Hydrothermally Synthesized Mg-Based Spinel Nanoferrites: Phase Formation and Study on Magnetic Features and Microwave Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Mg-Based Ferrite Nanoparticles
2.2. Characterization of Ferrtie Nanoparticles
3. Results and Discussion
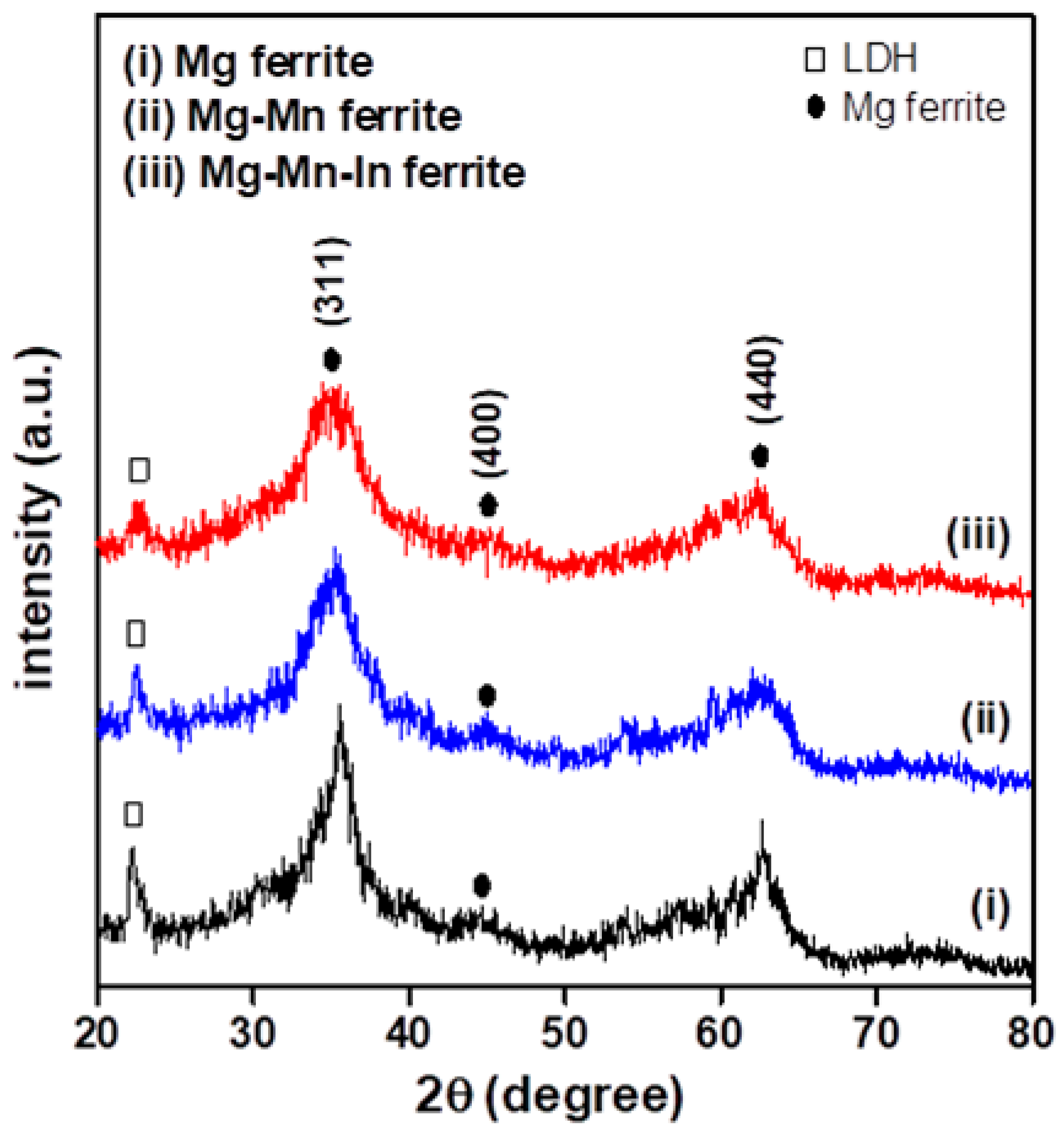
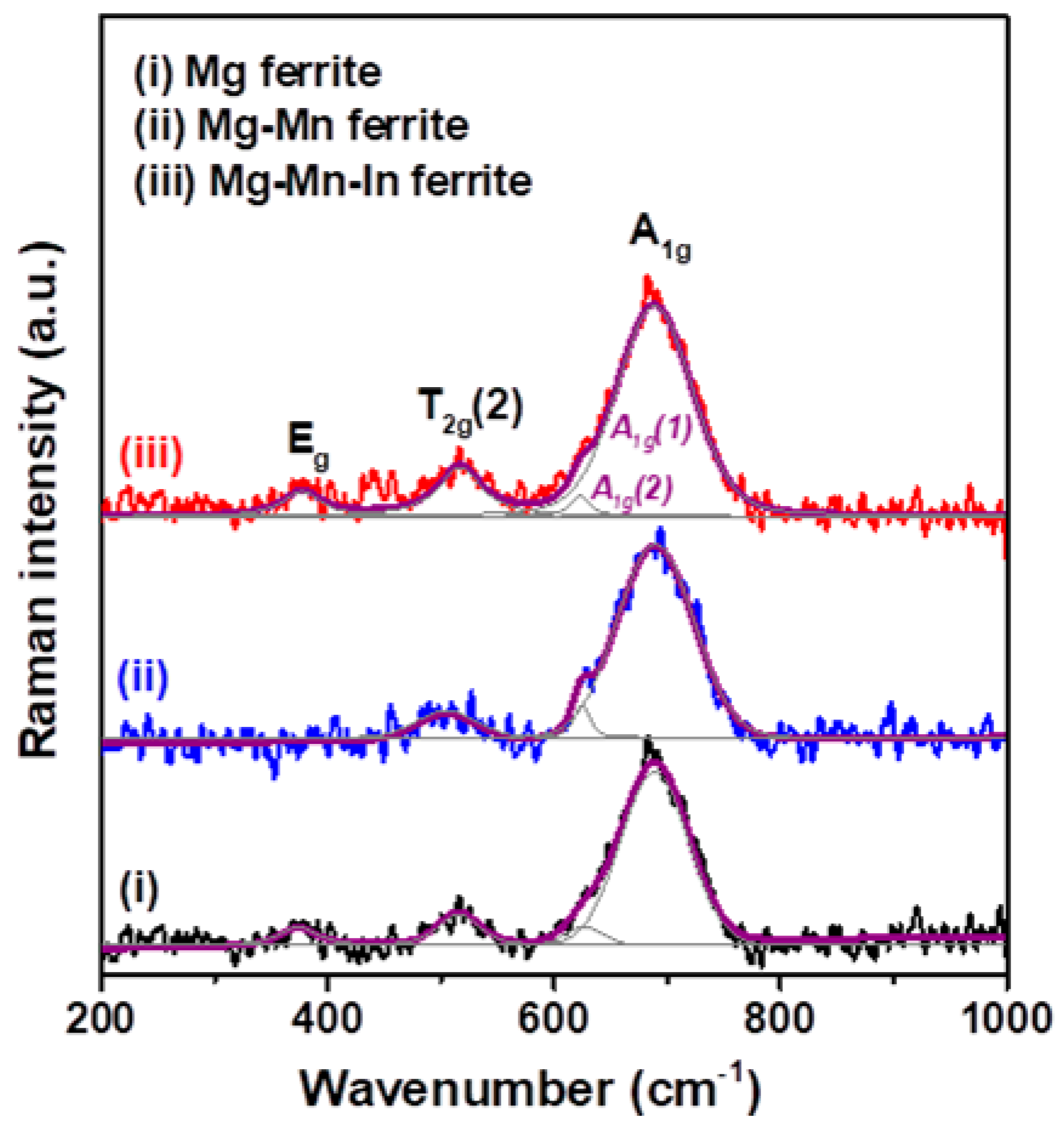
3.1. Structural and Morphological Characterization
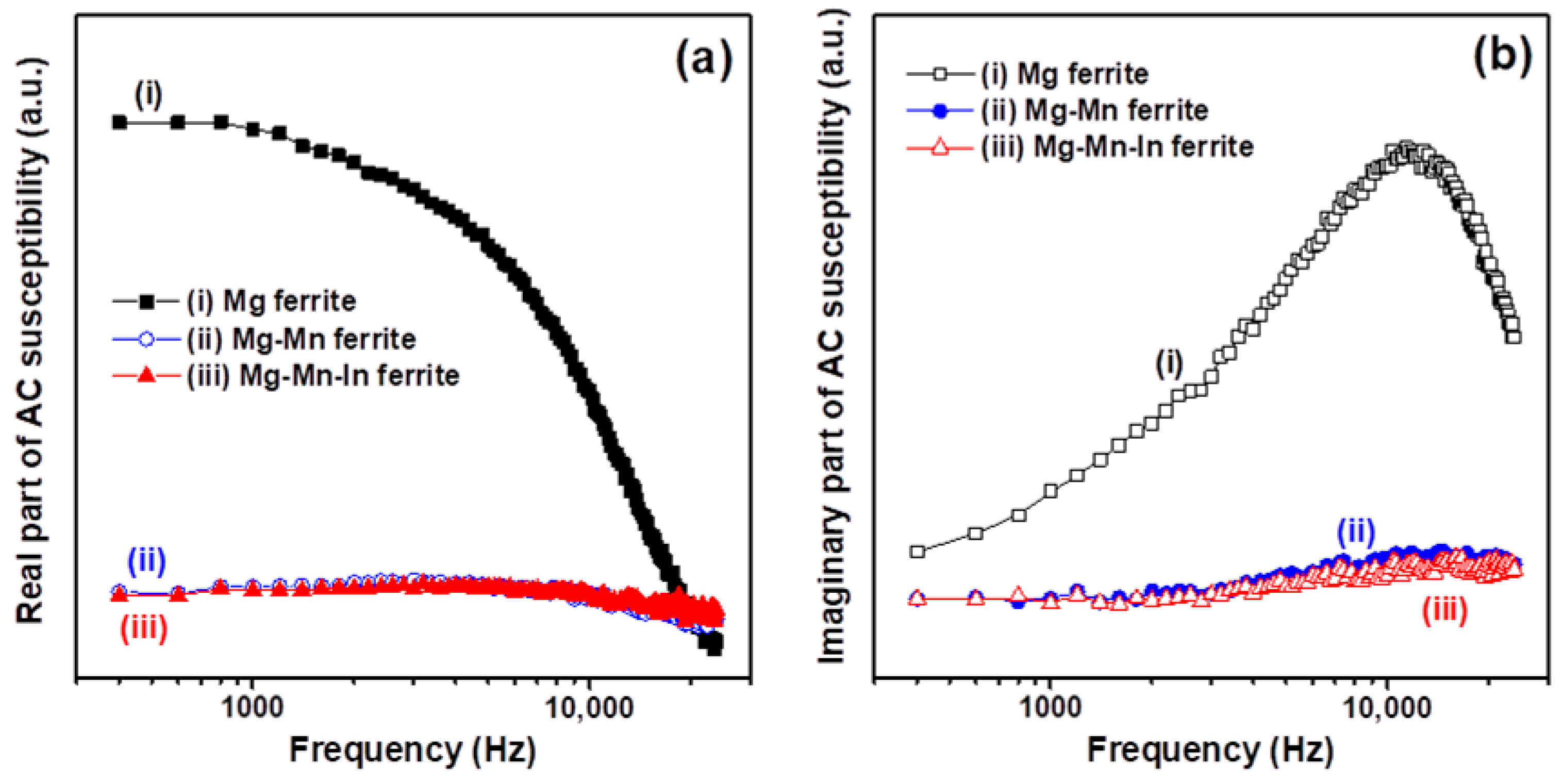
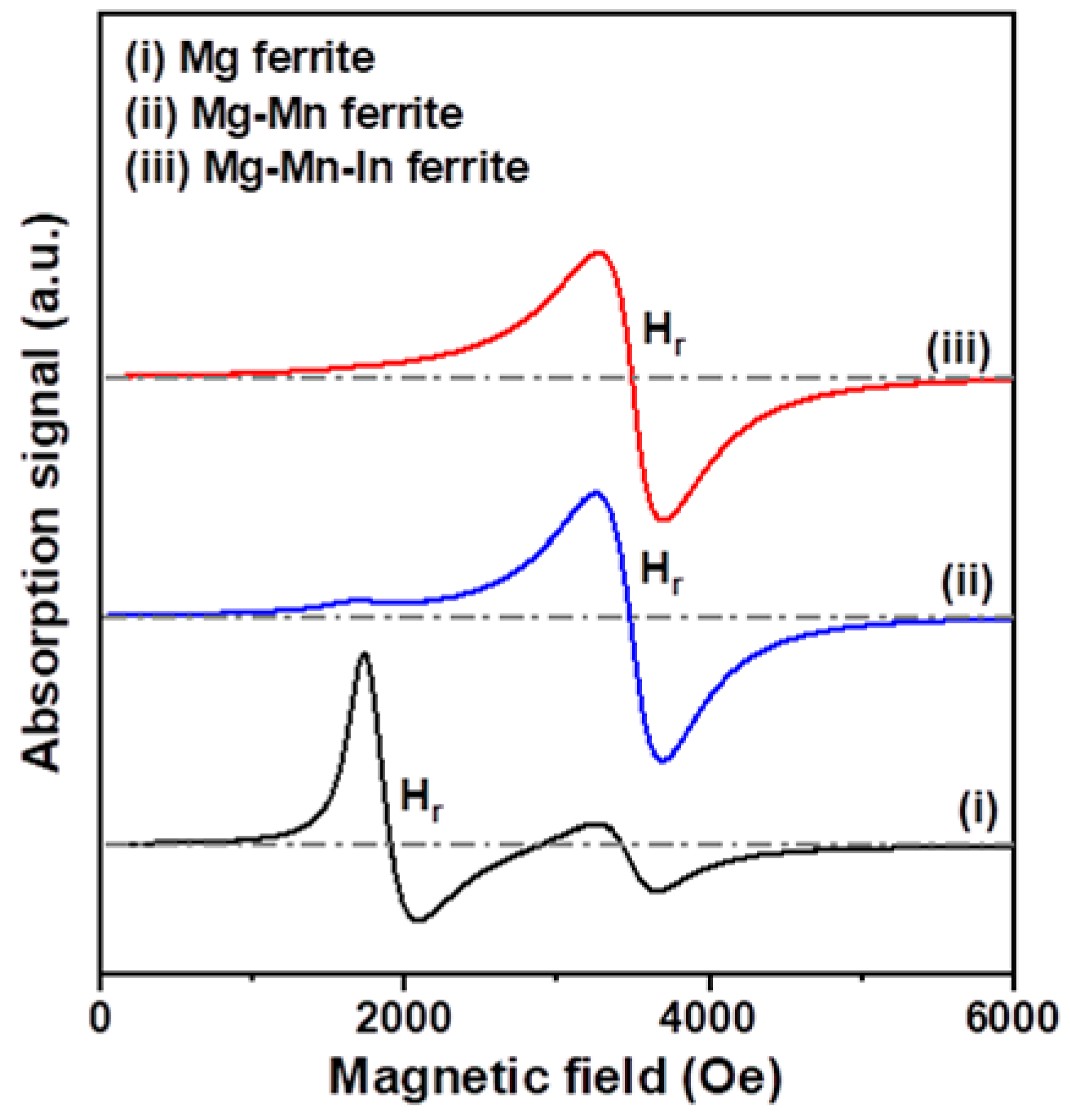
3.2. Magnetic and Microwave Proeprties Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lisjak, D.; Mertelj, A. Anisotropic magnetic nanoparticles: A review of their properties, syntheses and potential applications. Prog. Mater Sci. 2018, 95, 286–328. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Msagati, T.A.M.; Mamba, B.B. Ferrite nanoparticles: Synthesis, characterization and applications in electronic device. Mater. Sci. Eng. B 2017, 215, 37–55. [Google Scholar] [CrossRef]
- Obaidat, I.M.; Issa, B.; Haik, Y. Magnetic properties of magnetic nanoparticles for efficient hyperthermia. Nanomaterials 2015, 5, 63–89. [Google Scholar] [CrossRef] [PubMed]
- Pardavi-Horvath, M. Microwave applications of soft ferrites. J. Magn. Magn. Mater. 2000, 215–216, 171–183. [Google Scholar] [CrossRef]
