Analysis on the Influence of Component Ratio on Properties of Silica/Montmorillonite Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Silica/MMT Nanocomposites
2.3. FTIR Analysis
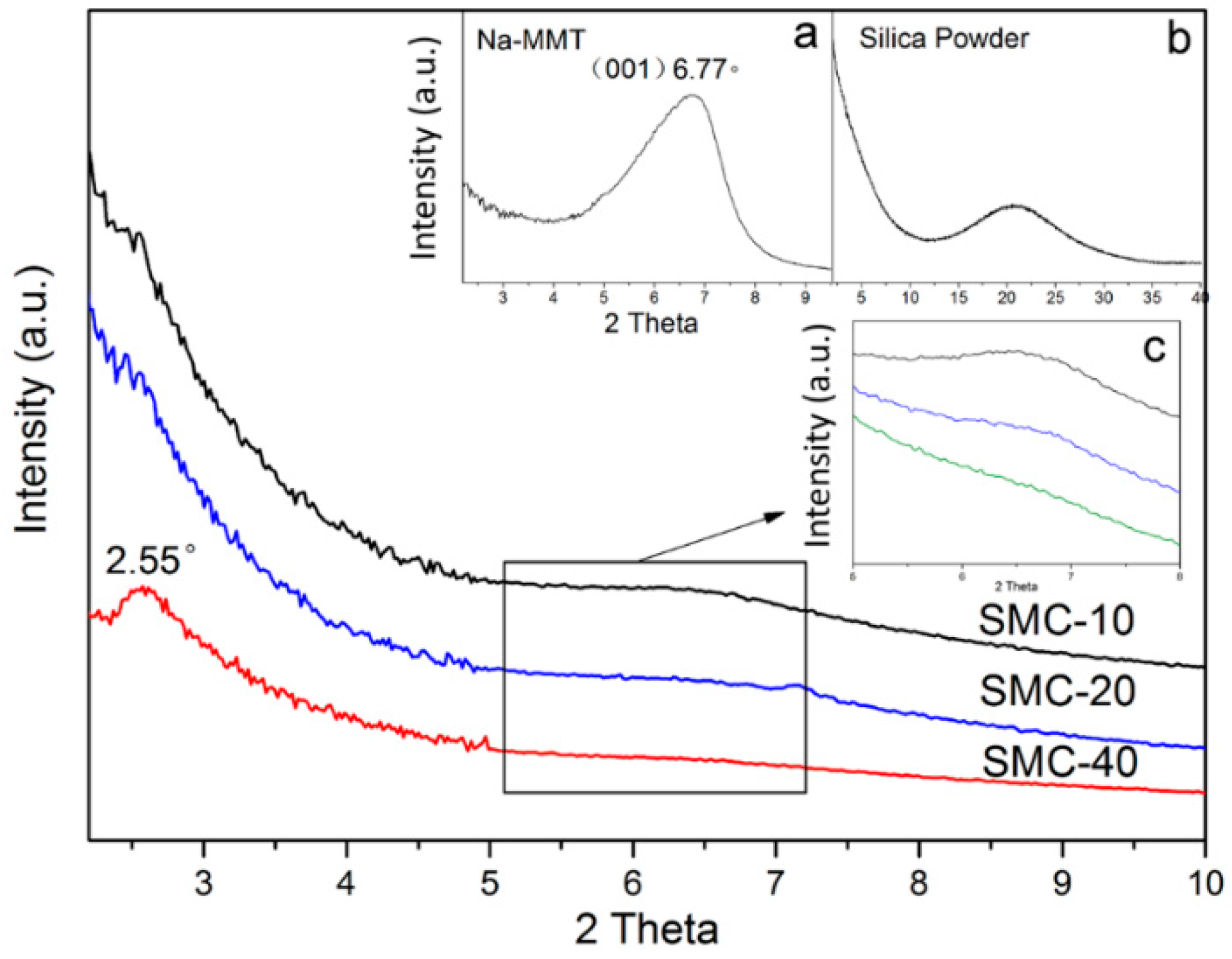
2.4. X-ray Diffraction Analysis
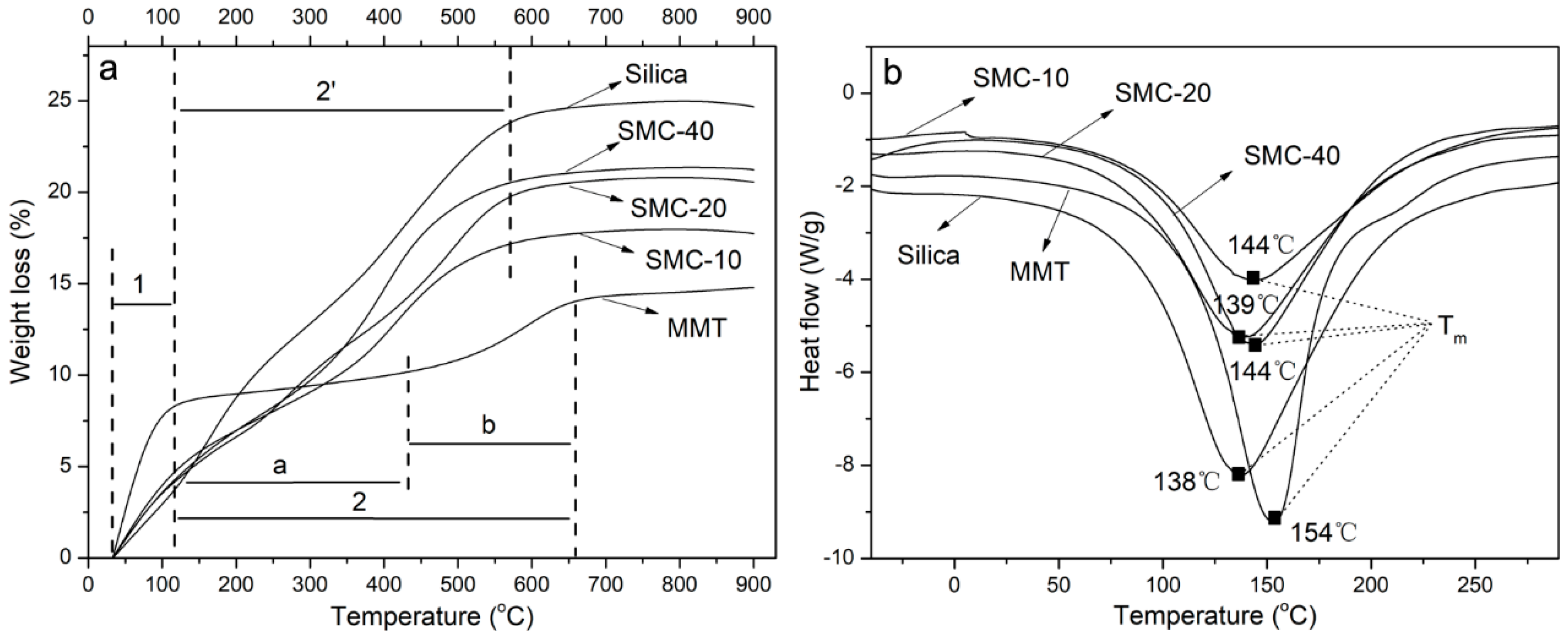
2.5. Thermal Characterization
2.6. Particulate Morphology
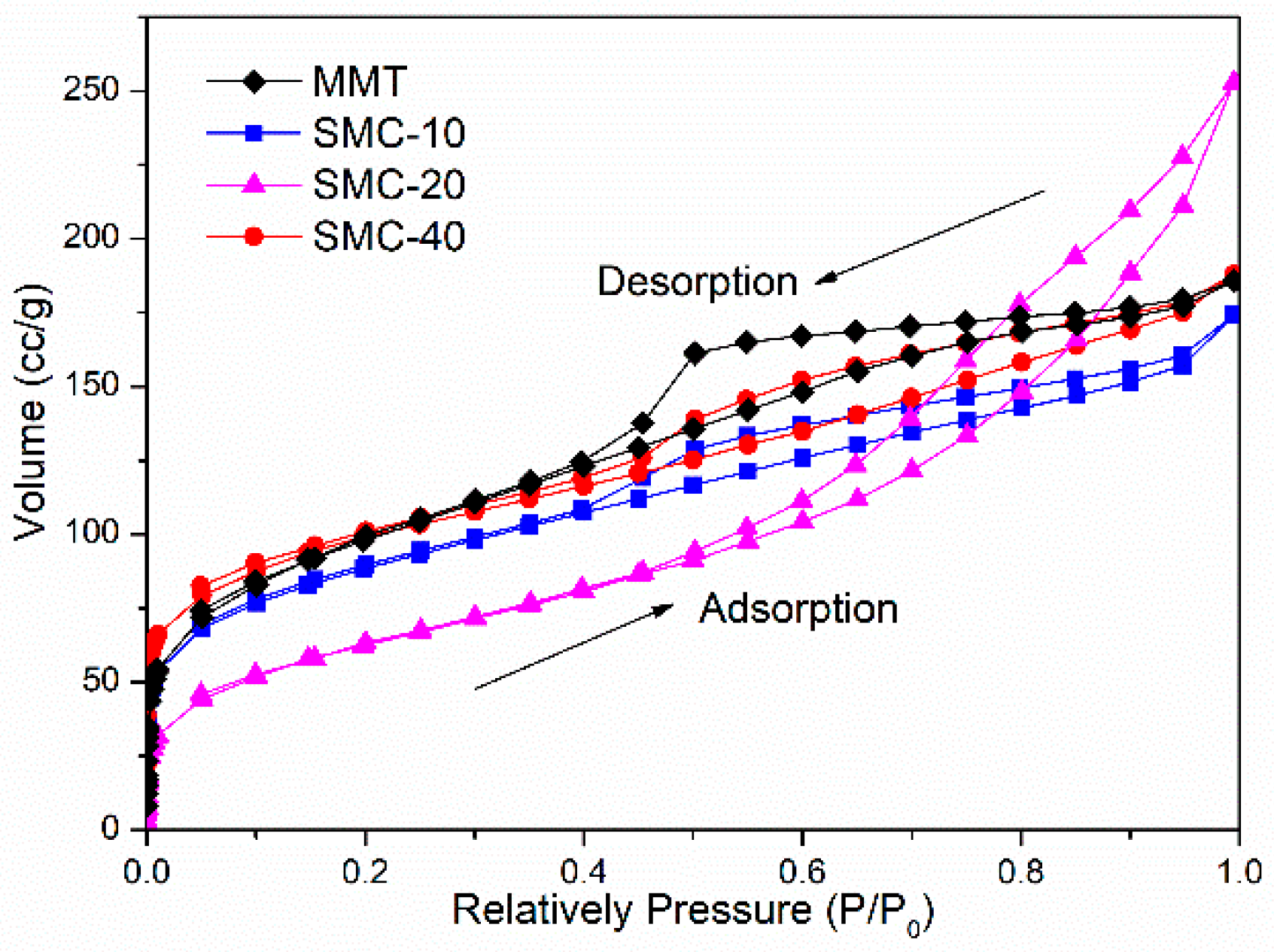
2.7. Pore Characteristics
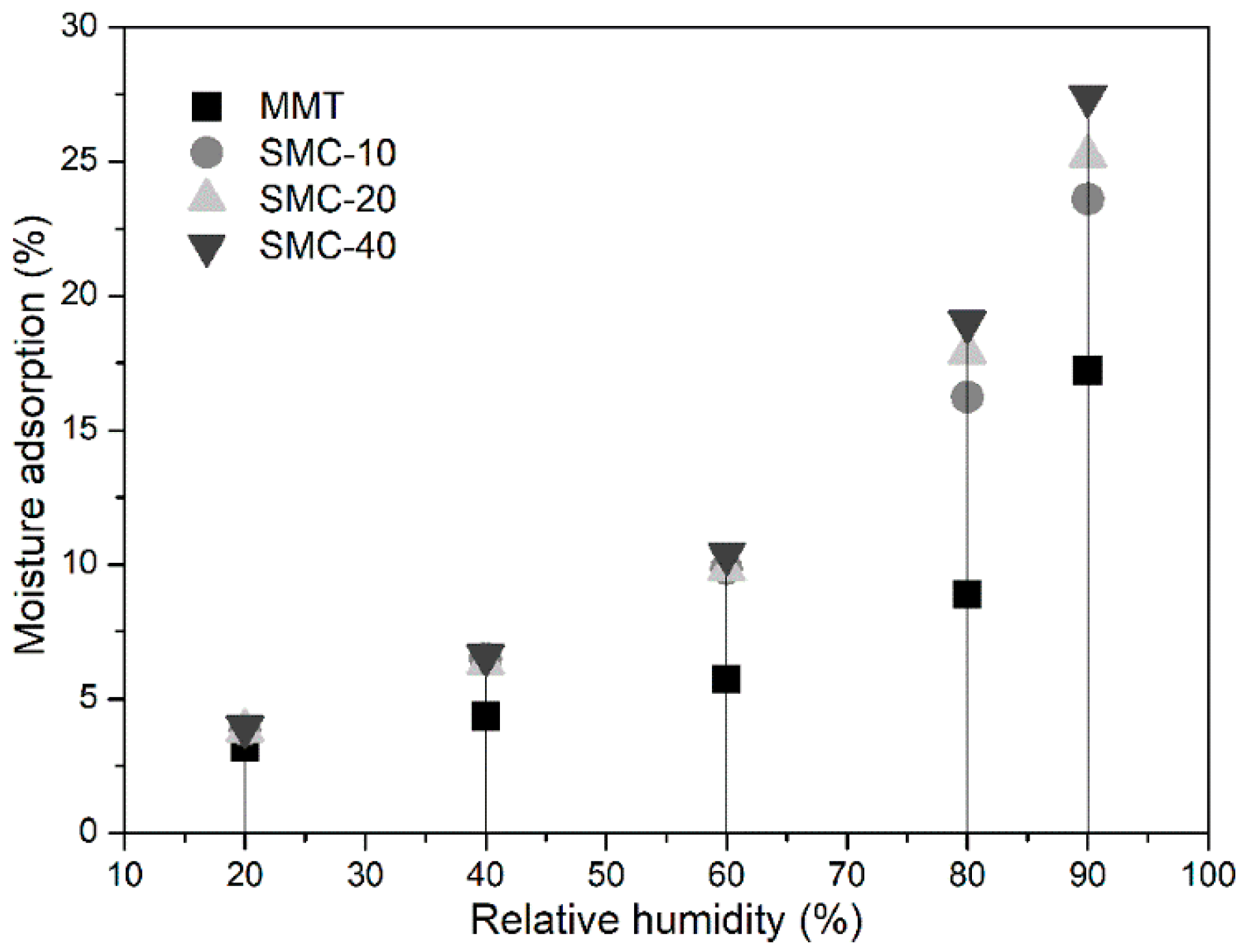
2.8. Moisture Adsorption
3. Results and Discussion
3.1. Characteristics of Surface Chemistry
3.2. XRD Analysis
3.3. Thermal Properties
3.4. Characteristics of Particulate Morphology
3.5. Characteristics of Pore Structure
3.6. Hygroscopicity
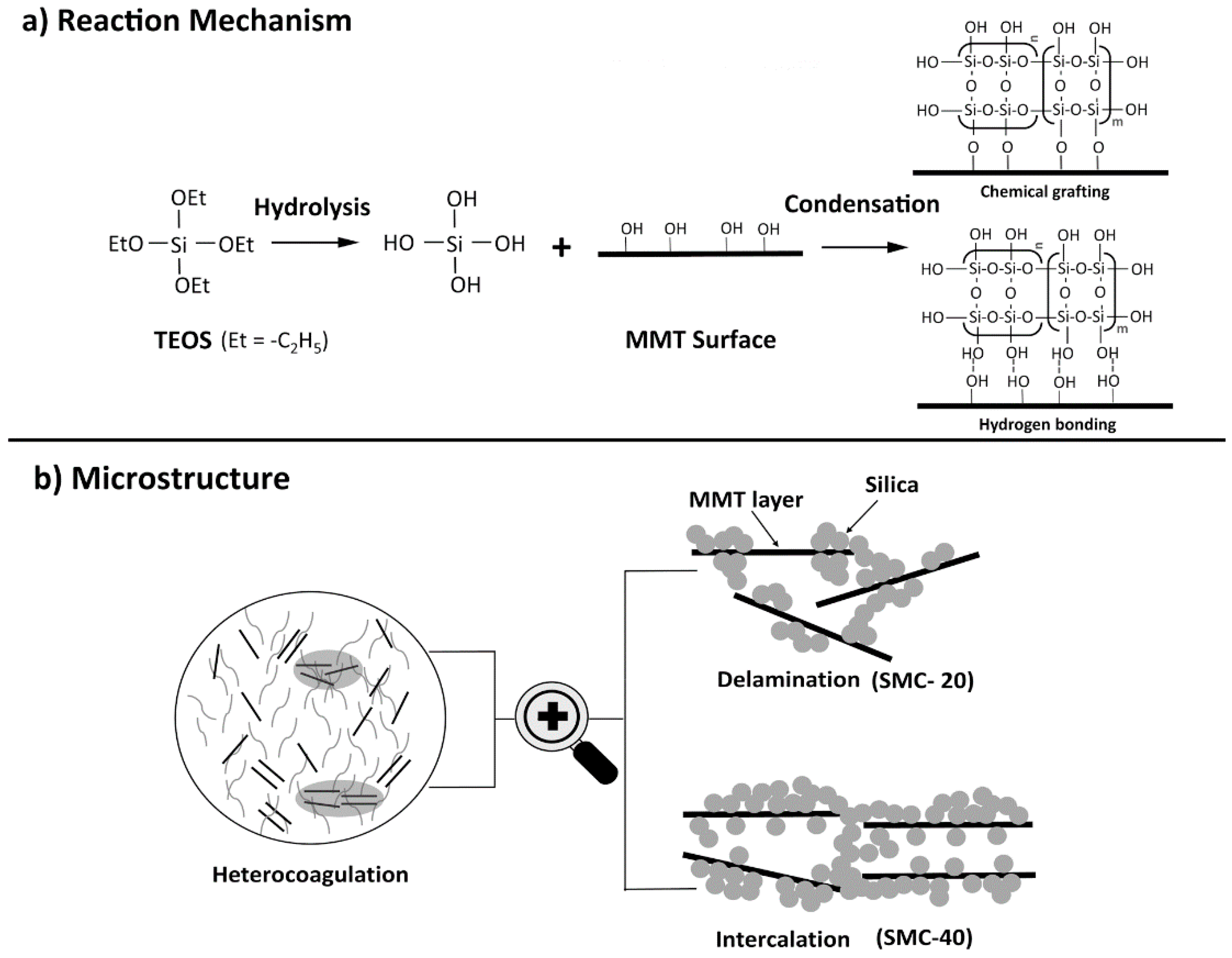
3.7. Synthesis Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ramaraj, B.; Jaisankar, S.N. Thermal and morphological properties of poly (vinyl alcohol) and layered double hydroxide (LDH) nanocomposites. Polym. Plast. Technol. Eng. 2008, 47, 733–738. [Google Scholar] [CrossRef]
