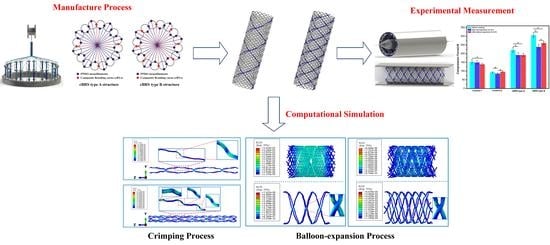
The Crimping and Expanding Performance of Self-Expanding Polymeric Bioresorbable Stents: Experimental and Computational Investigation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Composite Polymeric Bioresorbable Stents (cPBRSs)
2.3. Crimping and Expanding Performance
2.4. Numerical Analysis
2.4.1. Material Models
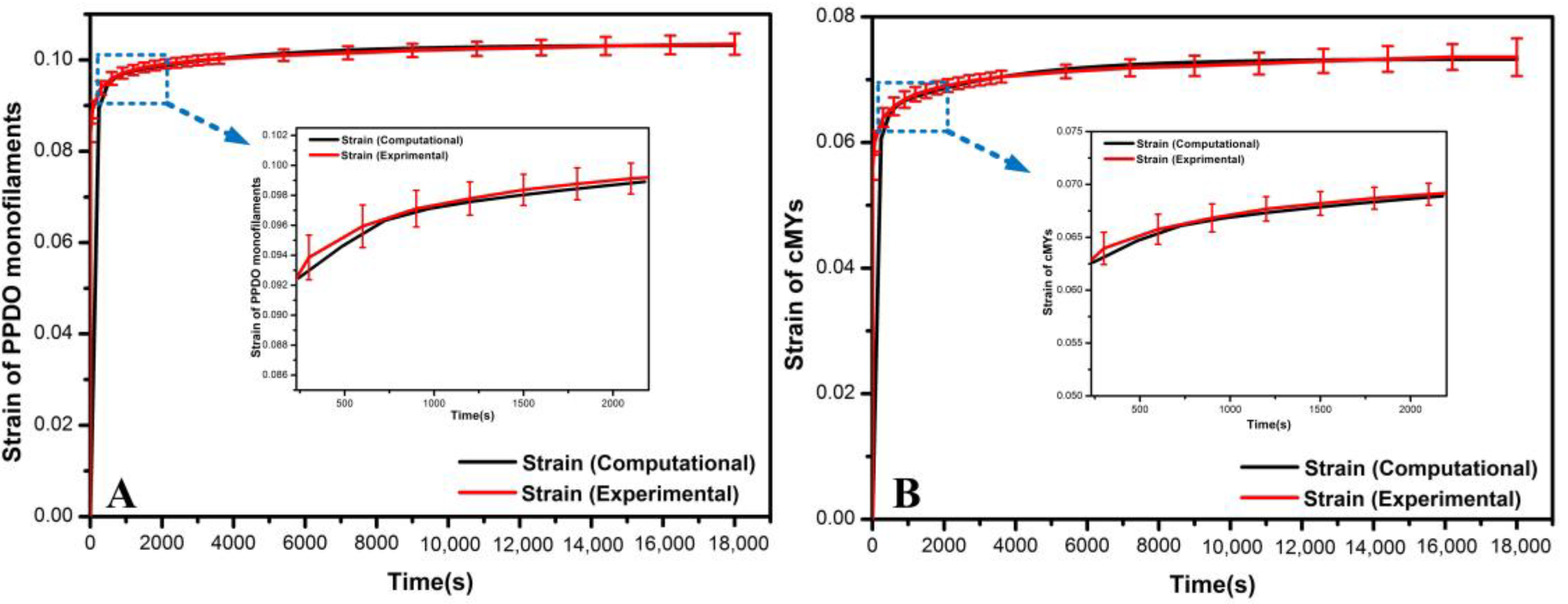
Viscoelastic Material Modeling
Validation of the Material Models
2.4.2. Finite Analysis Procedure
2.5. Statistical Analysis
3. Results
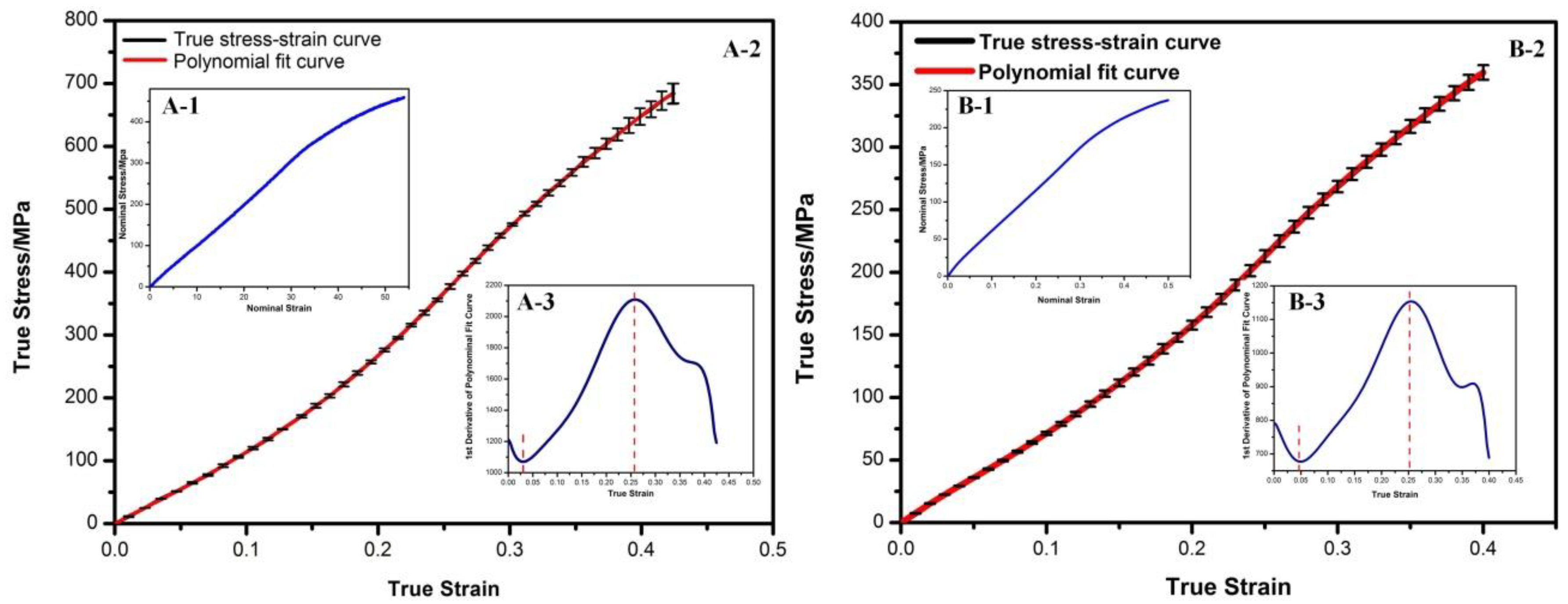
3.1. Stress-Strain Curves of PPDO Monofilaments and cMYs
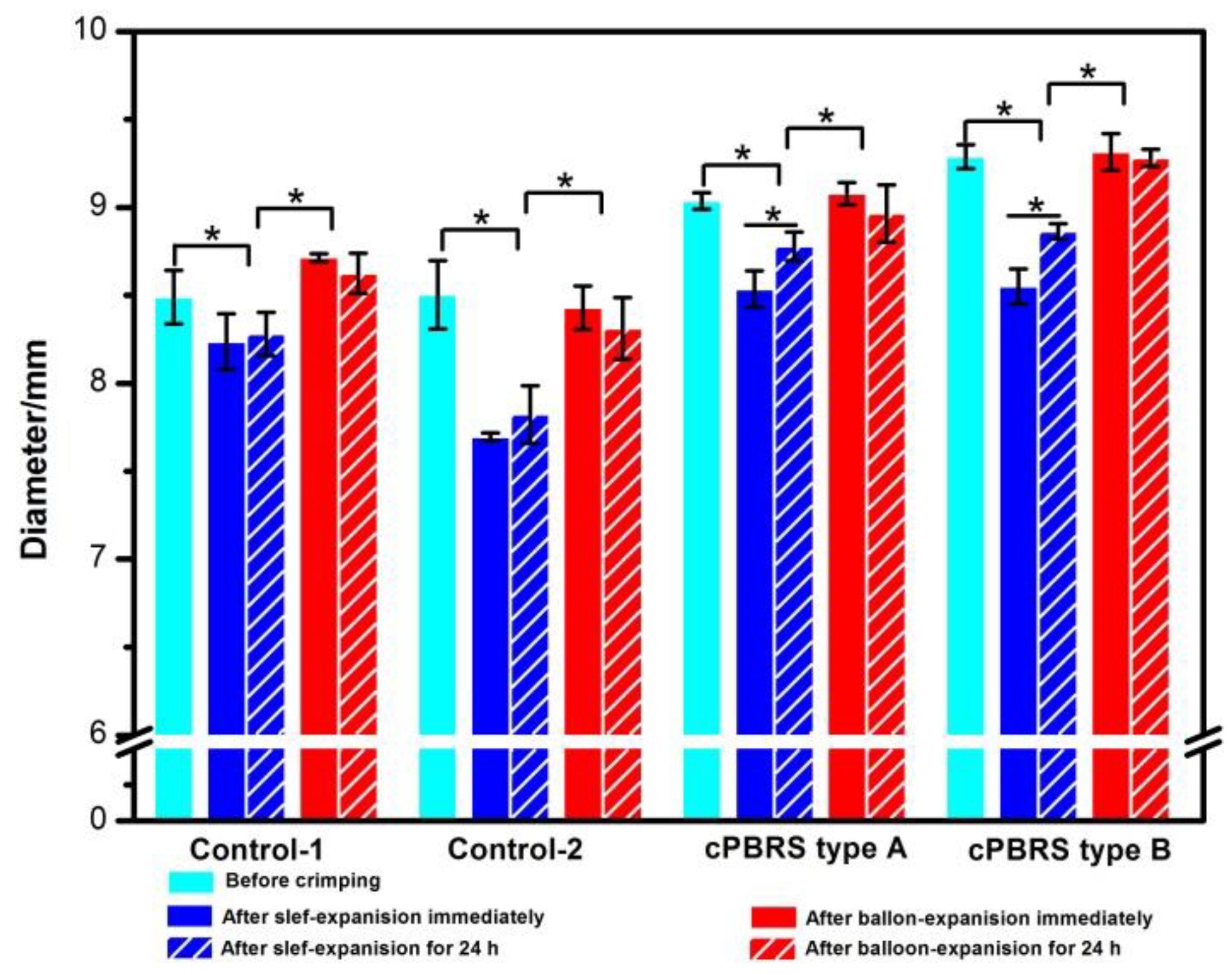
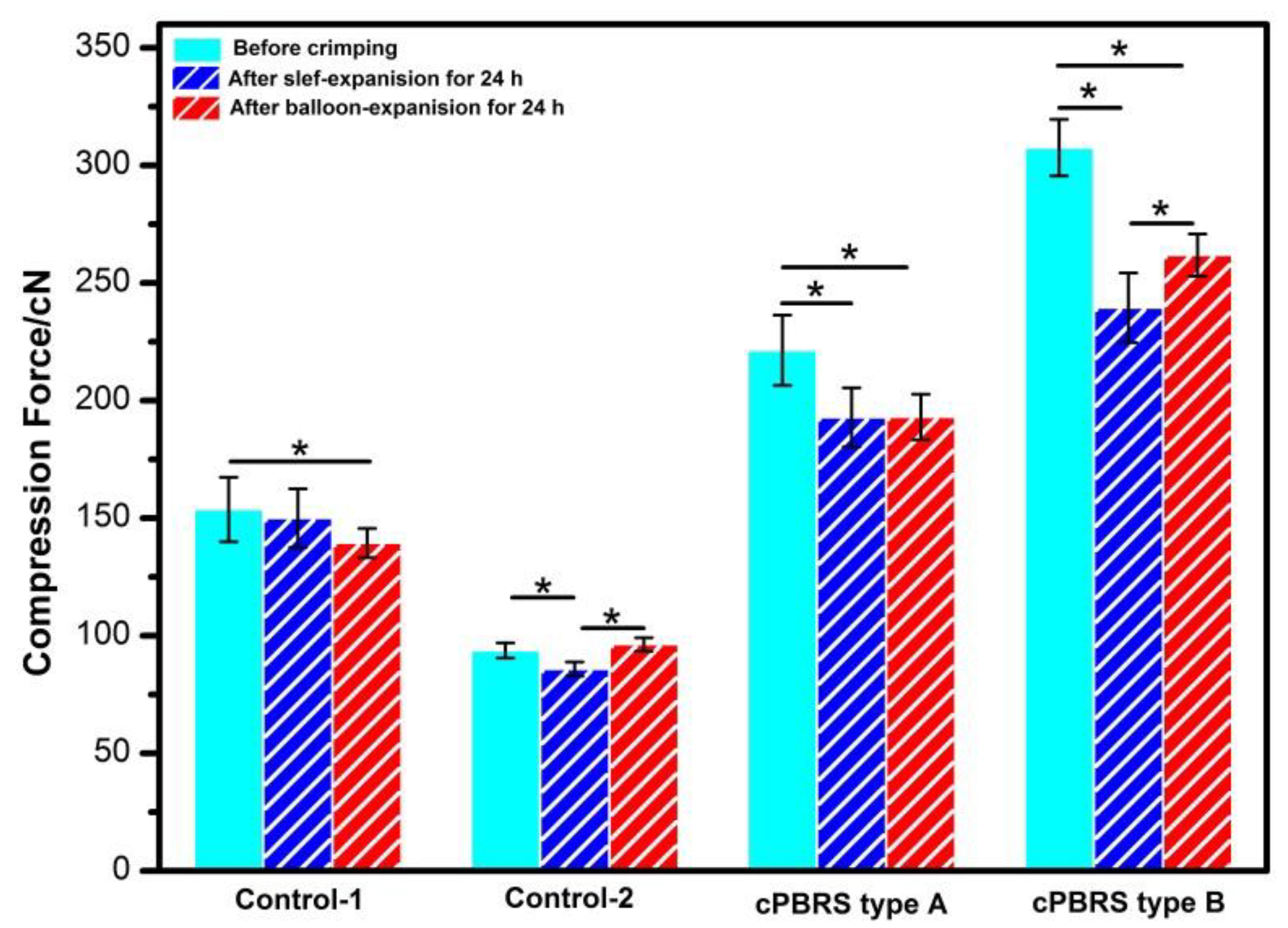
3.2. Crimping and Expanding Performance
3.3. Computational Results
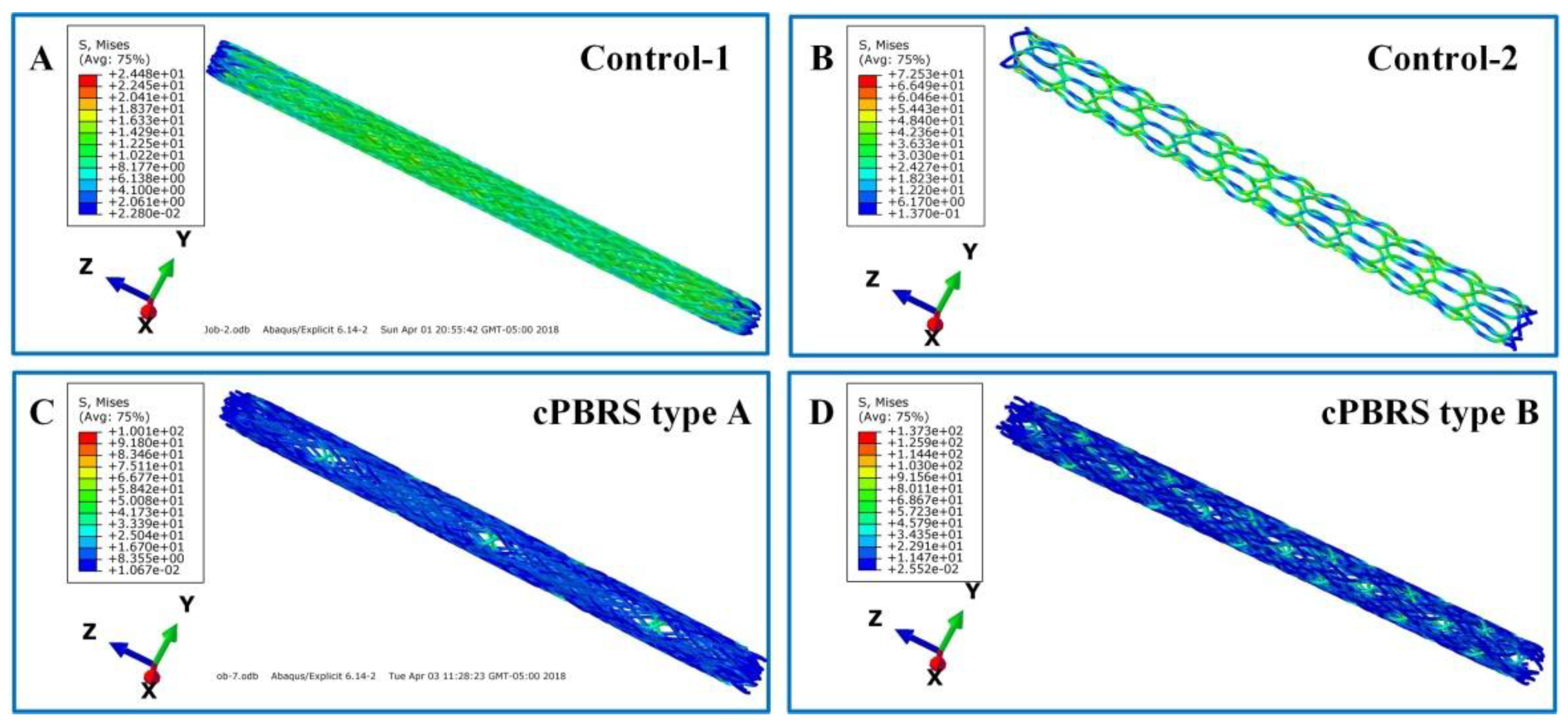
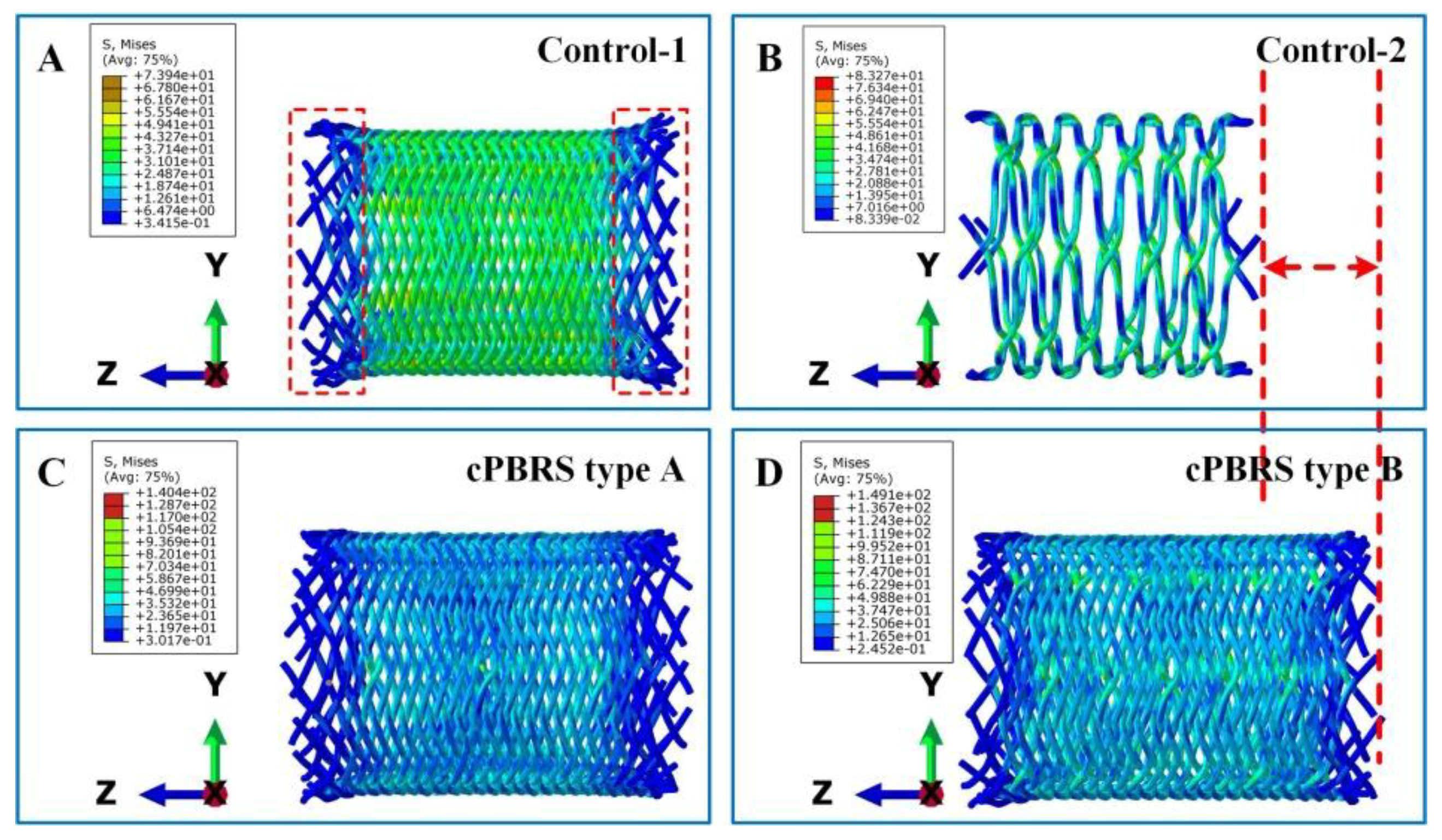
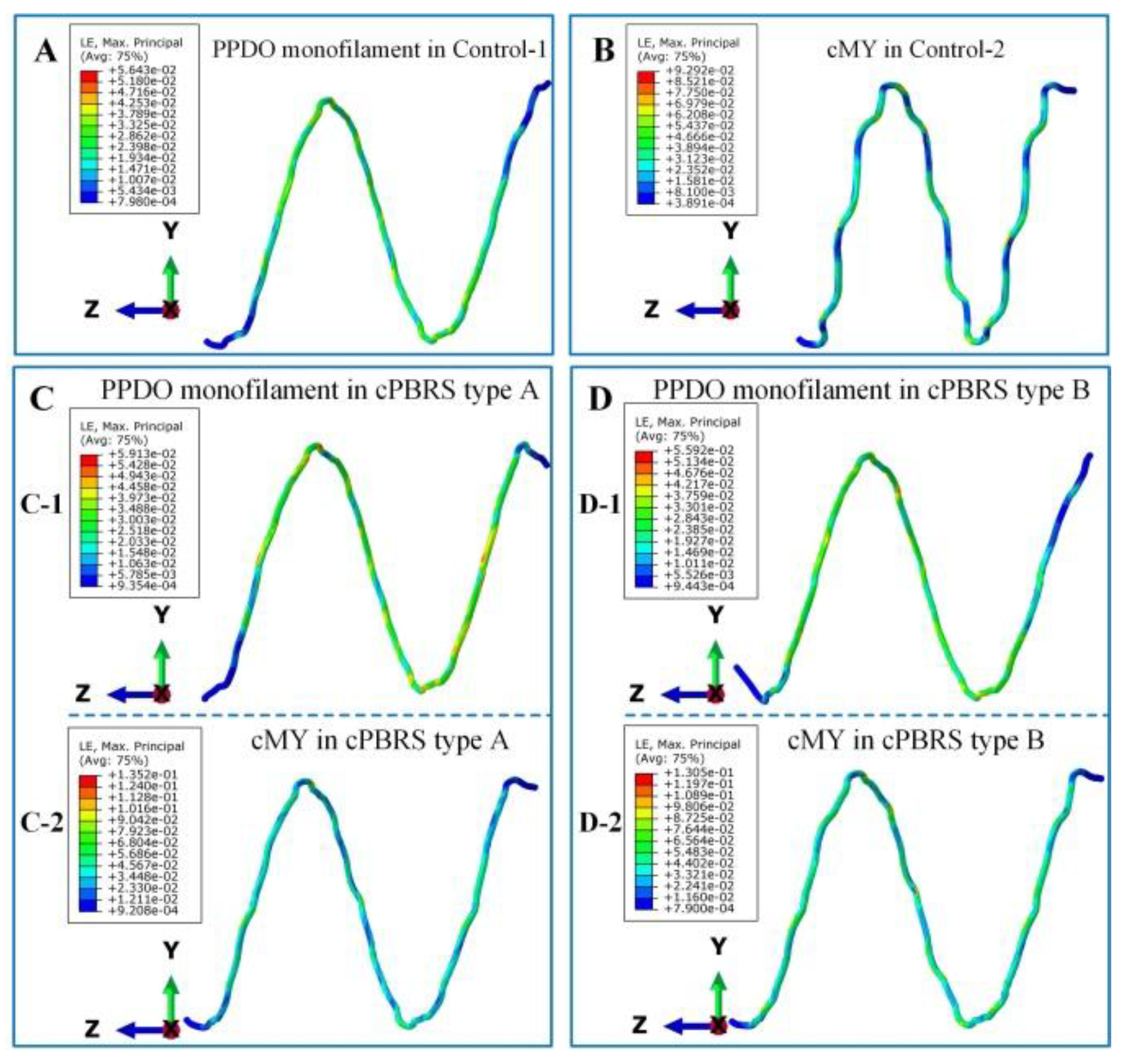
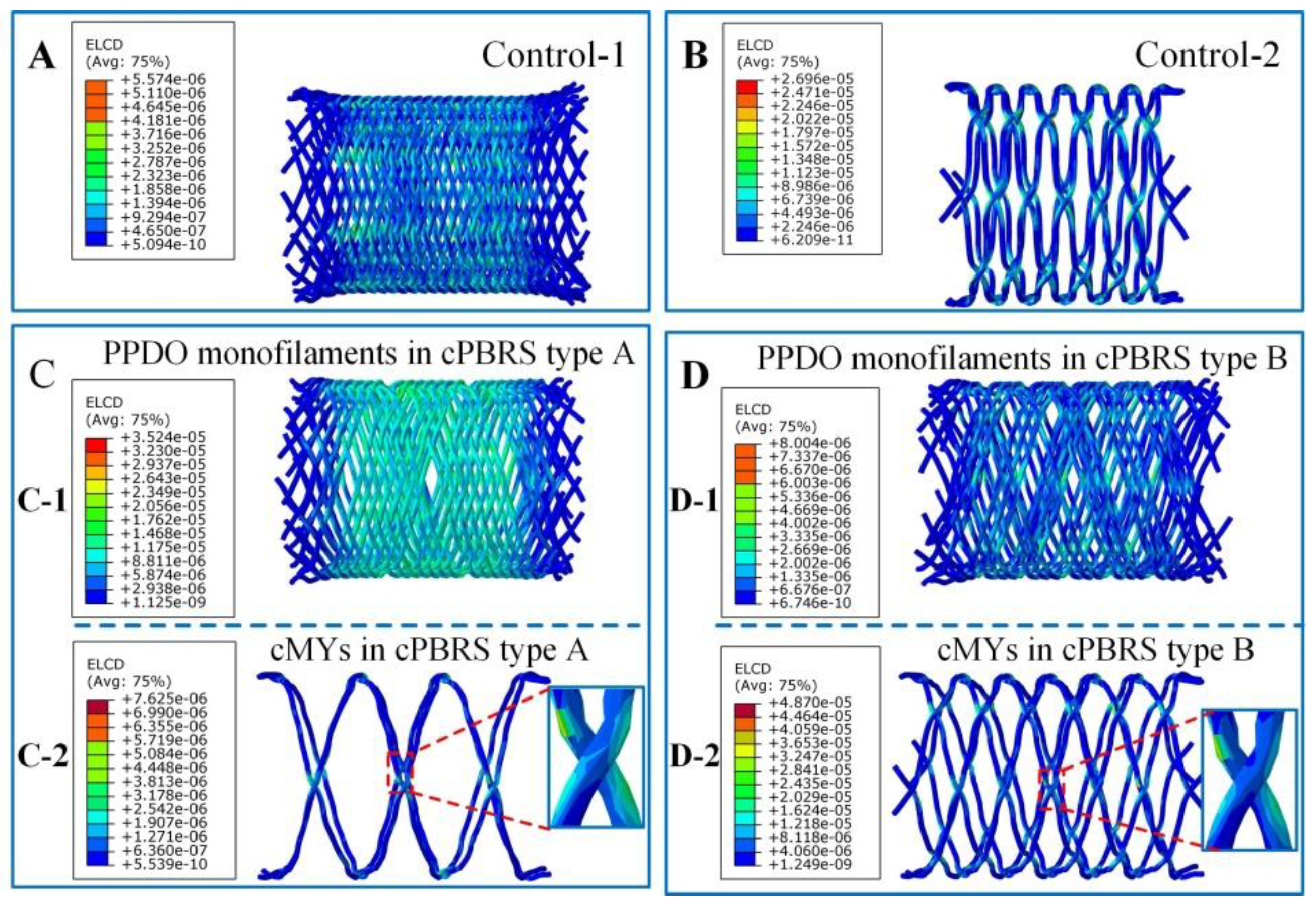
3.3.1. Crimping Simulation Results
