Preparation and Characterization of Expanded Clay-Paraffin Wax-Geo-Polymer Composite Material
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Materials Characterization
2.3. PCM Encapsulation
2.3.1. Immersion
2.3.2. Vacuum Impregnation
2.3.3. Coating
2.4. Testing of Thermal Stability
2.4.1. Weathering Test
2.4.2. Controlled Indoor Test
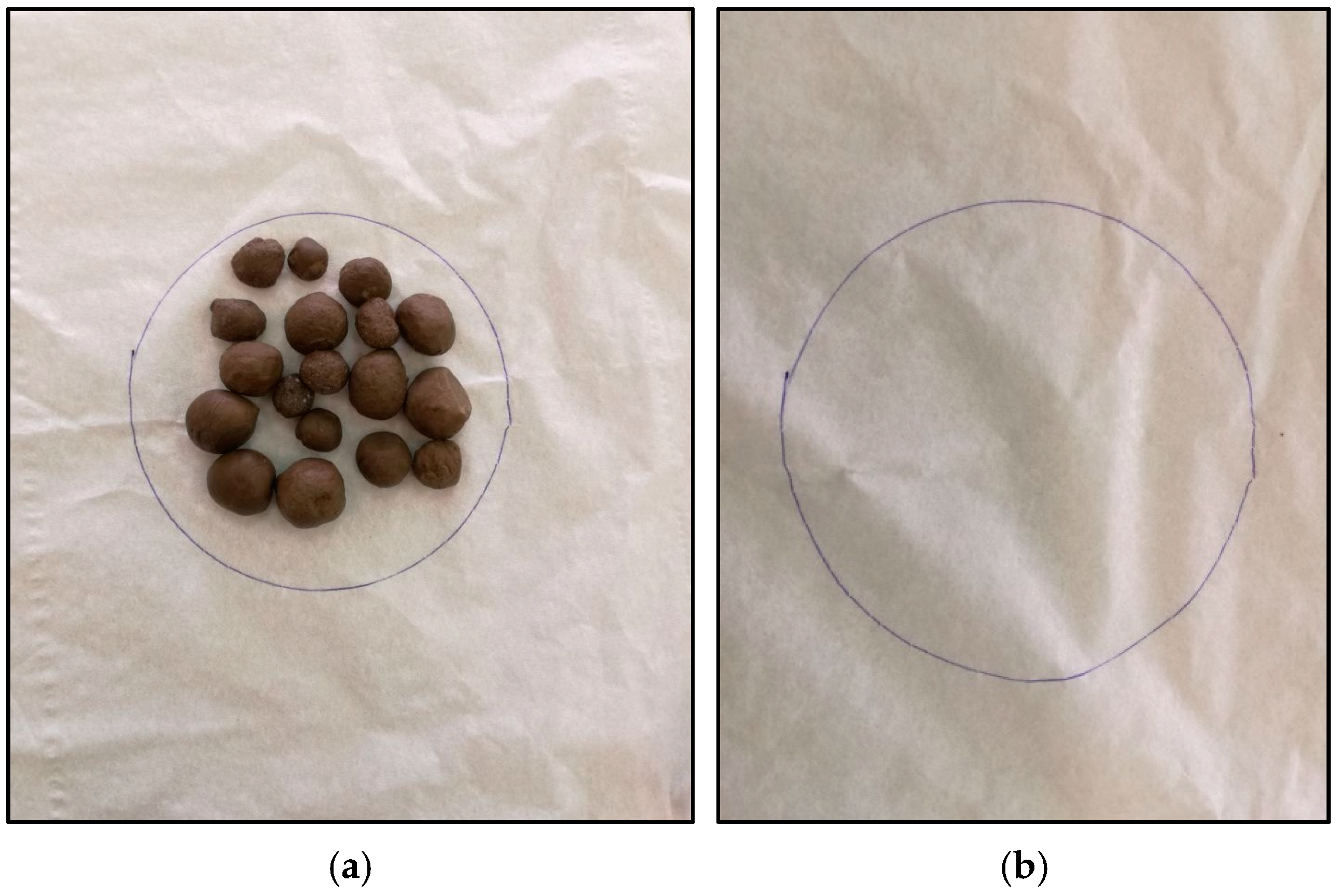
2.4.3. Diffusion-Oozing Circle Test
3. Results
3.1. Composition and Microstructure of the Materials
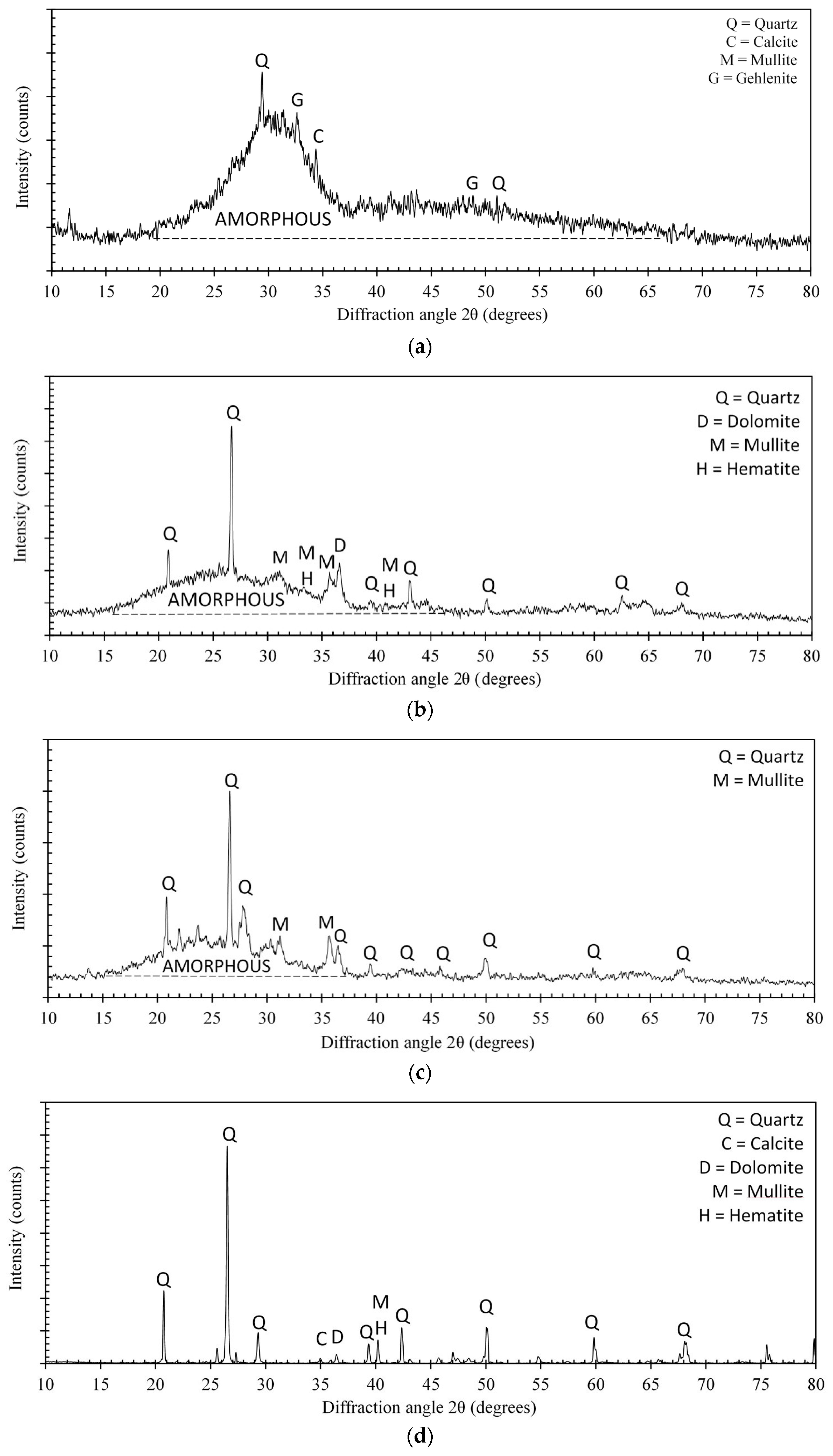
3.1.1. X-ray Diffraction Analysis
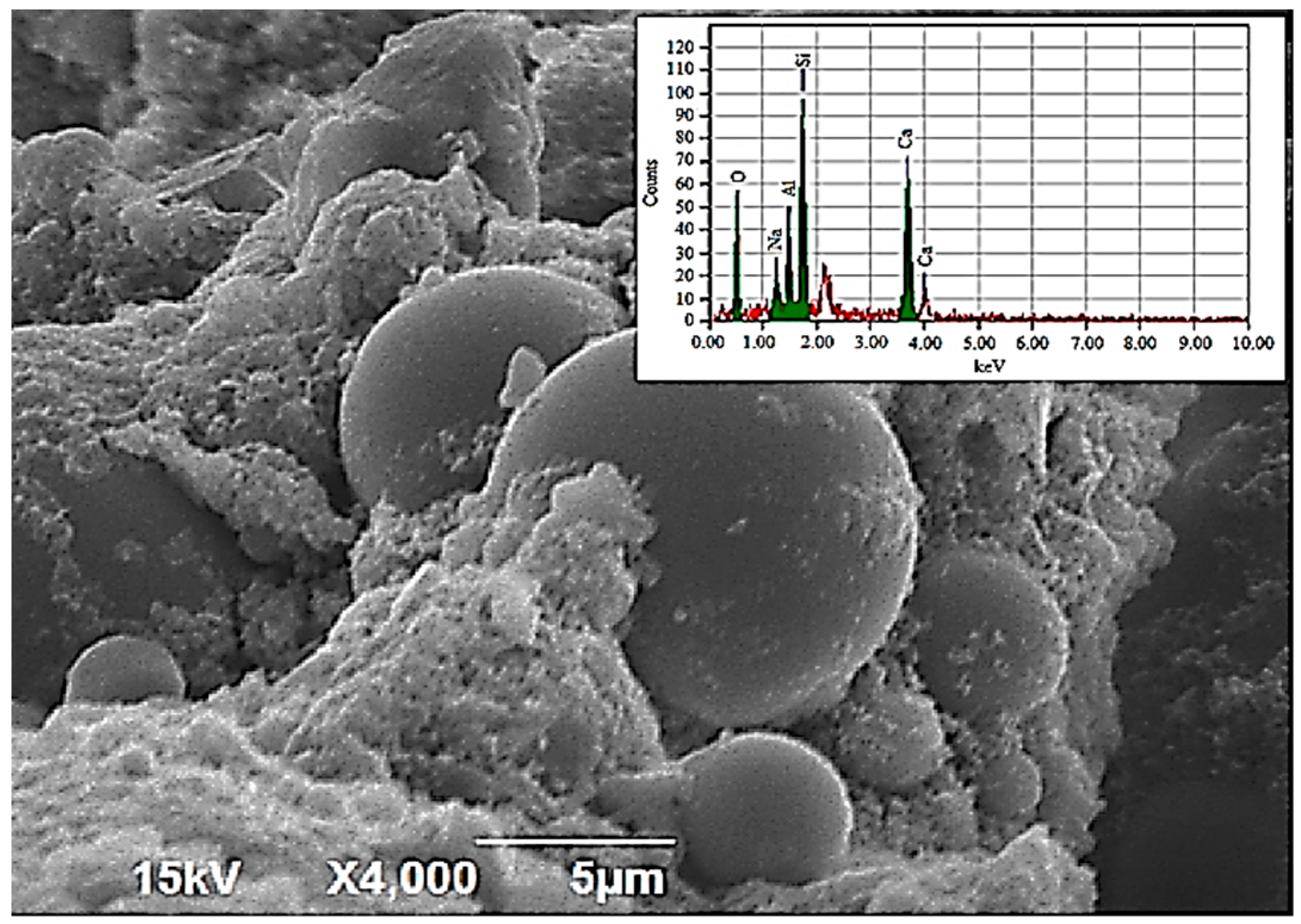
3.1.2. Scanning Electron Microscopy Analysis
3.1.3. X-ray Fluorescence Analysis
3.2. Thermo-Physical Properties of PCM
3.2.1. Differential Scanning Calorimetry
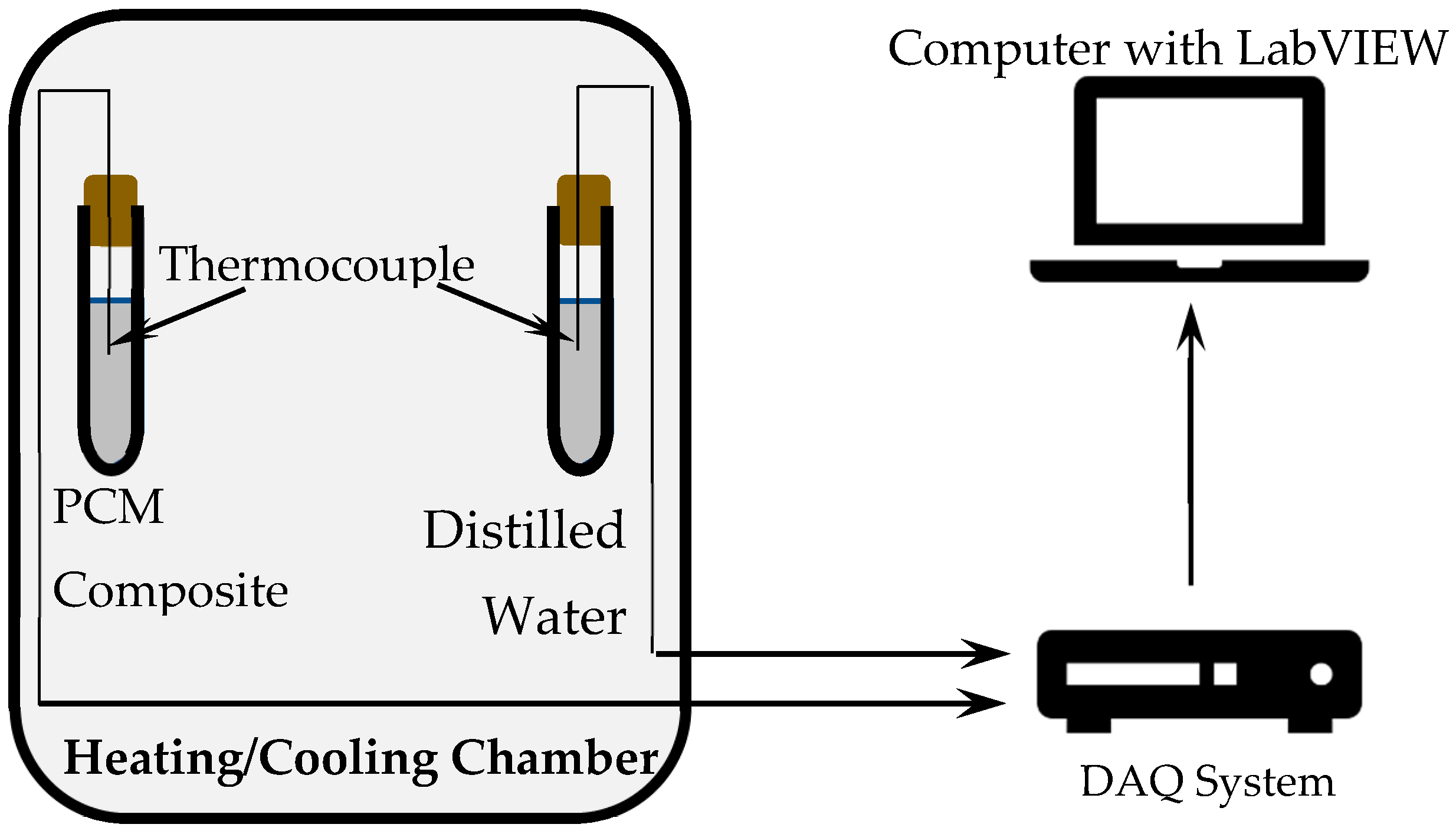
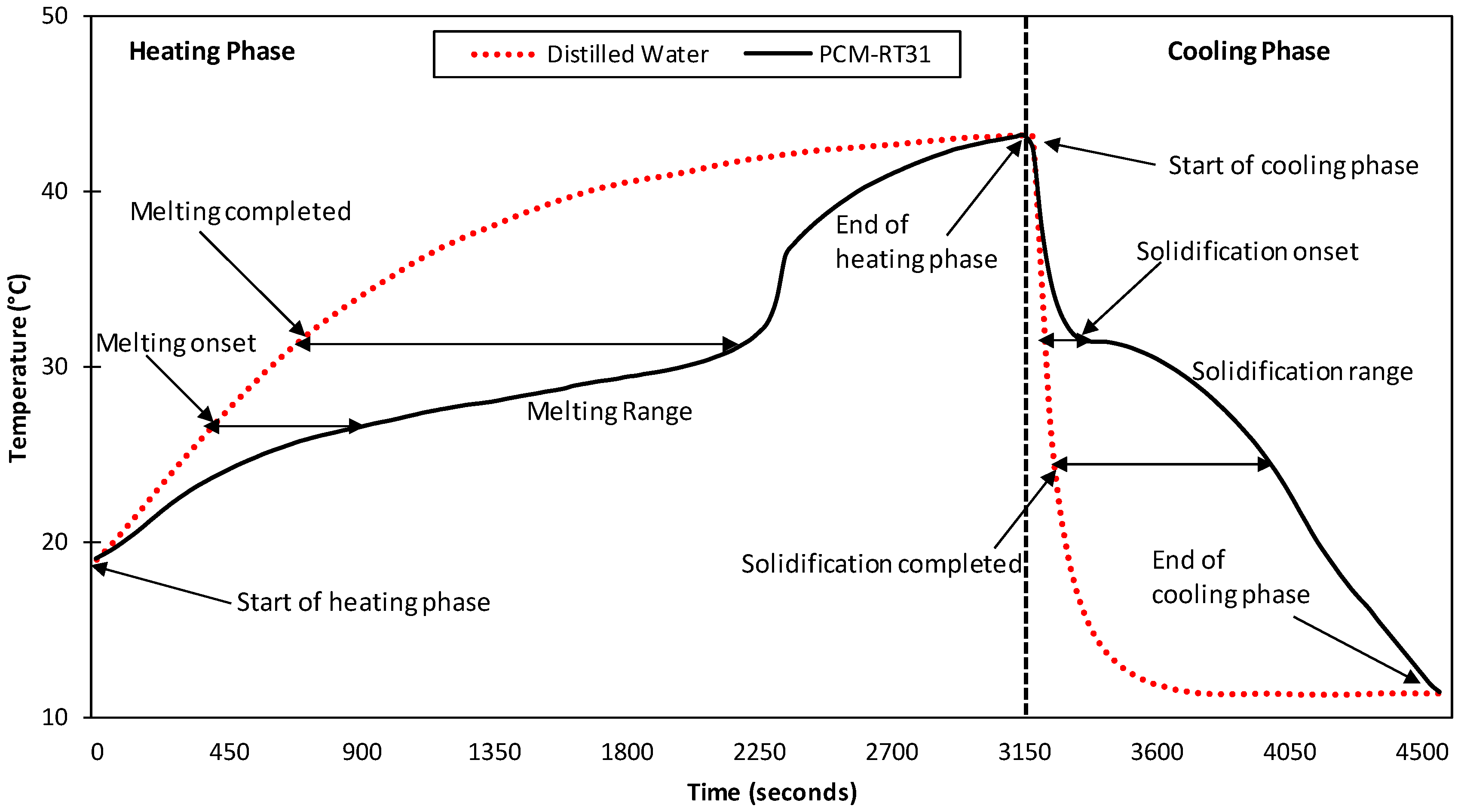
3.2.2. Temperature History Method
3.3. Impregnation Efficiency
3.3.1. Immersion
3.3.2. Vacuum Impregnation
3.4. Thermal Stability
3.4.1. Weather and Rapid Thermal Cycling
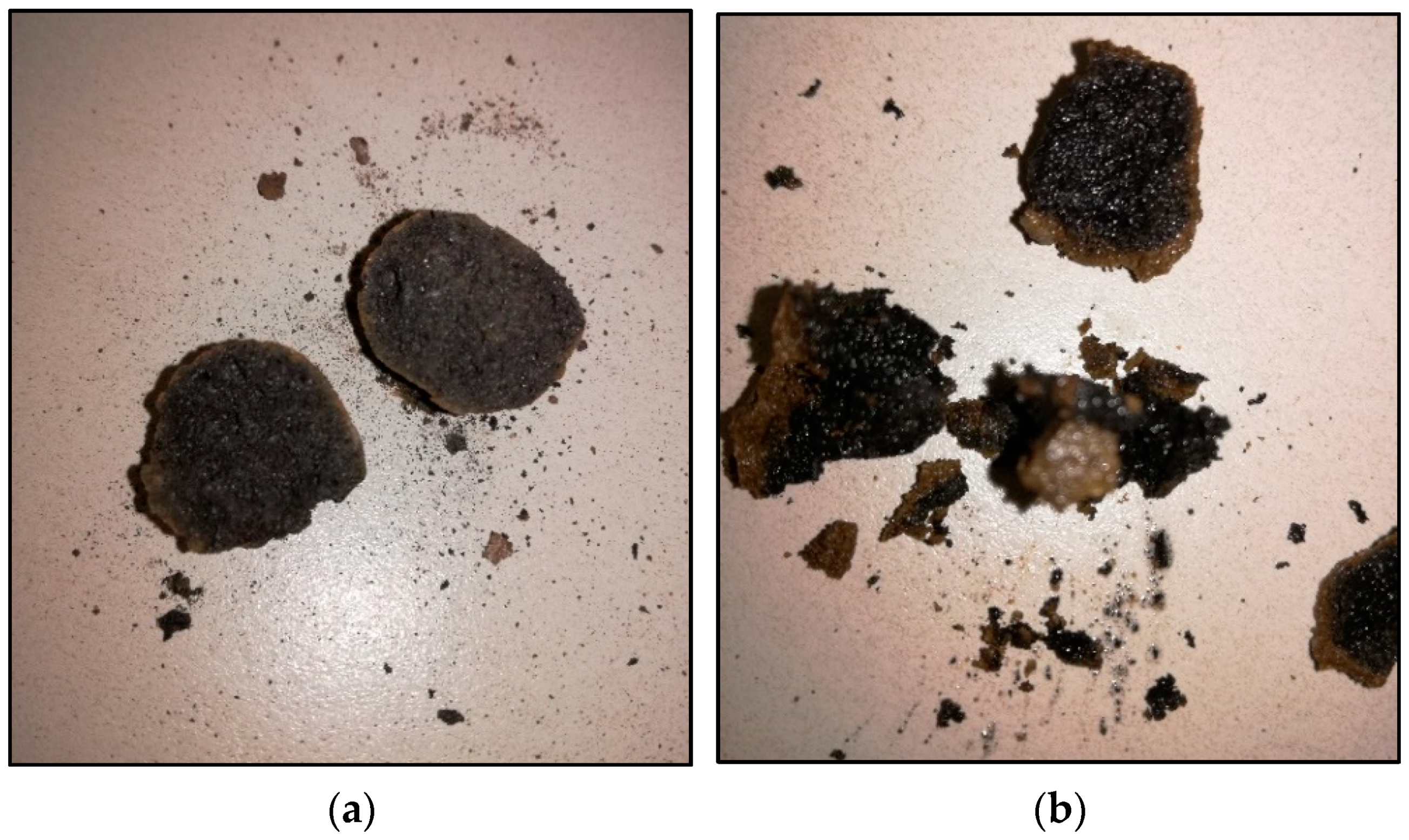
3.4.2. Diffusion-Oozing Circle Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hassan, A.; Shakeel Laghari, M.; Rashid, Y. Micro-Encapsulated Phase Change Materials: A Review of Encapsulation, Safety and Thermal Characteristics. Sustainability 2016, 8, 1046. [Google Scholar] [CrossRef]
- Sharifi, N.P.; Shaikh, A.A.N.; Sakulich, A.R. Application of phase change materials in gypsum boards to meet building energy conservation goals. Energy Build. 2017, 138, 455–467. [Google Scholar] [CrossRef]
- Hasan, A.; Al-Sallal, K.; Alnoman, H.; Rashid, Y.; Abdelbaqi, S. Effect of Phase Change Materials (PCMs) Integrated into a Concrete Block on Heat Gain Prevention in a Hot Climate. Sustainability 2016, 8, 1009. [Google Scholar] [CrossRef]
- Lei, J.; Kumarasamy, K.; Zingre, K.T.; Yang, J.; Wan, M.P.; Yang, E.-H. Cool colored coating and phase change materials as complementary cooling strategies for building cooling load reduction in tropics. Appl. Energy 2017, 190, 57–63. [Google Scholar] [CrossRef]