- Hemeda, O.M.; Mostafa, N.Y.; Elkader, O.H.A.; Ahmed, M.A. Solubility limits in Mn-Mg ferrites system under hydrothermal conditions. J. Magn. Magn. Mater. 2014, 364, 39–46. [Google Scholar] [CrossRef]
- Sharma, R.; Thakur, P.; Sharma, P. Mn2+ doped Mg-Zn ferrite nanoparticles for microwave device applications. IEEE Electron Device Lett. 2018, 39, 901–904. [Google Scholar] [CrossRef]
- Harris, V.G.; Geiler, A.; Chen, Y.; Yoon, S.D.; Wu, M.; Yang, A.; Chen, Z.; He, P.; Paeimi, P.V.; Zuo, X.; et al. Recent advances in processing and applications of microwave ferrites. J. Magn. Magn. Mater. 2009, 321, 2035–2047. [Google Scholar] [CrossRef]
- Harris, V.G. Modern microwave ferrites. IEEE Trans. Magn. 2012, 48, 1075–1104. [Google Scholar] [CrossRef]
- Msomi, J.Z.; Moyo, T.; Abdallah, H.M.I. Magnetic properties of MgxMn1−xFe2O4 nanoferrites. J. Supercond. Nov. Magn. 2012, 25, 2643–2646. [Google Scholar] [CrossRef]
- Kumar, G.; Chand, J.; Verma, S.; Singh, M. Mixed Mg-Mn ferrites for high frequency applications processed by citrate precursor technique. J. Phys. D-Appl. Phys. 2009, 42, 155001. [Google Scholar] [CrossRef]
- Jaswal, L.; Singh, B. Ferrite materials: A chronological review. J. Integr. Sci. Technol. 2014, 2, 69–71. [Google Scholar]