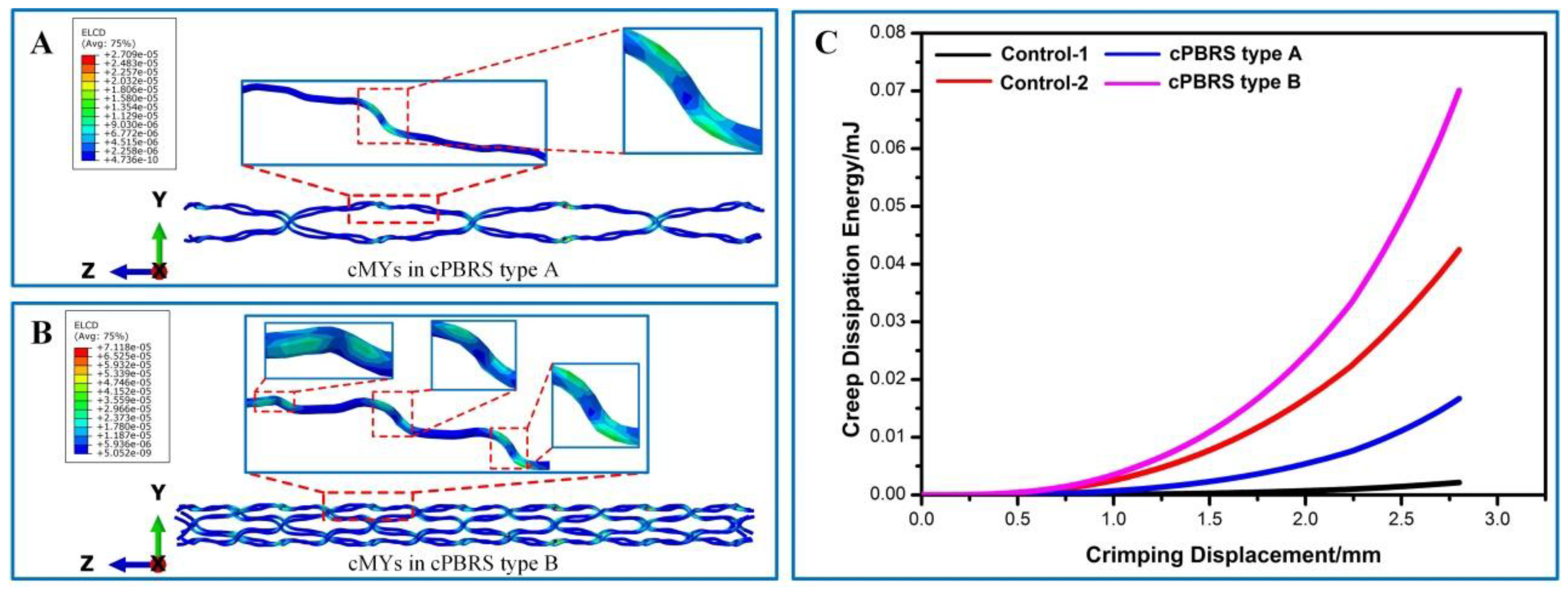
3.3.2. Balloon Expanding Simulation Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Time (s) | Normalized Shear Creep Compliance j(t) ± SD | |
|---|---|---|
| PPDO Monofilaments | cMYs | |
| 1 | 1.00 ± 0.024 | 1.00 ± 0.036 |
| 60 | 1.06 ± 0.023 | 1.07 ± 0.029 |
| 300 | 1.12 ± 0.018 | 1.14 ± 0.027 |
| 600 | 1.14 ± 0.017 | 1.17 ± 0.025 |
| 900 | 1.16 ± 0.015 | 1.19 ± 0.024 |
| 1200 | 1.16 ± 0.013 | 1.21 ± 0.021 |
| 1500 | 1.17 ± 0.012 | 1.22 ± 0.020 |
| 1800 | 1.18 ± 0.013 | 1.23 ± 0.019 |
| 2100 | 1.18 ± 0.012 | 1.23 ± 0.018 |
| 2400 | 1.18 ± 0.013 | 1.24 ± 0.016 |
| 2700 | 1.19 ± 0.013 | 1.24 ± 0.017 |
| 3000 | 1.19 ± 0.013 | 1.25 ± 0.016 |
| 3300 | 1.19 ± 0.012 | 1.25 ± 0.016 |
| 3600 | 1.19 ± 0.013 | 1.26 ± 0.017 |
| 5400 | 1.20 ± 0.015 | 1.27 ± 0.019 |
| 7200 | 1.21 ± 0.017 | 1.28 ± 0.024 |
| 9000 | 1.21 ± 0.017 | 1.29 ± 0.029 |
| 10800 | 1.22 ± 0.019 | 1.30 ± 0.031 |
| 12600 | 1.22 ± 0.020 | 1.30 ± 0.034 |
| 14400 | 1.23 ± 0.024 | 1.31 ± 0.037 |