- Kusama, Y.; Ishidoya, Y. Thermal effects of a novel phase change material (PCM) plaster under different insulation and heating scenarios. Energy Build. 2017, 141, 226–237. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Wang, X.; Sanjayan, J.; Wilson, J. Thermal performance assessment of phase change material integrated cementitious composites in buildings: Experimental and numerical approach. Appl. Energy 2017, 207, 654–664. [Google Scholar] [CrossRef]
- Li, M.G.; Zhang, Y.; Xu, Y.H.; Zhang, D. Effect of different amounts of surfactant on characteristics of nanoencapsulated phase-change materials. Polym. Bull. 2011, 67, 541–552. [Google Scholar] [CrossRef]
- Tang, B.; Cui, J.; Wang, Y.; Jia, C.; Zhang, S. Facile synthesis and performances of PEG/SiO2 composite form-stable phase change materials. Sol. Energy 2013, 97, 484–492. [Google Scholar] [CrossRef]
- Giro-Paloma, J.; Konuklu, Y.; Fernández, A.I. Preparation and exhaustive characterization of paraffin or palmitic acid microcapsules as novel phase change material. Sol. Energy 2015, 112, 300–309. [Google Scholar] [CrossRef]
- Konuklu, Y.; Paksoy, H.Ö. Polystyrene-based caprylic acid microencapsulation for thermal energy storage. Sol. Energy Mater. Sol. Cells 2017, 159, 235–242. [Google Scholar] [CrossRef]
- Kahraman Döğüşcü, D.; Kızıl, Ç.; Biçer, A.; Sarı, A.; Alkan, C. Microencapsulated n-alkane eutectics in polystyrene for solar thermal applications. Sol. Energy 2018, 160, 32–42. [Google Scholar] [CrossRef]
- Zhang, G.H.; Bon, S.A.F.; Zhao, C.Y. Synthesis, characterization and thermal properties of novel nanoencapsulated phase change materials for thermal energy storage. Sol. Energy 2012, 86, 1149–1154. [Google Scholar] [CrossRef]
- Ma, Y.; Chu, X.; Li, W.; Tang, G. Preparation and characterization of poly(methyl methacrylate-co-divinylbenzene) microcapsules containing phase change temperature adjustable binary core materials. Sol. Energy 2012, 86, 2056–2066. [Google Scholar] [CrossRef]
- Guo, X.; Cao, J.; Peng, Y.; Liu, R. Incorporation of microencapsulated dodecanol into wood flour/high-density polyethylene composite as a phase change material for thermal energy storage. Mater. Des. 2016, 89, 1325–1334. [Google Scholar] [CrossRef]