- Verma, S.; Chand, J.; Singh, M. Effect of In3+ ions doping on the structural and magnetic properties of Mg0.2Mn0.5Ni0.3InxFe2−xO4 spinel ferrites. J. Magn. Magn. Mater. 2012, 324, 3252–3260. [Google Scholar] [CrossRef]
- Tsay, C.Y.; Liang, S.C.; Lei, C.M.; Chang, C.C. A comparative study of the magnetic and microwave properties of Al3+ and In3+ substituted Mg-Mn ferrites. Ceram. Int. 2016, 42, 4748–4752. [Google Scholar] [CrossRef]
- Lwin, N.; Noor, A.F.M.; Sreekantan, S.; Othman, R.; Thant, A.A. A novel and simple process for nanosized Mg-Mn ferrite preparation from solution combustion method. Int. J. Appl. Ceram. Technol. 2013, 10, 1–7. [Google Scholar] [CrossRef]
- Lwin, N.; Ahmad Fauzi, M.N.; Sreekantan, S.; Othman, R. Physical and electromagnetic properties of nanosized Gd substituted Mg-Mn ferrites by solution combustion method. Physica B 2015, 461, 134–139. [Google Scholar] [CrossRef]
- Holec, P.; Plocek, J.; Nižňanský, D.; Vejpravová, J.P. Preparation of MgFe2O4 nanoparticles by microemulsion method and their characterization. J. Sol-Gel Sci. Technol. 2009, 51, 301–305. [Google Scholar] [CrossRef]
