Si-Based Materials for Thermoelectric Applications
Abstract
:1. Introduction
2. Bulk Nano-Si Thermoelectric Material
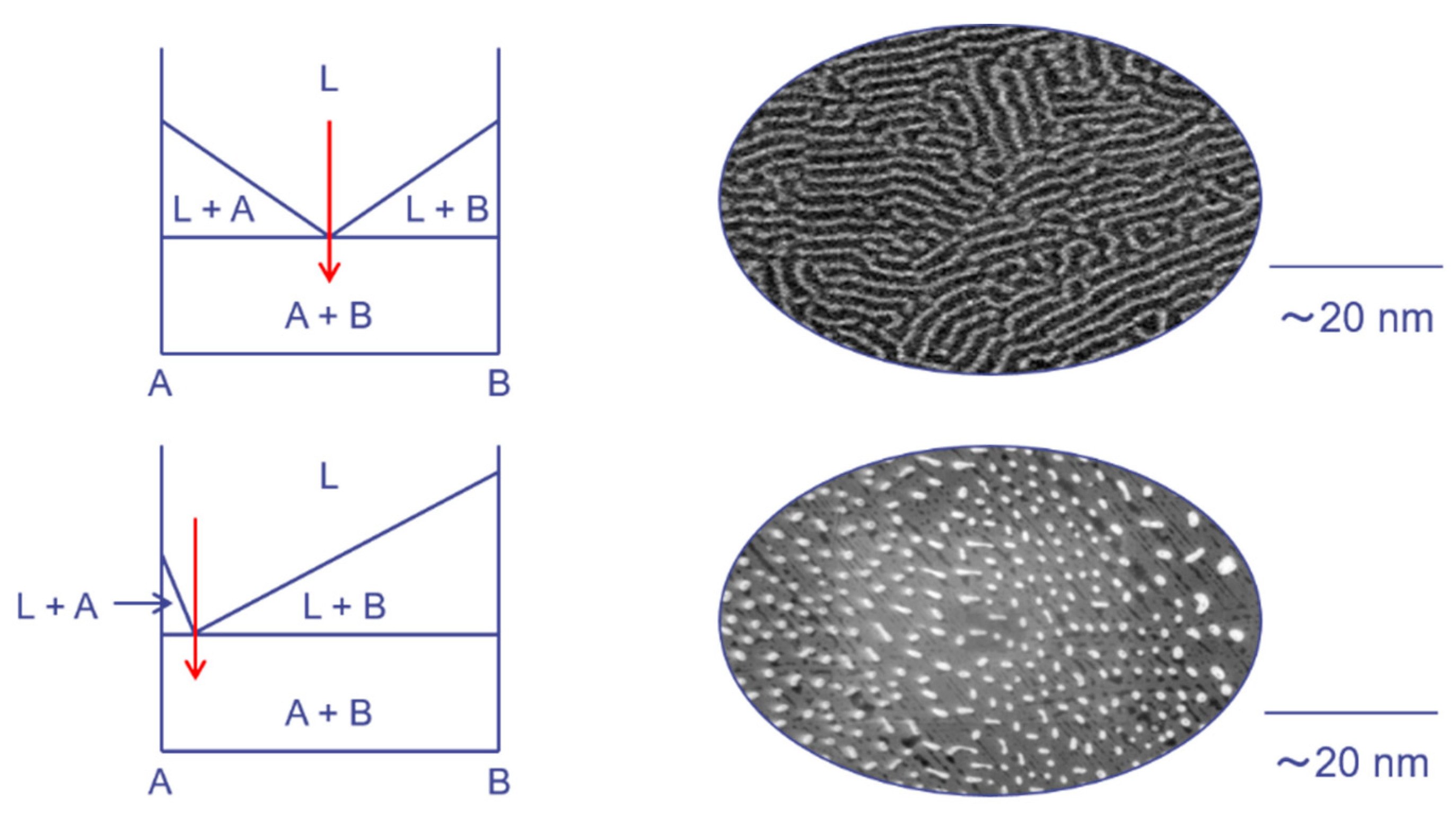
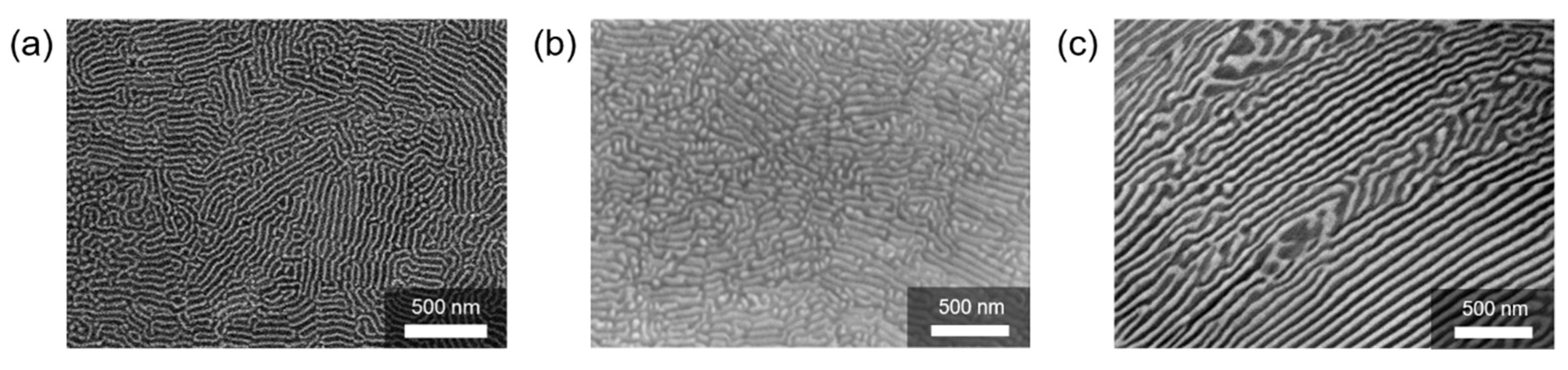
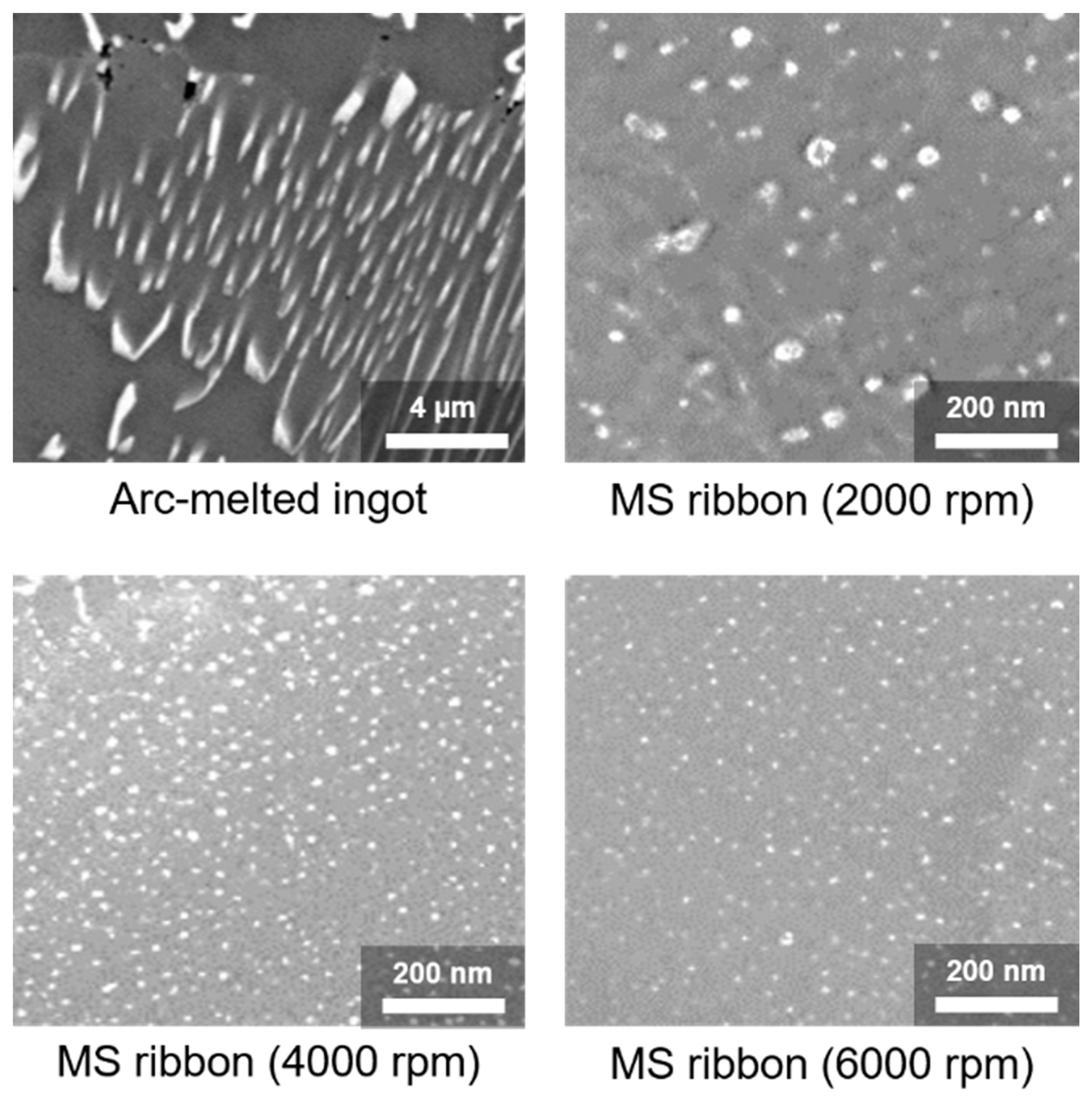
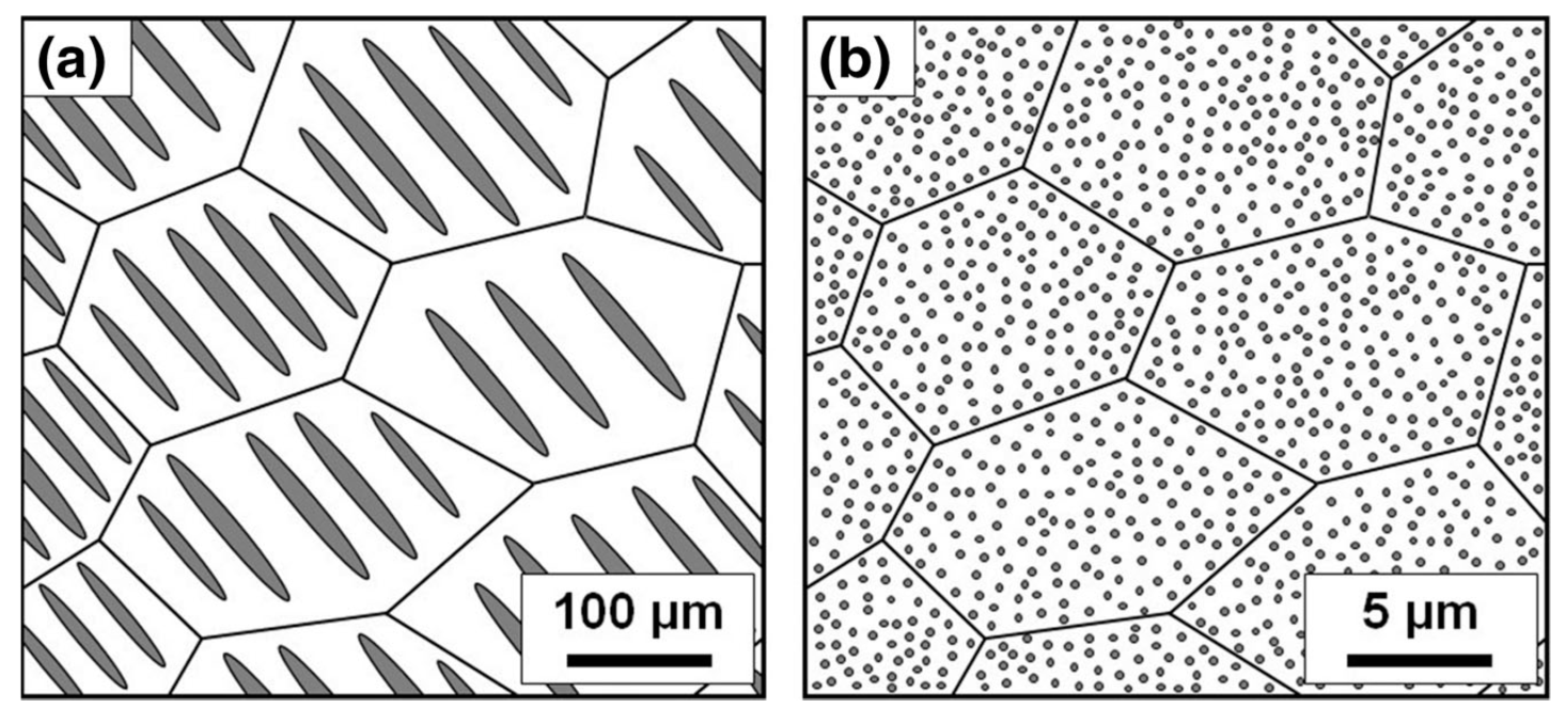
3. Synthesis and Size Control of Si and Metal Silicide Nanocomposites by Melt-Spinning
4. Promising New Metal Silicide for TE Devices
5. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bell, L.E. Cooling, Heating, Generating Power, and Recovering Waste Heat with Thermoelectric Systems. Science 2008, 321, 1457–1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snyder, G.J.; Toberer, E.S. Complex Thermoelectric Materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Venkatasubramanian, R.; Siivola, E.; Colpitts, T.; O’Quinn, B. Thin-Film Thermoelectric Devices with High Room-Temperature Figures of Merit. Nature 2001, 413, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Heremans, J.P.; Jovovic, V.; Toberer, E.S.; Saramat, A.; Kurosaki, K.; Charoenphakdee, A.; Yamanaka, S.; Snyder, G.J. Enhancement of Thermoelectric Efficiency in PbTe by Distortion of the Electronic Density of States. Science 2008, 321, 554–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steele, M.C.; Rosi, F.D. Thermal Conductivity and Thermoelectric Power of Germanium Silicon Alloys. J. App. Phys. 1958, 29, 1517–1520. [Google Scholar] [CrossRef]
