Effect of Oxygen Concentration and Tantalum Addition on the Formation of High Temperature Bismuth Oxide Phase by Mechanochemical Reaction
Abstract
:1. Introduction
2. Experimental Procedures
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Coomaraswamy, K.S.; Lumley, P.J.; Hofmann, M.P. Effect of bismuth oxide radioopacifier content on the material properties of an endodontic Portland cement-based (MTA-like) system. J. Endod. 2007, 33, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.S.; Yang, J.C.; Lai, F.C.; Chen, C.Y.; Hsieh, M.Y.; Fang, A.; Chen, S.H.; Lin, C.K. Radiopacity performances of precipitated ZrO2-doped Bi2O3 powders and the influences of dopant concentrations and sintering temperatures. Ceram. Int. 2017, 43, 14008–14014. [Google Scholar] [CrossRef]
- Chen, M.S.; Chen, S.H.; Lai, F.C.; Chen, C.Y.; Hsieh, M.Y.; Chang, W.J.; Yang, J.C.; Lin, C.K. Sintering temperature-dependence on radiopacity of Bi(2−x)ZrxO(3+x/2) powders prepared by sol-gel process. Materials 2018, 11, 1685. [Google Scholar] [CrossRef] [PubMed]
- Shuk, P.; Wiemhofer, H.D.; Guth, U.; Gopel, W.; Greenblatt, M. Oxide ion conducting solid electrolytes based on Bi2O3. Solid State Ion. 1996, 89, 179–196. [Google Scholar] [CrossRef]
- Weng, C.H.; Wei, W.C.J. Synthesis and Properties of Homogeneous Nb-Doped Bismuth Oxide. J. Am. Ceram. Soc. 2010, 93, 3124–3129. [Google Scholar] [CrossRef]
- Fruth, V.; Ianculescu, A.; Berger, D.; Preda, S.; Voicu, G.; Tenea, E.; Popa, M. Synthesis, structure and properties of doped Bi2O3. J. Eur. Ceram. Soc. 2006, 26, 3011–3016. [Google Scholar] [CrossRef]
- Wu, Y.S.; Hou, J.; Gong, Z.; Miao, L.N.; Tang, H.D.; Liu, W. High performance BaCe0.5Fe0.5-xBixO3-delta as cobalt-free cathode for proton-conducting solid oxide fuel cells. J. Alloy. Compd. 2019, 790, 551–557. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X.; Xie, S.; Chen, L.; Wang, Y.; Meng, J.; Zhou, D. Bismuth tungstate/neodymium-doped ceria composite electrolyte for intermediate-temperature solid oxide fuel cell: Sintering aid and composite effect. J. Power Sources 2019, 428, 105–114. [Google Scholar] [CrossRef]
- Aurivillius, B.; Sillen, L.G. Polymorphy of bismuth trioxide. Nature 1945, 155, 305–306. [Google Scholar] [CrossRef]