- Feczkó, T.; Kardos, A.F.; Németh, B.; Trif, L.; Gyenis, J. Microencapsulation of n-hexadecane phase change material by ethyl cellulose polymer. Polym. Bull. 2014, 71, 3289–3304. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Lu, W.; Luo, Z.; Zeng, Y. Synthesis and thermal properties of novel sodium nitrate microcapsules for high-temperature thermal energy storage. Sol. Energy Mater. Sol. Cells 2017, 159, 440–446. [Google Scholar] [CrossRef]
- Qiu, X.; Lu, L.; Zhang, Z.; Tang, G.; Song, G. Preparation, thermal property, and thermal stability of microencapsulated n-octadecane with poly(stearyl methacrylate) as shell. J. Therm. Anal. Calorim. 2014, 118, 1441–1449. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, T.; Chai, Y.; Wang, L. Preparation and Characterization of High Content Paraffin Wax Microcapsules and Micro/Nanocapsules with Poly Methyl Methacrylate Shell by Suspension-Like Polymerization. Chin. J. Chem. 2017, 35, 497–506. [Google Scholar] [CrossRef]
- Yang, Y.; Kuang, J.; Wang, H.; Song, G.; Liu, Y.; Tang, G. Enhancement in thermal property of phase change microcapsules with modified silicon nitride for solar energy. Sol. Energy Mater. Sol. Cells 2016, 151, 89–95. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Tang, B.; Lu, R.; Zhang, S. Form-stable phase change materials with high phase change enthalpy from the composite of paraffin and cross-linking phase change structure. Appl. Energy 2016, 184, 241–246. [Google Scholar] [CrossRef]
- Karaman, S.; Karaipekli, A.; Sarı, A.; Biçer, A. Polyethylene glycol (PEG)/diatomite composite as a novel form-stable phase change material for thermal energy storage. Sol. Energy Mater. Sol. Cells 2011, 95, 1647–1653. [Google Scholar] [CrossRef]
- Sarı, A.; Bicer, A.; Al-Sulaiman, F.A.; Karaipekli, A.; Tyagi, V.V. Diatomite/CNTs/PEG composite PCMs with shape-stabilized and improved thermal conductivity: Preparation and thermal energy storage properties. Energy Build. 2018, 164, 166–175. [Google Scholar] [CrossRef]
- Sarı, A.; Bicer, A.; Karaipekli, A.; Al-Sulaiman, F.A. Preparation, characterization and thermal regulation performance of cement based-composite phase change material. Sol. Energy Mater. Sol. Cells 2018, 174, 523–529. [Google Scholar] [CrossRef]
- Sarı, A.; Bicer, A.; Al-Ahmed, A.; Al-Sulaiman, F.A.; Zahir, M.H.; Mohamed, S.A. Silica fume/capric acid-palmitic acid composite phase change material doped with CNTs for thermal energy storage. Sol. Energy Mater. Sol. Cells 2018, 179, 353–361. [Google Scholar] [CrossRef]