- Reyes-Rodriguex, P.Y.; Cortés-Hernández, D.A.; Escobedo-Bocardo, J.C.; Almanza-Robles, J.M.; Sánchez-Fuentes, H.J.; Jasso-Terán, A.; León-Prado, L.E.D.; Méndez-Noell, J.; Hurtado-López, G.F. Structural and magnetic properties of Mg-Zn ferrites (Mg1−xZnxFe2O4) prepared by sol-gel method. J. Magn. Magn. Mater. 2017, 427, 268–271. [Google Scholar] [CrossRef]
- Yang, B.; Wang, Z. Structural and magnetic properties of Mg0.35Cu0.2Zn0.45Fe2O4 ferrite synthesized by co-precipitation method. AIP Adv. 2017, 7, 056114. [Google Scholar] [CrossRef]
- Modi, K.B.; Vasoya, N.H.; Lakhani, V.K.; Pathak, T.K. Magnetic phase evolution and particle size estimation study on nanocrystalline Mg-Mn ferrites. Appl. Nanosci. 2015, 5, 11–17. [Google Scholar] [CrossRef]
- Yan, Z.; Gao, J.; Li, Y.; Zhanh, M.; Guo, M. Hydrothermal synthesis and structure evolution of metal-doped magnesium ferrite from saprolite laterite. RSC Adv. 2015, 5, 92778–92787. [Google Scholar] [CrossRef]
- Kurian, J.; Mathew, M.J. Structural, magnetic and Mössbauer studies of magnesium ferrite nanoparticles prepared by hydrothermal method. Int. J. Nanosci. 2018, 17, 1760001. [Google Scholar] [CrossRef]
- Phumying, S.; Labuayai, S.; Swatsitang, E.; Amornkitbamrung, V.; Maensiri, S. Nanocrystalline spinel ferrite (MFe2O4, M = Ni, Co, Mn, Mg, Zn) powders prepared by a simple aloe vera plant-extracted solution hydrothermal route. Mater. Res. Bull. 2013, 48, 2060–2065. [Google Scholar] [CrossRef]