- Harwig, H.A.; Gerards, A.G. The polymorphism of bismuth sesquioxide. Thermochim. Acta 1979, 28, 121–131. [Google Scholar] [CrossRef]
- Zhou, W. Structural chemistry and physical properties of some ternary oxides in the Bi2O3-Ta2O5 system. J. Solid State Chem. 1992, 101, 1–17. [Google Scholar] [CrossRef]
- Jardiel, T.; Calatayud, D.G.; Rodríguez, M.; Fernández-Hevia, D.; Caballero, A.C. Synthesis of metastable Bi6Ti5WO22 phase by the mechanochemical method. Mater. Lett. 2013, 94, 58–60. [Google Scholar] [CrossRef]
- Zdujić, M.; Poleti, D.; Jovalekić, Č.; Karanović, L. Mechanochemical synthesis and electrical conductivity of nanocrystalline δ-Bi2O3 stabilized by HfO2 and ZrO2. J. Serb. Chem. Soc. 2009, 74, 1401–1411. [Google Scholar] [CrossRef]
- Asryan, N.A.; Kol’tsova, T.N.; Alikhanyan, A.S.; Nipan, G.D. Thermodynamics and Phase Diagram of the Bi2O3-SnO2 System. Inorg. Mater. 2002, 38, 1141–1147. [Google Scholar] [CrossRef]
- Ling, C.D.; Withers, R.L.; Schmid, S.; Thompson, J.G. A Review of Bismuth-Rich Binary Oxides in the Systems Bi2O3-Nb2O5, Bi2O3-Ta2O5, Bi2O3-MoO3, and Bi2O3-WO3. J. Solid State Chem. 1998, 137, 42–61. [Google Scholar] [CrossRef]
