Development of a Graphene-Based Surface Plasmon Resonance Optical Sensor Chip for Potential Biomedical Application
Abstract
:1. Introduction
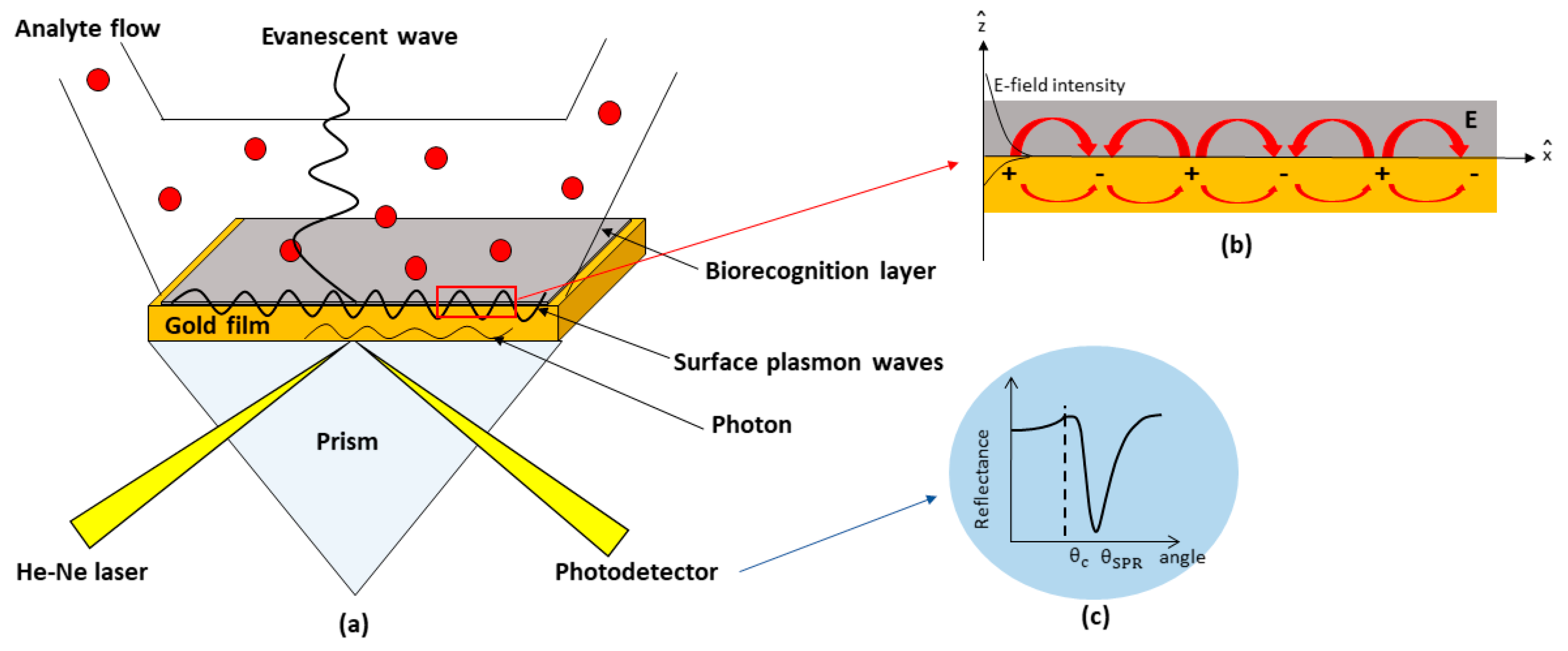
2. SPR-Based Kretschmann Configuration
2.1. Theory
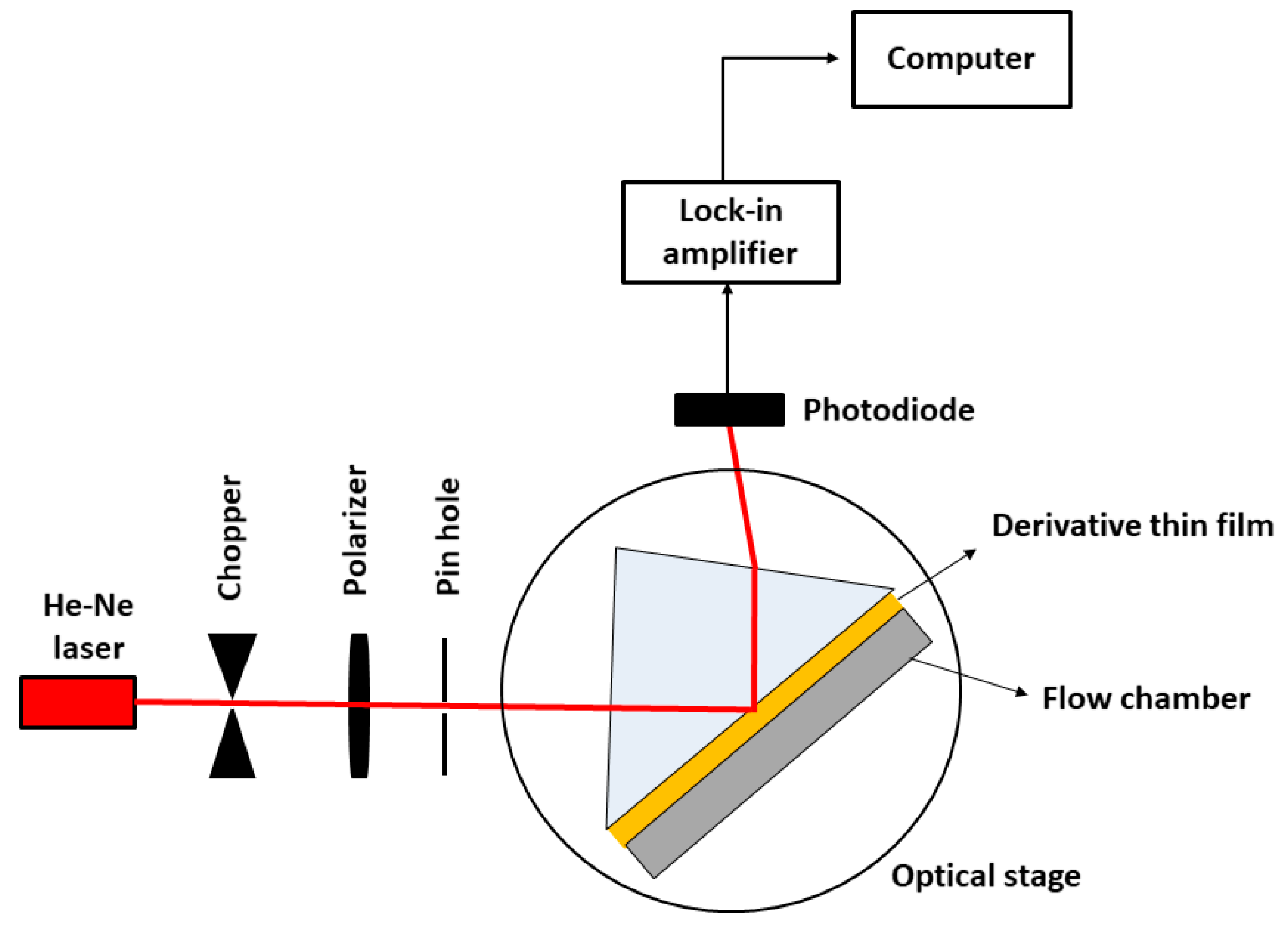
2.2. Experimental
3. Results and Discussions
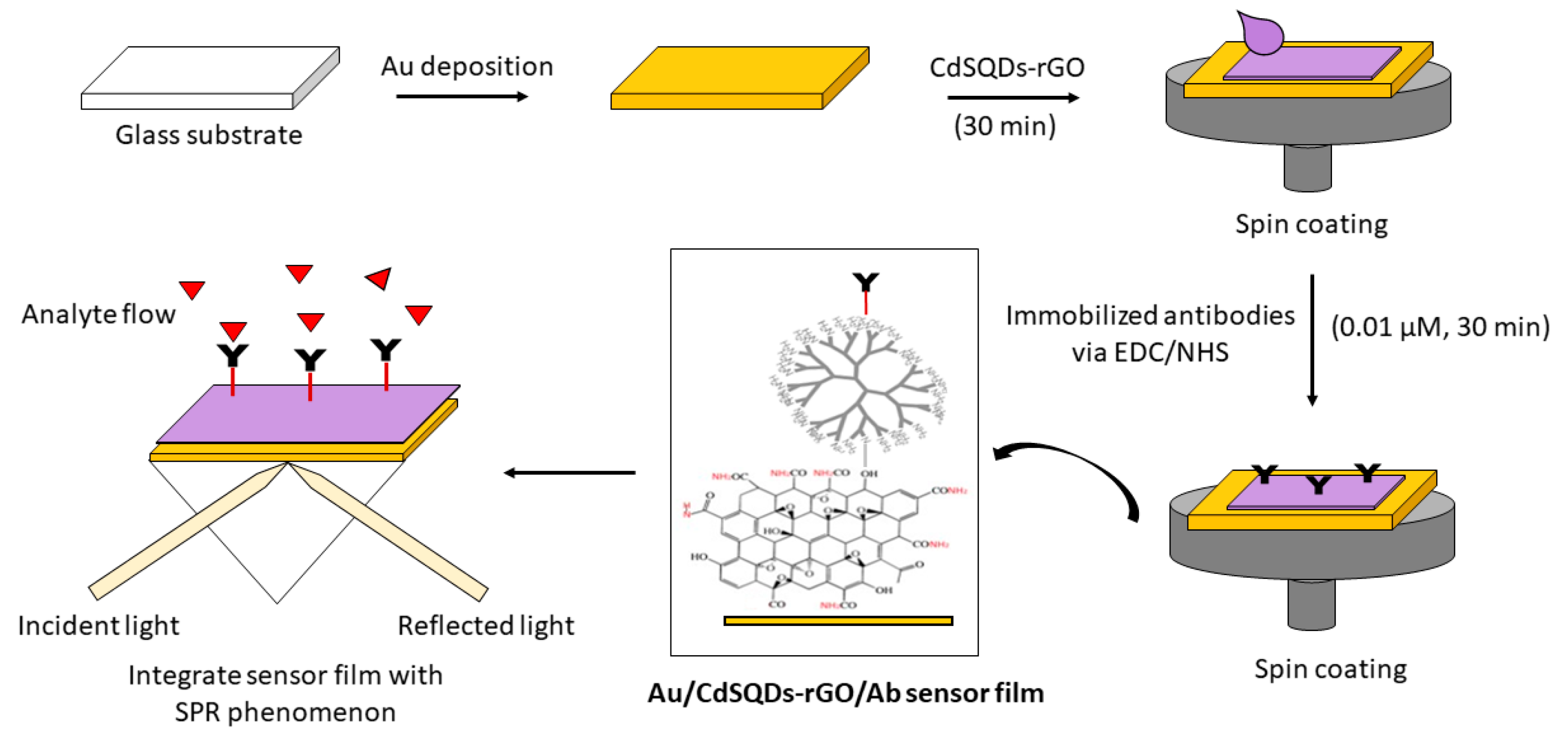
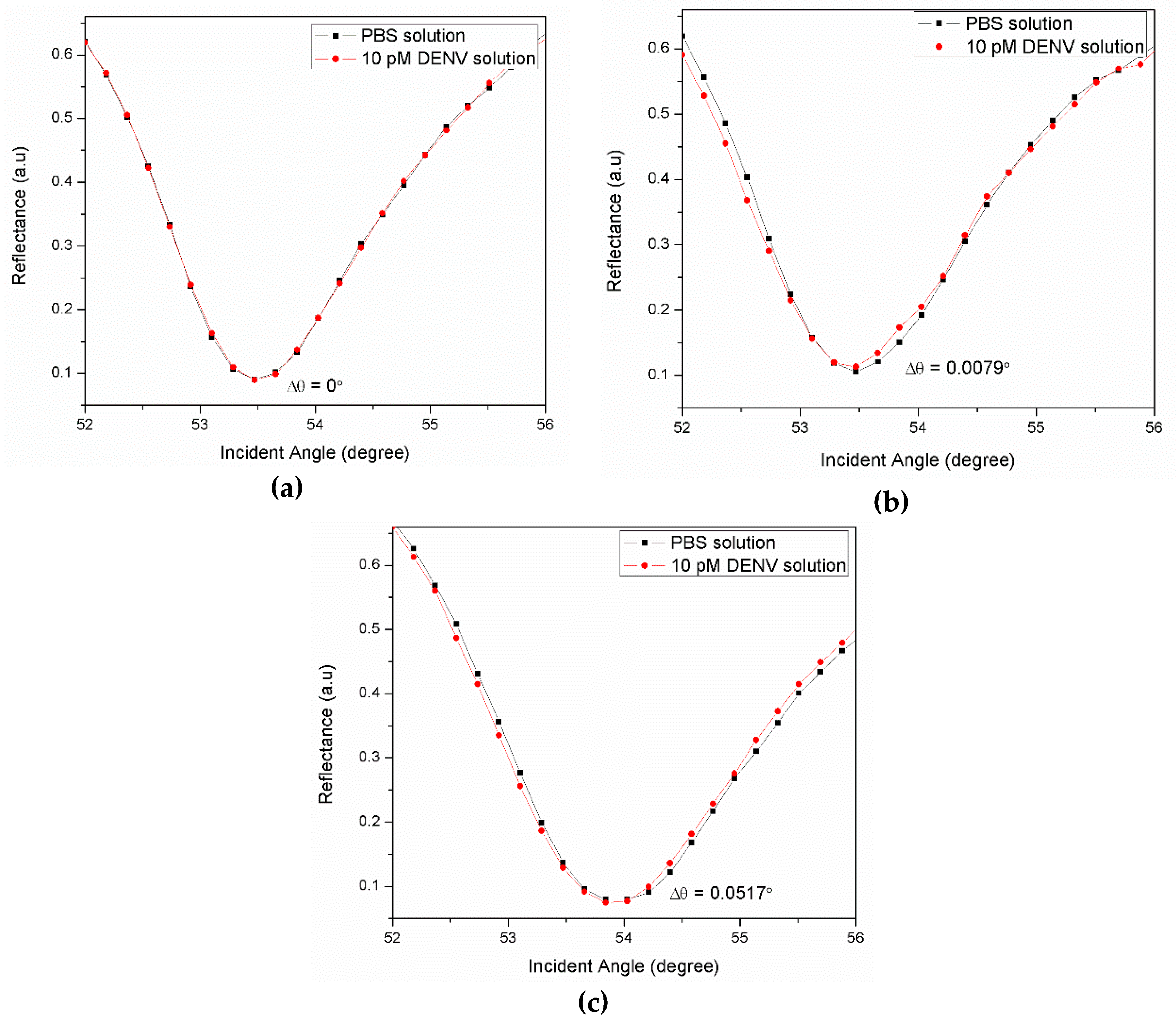
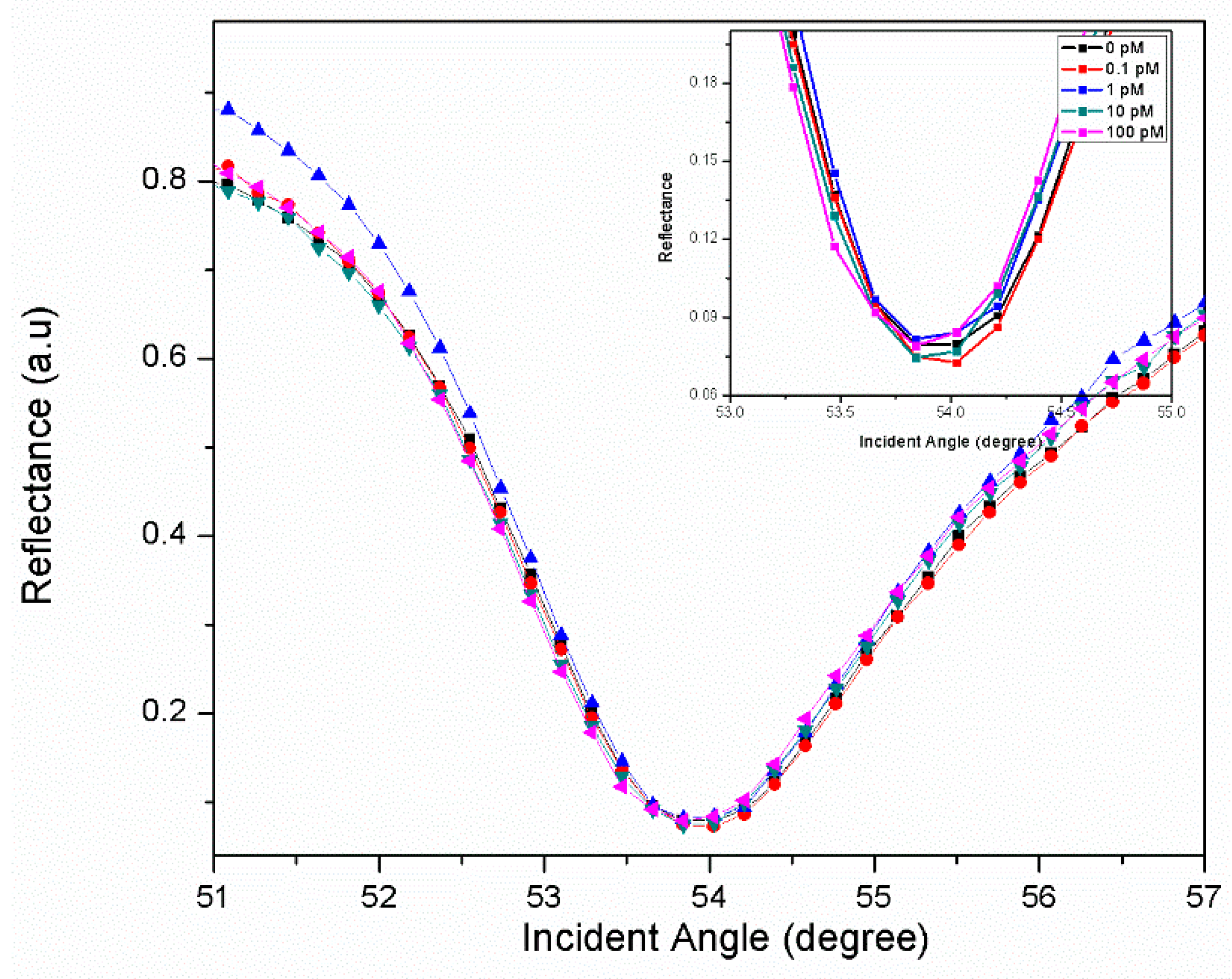
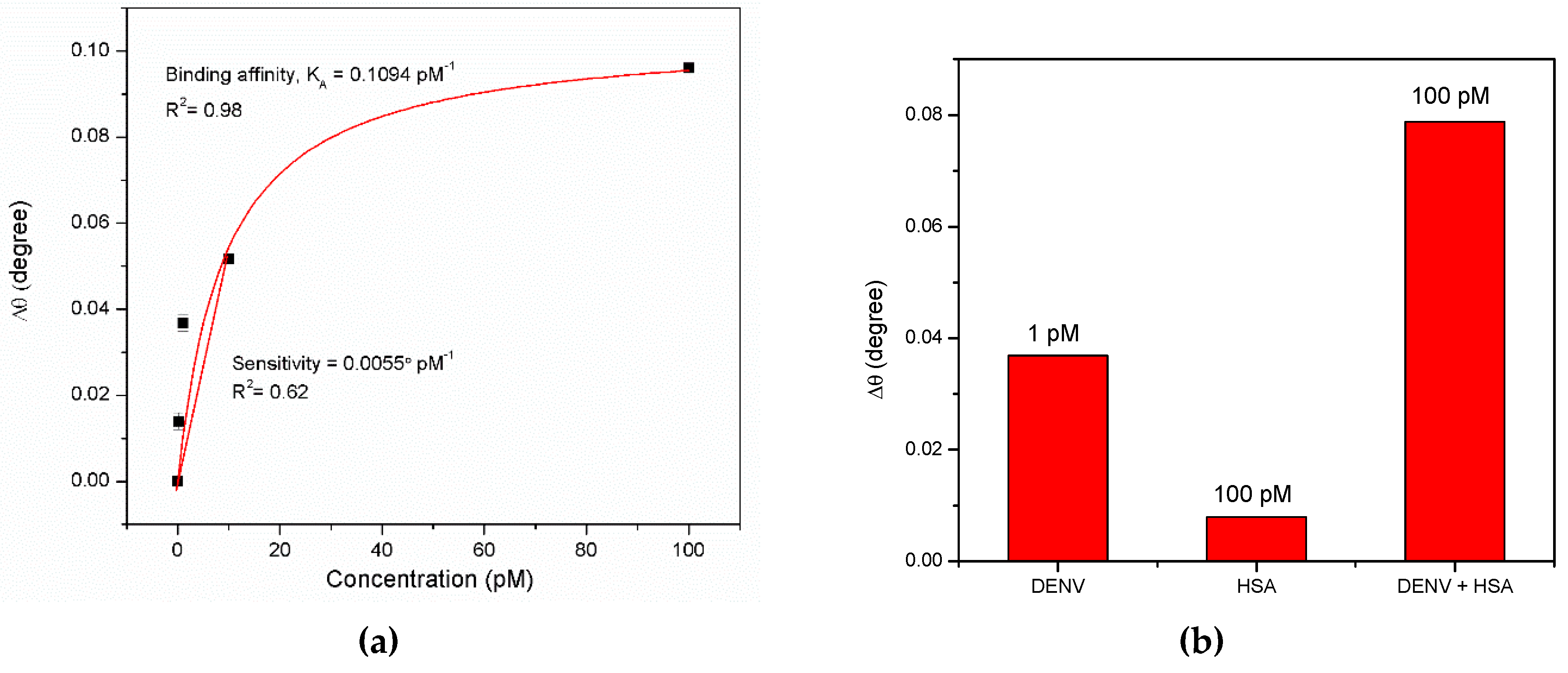
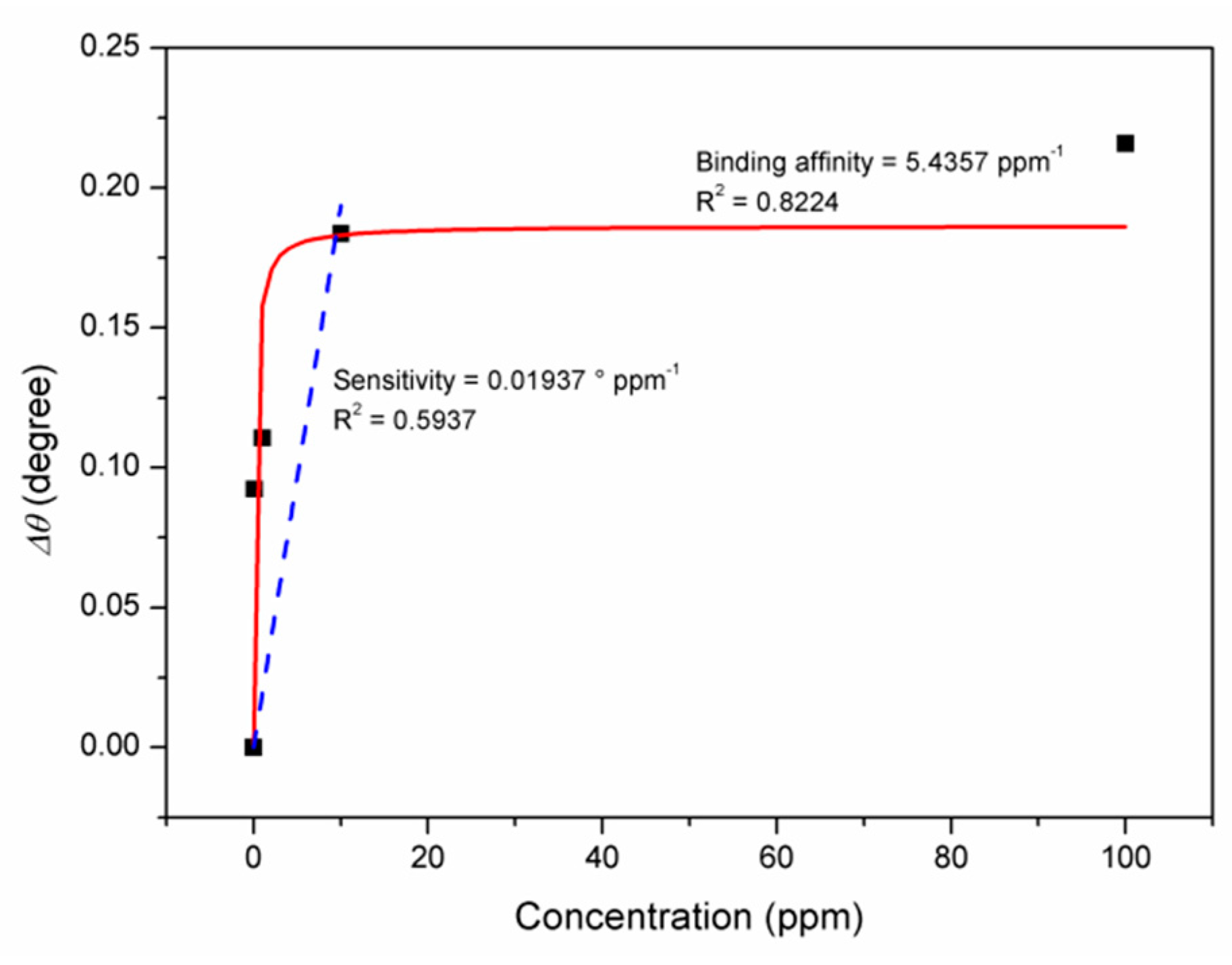
3.1. Graphene-SPR Based Materials for DENV Detection
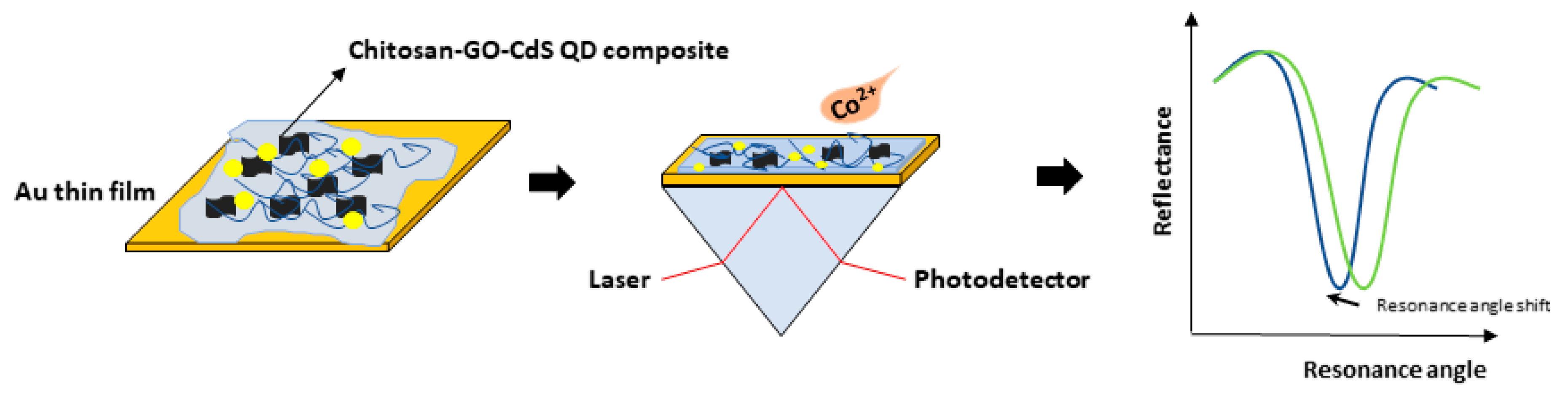
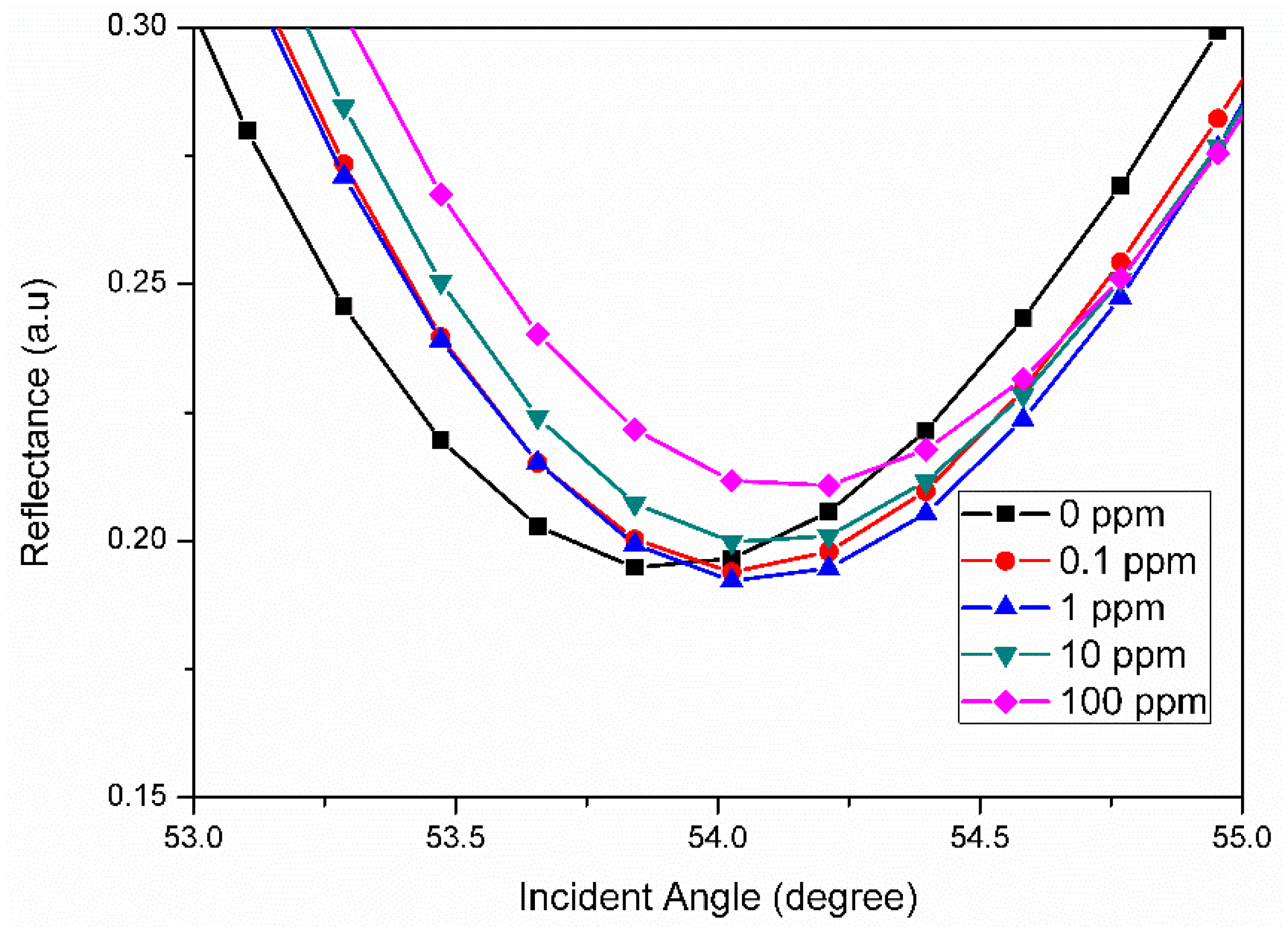
3.2. Graphene-SPR Based Materials for Metal Ion Detection
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wakida, S.I. Advanced sensing technology in environmental field. J. Environ. Sci. 2009, 21, 2–6. [Google Scholar] [CrossRef]
- Lu, Z.; Yang, S.; Yang, Q.; Luo, S.; Liu, C.; Tang, Y. A glassy carbon electrode modified with graphene, gold nanoparticles and chitosan for ultrasensitive determination of lead (II). Microchim. Acta. 2013, 180, 555–562. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M. Characterization of the optical properties of heavy metal ions using surface plasmon resonance technique. Opt. Photonics J. 2011, 1, 116. [Google Scholar] [CrossRef]
- Si, Y.; Liu, J.; Wang, A.; Niu, S.; Wan, J. A chitosan-graphene electrochemical sensor for the determination of copper (II). Instrum. Sci Technol. 2015, 43, 357–368. [Google Scholar] [CrossRef]
- Vanwambeke, S.O.; Lambin, E.F. Environmental change and vector-borne diseases: The contribution of remote sensing and spatial analyses. Med. Health Sci. 2010, 8, 231–239. [Google Scholar]
- Parham, P.E.; Waldock, J.; Christophides, G.K.; Hemming, D.; Agusto, F.; Evans, K.J.; Fefferman, N.; Gaff, H.; Gumel, A.; LaDeau, S.; et al. Climate, environmental and socio-economic change: Weighing up the balance in vector-borne disease transmission. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2015, 370, 1665–1682. [Google Scholar] [CrossRef] [PubMed]
- Batterman, S.; Eisenberg, J.; Hardin, R.; Kruk, M.E.; Lemos, M.C.; Michalak, A.M.; Mukherjee, B.; Renne, E.; Stein, H.; Watkins, C.; et al. Sustainable control of water-related infectious diseases: A review and proposal for interdisciplinary health-based systems research. Environ. Health Perspect. 2009, 117, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Smith, C.D.; Wang, L.; Li, Z.; Xiong, C.; Zhang, R. Combining Spatial Analysis and a Drinking Water Quality Index to Evaluate Monitoring Data. Int. J. Environ. Res. Public Health 2019, 16, 357–365. [Google Scholar] [CrossRef]
- McGrath, M.J.; Scanaill, C.N. Environmental monitoring for health and wellness. In Sensor technologies: Apress, Berkeley, CA, 2013, pp. 249–282. Apress: Berkeley, CA, USA, 2013; pp. 249–282. [Google Scholar]