- Naffakh, M.; Lopez, V.; Zamora, F.; Gomez, M.A. Novel melt-processable nanocomposites based on isotactic polypropylene and carbon nitride: Morphology, crystallization, and dynamic mechanical properties. Soft Mater. 2010, 8, 407–425. [Google Scholar] [CrossRef]
- Agarwal, G.K.; Titman, J.J. Characterization of vinyl polymer/silica colloidal nanocomposites using solid state NMR spectroscopy: Probing the interaction between the inorganic and organic phases on the molecular level. J. Phys. Chem. B 2003, 107, 12497–12502. [Google Scholar] [CrossRef]
- Stackhouse, S.; Coveney, P.V.; Sandre, E. Plane-wave density functional theoretic study of formation of clay-polymer nanocomposite materials by self-catalyzed in situ intercalative polymerization. J. Am. Chem. Soc. 2001, 123, 11764–11774. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.S.; Okamoto, M. Polymer/layered silicate nanocomposites: A review from preparation to processing. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar]
- Olivier, M.G.; Fedel, M.; Sciamanna, V.; Vandermiers, C.; Motte, C.; Poelman, M.; Deflorian, F. Study of the effect of nanoclay incorporation on the rheological properties and corrosion protection by a silane layer. Prog. Org. Coat. 2011, 72, 15–20. [Google Scholar] [CrossRef]