- Lin, S.E.; Wei, W.C.J. Long-term degradation of Ta2O5-doped Bi2O3 systems. J. Eur. Ceram. Soc. 2011, 31, 3081–3086. [Google Scholar] [CrossRef]
- Saito, T.; Miida, R. Crystal Structure and Ionic Conductivity in Bi2O3-Rich Bi2O3-Ta2O5 Sintered Oxides. Jpn. J. Appl. Phys. 1999, 38, 4838–4842. [Google Scholar] [CrossRef]
- Benjamin, J.S. Mechanical Alloying. Sci. Am. 1976, 234, 40–49. [Google Scholar] [CrossRef]
- Murty, B.S.; Ranganathan, S. Novel materials synthesis by mechanical alloying/milling. Int. Mater. Rev. 1998, 43, 101–141. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Lee, P.Y.; Koch, C.C. Formation of amorphous Ni-Zr alloy powder by mechanical alloying of intermetallic powder mixtures and mixtures of nickel or zirconium with intermetallics. J. Mater. Sci. 1988, 23, 2837–2845. [Google Scholar] [CrossRef]
- Koch, C.C.; Whittenberger, J.D. Mechanical milling/alloying of intermetallics. Intermetallics 1996, 4, 339–355. [Google Scholar] [CrossRef]