- Ushak, S.; Cruz, M.; Cabeza, L.; Grágeda, M. Preparation and Characterization of Inorganic PCM Microcapsules by Fluidized Bed Method. Materials 2016, 9, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moghaddam, M.K.; Mortazavi, S.M.; Khayamian, T. Preparation of calcium alginate microcapsules containing n-nonadecane by a melt coaxial electrospray method. J. Electrost. 2015, 73, 56–64. [Google Scholar] [CrossRef]
- Donkers, P.A.J.; Sögütoglu, L.C.; Huinink, H.P.; Fischer, H.R.; Adan, O.C.G. A review of salt hydrates for seasonal heat storage in domestic applications. Appl. Energy 2017, 199, 45–68. [Google Scholar] [CrossRef]
- Šavija, B. Smart Crack Control in Concrete through Use of Phase Change Materials (PCMs): A Review. Materials 2018, 11, 654. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Peng, B.; Yue, C.; Guo, M.; Zhang, M. Low-cost, shape-stabilized fly ash composite phase change material synthesized by using a facile process for building energy efficiency. Mater. Chem. Phys. 2019, 222, 87–95. [Google Scholar] [CrossRef]
- Jacob, R.; Trout, N.; Raud, R.; Clarke, S.; Steinberg, T.A.; Saman, W.; Bruno, F. Geopolymer encapsulation of a chloride salt phase change material for high temperature thermal energy storage. In Proceedings of the SolarPACES 2015, Cape Town, South Africa, 13–16 October 2015. [Google Scholar]
- Jacob, R.; Belusko, M.; Inés Fernández, A.; Cabeza, L.F.; Saman, W.; Bruno, F. Embodied energy and cost of high temperature thermal energy storage systems for use with concentrated solar power plants. Appl. Energy 2016, 180, 586–597. [Google Scholar] [CrossRef]
- Jacob, R.; Raud, R.; Trout, N.; Bell, S.; Clarke, S.; Will, G.; Saman, W.; Bruno, F. Effect of inner coatings on the stability of chloride-based phase change materials encapsulated in geopolymers. Sol. Energy Mater. Sol. Cells 2018, 174, 271–276. [Google Scholar] [CrossRef]
- Ismail, N.; El-Hassan, H. Development and Characterization of Fly Ash–Slag Blended Geopolymer Mortar and Lightweight Concrete. J. Mater. Civ. Eng. 2018, 30, 04018029. [Google Scholar] [CrossRef]