- Dismukes, J.P.; Ekstrom, L.; Steigmeier, E.F.; Kudman, I.; Beers, D.S. Thermal and Electrical Properties of Heavily Doped Ge-Si Alloys up to 1300 K. J. Appl. Phys. 1964, 35, 2899–2907. [Google Scholar] [CrossRef]
- Zaitsev, V.K.; Fedorov, M.I.; Gurieva, E.A.; Eremin, I.S.; Konstantinov, P.P.; Samunin, A.Y.; Vedernikov, M.V. Highly Effective Mg2Si1−xSnx Thermoelectrics. Phys. Rev. B 2006, 74, 045207. [Google Scholar] [CrossRef]
- Liu, W.; Tan, X.; Yin, K.; Liu, H.; Tang, X.; Shi, J.; Zhang, Q.; Uher, C. Convergence of Conduction Bands as a Means of Enhancing Thermoelectric Performance of n-Type Mg2Si1-xSnx Solid Solutions. Phys. Rev. Lett. 2012, 108, 166601. [Google Scholar] [CrossRef] [PubMed]
- Zaitsev, V.K. Thermoelectrics Handbook; Rowe, D.M., Ed.; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar]
- Fedorov, M.I.; Zaitsev, V.K. Thermoelectrics Handbook; Rowe, D.M., Ed.; CRC Press: Boca Raton, FL, USA, 2006; p. 3. [Google Scholar]
- Weber, L.; Gmelin, E. Transport Properties of Silicon. Appl. Phys. A 1991, 53, 136–140. [Google Scholar] [CrossRef]