- Tsuzuki, T.; McCormick, P.G. Nanopowders synthesized by mechanochemical processing. J. Mater. Sci. 2004, 39, 5143–5146. [Google Scholar] [CrossRef]
- Duan, C.W.; Hu, L.X.; Ma, J.L. Ionic liquids as an efficient medium for the mechanochemical synthesis of α-AlH3 nano-composites. J. Mater. Chem. A 2018, 6, 6309–6318. [Google Scholar] [CrossRef]
- Xiao, Z.; Li, X.; Xiao, Q.; Luo, W.; Zhang, H.; Wang, C.; Zhang, T.; Li, S.; Kong, L.B. Bismuth lanthanum titanate ceramics from amorphous precursors activated by using mechanochemical treatment. Ceram. Int. 2018, 44, 13106–13112. [Google Scholar] [CrossRef]
- Ding, J.; Miao, W.F.; McCormick, P.G.; Street, R. Mechanochemical synthesis of ultrafine Fe powder. Appl. Phys. Lett. 1995, 67, 3804. [Google Scholar] [CrossRef]
- Castro, A.; Palem, D. Study of fluorite phases in the system Bi2O3-Nb2O5-Ta2O5. Synthesis by mechanochemical activation assisted methods. J. Mater. Chem. 2002, 12, 2774–2780. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Dutta, S.; Dutta, A.; Pradhan, S.K. Mechanosynthesis of Nanocrystalline Fully Stabilized bcc γ-phase of Bi2O3 without Any Additive: Manifestation of Ferroelasticity in Microstructure, Optical, and Transport Properties. Cryst. Growth Des. 2018, 18, 6564–6572. [Google Scholar] [CrossRef]