- Guimarães, J.L.; Peralta-Zamora, P.; Wypych, F. Covalent grafting of phenylphosphonate groups onto the interlamellar aluminol surface of kaolinite. J. Colloid Interface Sci. 1998, 206, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.E.; Olin, T.J.; Bricka, R.M.; Adrian, D.D. A review of potentially low-cost sorbents for heavy metals. Water Res. 1999, 33, 2469–2479. [Google Scholar] [CrossRef]
- Mercier, L.; Detellier, C. Preparation, characterization, and applications as heavy metals sorbents of covalently grafted thiol functionalities on the interlamellar surface of montmorillonite. Environ. Sci. Technol. 1995, 29, 1318–1323. [Google Scholar] [CrossRef] [PubMed]
- Herrera, N.N.; Letoffe, J.M.; Reymondc, J.P.; Bourgeat-Lami, E. Silylation of laponite clay particles with monofunctional and trifunctional vinyl alkoxysilanes. J. Mater. Chem. 2005, 15, 863–871. [Google Scholar] [CrossRef]
- Legrand, A.P. The Surface Properties of Silicas; Wiley: London, UK, 1998. [Google Scholar]
- Dai, J.C.; Huang, J.T. Surface modification of clays and clay–rubber composite. Appl. Clay Sci. 1999, 15, 51–65. [Google Scholar] [CrossRef]
- Herrera, N.N.; Letoffe, J.M.; Putaux, J.L.; David, L.; Bourgeat-Lami, E. Aqueous dispersions of silane-functionalized laponite clay platelets. A first step toward the elaboration of water-based polymer/clay nanocomposites. Langmuir 2004, 20, 1564–1571. [Google Scholar]
- Mercier, L.; Pinnavaia, T.J. A functionalized porous clay heterostructure for heavy metal ion (Hg2+) trapping. Microporous Mesoporous Mater. 1998, 20, 101–106. [Google Scholar] [CrossRef]
- Ogiso, K.; Saka, S. Wood-inorganic composites prepared by sol-gel process. II. Effects of ultrasonic treatments on preparation of wood-inorganic composites. Mokuzai Gakkaishi 1993, 39, 301–307. [Google Scholar]
- Kanokwijitsilp, T.; Traiperm, P.; Osotchan, T.; Srikhirin, T. Development of abrasion resistance SiO2 nanocomposite coating for teak wood. Prog. Org. Coat. 2016, 93, 118–126. [Google Scholar] [CrossRef]