- Zhu, G.H.; Lee, H.; Lan, Y.; Wang, X.; Joshi, G.; Wang, D.; Yang, J.; Vashaee, D.; Guilbert, H.; Pillitteri, A.; et al. Increased Phonon Scattering by Nanograins and Point Defects in Nanostructured Silicon with a Low Concentration of Germanium. Phys. Rev. Lett. 2009, 102, 196803. [Google Scholar] [CrossRef] [PubMed]
- Tritt, T.M. Holey and Unholey Semiconductors. Science 1999, 283, 804–805. [Google Scholar] [CrossRef]
- Xie, H.H.; Wang, H.; Pei, Y.Z.; Fu, C.G.; Liu, X.H.; Snyder, G.J.; Zhao, X.B.; Zhu, T.J. Beneficial Contribution of Alloy Disorder to Electron and Phonon Transport in Half-Heusler Thermoelectric Materials. Adv. Funct. Mater. 2013, 23, 5123–5130. [Google Scholar] [CrossRef]
- Sales, B.C.; Mandrus, D.; Williams, R.K. Filled Skutterudite Antimonides: A New Class of Thermoelectric Materials. Science 1996, 272, 1325–1328. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Yang, J.; Salvador, J.R.; Chi, M.; Cho, J.Y.; Wang, H.; Bai, S.; Yang, J.; Zhang, W.; Chen, L. Multiple-Filled Skutterudites: High Thermoelectric Figure of Merit through Separately Optimizing Electrical and Thermal Transports. J. Am. Chem. Soc. 2011, 133, 7837–7846. [Google Scholar] [CrossRef]