- Mazo, J.; Delgado, M.; Peñalosa, C.; Dolado, P.; Miranda, I.; Lázaro, A.; Marín, J.M.; Zalba, B. Evaluation of the suitability of different calorimetric methods to determine the enthalpy-temperature curve of granular PCM composites. Appl. Therm. Eng. 2017, 125, 306–316. [Google Scholar] [CrossRef]
- Lazaro, A.; Peñalosa, C.; Solé, A.; Diarce, G.; Haussmann, T.; Fois, M.; Zalba, B.; Gshwander, S.; Cabeza, L.F. Intercomparative tests on phase change materials characterisation with differential scanning calorimeter. Appl. Energy 2013, 109, 415–420. [Google Scholar] [CrossRef]
- Mehling, H.; Cabeza, L.F. Heat and Cold Storage with PCM; Springer: Berlin, Germany, 2008; ISBN 978-3-540-68556-2. [Google Scholar]
- Hasan, A.; McCormack, S.J.; Huang, M.J.; Norton, B. Characterization of phase change materials for thermal control of photovoltaics using Differential Scanning Calorimetry and Temperature History Method. Energy Convers. Manag. 2014, 81, 322–329. [Google Scholar] [CrossRef]
- Hahn, D.W. Heat Conduction; Wiley: Hoboken, NJ, USA, 2012; ISBN 978-0-470-90293-6. [Google Scholar]
- Ma, B.; Adhikari, S.; Chang, Y.; Ren, J.; Liu, J.; You, Z. Preparation of composite shape-stabilized phase change materials for highway pavements. Constr. Build. Mater. 2013, 42, 114–121. [Google Scholar] [CrossRef]
- Kong, X.; Zhong, Y.; Rong, X.; Min, C.; Qi, C. Building Energy Storage Panel Based on Paraffin/Expanded Perlite: Preparation and Thermal Performance Study. Materials 2016, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- ASTM C618-17a; ASTM International: West Conshohocken, PA, USA, 2017.
- Material Data Sheet of PCM RT-31. Available online: https://www.rubitherm.eu/media/products/datasheets/Techdata_-RT31_EN_31052016.PDF (accessed on 23 October 2018).
- RS Pro K Type Thermocouple 1 m Length, 0.6 mm Diameter, −50 °C → +250 °C. Available online: https://uk.rs-online.com/web/p/thermocouples/6212158 (accessed on 13 March 2018).
- Alazhari, M.; Sharma, T.; Heath, A.; Cooper, R.; Paine, K. Application of expanded perlite encapsulated bacteria and growth media for self-healing concrete. Constr. Build. Mater. 2018, 160, 610–619. [Google Scholar] [CrossRef]
- Alghamri, R.; Kanellopoulos, A.; Al-Tabbaa, A. Impregnation and encapsulation of lightweight aggregates for self-healing concrete. Constr. Build. Mater. 2016, 124, 910–921. [Google Scholar] [CrossRef]