- Snyder, E.G.; Watkins, T.H.; Solomon, P.A.; Thoma, E.D.; Williams, R.W.; Hagler, G.S.; Shelow, D.; Hindin, D.A.; Kilaru, V.J.; Preuss, P.W. The changing paradigm of air pollution monitoring. Environ. Sci. Technol. 2013, 47, 11369–11377. [Google Scholar] [CrossRef]
- Westaway, E.G.; Brinton, M.A.; Gaidamovich, S.Y.; Horzinek, M.C.; Igarashi, A.; Kääriäinen, L.; Lvov, D.K.; Porterfield, J.S.; Russell, P.K.; Trent, D.W. Flaviviridae. Intervirology 1985, 24, 183–192. [Google Scholar] [CrossRef]
- Wang, E.; Ni, H.; Xu, R.; Barrett, A.D.; Watowich, S.J.; Gubler, D.J.; Weaver, S.C. Evolutionary relationships of endemic/epidemic and sylvatic dengue viruses. J. Virol. 2000, 74, 3227–3234. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, M.G.; Kouri, G. Dengue: An update. Lancet Infect. Dis. 2002, 2, 33–42. [Google Scholar] [CrossRef]
- Fry, S.R.; Meyer, M.; Semple, M.G.; Simmons, C.P.; Sekaran, S.D.; Huang, J.X.; McElnea, C.; Huang, C.Y.; Valks, A.; Young, P.R.; et al. The diagnostic sensitivity of dengue rapid test assays is significantly enhanced by using a combined antigen and antibody testing approach. PLoS Negl. Trop. Dis. 2011, 5, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Kumbhat, S.; Sharma, K.; Gehlot, R.; Solanki, A.; Joshi, V. Surface plasmon resonance based immunosensor for serological diagnosis of dengue virus infection. J. Pharm. Biomed. Anal. 2010, 52, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Beatty, M.E.; Stone, A.; Fitzsimons, D.W.; Hanna, J.N.; Lam, S.K.; Vong, S.; Guzman, M.G.; Mendez-Galvan, J.F.; Halstead, S.B.; Letson, G.W.; et al. Best practices in dengue surveillance: A report from the Asia-Pacific and Americas Dengue Prevention Boards. PLoS Negl. Trop. Dis. 2010, 4, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Jahanshahi, P.; Zalnezhad, E.; Sekaran, S.D.; Adikan, F.R.M. Rapid immunoglobulin M-based dengue diagnostic test using surface plasmon resonance biosensor. Sci. Rep. 2014, 4, 3851–3857. [Google Scholar] [CrossRef] [PubMed]
- Kamil, Y.M.; Bakar, M.H.A.; Yaacob, M.H.; Syahir, A.; Lim, H.N.; Mahdi, M.A. Dengue E Protein Detection Using a Graphene Oxide Integrated Tapered Optical Fiber Sensor. IEEE J. Sel. Top. Quantum. Electron. 2019, 1, 1–8. [Google Scholar]
- Fang, X.; Tan, O.K.; Tse, M.S.; Ooi, E.E. A label-free immunosensor for diagnosis of dengue infection with simple electrical measurements. Biosens. Bioelectron. 2010, 25, 1137–1142. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W. Recent development of SPR spectroscopy as potential method for diagnosis of dengue virus E-protein. Sens. Rev. 2018, 38, 106–116. [Google Scholar] [CrossRef]
- Fletcher, S.J.; Phillips, L.W.; Milligan, A.S.; Rodda, S.J. Toward specific detection of Dengue virus serotypes using a novel modular biosensor. Biosens. Bioelectron. 2010, 26, 1696–1700. [Google Scholar] [CrossRef]