- Byrappa, K.; Adschiri, T. Hydrothermal technology for nanotechnology. Prog. Cryst. Growth Charact. Mater. 2007, 53, 117–166. [Google Scholar] [CrossRef] [Green Version]
- Pathak, T.K.; Buch, J.J.; Trivedi, U.N.; Joshi, H.H.; Modi, K.B. Infrared spectroscopy and elastic properties of nanocrystalline Mg-Mn ferrites prepared by co-precipitation technique. J. Nanosci. Nanotechnol. 2008, 8, 4181–4187. [Google Scholar] [CrossRef] [PubMed]
- Cullity, B.D.; Stock, S.R. Elements of X-ray Diffraction, 3rd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2001; p. 170. ISBN 0-201-61091-4. [Google Scholar]
- Okasha, N. Enhancement of magnetization of Mg-Mn nanoferrites by γ-irradiation. J. Alloys Compd. 2010, 490, 307–310. [Google Scholar] [CrossRef]
- Wang, Z.; Lazor, P.; Saxena, S.K.; O’Neill, H.S.C. High pressure Raman spectroscopy of ferrite MgFe2O4. Mater. Res. Bull. 2002, 37, 1589–1602. [Google Scholar] [CrossRef]
- Kreisel, J.; Lucazeau, G.; Vincenta, H. Raman spectra and vibrational analysis of BaFe12O19 hexagonal ferrite. J. Solid State Chem. 1998, 137, 127–137. [Google Scholar] [CrossRef]
- Nakagomi, F.; da Silva, S.W.; Garg, V.K.; Oliveira, A.C.; Morais, P.C.; Franco, A. Influence of the Mg-content on the cation distribution in cubic MgxFe3−xO4 nanoparticles. J. Solid State Chem. 2009, 182, 2423–2429. [Google Scholar] [CrossRef]