- Nielsen, M.D.; Ozolins, V.; Heremans, J.P. Lone Pair Electrons Minimize Lattice Thermal Conductivity. Energy Environ. Sci. 2013, 6, 570–578. [Google Scholar] [CrossRef]
- Zhao, L.D.; Lo, S.H.; Zhang, Y.; Sun, H.; Tan, G.; Uher, C.; Wolverton, C.; Dravid, V.P.; Kanatzidis, M.G. Ultralow Thermal Conductivity and High Thermoelectric Figure of Merit in SnSe Crystals. Nature 2014, 508, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Poudel, B.; Hao, Q.; Ma, Y.; Lan, Y.; Minnich, A.; Yu, B.; Yan, X.; Wang, D.; Muto, A.; Vashaee, D.; et al. High-Thermoelectric Performance of Nanostructured Bismuth Antimony Telluride Bulk Alloys. Science 2008, 320, 634–638. [Google Scholar] [CrossRef] [Green Version]
- Biswas, K.; He, J.; Blum, I.D.; Wu, C.I.; Hogan, T.P.; Seidman, D.N.; Dravid, V.P.; Kanatzidis, M.G. High-Performance Bulk Thermoelectrics with All-Scale Hierarchical Architectures. Nature 2012, 489, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Hochbaum, A.I.; Chen, R.; Delgado, R.D.; Liang, W.; Garnett, E.C.; Najarian, M.; Majumdar, A.; Yang, P. Enhanced Thermoelectric Performance of Rough Silicon Nanowires. Nature 2008, 451, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Boukai, A.I.; Bunimovich, Y.; Tahir-Kheli, J.; Yu, J.K.; Goddard, W.A.; Heath, J.R. Silicon Nanowires as Efficient Thermoelectric Materials. Nature 2008, 451, 168–171. [Google Scholar] [CrossRef]