- Wang, R.; Ren, M.; Gao, X.; Qin, L. Preparation and properties of fatty acids based thermal energy storage aggregate concrete. Constr. Build. Mater. 2018, 165, 1–10. [Google Scholar] [CrossRef]
- Aguayo, M.; Das, S.; Castro, C.; Kabay, N.; Sant, G.; Neithalath, N. Porous inclusions as hosts for phase change materials in cementitious composites: Characterization, thermal performance, and analytical models. Constr. Build. Mater. 2017, 134, 574–584. [Google Scholar] [CrossRef]
- Sun, J.; Wu, Z. Study on evaluation method of exudation of phase transition working substance for building materials. New Build. Mater. 2004, 7, 43–46. [Google Scholar]







| Production Process | Core Material | Shell Material | Findings |
|---|---|---|---|
| Sol–gel method [8] | Polyethylene glycol | Silicon dioxide | Hm was in the range of 102.8–111.1 J/g. There was no change on enthalpy and transition temperature after 50 thermal cycles. Polyethylene glycol decomposes at 410 °C. |
| Emulsion polymerization [9] | Paraffin and palmitic acid | Styrene and ethyl acrylate | η was successful with 32.7 wt % of paraffin and 47.8 wt % palmitic acid. Φ was 165 nm and 265 nm for both CMs and they don’t decompose up to 200 °C. |
| Emulsion polymerization [10] | Caprylic (octanoic) acid | Polystyrene | Crosslinking agent had a direct impact on the encapsulation efficiency. Efficiency was compromised with repeatability. |
| Miniemulsion polymerization [11] | n-alkanes | Polystyrene | Thermal stability after RTC was reported. Mp range was from 20 °C to 35.9 °C and Hm range was 61.2 J/g to 46.1 J/g. |
| Mini-emulsion polymerization [7] | Hexadecane | Urea–formaldehyde resin | The results indicated that the nano-capsules have a smooth surface and Φ was 270 nm. Capsules were stable when heated at 100 °C for 72 h after encapsulation decreased the undercooling of hexadecane by 94%. |
| Mini-emulsion polymerization [12] | n-octadecane | Poly (ethyl methacrylate) + poly (methyl methacrylate) | Φ was 119 nm, Mp and Hm were 32.7 °C and 198.5 J/g respectively. The capsules have η of 89.5%. |
| In situ polymerization [13] | Butyl stearate and paraffin | Poly (methyl methacrylate-co-divinylbenzene) | Φ was 5–10 μm. Capsules decomposes at temperatures above 200 °C. Capsules were thermally stable after 50 cycles. |