- Iftikhar, A.; Islam, M.U.; Awan, M.S.; Ahmad, M.; Naseem, S.; Asif Iqbal, M. Synthesis of super paramagnetic particles of Mn1−xMgxFe2O4 ferrites for hyperthermia applications. J. Alloys Compd. 2014, 601, 116–119. [Google Scholar] [CrossRef]
- Verma, S.; Chand, J.; Batoo, K.M.; Singh, M. Cation distribution and Mössbauer spectral studied of Mg0.2Mn0.5Ni0.3InxFe2−xO4 ferrites (x = 0.0, 0.05 and 0.10). J. Alloys Compd. 2013, 565, 148–153. [Google Scholar] [CrossRef]
- Sabale, S.; Jadhav, V.; Khot, V.; Zhu, X.; Xin, M.; Chen, H. Superparamagnetic MFe2O4 (M = Ni, Co, Zn, Mn) nanoparticles: Synthesis, characterization, induction heating and cell viability studies for cancer hyperthermia applications. J. Mater. Sci. Mater. Med. 2015, 26, 127. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, H.M.I.; Moyo, T. Superparamagnetic behavior of MnxNi1−xFe2O4 spinel nanoferrites. J. Magn. Magn. Mater. 2014, 361, 170–174. [Google Scholar] [CrossRef]
- Pileni, M.P. Magnetic fluids: Fabrication, magnetic properties, and organization of nanocrystals. Adv. Funct. Mater. 2001, 11, 323–336. [Google Scholar] [CrossRef]