- Jin, S.A.; Poudyal, S.; Marinero, E.E.; Kuhn, R.J.; Stanciu, L.A. Impedimetric dengue biosensor based on functionalized graphene oxide wrapped silica particles. Electrochim. Acta. 2016, 194, 422–430. [Google Scholar] [CrossRef]
- Rahman, S.A.; Saadun, R.; Azmi, N.E.; Ariffin, N.; Abdullah, J.; Yusof, N.A.; Sidek, H.; Hajian, R. Label-free dengue detection utilizing PNA/DNA hybridization based on the aggregation process of unmodified gold nanoparticles. J. Nanomater. 2014, 2014, 106. [Google Scholar]
- Omar, N.A.S.; Fen, Y.W.; Abdullah, J.; Zaid, M.H.M.; Daniyal, W.M.E.M.M.; Mahdi, M.A. Sensitive surface plasmon resonance performance of cadmium sulfide quantum dots-amine functionalized graphene oxide based thin film towards dengue virus E-protein. Opt. Laser Technol. 2019, 114, 204–208. [Google Scholar] [CrossRef]
- Ogunlana, O. Heavy Metal Analysis of Selected Soft Drinks in Nigeria. J. Glob. Biosci. 2015, 4, 1335–1338. [Google Scholar]
- Magomya, A.M.; Yebpella, G.G.; Okpaegbe, U.C. Asssessment of metal contaminant levels in selected soft drinks sold in Nigeria. Int. J. Innov. Res. Sci. Eng. Technol. 2015, 10, 517–522. [Google Scholar]
- Lough, G.C.; Schauer, J.J.; Park, J.-S.; Shafer, M.M.; Deminter, J.T.; Weinstein, J.P. Emissions of metals associated with motor vehicle roadways. Environ. Sci. Technol. 2005, 39, 826–836. [Google Scholar] [CrossRef]
- Ullah, H.; Ahmad, I. Comparative study of heavy metals content in cosmetic products of different countries marketed in Khyber Pakhtunkhwa, Pakistan. Arab. J. Chem. 2017, 10, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Alsaffar, N.M.; Hussein, H.J. Determination of heavy metals in some cosmetics available in locally markets. IOSR J. Environ. Sci. Toxicol. Food Technol. 2014, 8, 9–12. [Google Scholar] [CrossRef]
- Arruti, A.; Fernández-Olmo, I.; Irabien, A. Evaluation of the contribution of local sources to trace metals levels in urban PM2.5 and PM10 in the Cantabria region (Northern Spain). J. Environ. Monit. 2010, 12, 1451–1458. [Google Scholar] [CrossRef]
- Rajeswari, T.R.; Sailaja, N. Impact of Heavy Metals on Environment Pollution. J. Chem. Pharm. Sci. 2014, 3, 175–181. [Google Scholar]
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry Risks and Best Availble Strategies for Remediation. ISRN Ecol. 2011, 2011, 1–20. [Google Scholar] [CrossRef]
- Masindi, V.; Muedi, K.L. Environmental Contamination by Heavy Metals. In Heavy Metals; IntechOpen: Aglan, France, 2018; pp. 115–133. [Google Scholar]
- Rare Disease Database. Available online: https://rarediseases.org/rare-diseases/heavy-metal-poisoning/ (accessed on 27 April 2019).
- Li, S.; Zhang, C.; Wang, S.; Lui, Q.; Feng, H.; Ma, X.; Guo, J. Electrochemical Microfluidics Techniques for Heavy Metal Ions Detection. Analyst 2018, 1039, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Acha, N.D.; Elosua, C.; Corres, J.M.; Arregui, F.J. Fluorescent Sensors for the Detection Heavy Metal Ions in Aqueous Media. Sensors 2019, 19, 1–34. [Google Scholar]