| 16200 | 1.23 ± 0.025 | 1.31 ± 0.037 |
| 18000 | 1.23 ± 0.028 | 1.31 ± 0.053 |
References
- Ailianou, A.; Ramachandran, K.; Kossuth, M.B.; Oberhauser, J.P.; Kornfield, J.A. Multiplicity of morphologies in poly (l-lactide) bioresorbable vascular scaffolds. Proc. Natl. Acad. Sci. USA 2016, 113, 11670. [Google Scholar] [CrossRef] [PubMed]
- Ang, H.Y.; Bulluck, H.; Wong, P.; Venkatraman, S.S.; Huang, Y.; Foin, N. Bioresorbable stents: current and upcoming bioresorbable technologies. Int. J. Cardiol. 2017, 228, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Bangalore, S.; Bezerra, H.G.; Rizik, D.G.; Armstrong, E.J.; Samuels, B.; Naidu, S.S.; Grines, C.L.; Foster, M.T.; Choi, J.W.; Bertolet, B.D.; Shah, A.P. The state of the absorb bioresorbable scaffold: consensus from an expert panel. JACC Cardiovasc. Interv. 2017, 10, 2349–2359. [Google Scholar] [CrossRef] [PubMed]
- Bergström, J.S.; Hayman, D. An overview of mechanical properties and material modeling of polylactide (PLA) for medical applications. Ann. Biomed. Eng. 2016, 44, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Debusschere, N.; Segers, P.; Dubruel, P.; Verhegghe, B.; De Beule, M. A finite element strategy to investigate the free expansion behaviour of a biodegradable polymeric stent. J. Biomech. 2015, 48, 2012–2018. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Zhou, J.; Chen, G.; Burkley, D.; Xu, Y.; Jamiolkowski, D.; Barbolt, T. Effect of load and temperature on in vitro degradation of poly(glycolide-co-l-lactide) multifilament braids. Biomaterials 2005, 26, 4327. [Google Scholar] [CrossRef] [PubMed]
- Joner, M.; Koppara, T.; Virmani, R.; Byrne, R.A. Improved vessel healing with fully bioresorbable drug-eluting stents: More than a pipe dream. Eur. Heart J. 2016, 37, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Drozdov, A.D.; Christiansen, J.D.C. The effect of strain rate on the viscoplastic behavior of isotactic polypropylene at finite strains. Polymer 2003, 44, 1211–1228. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.; Xue, W.; Wang, F.; Liu, L.; Shi, H.; Wang, L. Composite self-expanding bioresorbable prototype stents with reinforced compression performance for congenital heart disease application: computational and experimental investigation. J. Mech. Behav. Biomed. Mater. 2018, 84, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zheng, F.; Qiu, X.; Wan, P.; Tan, L.; Yang, K. Finite element analyses for optimization design of biodegradable magnesium alloy stent. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 42, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Feng, X.; Jia, X.; Fan, Y. Influences of tensile load on in vitro degradation of an electrospun poly(l-lactide-co-glycolide) scaffold. Acta Biomater. 2010, 6, 2991. [Google Scholar] [CrossRef] [PubMed]
- McGarry, J.P.; O’donnell, B.P.; McHugh, P.E.; McGarry, J.G. Analysis of the mechanical performance of a cardiovascular stent design based on micromechanical modelling. Comput. Mater. Sci. 2004, 31, 421–438. [Google Scholar] [CrossRef]
- Nicodemus, G.D.; Shiplet, K.A.; Kaltz, S.R.; Bryant, S.J. Dynamic compressive loading influences degradation behavior of peg-Pla hydrogels. Biotechnol. Bioeng. 2009, 102, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Nishio, S.; Kosuga, K.; Igaki, K.; Okada, M.; Kyo, E.; Tsuji, T.; Takeuchi, E.; Inuzuka, Y.; Takeda, S.; Hata, T. Long-term (>10 Years) clinical outcomes of first-in-human biodegradable poly-l-lactic acid coronary stents: Igaki-Tamai stents. Circulation 2012, 125, 2343–2353. [Google Scholar] [CrossRef] [PubMed]
- Onuma, Y.; Serruys, P.W.; Ormiston, J.A.; Regar, E.; Webster, M.; Thuesen, L.; Dudek, D.; Veldhof, S.; Rapoza, R. Three-year results of clinical follow-up after a bioresorbable everolimus-eluting scaffold in patients with de novo coronary artery disease: The absorb trial. EuroInterv. J. Europcr Collab. Work Group Int. Cardiol. Eur. Soc. Cardiol. 2010, 6, 447. [Google Scholar] [CrossRef] [PubMed]
- Ormiston, J.A.; Serruys, P.W. Bioabsorbable coronary stents. Lancet 2009, 369, 1839–1840. [Google Scholar] [CrossRef]
- Mathias, P. Design of Biodegradable Esophageal Stents; Universiteit Gent: Ghent, Belgium, 2013. [Google Scholar]
- Rajagopal, K.R.; Srinivasa, A.R. On the thermomechanics of materials that have multiple natural configurations part I: Viscoelasticity and classical plasticity. J. Appl. Math. Phys. 2004, 55, 1074–1093. [Google Scholar] [CrossRef]
- Schiavone, A.; Zhao, L.G. A computational study of stent performance by considering vessel anisotropy and residual stresses. Mater. Sci. Eng. C 2016, 62, 307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiavone, A.; Qiu, T.; Zhao, L. Crimping and Deployment of Metallic and Polymeric Stents—Finite Element Modelling. Available online: https://dspace.lboro.ac.uk/dspace-jspui/bitstream/2134/23975/3/Zhao_1777-7383-1-PB.pdf (accessed on 2 November 2018).