- Hasanpour, A.; Mozaffari, M.; Amighian, J. Preparation of Bi–Fe3O4 nanocomposite through reduction of Bi2O3 with Fe via high-energy ball milling. Phys. B Condens. Matter 2007, 387, 298–301. [Google Scholar] [CrossRef]
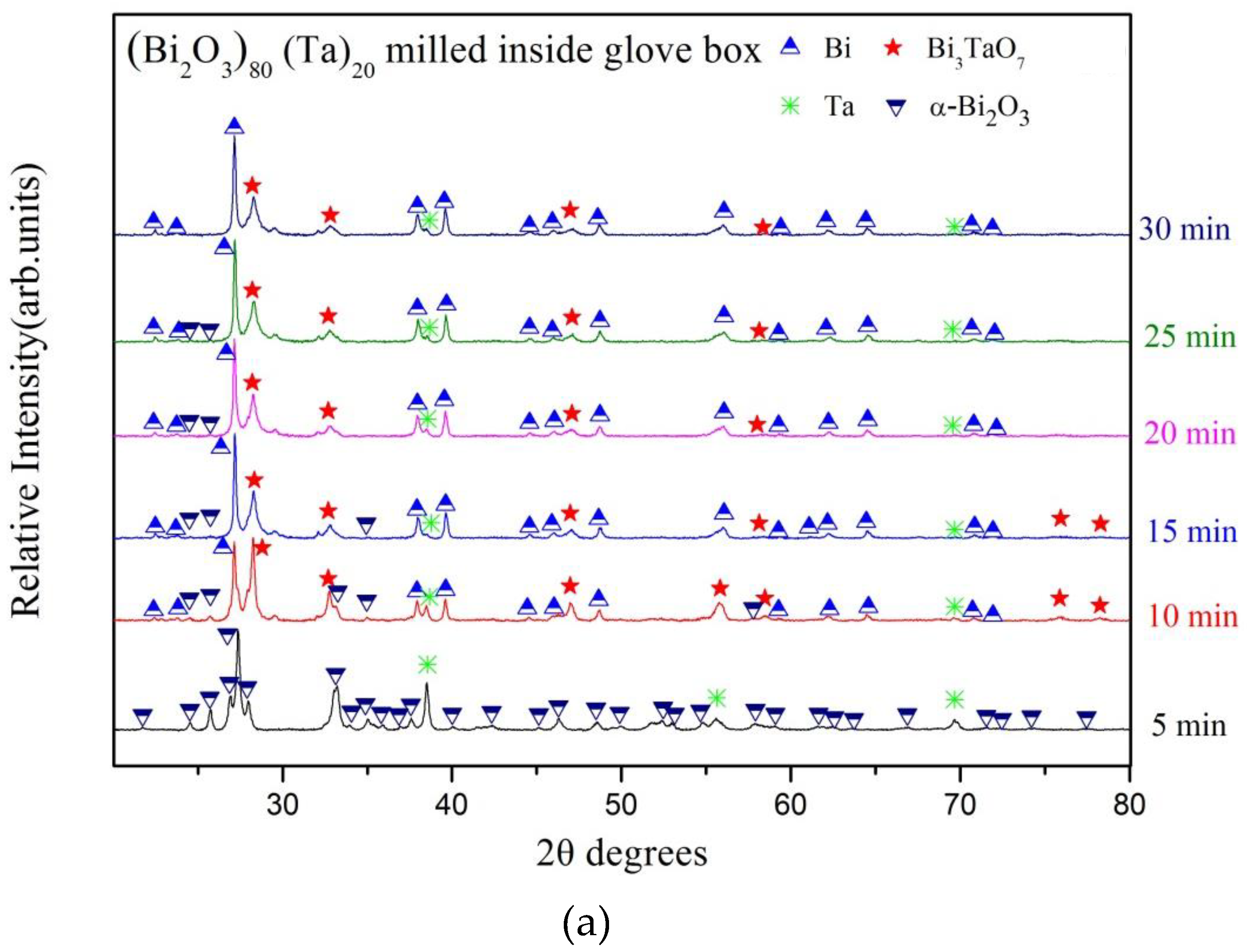
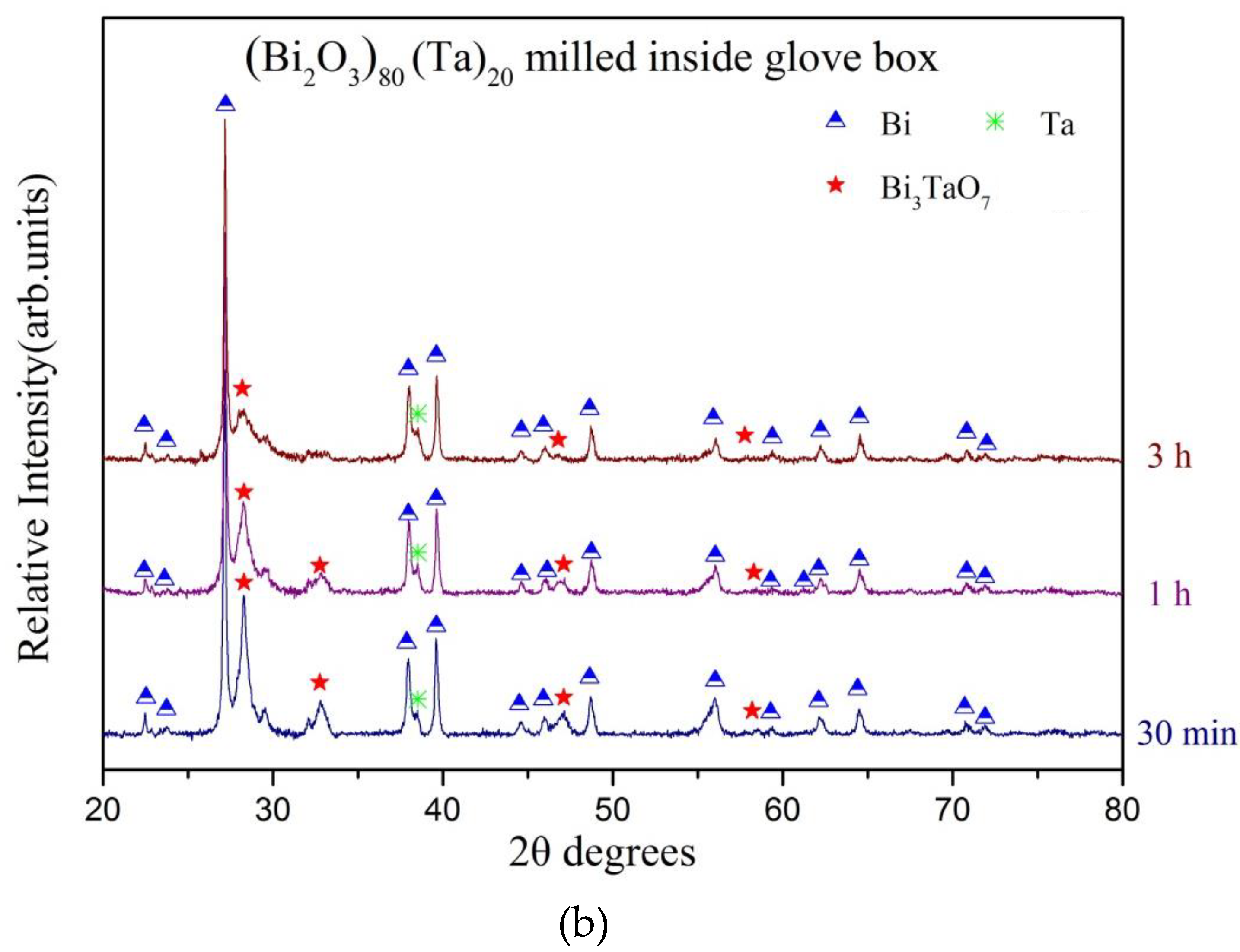
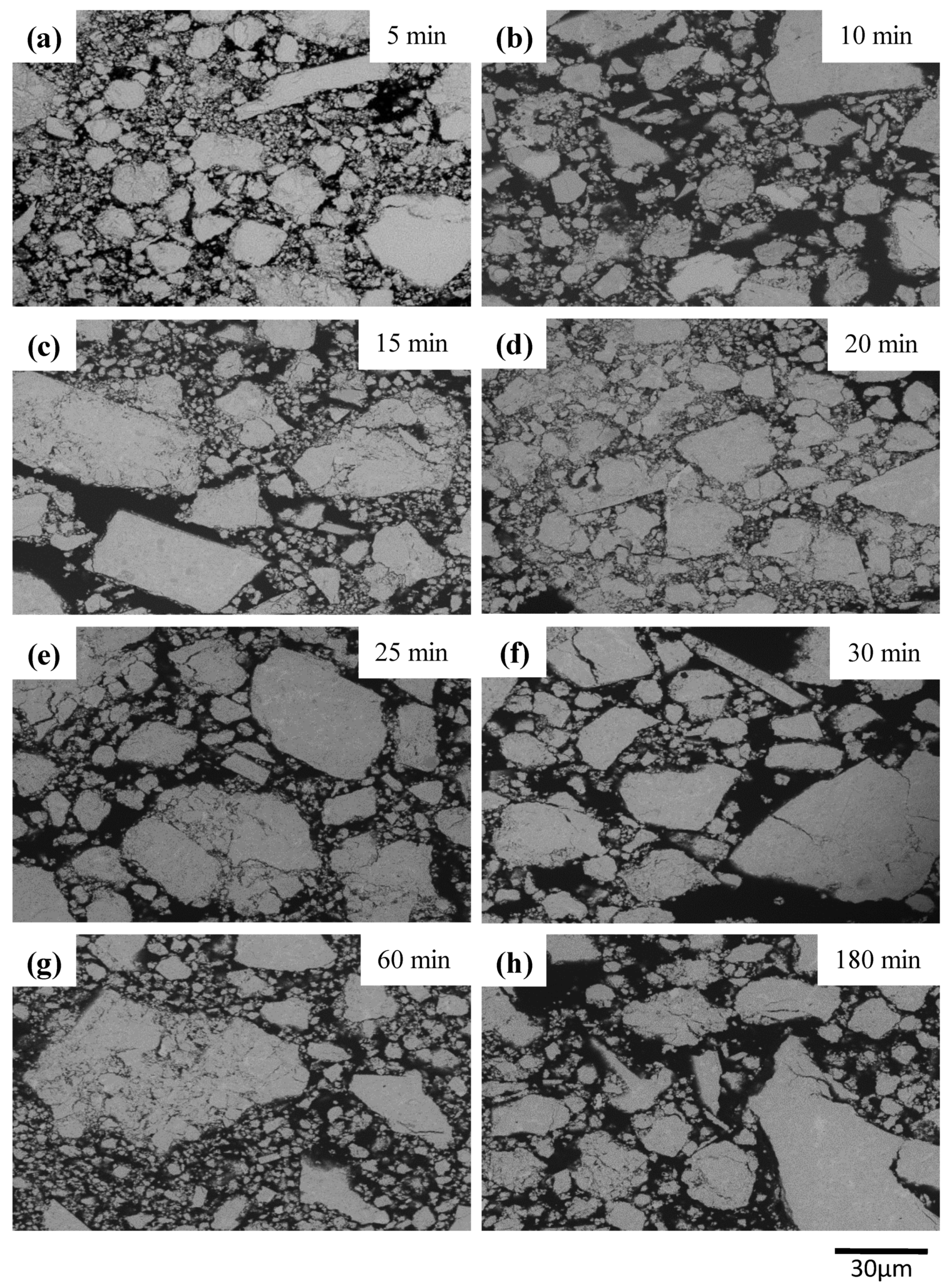
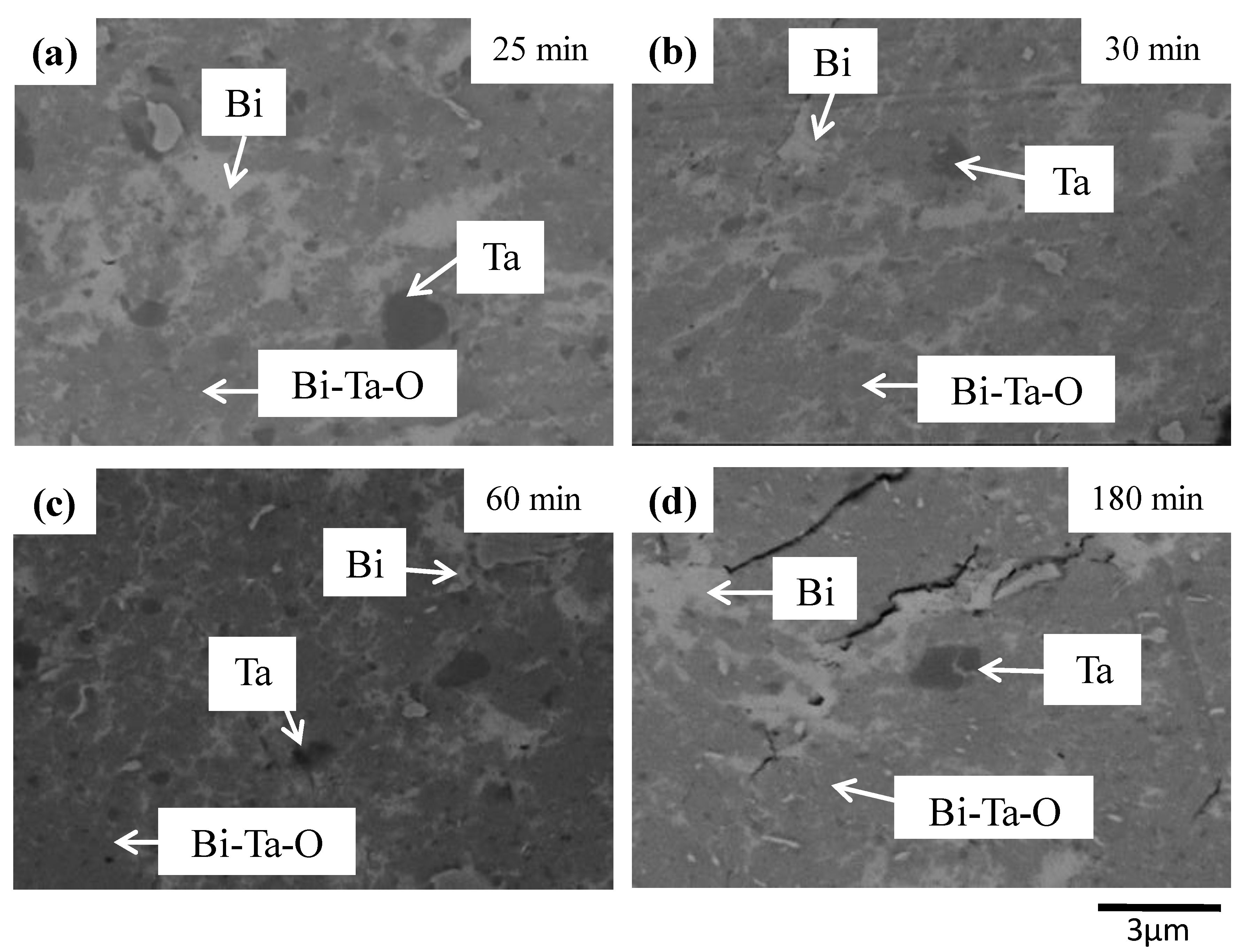
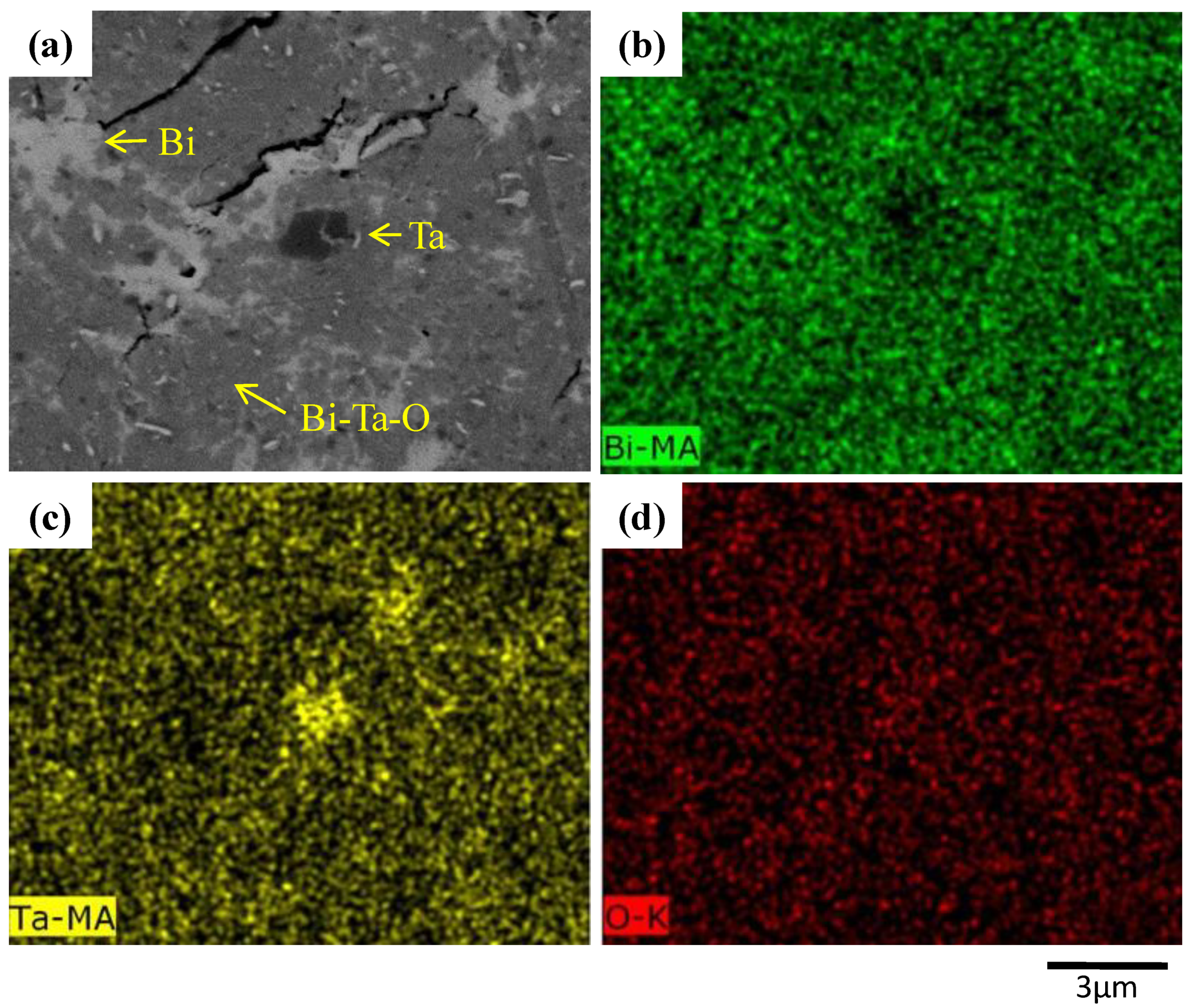
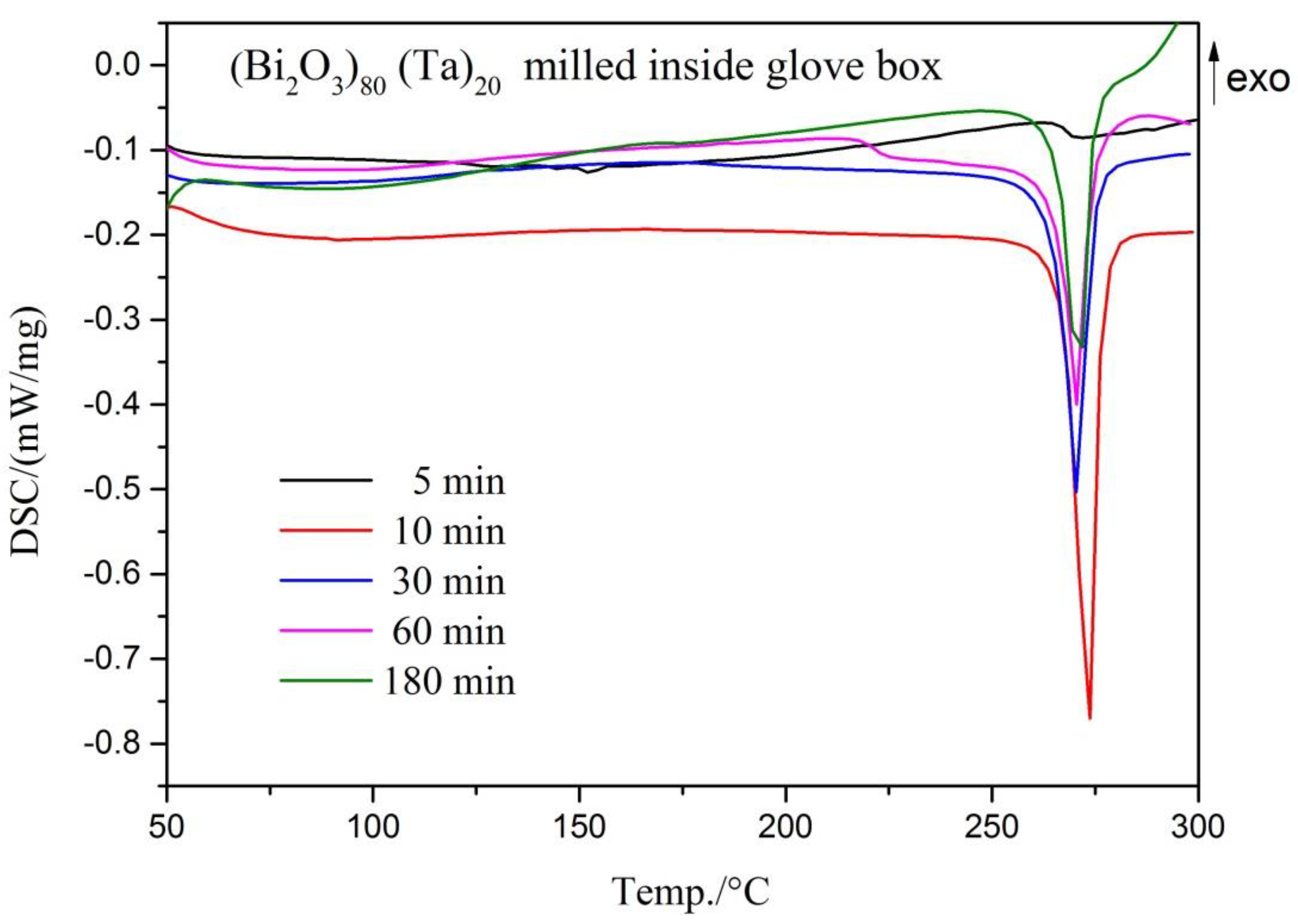
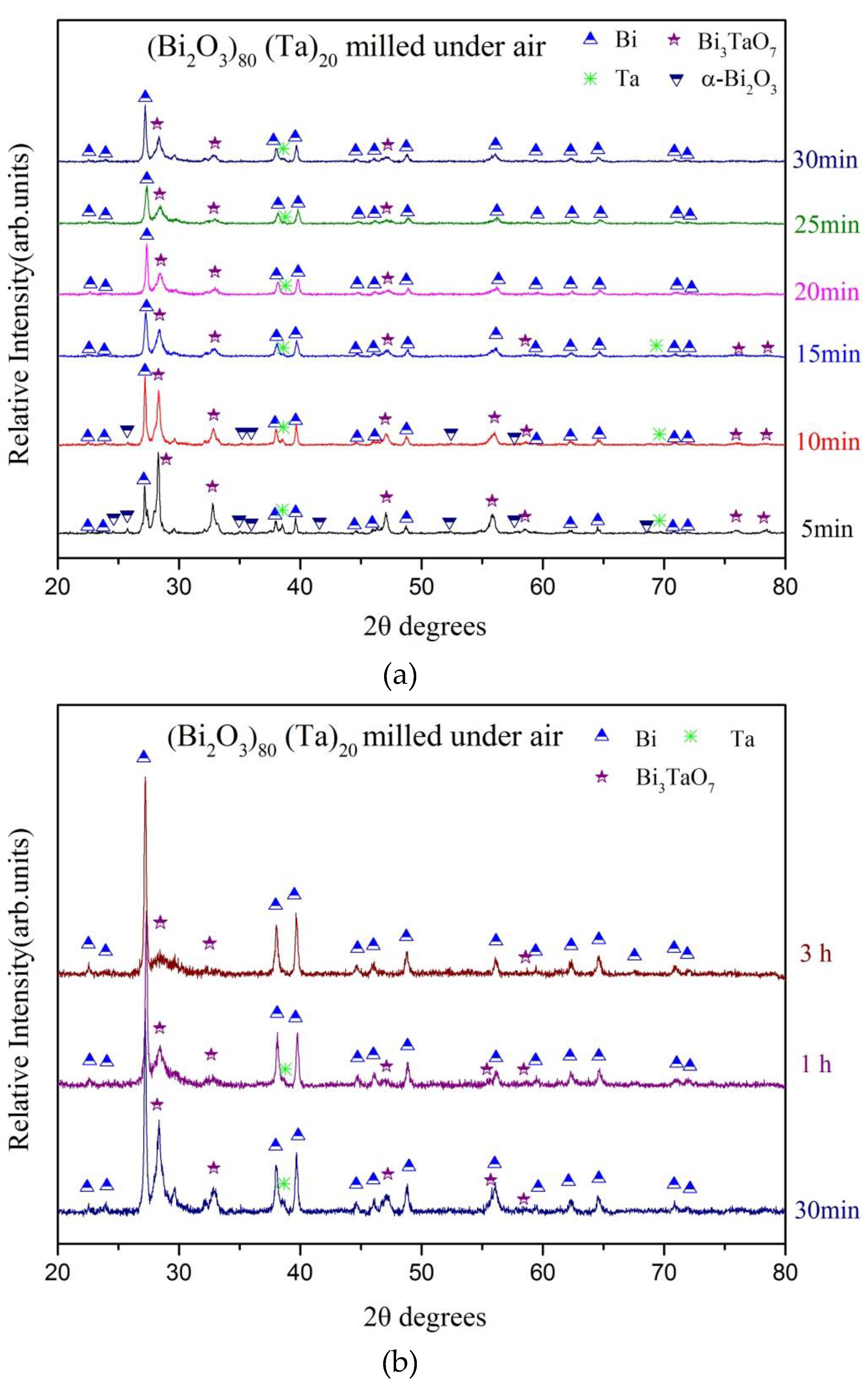
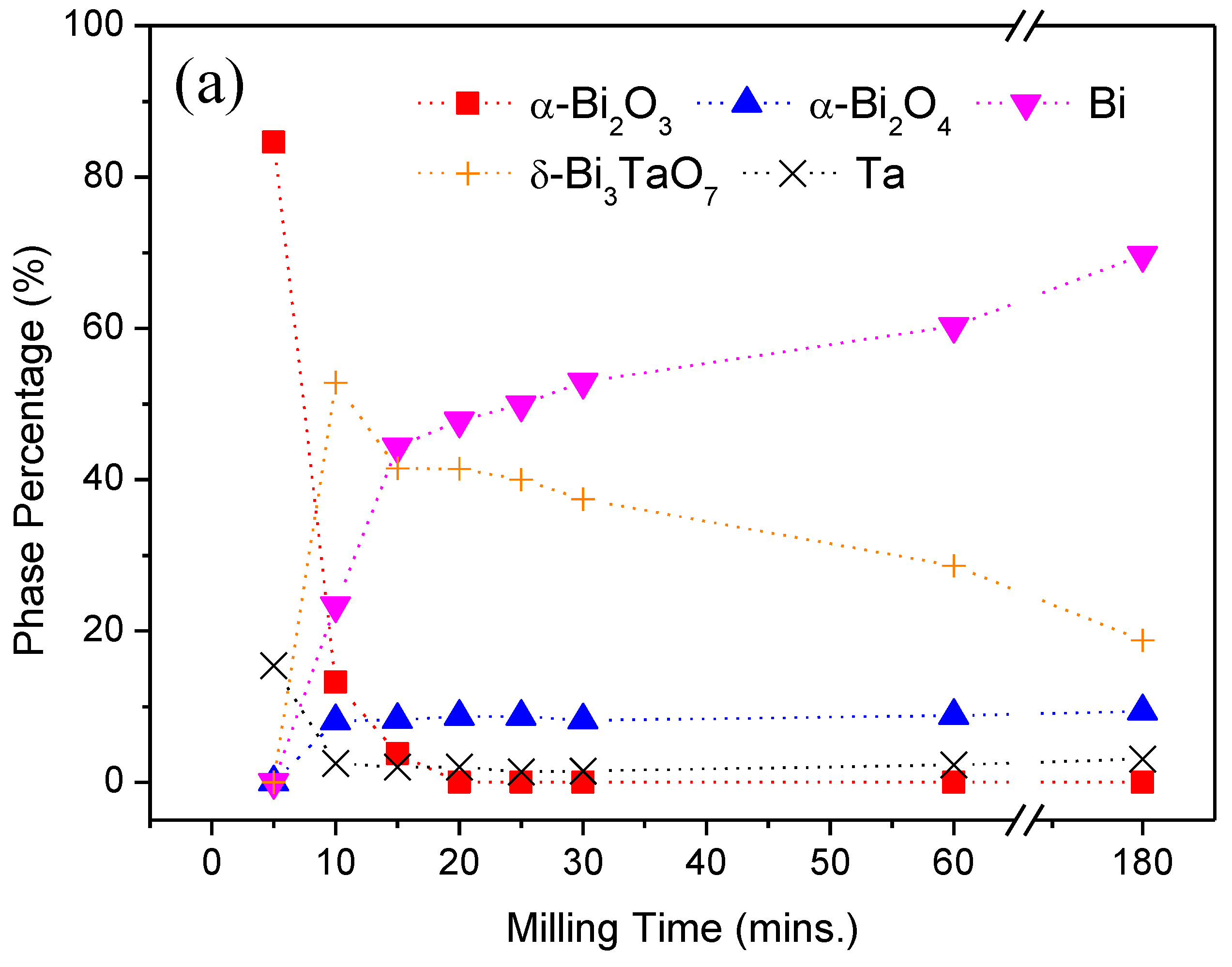

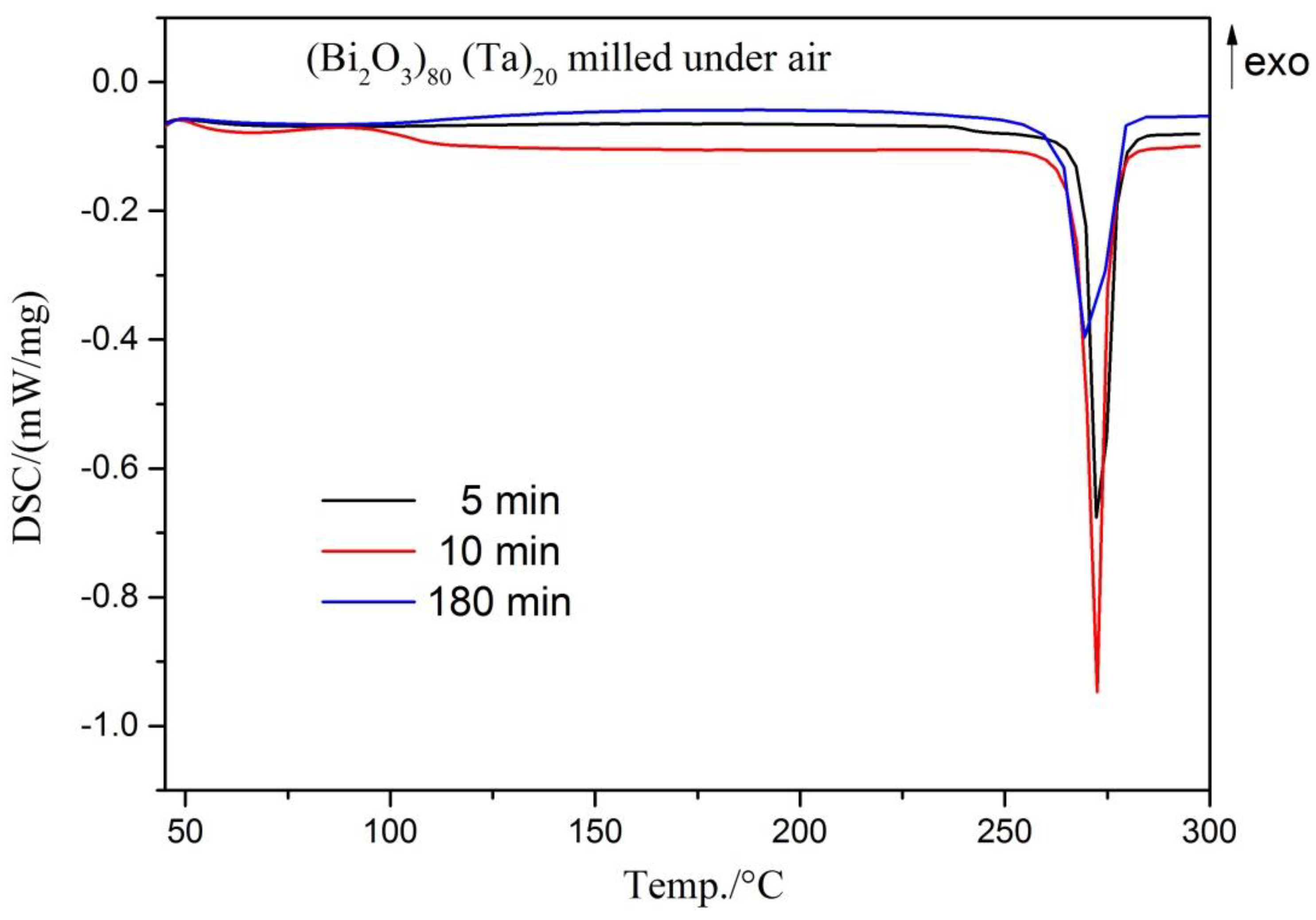
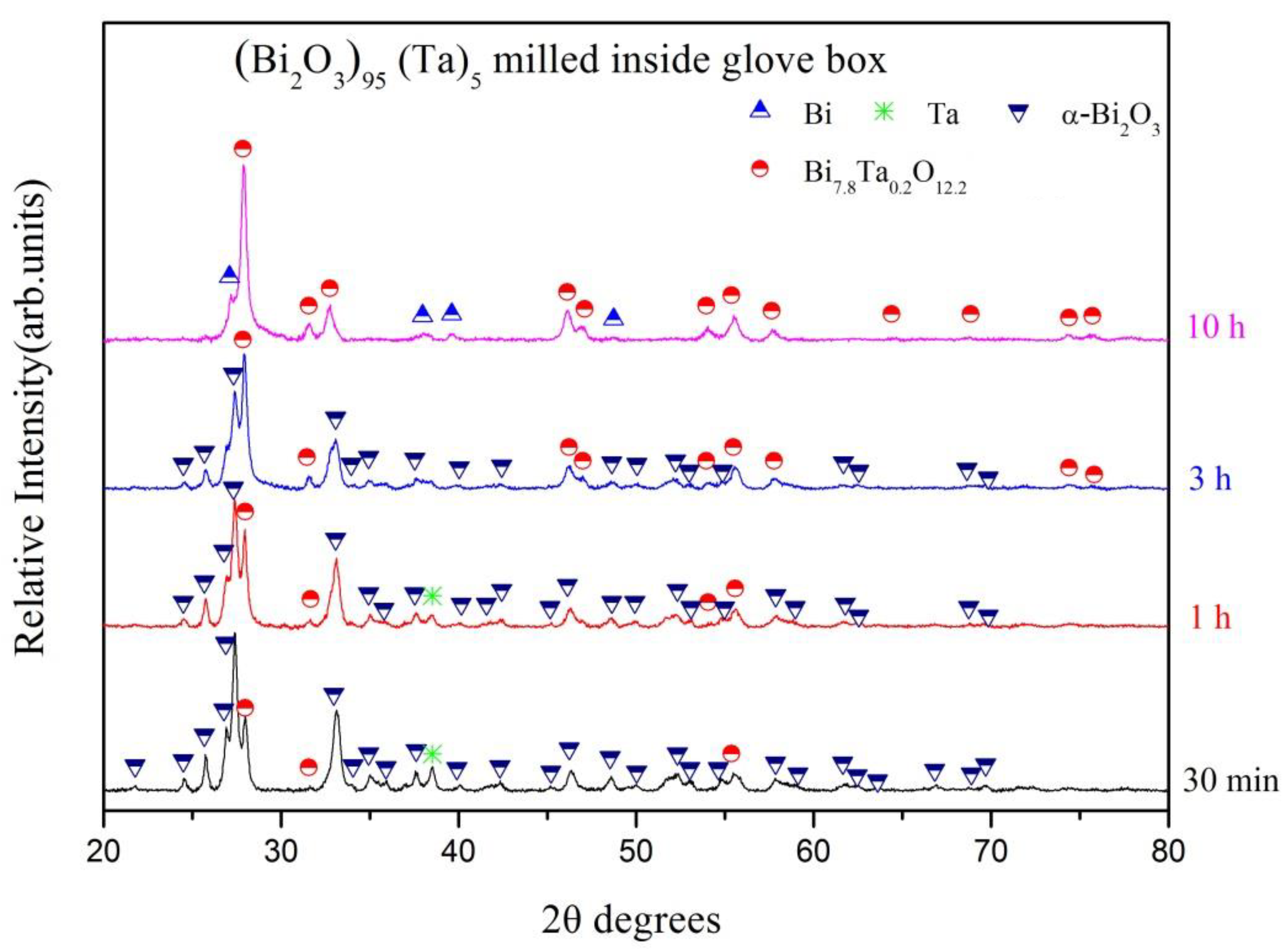

| Milling Condition | Milling Time | Crystalline Phases |
|---|---|---|
| Inside Ar-filled glove box | 5 min | α-Bi2O3 (84.6%) * + Ta (15.4%) |
| 10 min | α-Bi2O4 (8.1%) + α-Bi2O3 (13.2%) + Bi (23.4%) + δ-Bi3TaO7 (52.8%) + Ta (2.5%) | |
| 15 min | α-Bi2O4 (8.3%) + α-Bi2O3 (3.8%) + Bi (44.4%) + δ-Bi3TaO7 (41.5%) + Ta (2.0%) | |