- Terra, I.A.A.; Mercante, L.A.; Andre, R.S.; Correa, D.S. Fluorescent and Colorimeter Electrospun Nanifibers for Heavy-Metal Sensing. Biosensors 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Turdean, G.L. Design and Development of Biosensors for the Detection of Heavy Metal Toxicity. Int. J. Electrochem. 2011, 2011, 1–15. [Google Scholar] [CrossRef]
- Berezhetskyy, A.L.; Sosovska, O.F.; Durrieu, C.; Chovelon, J.M.; Dzyadevych, S.V.; Tran-Minh, C. Alkaline phosphatase conductometric biosensor for heavy-metal ions determination. ITBM-RBM 2008, 29, 136–140. [Google Scholar] [CrossRef]
- Zheng, G.; Chen, Y.; Bu, L.; Xu, L.; Su, W. Waveguide-coupled surface phonon resonance sensors with super-resolution in the mid-infrared region. Opt. Lett. 2016, 41, 1582–1585. [Google Scholar] [CrossRef]
- Ozbay, E. Plasmonics: Merging photonics and electronics at nanoscale dimensions. Science 2006, 311, 189–193. [Google Scholar] [CrossRef]
- Gupta, G.; Kondoh, J. Tuning and sensitivity enhancement of surface plasmon resonance sensor. Sens. Actuators B Chem. 2007, 122, 381–388. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Verma, R.; Gupta, B.D. Theoretical modeling of a self-referenced dual mode SPR sensor utilizing indium tin oxide film. Opt. Commun. 2016, 369, 131–137. [Google Scholar] [CrossRef]
- Zeng, S.; Baillargeat, D.; Ho, H.P.; Yong, K.T. Nanomaterials enhanced surface plasmon resonance for biological and chemical sensing applications. Chem. Soc. Rev. 2014, 43, 3426–3452. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Q.; Zeng, S.; Jiang, L.; Hong, L.; Xu, G.; Dinh, X.Q.; Qian, J.; He, S.; Qu, J.; Coquet, P.; et al. Sensitivity enhancement of transition metal dichalcogenides/silicon nanostructure-based surface plasmon resonance biosensor. Sci. Rep. 2016, 6, 28190–28202. [Google Scholar] [CrossRef] [PubMed]
- Maharana, P.K.; Jha, R.; Padhy, P. ; On the electric field enhancement and performance of SPR gas sensor based on graphene for visible and near infrared. Sens. Actuators B Chem. 2015, 207, 117–122. [Google Scholar] [CrossRef]
- Zhang, C.G.; Chang, S.J.; Settu, K.; Chen, C.J.; Liu, J.T. High-sensitivity glycated hemoglobin (HbA1c) aptasensor in rapid-prototyping surface plasmon resonance. Sens. Actuators B Chem. 2019, 279, 267–273. [Google Scholar] [CrossRef]
- Daniyal, W.M.E.M.M.; Saleviter, S.; Fen, Y.W. Development of surface plasmon resonance spectroscopy for metal ion detection. Sens. Mater. 2018, 30, 2023–2038. [Google Scholar] [CrossRef]
- Roshidi, M.D.A.; Fen, Y.W.; Daniyal, W.M.E.M.M.; Omar, N.A.S.; Zulholinda, M. Structural and optical properties of chitosan–poly (amidoamine) dendrimer composite thin film for potential sensing Pb2+ using an optical spectroscopy. Optik 2019, 185, 351–358. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W.; Abdullah, J.; Chik, C.E.N.C.E.; Mahdi, M.A. Development of an optical sensor based on surface plasmon resonance phenomenon for diagnosis of dengue virus E-protein. Sens. Biosensing Res. 2018, 20, 16–21. [Google Scholar] [CrossRef]
