zuo, Y.; Feng, S.; Liu, H. POSS-based luminescent porous polymers for carbon dioxide sorption and nitroaromatic explosives detection. RSC Adv. 2014, 4, 59877–59884. [Google Scholar] [CrossRef]
- Wang, D.; Yang, W.; Feng, S.; Liu, H. Amine post-functionalized POSS-based porous polymers exhibiting simultaneously enhanced porosity and carbon dioxide adsorption properties. RSC Adv. 2016, 6, 13749–13756. [Google Scholar] [CrossRef]
- Wu, Y.; Li, L.; Yang, W.; Feng, S.; Liu, H. Hybrid nanoporous polystyrene derived from cubic octavinylsilsesquioxane and commercial polystyrene via the Friedel–Crafts reaction. RSC Adv. 2015, 5, 12987–12993. [Google Scholar] [CrossRef]
- Chen, G.; Huang, X.; Zhang, Y.; Sun, M.; Shen, J.; Huang, R.; Tong, M.; Long, Z.; Wang, X. Constructing POSS and viologen-linked porous cationic frameworks induced by the Zincke reaction for efficient CO2 capture and conversion. Chem. Commun. 2018, 54, 12174–12177. [Google Scholar] [CrossRef]
- Lou, S.; Di, D. Synthesis of Resins with Ionic Liquids for Purification of Flavonoids from Hippophae rhamnoides L. Leaves. J. Agric. Food Chem. 2012, 60, 6546–6558. [Google Scholar] [CrossRef]
- Yin, G.; Zhang, L.; Li, Q. Preparation and characterization of POSS-crosslinked PCL based hybrid materials. J. Polym. Res. 2016, 23, 138. [Google Scholar] [CrossRef]