| 20 min | α-Bi2O4 (8.7%) + Bi (47.8%) + δ-Bi3TaO7 (41.4%) + Ta (2.0%) | |
| 25 min | α-Bi2O4 (8.7%) + Bi (49.9%) + δ-Bi3TaO7 (40.0%) + Ta (1.4%) | |
| 30 min | α-Bi2O4 (8.2%) + Bi (52.9%) + δ-Bi3TaO7 (37.4%) + Ta (1.5%) | |
| 1 h | α-Bi2O4 (8.8%) + Bi (60.3%) + δ-Bi3TaO7 (28.6%) + Ta (2.3%) | |
| 3 h | α-Bi2O4 (9.4%) + Bi (69.7%) + δ-Bi3TaO7 (18.8%) + Ta (3.1%) | |
| Under air | 5 min | α-Bi2O4 (5.5%) + α-Bi2O3 (13.0%) + Bi (21.2%) + δ-Bi3TaO7 (58.7%) + Ta (1.6%) |
| 10 min | α-Bi2O4 (4.5%) + α-Bi2O3 (10.0%) +Bi (33.8%) + δ-Bi3TaO7 (50.5%) + Ta (1.2%) | |
| 15 min | α-Bi2O4 (5.3%) + Bi (39.5%) + δ-Bi3TaO7 (54.5%) + Ta (0.7%) | |
| 20 min | α-Bi2O4 (3.0%) + Bi (38.0%) + δ-Bi3TaO7 (58.6%) + Ta (0.4%) | |
| 25 min | α-Bi2O4 (1.8%) + Bi (42.0%) + δ-Bi3TaO7 (54.1%) + Ta (2.1%) | |
| 30 min | α-Bi2O4 (5.1%) + Bi (52.8%) + δ-Bi3TaO7 (40.6%) + Ta (1.5%) | |
| 1 h | α-Bi2O4 (2.9%) + Bi (56.0%) + δ-Bi3TaO7 (40.3%) + Ta (0.8%) | |
| 3 h | α-Bi2O4 (2.7%) + Bi (71.9%) + δ-Bi3TaO7 (25.4%) |
| Milling Condition | Milling Time | Crystalline Phases |
|---|---|---|