- Squires, T.M.; Messinger, R.J.; Manalis, S.R. Making it stick: Convection, reaction and diffusion in surface-based biosensors. Nat. Biotechnol. 2008, 26, 417–426. [Google Scholar] [CrossRef]
- Eftekhari, F.; Escobedo, C.; Ferreira, J.; Duan, X.; Girotto, E.M.; Brolo, A.G.; Gordon, R.; Sinton, D. Nanoholes as nanochannels: Flow-through plasmonic sensing. Anal. Chem. 2009, 81, 4308–4311. [Google Scholar] [CrossRef]
- Kumar, S.; Wittenberg, N.J.; Oh, S.H. Nanopore-induced spontaneous concentration for optofluidic sensing and particle assembly. Anal. Chem. 2012, 85, 971–977. [Google Scholar] [CrossRef]
- Fabini, E.; Danielson, U.H. Monitoring drug–serum protein interactions for early ADME prediction through Surface Plasmon Resonance technology. J. Pharm. Biomed. Anal. 2017, 144, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Ong, B.H.; Yuan, X.; Tjin, S.C.; Zhang, J.; Ng, H.M. Optimised film thickness for maximum evanescent field enhancement of a bimetallic film surface plasmon resonance biosensor. Sens. Actuators B Chem. 2006, 114, 1028–1034. [Google Scholar] [CrossRef]
- Piliarik, M.; Homola, J. Surface plasmon resonance (SPR) sensors: Approaching their limits? Opt. Express 2009, 17, 16505–16517. [Google Scholar] [CrossRef] [PubMed]
- Szunerits, S.; Maalouli, N.; Wijaya, E.; Vilcot, J.P.; Boukherroub, R. Recent advances in the development of graphene-based surface plasmon resonance (SPR) interfaces. Anal. Bioanal. Chem. 2013, 405, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Wu, T.; Bao, H.; Li, L. Green fabrication of chitosan films reinforced with parallel aligned graphene oxide. Carbohyd. Polym. 2011, 83, 1908–1915. [Google Scholar] [CrossRef]
- Krishnan, S.K.; Singh, E.; Singh, P.; Meyyappan, M.; Nalwa, H.S. A Review on Graphene-Based Nanocomposites for Electrochemical and Fluorescent Biosensors. RSC Adv. 2019, 9, 8778–8781. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, Y.; Wang, C.; He, M.; Lin, Q. Selective Detection of Water Pollutants Using a Differential Aptamer-Based Graphene Biosensor. Biosens. Bioelectron. 2019, 126, 59–67. [Google Scholar] [CrossRef]
- Taniselass, S.; Arshad, M.K.M.; Gopinath, S.C.B. Graphene-Based Electrochemical Biosensors for Monitoring Noncommunicable Disease Biomarkers. Biosens. Bioelectron. 2019, 130, 276–292. [Google Scholar] [CrossRef]
- Koppens, F.H.L.; Chang, D.E.; De Abajo, F.J.G. Graphene Plasmonics: A Platform for Strong Light-Matter Interactions. Nano Lett. 2011, 11, 3370–3377. [Google Scholar] [CrossRef]
- Maier, S.A. Graphene Plasmonics: All Eyes on Flatland. Nat. Phys. 2012, 8, 581–582. [Google Scholar] [CrossRef]
- Stauber, T. Plasmonics in Dirac Systems: From Graphene to Topological Insulators. J. Phys. Condens. Matter 2014, 26, 123201. [Google Scholar] [CrossRef] [PubMed]