- Letaïef, S.; Martín-Luengo, M.A.; Aranda, P.; Ruiz-Hitzky, E. A Colloidal Route for Delamination of Layered Solids: Novel Porous-Clay Nanocomposites. Adv. Funct. Mater. 2006, 16, 401–409. [Google Scholar] [CrossRef] [Green Version]
- Akkari, M.; Aranda, P.; Rhaiem, H.B.; Amara, A.B.H.; Ruiz-Hitzky, E. ZnO/clay nanoarchitectures: Synthesis, characterization and evaluation as photocatalysts. Appl. Clay Sci. 2016, 131, 131–139. [Google Scholar] [CrossRef]
- Binitha, N.N.; Sugunan, S. Preparation, characterization and catalytic activity of titania pillared montmorillonite clays. Microporous Mesoporous Mater. 2006, 93, 82–89. [Google Scholar] [CrossRef]
- Manova, E.; Aranda, P.; Martín-Luengo, M.A.; Letaïef, S.; Ruiz-Hitzky, E. New titania-clay nanostructured porous materials. Microporous Mesoporous Mater. 2010, 131, 252–260. [Google Scholar] [CrossRef]
- Sanchez, C.; Julián, B.; Belleville, P.; Popall, M. Applications of hybrid organic–inorganic nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, W.; Shen, H.; Wang, J.; Cao, J. Characterization of silica particles modified with γ-methacryloxypropyltrimethoxysilane. Appl. Surf. Sci. 2017, 397, 104–111. [Google Scholar] [CrossRef]