| Inside Ar-filled glove box | 30 min | α-Bi2O3 (56.0%) * + Ta (2.4%) + β-Bi7.8Ta0.2O12.2 (41.6%) |
| 1 h | α-Bi2O3 (46.2%) + Ta (1.7%) + β-Bi7.8Ta0.2O12.2 (52.1%) | |
| 3 h | α-Bi2O3 (32.8%) + β-Bi7.8Ta0.2O12.2 (67.2%) | |
| 10 h | β-Bi7.8Ta0.2O12.2 (90.8%) + Bi (9.2%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.-N.; Chen, M.-S.; Chang, Y.-H.; Lee, P.-Y.; Lin, C.-K. Effect of Oxygen Concentration and Tantalum Addition on the Formation of High Temperature Bismuth Oxide Phase by Mechanochemical Reaction. Materials 2019, 12, 1947. https://doi.org/10.3390/ma12121947
Lin H-N, Chen M-S, Chang Y-H, Lee P-Y, Lin C-K. Effect of Oxygen Concentration and Tantalum Addition on the Formation of High Temperature Bismuth Oxide Phase by Mechanochemical Reaction. Materials. 2019; 12(12):1947. https://doi.org/10.3390/ma12121947
Chicago/Turabian StyleLin, Hsiu-Na, May-Show Chen, Yu-Hsueh Chang, Pee-Yew Lee, and Chung-Kwei Lin. 2019. "Effect of Oxygen Concentration and Tantalum Addition on the Formation of High Temperature Bismuth Oxide Phase by Mechanochemical Reaction" Materials 12, no. 12: 1947. https://doi.org/10.3390/ma12121947