- Politano, A.; Chiarello, G. Plasmon Modes in Graphene: Status and Prospect. Nanoscale 2014, 6, 10927–10940. [Google Scholar] [CrossRef] [PubMed]
- Barik, A.; Zhang, Y.; Grassi, R.; Nadappuram, B.P.; Edel, J.B.; Low, T.; Koester, S.J.; Oh, S.H. Graphene-edge dielectrophoretic tweezers for trapping of biomolecules. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nayak, J.K.; Parhi, P.; Jha, R. Graphene oxide encapsulated gold nanoparticle based stable fibre optic sucrose sensor. Sens. Actuators B Chem. 2015, 221, 835–841. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, C.; Han, L.; Jin, L.; Zhou, M.; Dong, S. Label-free, regenerative and sensitive surface plasmon resonance and electrochemical aptasensors based on graphene. Chem. Commun. 2011, 47, 7794–7796. [Google Scholar] [CrossRef] [PubMed]
- Chiu, N.F.; Huang, T.Y.; Kuo, C.C.; Lee, W.C.; Hsieh, M.H.; Lai, H.C. Single-layer graphene based SPR biochips for tuberculosis bacillus detection. In Proceedings of the Biophotonics: Photonic Solutions for Better Health Care III: International Society for Optics and Photonics, Brussels, Belgium, 16–19 April 2012; Volume 8427, pp. 84273M-1–84273M-7. [Google Scholar]
- Aksimsek, S.; Sun, Z. Graphene-MoS 2 heterostructure based surface plasmon resonance biosensor. In Proceedings of the 2016 URSI International Symposium on Electromagnetic Theory (EMTS): IEEE, Espoo, Finland, 14–18 August 2016; pp. 180–181. [Google Scholar]
- Prabowo, B.A.; Alom, A.; Secario, M.K.; Masim, F.C.P.; Lai, H.C.; Hatanaka, K.; Liu, K.C. Graphene-based portable SPR sensor for the detection of Mycobacterium tuberculosis DNA strain. Procedia Eng. 2016, 168, 541–545. [Google Scholar] [CrossRef]
- Jiang, W.S.; Xin, W.; Xun, S.; Chen, S.N.; Gao, X.G.; Liu, Z.B.; Tian, J.G. Reduced graphene oxide-based optical sensor for detecting specific protein. Sens. Actuators B Chem. 2017, 249, 142–148. [Google Scholar] [CrossRef]
- Chiu, N.F.; Fan, S.Y.; Yang, C.D.; Huang, T.Y. Carboxyl-functionalized graphene oxide composites as SPR biosensors with enhanced sensitivity for immunoaffinity detection. Biosens. Bioelectron. 2017, 89, 370–376. [Google Scholar] [CrossRef]
- Jamil, N.A.; Menon, P.S.; Said, F.A.; Tarumaraja, K.A.; Mei, G.S.; Majlis, B.Y. Graphene-based surface plasmon resonance urea biosensor using Kretschmann configuration. In Proceedings of the 2017 IEEE Regional Symposium on Micro and Nanoelectronics (RSM): IEEE, Penang, Malaysia, 23–25 August 2017; pp. 112–115. [Google Scholar]
- Primo, E.N.; Kogan, M.J.; Verdejo, H.E.; Bollo, S.; Rubianes, M.D.; Rivas, G.A. Label-free graphene oxide-based surface plasmon resonance immunosensor for the quantification of galectin-3, a novel cardiac biomarker. ACS Appl. Mater. Interfaces 2018, 10, 23501–23508. [Google Scholar] [CrossRef]