- Xi, W.-S.; Song, Z.-M.; Chen, Z.; Chen, N.; Yan, G.-H.; Gao, Y.; Cao, A.; Liu, Y.; Wang, H. Short-term and long-term toxicological effects of vanadium dioxide nanoparticles on A549 cells. Environ. Sci. Nano 2019, 6, 565–579. [Google Scholar] [CrossRef]
- Dong, H.; Brennan, J.D. Macroporous Monolithic Methylsilsesquioxanes Prepared by a Two-Step Acid/Acid Processing Method. Chem. Mater. 2006, 18, 4176–4182. [Google Scholar] [CrossRef]
- Kytökivi, A.; Haukka, S. Reactions of HMDS, TiCl4, ZrCl4, and AlCl3 with Silica As Interpreted from Low-Frequency Diffuse Reflectance Infrared Spectra. J. Phys. Chem. B 1997, 101, 10365–10372. [Google Scholar] [CrossRef]
- Cordes, D.B.; Lickiss, P.D.; Rataboul, F. Recent Developments in the Chemistry of Cubic Polyhedral Oligosilsesquioxanes. Chem. Rev. 2010, 110, 2081–2173. [Google Scholar] [CrossRef] [PubMed]
- Simonutti, R.; Comotti, A.; Bracco, S.; Sozzani, P. Surfactant Organization in MCM-41 Mesoporous Materials As Studied by 13C and 29Si Solid-State NMR. Chem. Mater. 2001, 13, 771–777. [Google Scholar] [CrossRef]
- Widgeon, S.J.; Sen, S.; Mera, G.; Ionescu, E.; Riedel, R.; Navrotsky, A. 29Si and 13C Solid-State NMR Spectroscopic Study of Nanometer-Scale Structure and Mass Fractal Characteristics of Amorphous Polymer Derived Silicon Oxycarbide Ceramics. Chem. Mater. 2010, 22, 6221–6228. [Google Scholar] [CrossRef]