| In situ polymerization [14] | Dodecanol | High-density polyethylene | η was successful yielding different sizes of the capsules in the range of 0.83 μm to 14.4 μm. Excellent thermal storage ability was good thermal stability reported. |
| Emulsion-solvent evaporation [15] | n-hexadecane | Ethyl cellulose | Mp ranges from 18.5 °C to 19.5 °C while Hm ranges from 137.8 J/g to 147.1 J/g. The shell had porosity and leakage was observed. |
| Solvent extraction [16] | Sodium nitrate | Perhydropolysilazane | SM in the capsules was 85 wt % while Φ was non-uniform with the range of 0.4 μm to 140 μm. SM was very stable at high temperatures of 350 °C. |
| Suspension-like polymerization [17] | n-octadecane | Poly (stearyl methacrylate) | Particles have a spherical profile with an average φ of 5 μm and 21 μm. Good thermal energy storage and thermal regulation potential was reported. |
| Suspension-like polymerization [18] | Paraffin | Poly Methyl Methacrylate | 89.5 wt % of CM was encapsulated successfully with good thermal stability. Nano particles of 0.1 μm to 19 μm and micro particles of 94 μm were produced. |
| Suspension-like polymerization [19] | n-octadecane | Polymethylmethacrylate | Microcapsules have a high thermal storage capability, enhanced thermal reliability and stability, and increased thermal conductivity. |
| Crosslinking and blending [20] | Paraffin | Cross-linking structure | 74 wt % of the CM was contained in the matrix successfully with the Hm of 210.6 J/g. The samples were observed to be dry when heated up to 100 °C. |
| Vacuum Impregnation [21] | Polyethylene glycol | Diatomite | Mp of the composite PCM was 27.7 °C and Hm of 87.09 J/g. An addition of expanded graphite increased the thermal conductivity of the composite. |
| Vacuum impregnation [22] | Polyethylene glycol | Diatomite/carbon nanotubes | No leakage of PCM was observed. Mp of the composite was 8 °C with Hm of 62.9 J/g. |
| Vacuum impregnation [23] | Capric acid-myristic acid | Cement | Composite’s Mp and Hm were 21.13 °C and 41.78 J/g, respectively. A temperature difference of 0.78 °C in the indoor space was measured by using this composite. |