- Bux, S.K.; Blair, R.G.; Gogna, P.K.; Lee, H.; Chen, G.; Dresselhaus, M.S.; Kaner, R.B.; Fleurial, J.P. Nanostructured Bulk Silicon as an Effective Thermoelectric Material. Adv. Funct. Mater. 2009, 19, 2445–2452. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Chen, G.; Tang, M.Y.; Yang, R.; Lee, H.; Wang, D.; Ren, Z.; Fleurial, J.P.; Gogna, P. New Directions for Low-Dimensional Thermoelectric Materials. Adv. Mater. 2007, 19, 1043–1053. [Google Scholar] [CrossRef]
- Kurosaki, K.; Yusufu, A.; Miyazaki, Y.; Ohishi, Y.; Muta, H.; Yamanaka, S. Enhanced Thermoelectric Properties of Silicon via Nanostructuring. Mater. Trans. 2016, 57, 1018–1021. [Google Scholar] [CrossRef]
- Yusufu, A.; Kurosaki, K.; Miyazaki, Y.; Ishimaru, M.; Kosuga, A.; Ohishi, Y.; Muta, H.; Yamanaka, S. Bottom-Up Nanostructured Bulk Silicon: A Practical High-Efficiency Thermoelectric Material. Nanoscale 2014, 6, 13921–13927. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; He, J.; Kang, H.J.; Tang, X.; Zhu, S.; Laver, M.; Wang, S.; Copley, J.R.D.; Brown, C.M.; Zhang, Q.; et al. Identifying the Specific Nanostructures Responsible for the High Thermoelectric Performance of (Bi,Sb)2Te3 Nanocomposites. Nano Lett. 2010, 10, 3283–3289. [Google Scholar] [CrossRef]