- Shanahan, C.; Tofail, S.A.M.; Tiernan, P. Viscoelastic braided stent: finite element modelling and validation of crimping behavior. Mater. Des. 2017, 121, 143–153. [Google Scholar] [CrossRef]
- Soares, J.S.; Moore, J.E. Biomechanical challenges to polymeric biodegradable stents. Ann. Biomed. Eng. 2015, 44, 560–579. [Google Scholar] [CrossRef] [PubMed]
- Stoeckel, D.; Pelton, A.; Duerig, T. Self-expanding nitinol stents: Material and design considerations. Eur. Radiol. 2004, 14, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Virmani, R. Self-expanding stent deployment strategies may be the key to reducing in-stent restenosis. Catheter. Cardiovasc. Interv. 2002, 56, 487–488. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Fang, G.; Zhao, Y.; Wang, G.; Cai, T. Computational and experimental investigation into mechanical performances of poly-l-lactide acid (PLLA) coronary stents. J. Mech. Behav. Biomed. Mater. 2016, 65, 415. [Google Scholar] [CrossRef] [PubMed]
- Ward, I.M.; Sweeney, J. Mechanical Properties of Solid Polymers; John Wiley & Sons: Hoboken, NJ, USA, 2013; p. 694. [Google Scholar]
- Welch, T.; Eberhart, R.C.; Chuong, C.J. Characterizing the expansive deformation of a bioresorbable polymer fiber stent. Ann. Biomed. Eng. 2008, 36, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Wiebe, J.; Nef, H.M.; Hamm, C.W. Current status of bioresorbable scaffoldsin the treatment of coronaryartery disease. J. Am. Coll. Cardiol. 2014, 64, 2541–2551. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.; Lu, W.; Li, W.; Wang, W.; Fang, C.; King, M.W. A resorbable bicomponent braided ureteral stent with improved mechanical performance. J. Mech. Behav. Biomed. Mater. 2014, 38, 17. [Google Scholar] [CrossRef] [PubMed]
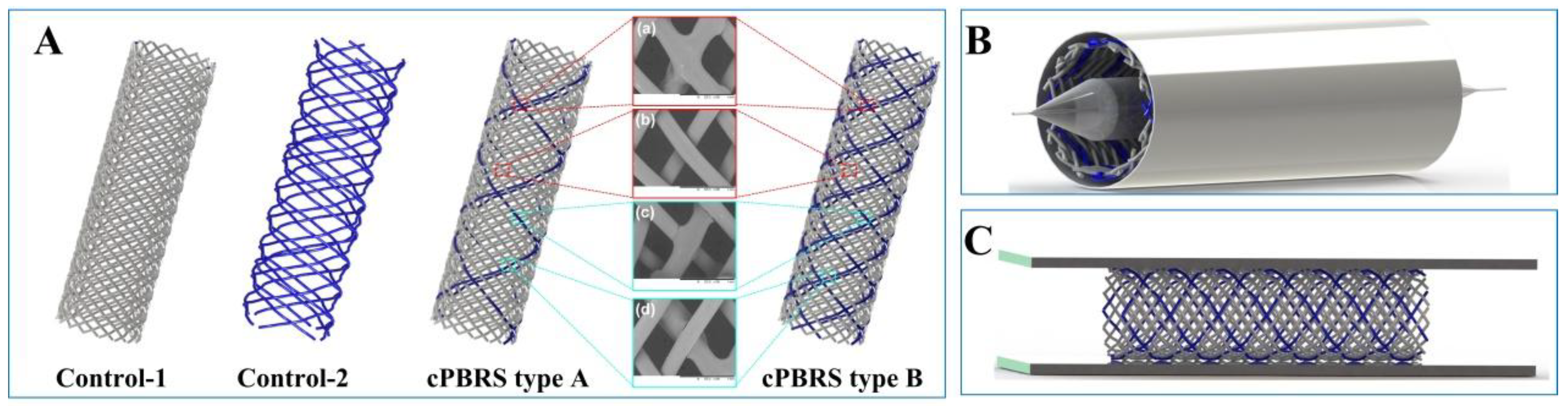

| Type of Yarns | Diameter/mm | Breaking Strength/MPa | Breaking Elongation/% | Melting Temperature/°C |
|---|---|---|---|---|
| PPDO monofilaments | 0.30 ± 0.01 | 433.17 ± 21.71 | 53.50 ± 1.09 | 102.73 ± 0.15 |
| PCL multifilament | 0.14 ± 0.02 | 199.02 ± 0.01 | 47.64 ± 0.03 | 56.03 ± 0.21 |
| cBYs | 0.48 ± 0.01 | 182.72 ± 15.92 | 55.61 ± 3.70 | - |
| cMYs | 0.43 ± 0.01 | 221.49 ± 4.01 | 61.58 ± 6.20 | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, F.; Liu, L.; Yang, Y.; Wang, F.; Wang, L. The Crimping and Expanding Performance of Self-Expanding Polymeric Bioresorbable Stents: Experimental and Computational Investigation. Materials 2018, 11, 2184. https://doi.org/10.3390/ma11112184
Zhao F, Liu L, Yang Y, Wang F, Wang L. The Crimping and Expanding Performance of Self-Expanding Polymeric Bioresorbable Stents: Experimental and Computational Investigation. Materials. 2018; 11(11):2184. https://doi.org/10.3390/ma11112184
Chicago/Turabian StyleZhao, Fan, Laijun Liu, Yang Yang, Fujun Wang, and Lu Wang. 2018. "The Crimping and Expanding Performance of Self-Expanding Polymeric Bioresorbable Stents: Experimental and Computational Investigation" Materials 11, no. 11: 2184. https://doi.org/10.3390/ma11112184