- Gong, W.; Jiang, S.; Li, Z.; Li, C.; Xu, J.; Pan, J.; Huo, Y.; Man, B.; Liu, A.; Zhang, C. Experimental and theoretical investigation for surface plasmon resonance biosensor based on graphene/Au film/D-POF. Opt. Express 2019, 27, 3483–3495. [Google Scholar] [CrossRef]
- Lokman, N.F.; Bakar, A.A.A.; Suja, F.; Abdullah, H.; Rahman, W.B.W.A.; Huang, N.M.; Yaacob, M.H. Highly sensitive SPR response of Au/chitosan/graphene oxide nanostructured thin films toward Pb (II) ions. Sens. Actuators B Chem. 2014, 195, 459–466. [Google Scholar] [CrossRef]
- Nawi, N.M.; Abdullah, S.; Bakar, A.A.A. Gold nanoparticles/graphene oxide/polyaniline nanocomposites film as sensitive LSPR-based sensor for Pb(II) ions detection. In Proceedings of the 2014 IEEE 5th International Conference on Photonics (ICP), Kuala Lumpur, Malaysia, 2–4 September 2014. [Google Scholar]
- Kamaruddin, N.H.; Bakar, A.A.A.; Yaacob, M.H.; Mahdi, M.A.; Zan, M.S.D.; Shaari, S. Enhancement of chitosan-graphene oxide SPR sensor with a multi-metallic layers of Au-Ag-Au nanostructure for lead(II) ion detection. Appl. Surf. Sci. 2016, 361, 177–184. [Google Scholar] [CrossRef]
- Kamaruddin, N.H.; Bakar, A.A.A.; Mobarak, N.N.; Zan, M.S.D.; Arsad, N. Binding affinity of a highly sensitive Au/Ag/Au/chitosan-graphene oxide sensor based on direct detection of Pb2+ and Hg2+ ions. Sensors 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Zainudin, A.A.; Fen, Y.W.; Yusof, N.A.; Al-Rekabi, S.H.; Mahdi, M.A.; Omar, N.A.S. Incorporation of surface plasmon resonance with novel valinomycin doped chitosan-graphene oxide thin film for sensing potassium ion. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 191, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Daniyal, W.M.E.M.M.; Fen, Y.W.; Abdullah, J.; Sadrolhosseini, A.R.; Saleviter, S.; Omar, N.A.S. Exploration of surface plasmon resonance for sensing copper ion based on nanocrystalline cellulose-modified thin film. Opt. Express 2018, 26, 34880–34894. [Google Scholar] [CrossRef]
- Daniyal, W.M.E.M.M.; Fen, Y.W.; Abdullah, J.; Sadrolhosseini, A.R.; Saleviter, S.; Omar, N.A.S. Label-free optical spectroscopy for characterizing binding properties of highly sensitive nanocrystalline cellulose-graphene oxide based nanocomposite towards nickel ion. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 212, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Ramdzan, N.S.M.; Fen, Y.W.; Omar, N.A.S.; Anas, N.A.A.; Daniyal, W.M.E.M.M.; Saleviter, S.; Zainudin, A.A. Optical and surface plasmon resonance sensing properties for chitosan/carboxyl-functionalized graphene quantum dots thin film. Optik 2019, 17, 802–812. [Google Scholar] [CrossRef]
- Wang, D.S.; Fan, S.K. Microfluidic surface plasmon resonance sensors: From principles to point-of-care applications. Sensors 2016, 16, 1175–1192. [Google Scholar] [CrossRef]