- Leslie-Pelecky, D.L.; Ricke, R.D. Magnetic properties of nanostructured Materials. Chem. Mater. 1996, 8, 1770–1783. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Z.J. Size-dependent superparamagnetic properties of MgFe2O4 spinel ferrite nanocrystallites. Appl. Phys. Lett. 1998, 73, 3156–3158. [Google Scholar] [CrossRef]
- Köseoglu, Y. Structural, magnetic electrical and dielectric properties of MnxNi1−xFe2O4 spinel nanoferrites prepared by PEG assisted hydrothermal method. Ceram. Int. 2013, 39, 4221–4230. [Google Scholar] [CrossRef]
- Mohseni, H.; Shokrollahi, H.; Sharifi, I.; Gheisari, K. Magnetic and structural studies of the Mn-doped Mg-Zn ferrite nanoparticles synthesized by the glycine nitrate process. J. Magn. Magn. Mater. 2012, 324, 3741–3747. [Google Scholar] [CrossRef]
- Chu, P.; Mills, D.L. Exchange/dipole collective spin-wave modes of ferromagnetic nanosphere arrays. Phys. Rev. 2006, 73, 094405. [Google Scholar] [CrossRef]
- Guskos, N.; Zolnierkiewicz, G.; Typek, J.; Sibera, D.; Narkiewicz, U. Magnetic resonance study of ZnO-Fe2O3-ZnFe2O4 system. Rev. Adv. Mater. Sci. 2010, 23, 224–228. [Google Scholar]
- Thota, S.; Kashyap, S.C.; Shaema, S.K.; Reddy, V.R. Micro Raman, Mössbauer and magnetic studies of manganese substituted zinc ferrite nanoparticles: Role of Mn. J. Phys. Chem. Solids 2016, 91, 136–144. [Google Scholar] [CrossRef]