- Simonutti, R.; Comotti, A.; Negroni, F.; Sozzani, P. 13C and 29Si Solid-State NMR of Rubber−Silica Composite Materials. Chem. Mater. 1999, 11, 822–828. [Google Scholar] [CrossRef]
- Kar, M.; Pauline, M.; Sharma, K.; Kumaraswamy, G.; Sen Gupta, S. Synthesis of Poly-l-glutamic Acid Grafted Silica Nanoparticles and Their Assembly into Macroporous Structures. Langmuir 2011, 27, 12124–12133. [Google Scholar] [CrossRef]
- Wang, S.; Tan, L.; Zhang, C.; Hussain, I.; Tan, B. Novel POSS-based organic–inorganic hybrid porous materials by low cost strategies. J. Mater. Chem. A 2015, 3, 6542–6548. [Google Scholar] [CrossRef]
- Jing, X.; Sun, F.; Ren, H.; Tian, Y.; Guo, M.; Li, L.; Zhu, G. Targeted synthesis of micro–mesoporous hybrid material derived from octaphenylsilsesquioxane building units. Microporous Mesoporous Mater. 2013, 165, 92–98. [Google Scholar] [CrossRef]
- Wang, D.; Yang, W.; Li, L.; Zhao, X.; Feng, S.; Liu, H. Hybrid networks constructed from tetrahedral silicon-centered precursors and cubic POSS-based building blocks via Heck reaction: Porosity, gas sorption, and luminescence. J. Mater. Chem. A 2013, 1, 13549–13558. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, D.; Li, L.; Yang, W.; Feng, S.; Liu, H. Hybrid porous polymers constructed from octavinylsilsesquioxane and benzene via Friedel–Crafts reaction: Tunable porosity, gas sorption, and postfunctionalization. J. Mater. Chem. A 2014, 2, 2160–2167. [Google Scholar] [CrossRef]
- Yuan, Z.-Y.; Ren, T.-Z.; Azioune, A.; Pireaux, J.-J.; Su, B.-L. Self-Assembly of Hierarchically Mesoporous−Macroporous Phosphated Nanocrystalline Aluminum (Oxyhydr)oxide Materials. Chem. Mater. 2006, 18, 1753–1767. [Google Scholar] [CrossRef]
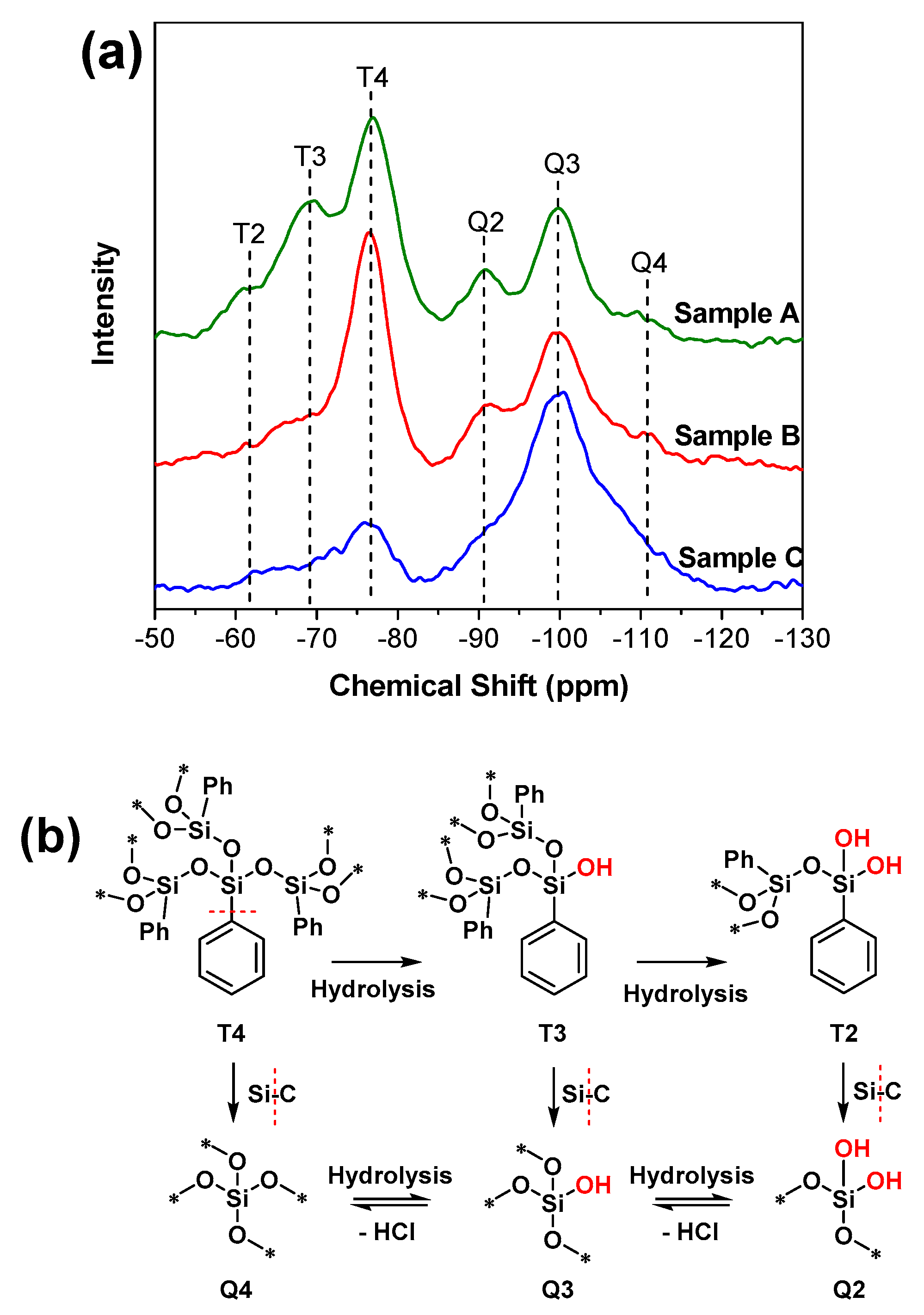
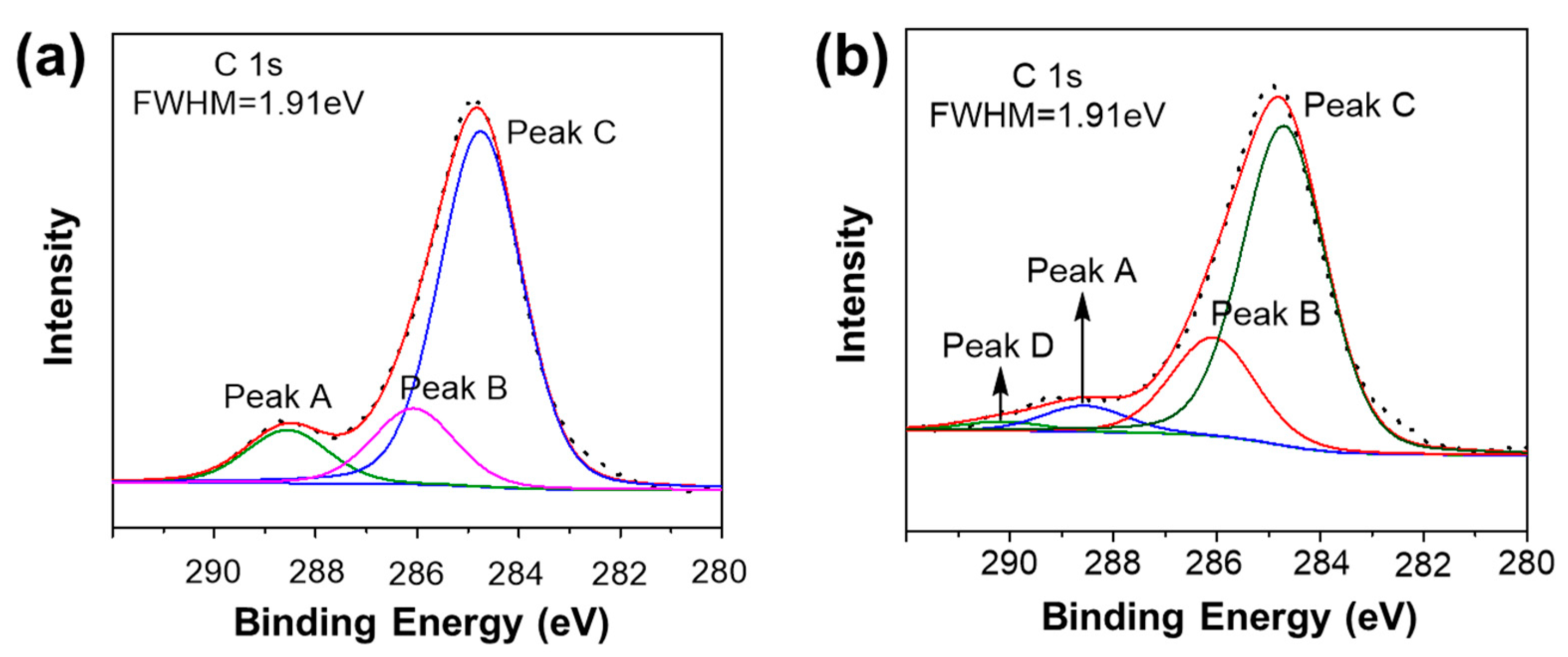
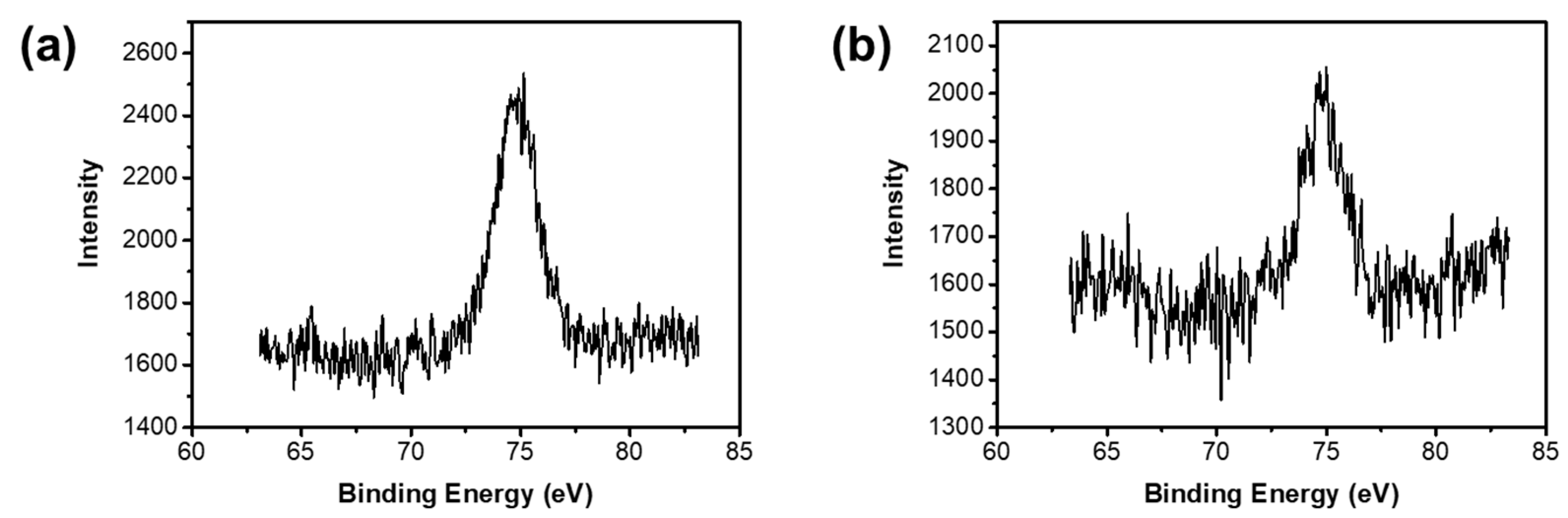

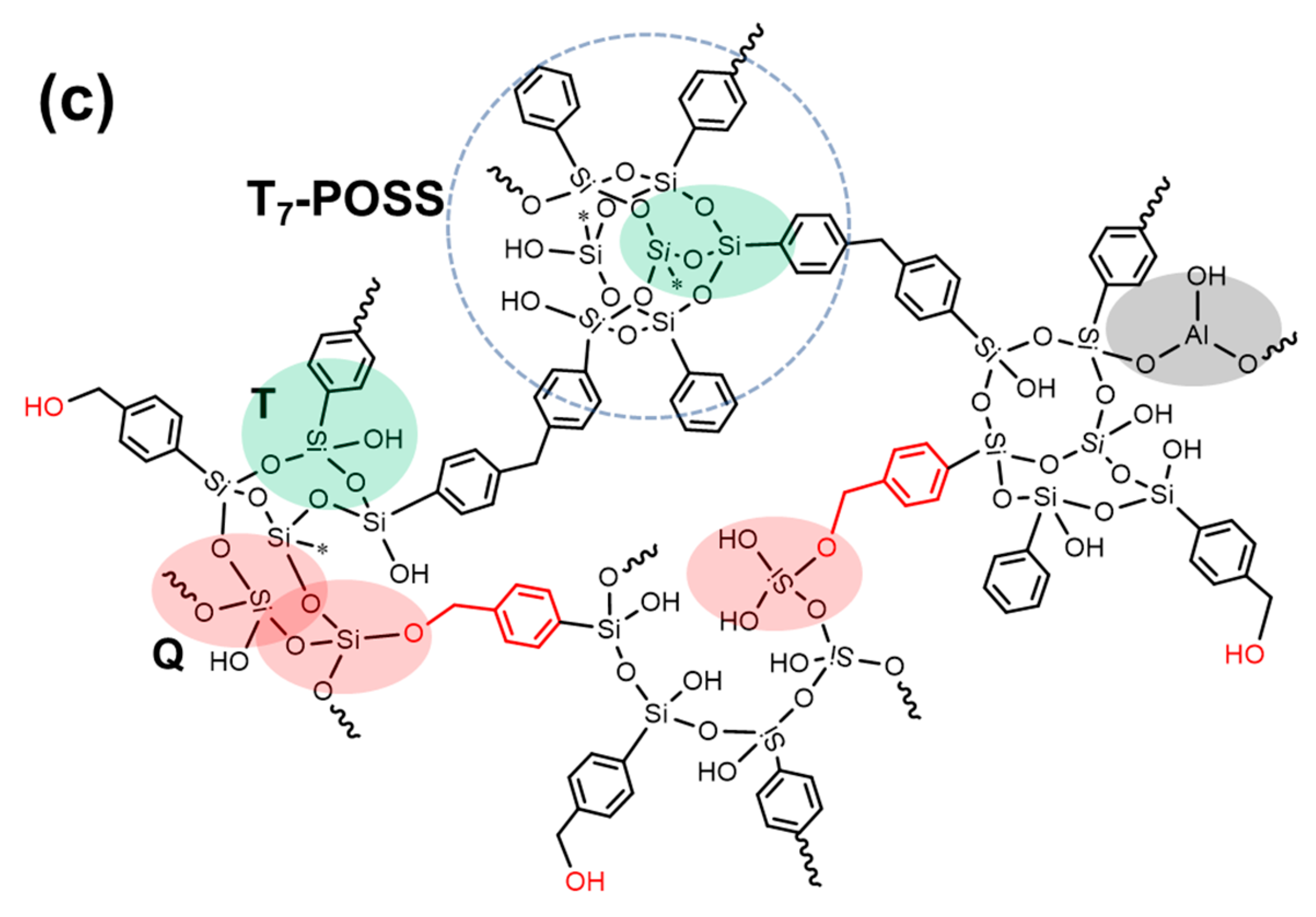
| Samples | Raw Materials | Reaction Time (h) | Active Hydrogen Source | Reaction Temperature (°C) |
|---|---|---|---|---|
| A | OPS | 20 | H2O | 40 |
| B | OPS | 20 | CH3OH | 40 |
| C | T7-POSS | 20 | H2O | 40 |
| Signal | Structure | Binding Energy (eV) |
|---|---|---|
| Peak A | Ph–CH2–OH | 288.56 |
| Peak B | Ph–CH2–Ph | 286.07 |
| Peak C | –C6H6 | 284.66 |
| Peak D | Si–OCH2–Ph | 290.15 |
| Samples | T90 (°C) | Tmax (°C) |
|---|---|---|
| OPS | 459.84 | 456.02/539.18 |
| A | 402.08 | 550.42 |
| T7-POSS | 564.50 | 211.45/597.73 |