| Vacuum impregnation [24] | Capric acid-palmitic acid | Silica fume, carbon nano tube | Mp range of different compositions was 19 to 26 °C and Hm was 46 to 49 J/g. Good thermal and chemical stability was reported after 1000 cycles. |
| Fluidized bed method [25] | Bischofite | Acrylic | Encapsulation efficiency of up to 95% was achieved. Microcapsules had excellent Mp and Hm compared to the original PCM. |
| Melt coaxial electrospray [26] | n-octadecane | Sodium alginate | 56 wt % of paraffin was contained in the microcapsules with Φ less than 100 μm. This technique offers good results regarding the encapsulation of PCMs. |
| Materials | Density | Particle Size |
|---|---|---|
| Lightweight expanded clay aggregate (LECA1) | 421 kg/m3 | 1–4 mm |
| Lightweight expanded clay aggregate (LECA2) | 369 kg/m3 | 4–10 mm |
| Lightweight expanded clay aggregate (LECA3) | 340 kg/m3 | 4–10 mm |
| Paraffin-based phase change material | 0.88 kg/L for solid 0.76 kg/L for liquid | Liquid/solid |
| Sodium Hydroxide (NaOH) | 1.19 kg/L | Liquid |
| Sodium silicate (Na2SiO3) | 1.39 kg/L | Liquid |
| Ground granulated blast furnace slag (GGBS) | 1236 kg/m3 | 0.2–70 µm |
| Fly Ash (FA) | 1262 kg/m3 | 3–70 µm |
| Dune Sand (DS) | 1693 kg/m3 | 80–500 µm |
| Constituent | SiO2 % | Al2O3 % | Fe2O3 % | CaO % | MgO % | LOI % |
|---|---|---|---|---|---|---|
| Fly ash | 48 | 23 | 12.5 | 3.2 | 1.5 | 1.1 |
| Slag | 34.7 | 14.4 | 0.8 | 41.9 | 6.8 | 1.1 |
| Dune sand | 64.9 | 3 | 0.7 | 14.1 | 1.3 | 0.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, A.; Ismail, N.; Mourad, A.-H.I.; Rashid, Y.; Laghari, M.S. Preparation and Characterization of Expanded Clay-Paraffin Wax-Geo-Polymer Composite Material. Materials 2018, 11, 2191. https://doi.org/10.3390/ma11112191
Hassan A, Ismail N, Mourad A-HI, Rashid Y, Laghari MS. Preparation and Characterization of Expanded Clay-Paraffin Wax-Geo-Polymer Composite Material. Materials. 2018; 11(11):2191. https://doi.org/10.3390/ma11112191
Chicago/Turabian StyleHassan, Ahmed, Najif Ismail, Abdel-Hamid I. Mourad, Yasir Rashid, and Mohammad S. Laghari. 2018. "Preparation and Characterization of Expanded Clay-Paraffin Wax-Geo-Polymer Composite Material" Materials 11, no. 11: 2191. https://doi.org/10.3390/ma11112191