- De Biasi, R.S.; Devezas, T.C. Anisotropy field of small magnetic particles as measured by resonance. J. Appl. Phys. 1978, 49, 2466–2469. [Google Scholar] [CrossRef]

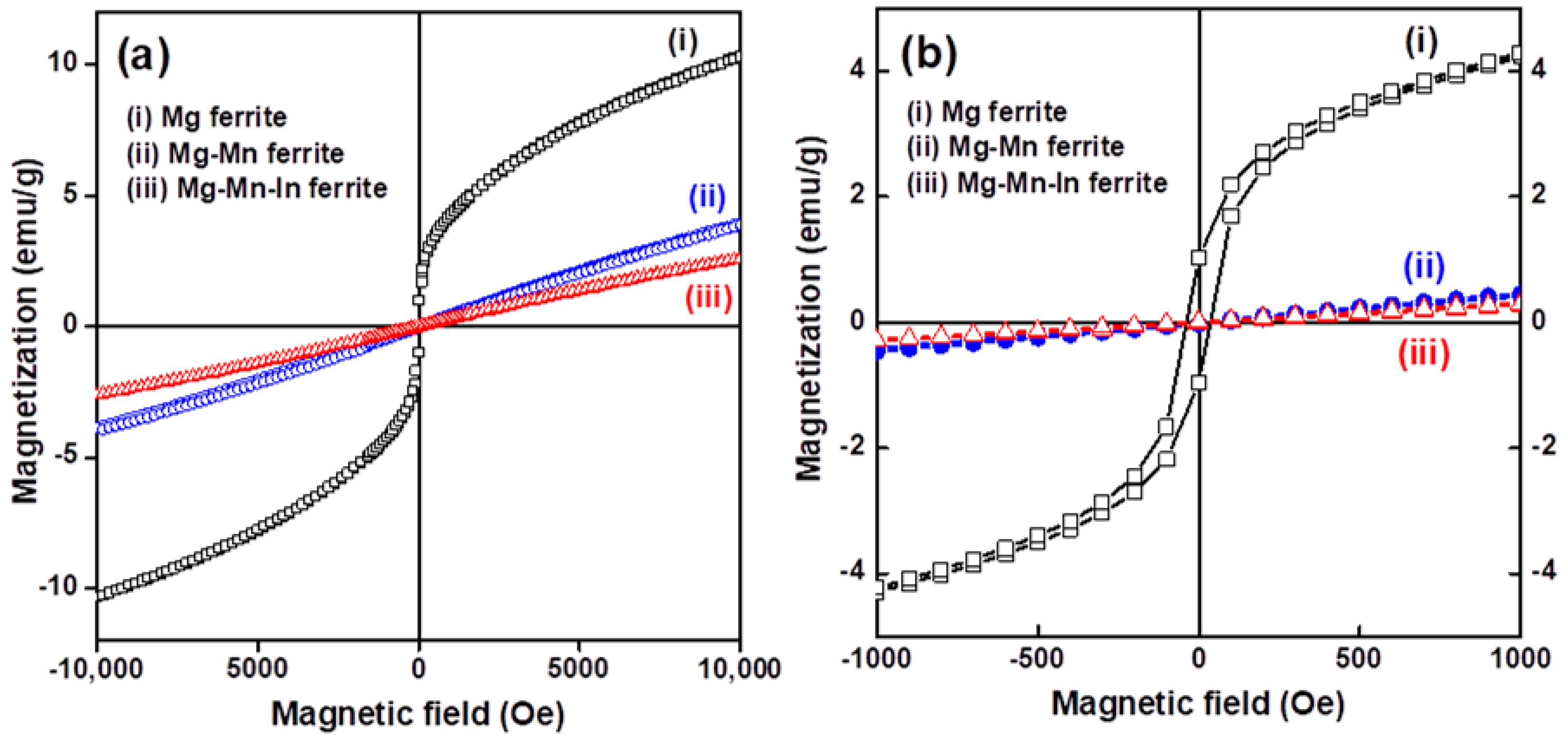
| Nanoferrite Sample | M (emu/g) | Hc (Oe) | Mr (emu/g) | ΔH (Oe) | Hr (Oe) | g-Factor |
|---|---|---|---|---|---|---|
| Mg | 10.36 | 37.8 | 1.0 | 355 | 1910 | 3.690 |
| Mg-Mn | 3.97 | - | - | 440 | 3480 | 2.024 |
| Mg-Mn-In | 2.63 | - | - | 420 | 3495 | 2.016 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsay, C.-Y.; Chiu, Y.-C.; Lei, C.-M. Hydrothermally Synthesized Mg-Based Spinel Nanoferrites: Phase Formation and Study on Magnetic Features and Microwave Characteristics. Materials 2018, 11, 2274. https://doi.org/10.3390/ma11112274
Tsay C-Y, Chiu Y-C, Lei C-M. Hydrothermally Synthesized Mg-Based Spinel Nanoferrites: Phase Formation and Study on Magnetic Features and Microwave Characteristics. Materials. 2018; 11(11):2274. https://doi.org/10.3390/ma11112274
Chicago/Turabian StyleTsay, Chien-Yie, Yi-Chun Chiu, and Chien-Ming Lei. 2018. "Hydrothermally Synthesized Mg-Based Spinel Nanoferrites: Phase Formation and Study on Magnetic Features and Microwave Characteristics" Materials 11, no. 11: 2274. https://doi.org/10.3390/ma11112274