| C | 343.47 | 420.91 |
| No. | Reaction Time (h) | Reaction Temperature (°C) | Raw Materials (POSS) | Solvents | Other Conditions | References |
|---|---|---|---|---|---|---|
| 1 | 48 | 80 | OVS | THF/Et3N | Pd(OAc)2/PPh3 | [24] |
| 2 | 48 | 100 | OVS | DMF/Et3N | Pd(OAc)2/P(o-CH3Ph)3 | [25] |
| 3 | 24 | Reflux | OVS | CS2 | AlCl3, Argon | [26] |
| 4 | 48 | 100 | OVS | DMF/Et3N | Pd(OAc)2/P(o-CH3Ph)3 | [40] |
| 5 | 24 | 60 | OPS | CHCl3 | AlCl3 | [38] |
| 6 | 24 | Reflux | OVS | CS2 | AlCl3, Stoichiometric benzene | [41] |
| 7 | 20 | 40 | OPS or T7-POSS | DCM | AlCl3 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Yin, G.; Li, Z.; Wu, P.; Jin, X.; Li, Q. The Preparation and Chemical Structure Analysis of Novel POSS-Based Porous Materials. Materials 2019, 12, 1954. https://doi.org/10.3390/ma12121954
Yang X, Yin G, Li Z, Wu P, Jin X, Li Q. The Preparation and Chemical Structure Analysis of Novel POSS-Based Porous Materials. Materials. 2019; 12(12):1954. https://doi.org/10.3390/ma12121954
Chicago/Turabian StyleYang, Xiaomei, Guangzhong Yin, Zhiyong Li, Pengfei Wu, Xiaopei Jin, and Qifang Li. 2019. "The Preparation and Chemical Structure Analysis of Novel POSS-Based Porous Materials" Materials 12, no. 12: 1954. https://doi.org/10.3390/ma12121954