- Zainuddin, N.H.; Fen, Y.W.; Alwahib, A.A.; Yaacob, M.H.; Bidin, N.; Omar, N.A.S.; Mahdi, M.A. Detection of adulterated honey by surface plasmon resonance optical sensor. Optik 2018, 168, 134–139. [Google Scholar] [CrossRef]
- Unser, S.; Bruzas, I.; He, J.; Sagle, L. Localized surface plasmon resonance biosensing: Current challenges and approaches. Sensors 2015, 15, 15684–15716. [Google Scholar] [CrossRef]
- Sheikh, I. Cobalt poisoning: A comprehensive review of the literature. J. Med. Toxicol. Clinical Forensic Med. 2016, 2, 1–6. [Google Scholar] [CrossRef]
- Leyssens, L.; Vinck, B.; Straeten, C.V.D.; Wuyts, F.; Maes, L. Cobalt toxic in humans. A review of the potential sources and systemic health effects. Toxicology 2017, 387, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Noboru, Y.; Sadao, M.; Torii, T. The cobalt content of human body. J. Radiat. 1962, 3, 4–8. [Google Scholar]
| Sensor Layer | Target | Limit of Detection | Sensitivity | Reference |
|---|---|---|---|---|
| Graphene | α-thrombin | 0.05 nM | - | [68] |
| Au/SAM/Graphene/ | Tuberculosis bacillus | - | - | [69] |
| Ag/Graphene-MoS2 | ssDNA | - | - | [70] |
| Graphene | Mycobacterium tuberculosis (cssDNA) | 28 fM | - | [71] |
| RGO | Rabbit IgG | 0.0625 µg/mL | - | [72] |
| Au/GO-COOH | Anti BSA | 0.01 pg/mL | - | [73] |
| Cr/Au/MoS2/Graphene | Urea | - | 230°/RIU | [74] |
| Au/SAM/GO/3ABA | Galectin-3 | 2.0 ng/mL | - | [75] |
| Au/Graphene | Glucose | - | 1227 nm/RIU | [76] |
| DNA | 0.1 nM | - |
| Active Layer | Metal Ions | Limit of Detection | Sensitivity | References |
|---|---|---|---|---|
| Au/CS/GO | Pb2+ | 0.03 ppm | 1.11200° ppm−1 | [77] |
| AuNPs/GO/PANI | Pb2+ | 0.03 ppm | - | [78] |
| Au-Ag-Au CS-GO | Pb2+ | 0.1 ppm | 2.05° ppm−1 | [79] |
| Au/Ag/Au/CS-GO | Hg2+ | 0.1 ppm | 1.66° ppm−1 | [80] |
| C-GO-V | K+ | 0.001 ppm | 0.00948° ppm−1 | [81] |
| CTA-NCC/GO | Cu2+ | 0.01 ppm | 3.271° ppm−1 | [82] |
| CTA-NCC/GO | Ni2+ | 0.01 ppm | 1.509° ppm−1 | [83] |
| Cs/CGQDs | Hg2+ | 0.5 ppm | 0.00062° ppm−1 | [84] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omar, N.A.S.; Fen, Y.W.; Saleviter, S.; Daniyal, W.M.E.M.M.; Anas, N.A.A.; Ramdzan, N.S.M.; Roshidi, M.D.A. Development of a Graphene-Based Surface Plasmon Resonance Optical Sensor Chip for Potential Biomedical Application. Materials 2019, 12, 1928. https://doi.org/10.3390/ma12121928
Omar NAS, Fen YW, Saleviter S, Daniyal WMEMM, Anas NAA, Ramdzan NSM, Roshidi MDA. Development of a Graphene-Based Surface Plasmon Resonance Optical Sensor Chip for Potential Biomedical Application. Materials. 2019; 12(12):1928. https://doi.org/10.3390/ma12121928
Chicago/Turabian StyleOmar, Nur Alia Sheh, Yap Wing Fen, Silvan Saleviter, Wan Mohd Ebtisyam Mustaqim Mohd Daniyal, Nur Ain Asyiqin Anas, Nur Syahira Md Ramdzan, and Mohammad Danial Aizad Roshidi. 2019. "Development of a Graphene-Based Surface Plasmon Resonance Optical Sensor Chip for Potential Biomedical Application" Materials 12, no. 12: 1928. https://doi.org/10.3390/ma12121928