- Tang, X.; Xie, W.; Li, H.; Zhao, W.; Zhang, Q.; Niino, M. Preparation and Thermoelectric Transport Properties of High-Performance p-type Bi2Te3 with Layered Nanostructure. Appl. Phys. Lett. 2007, 90, 012102. [Google Scholar] [CrossRef]
- Li, H.; Tang, X.; Su, X.; Zhang, Q. Preparation and Thermoelectric Properties of High-Performance Sb Additional Yb0.2Co4Sb12+y Bulk Materials with Nanostructure. Appl. Phys. Lett. 2008, 92, 202114. [Google Scholar] [CrossRef]
- Li, H.; Tang, X.; Zhang, Q.; Uher, C. Rapid Preparation Method of Bulk Nanostructured Yb0.3Co4Sb12+y Compounds and Their Improved Thermoelectric Performance. Appl. Phys. Lett. 2008, 93, 252109. [Google Scholar] [CrossRef]
- Tan, L.P.; Sun, T.; Fan, S.; Ramanujan, R.V.; Hng, H.H. Facile Precipitation of Two Phase Alloys in SnTe0.75Se0.25 with Improved Power Factor. J. Alloys Compd. 2014, 587, 420–427. [Google Scholar] [CrossRef]
- Norizan, M.N.; Miyazaki, Y.; Ohishi, Y.; Muta, H.; Kurosaki, K.; Yamanaka, S. The Nanometer-Sized Eutectic Structure of Si/CrSi2 Thermoelectric Materials Fabricated by Rapid Solidification. J. Electron. Mater. 2018, 47, 2330–2336. [Google Scholar] [CrossRef]
- Xie, J.; Ohishi, Y.; Ichikawa, S.; Muta, H.; Kurosaki, K.; Yamanaka, S. Thermoelectric Properties of Si/CoSi2 Sub-Micrometer Composites Prepared by Melt-Spinning Technique. J. Appl. Phys. 2017, 121, 205107. [Google Scholar] [CrossRef]
- Tanusilp, S.; Kurosaki, K.; Yusufu, A.; Ohishi, Y.; Muta, H.; Yamanaka, S. Enhancement of Thermoelectric Properties of Bulk Si by Dispersing Size-Controlled VSi2. J. Electron. Mater. 2017, 46, 3249–3255. [Google Scholar] [CrossRef]
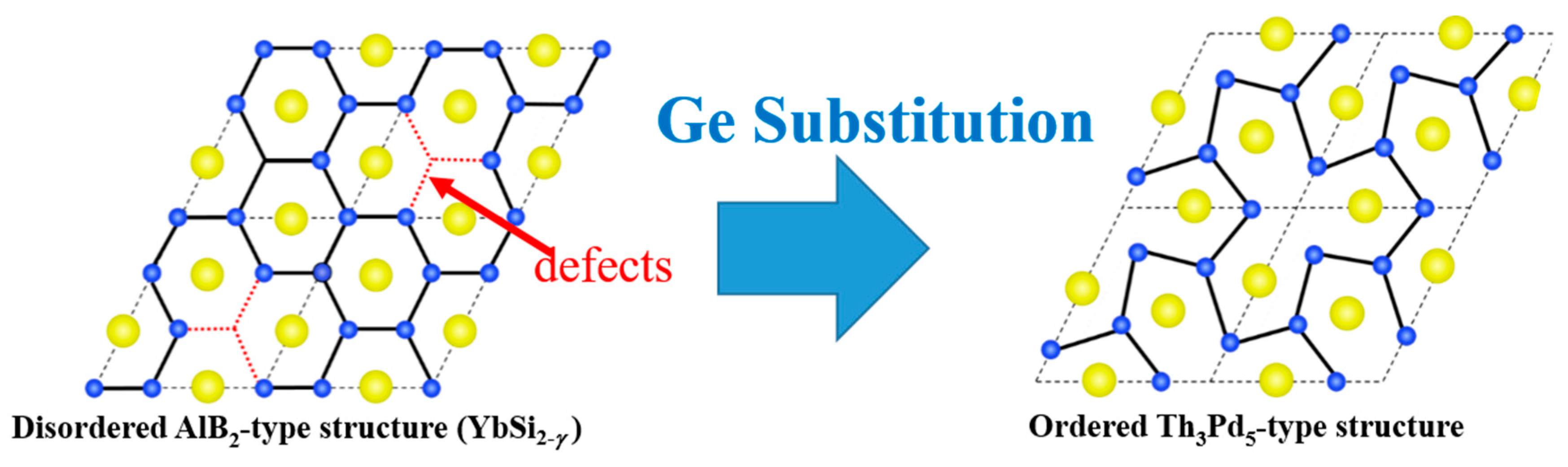
- Tanusilp, S.; Ohishi, Y.; Muta, H.; Yamanaka, S.; Nishide, A.; Hayakawa, J.; Kurosaki, K. Ytterbium Silicide (YbSi2): A Promising Thermoelectric Material with a High Power Factor at Room Temperature. Phys. Status Solidi RRL 2018, 12, 1700372. [Google Scholar] [CrossRef]
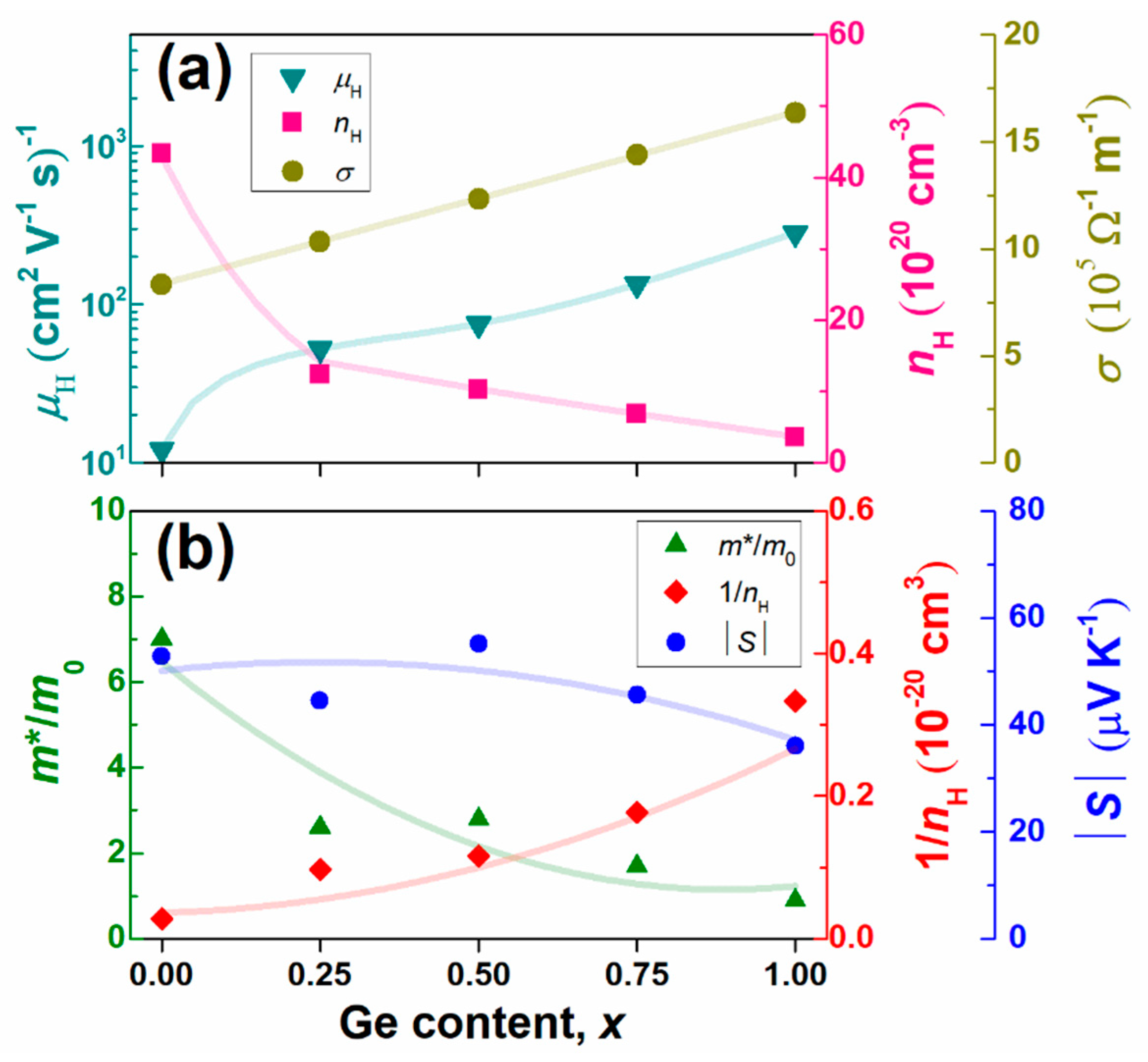
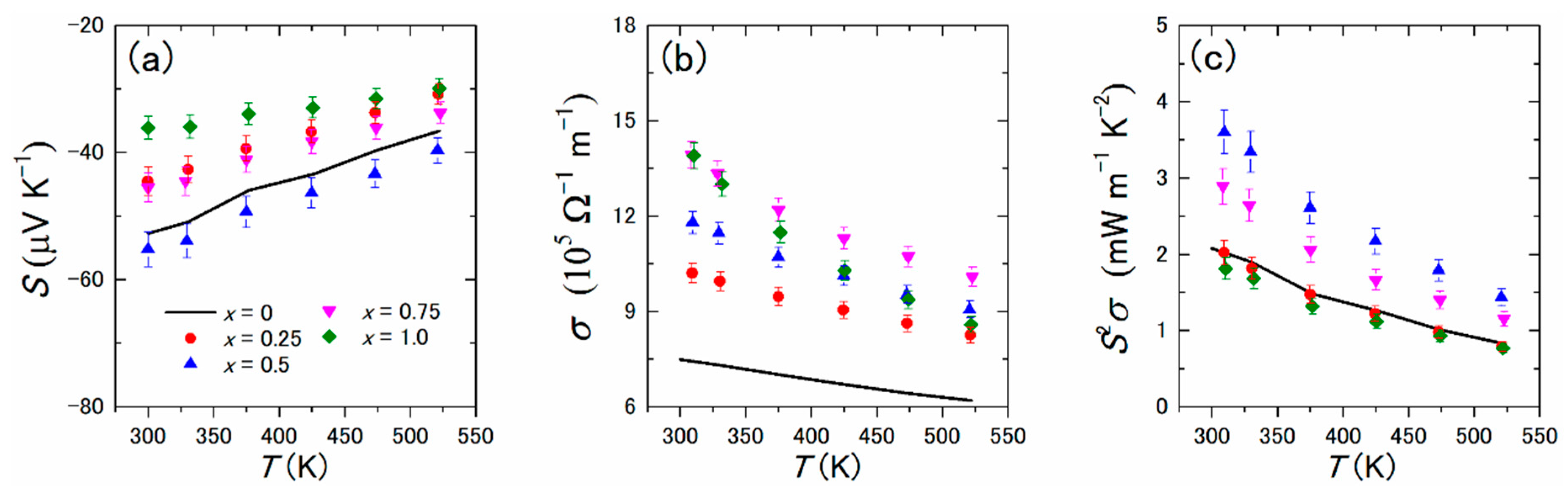
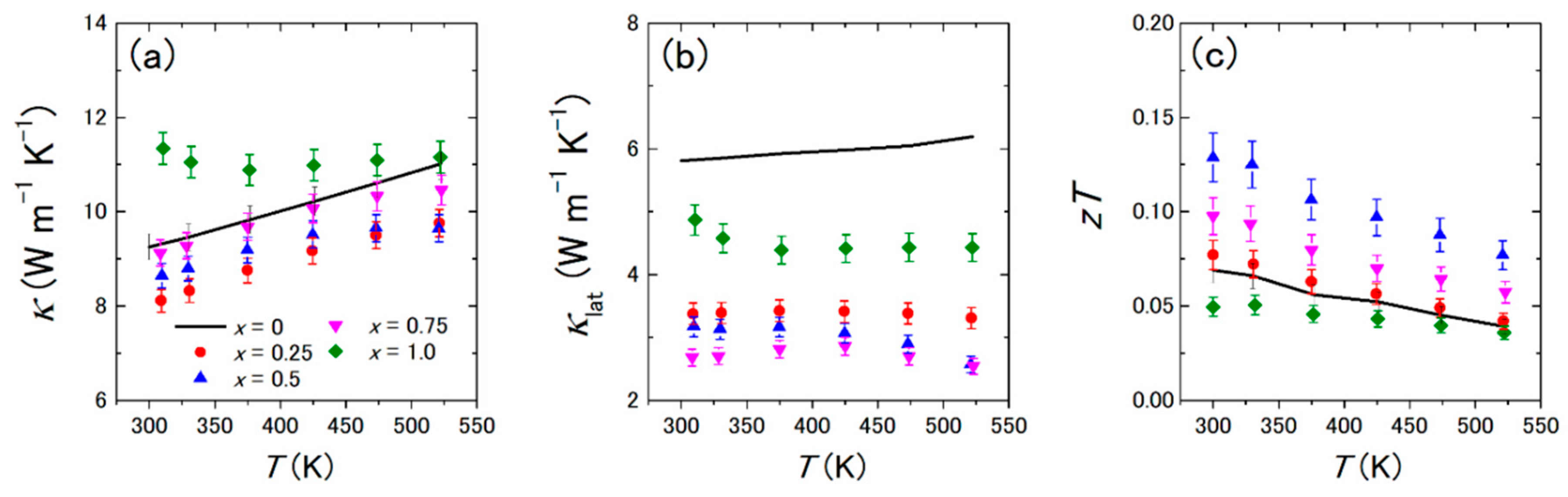
- Tanusilp, S.; Ohishi, Y.; Muta, H.; Nishide, A.; Hayakawa, J.; Kurosaki, K. High Thermoelectric Power Factor of Ytterbium Silicon-Germanium. Appl. Phys. Lett. 2018, 113, 193901. [Google Scholar] [CrossRef]
- Olesinski, R.W.; Kanani, N.; Abbaschian, G.J. The P-Si (Phosphorus-Silicon) System. Bull. Alloy Phase Diagr. 1985, 6, 128–130. [Google Scholar] [CrossRef]
- Golikova, O.A.; Iordanishvili, E.K.; Petrov, A.V.; Tela, F.T. Electrical properties of solid solutions in the Si-Ge system (Electric conductivity and thermal emf of solid solutions of silicon-germanium with near silicon composition and various current-carrier concentrations and test temperatures). Sov. Phys. Solid State 1966, 8, 397–401. [Google Scholar]
- Masetti, G.; Severi, M.; Solmi, S. Modeling of Carrier Mobility against Carrier Concentration in Arsenic-, Phosphorus-, and Boron-Doped Silicon. IEEE Trans. Electron Devices 1983, 30, 764–769. [Google Scholar] [CrossRef]
- Morin, F.; Maita, J. Electrical Properties of Silicon Containing Arsenic and Boron. Phys. Rev. 1954, 96, 28–35. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanusilp, S.-a.; Kurosaki, K. Si-Based Materials for Thermoelectric Applications. Materials 2019, 12, 1943. https://doi.org/10.3390/ma12121943
Tanusilp S-a, Kurosaki K. Si-Based Materials for Thermoelectric Applications. Materials. 2019; 12(12):1943. https://doi.org/10.3390/ma12121943
Chicago/Turabian StyleTanusilp, Sora-at, and Ken Kurosaki. 2019. "Si-Based Materials for Thermoelectric Applications" Materials 12, no. 12: 1943. https://doi.org/10.3390/ma12121943