- Madejova, J. FTIR techniques in clay mineral studies. Vib. Spectrosc. 2003, 31, 1–10. [Google Scholar] [CrossRef]
- Bishop, J.; Madejova, J.; Komadel, P.; Fröschl, H. The influence of structural Fe, Al and Mg on the infrared OH bands in spectra of dioctahedral smectites. Clay Miner. 2002, 37, 607–616. [Google Scholar] [CrossRef]
- Wei, B.; Chang, Q.; Bao, C.; Dai, L.; Zhang, G.; Wu, F. Surface modification of filter medium particles with silane coupling agent KH550. Colloids Surf. A Physicochem. Eng. Asp. 2013, 434, 276–280. [Google Scholar] [CrossRef]
- Khanmohammadi, S.; Taheri-Nassaj, E. Micro-porous silica–yttria membrane by sol–gel method: Preparation and characterization. Ceram. Int. 2014, 40, 9403–9411. [Google Scholar] [CrossRef]
- Tonle, I.K.; Ngameni, E.; Njopwouo, D.; Carteret, C.; Walcarius, A. Functionalization of natural smectite-type clays by grafting with organosilanes: Physico-chemical characterization and application to mercury (II) uptake. Phys. Chem. Chem. Phys. 2003, 5, 4951–4961. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Y.; Li, X.; Luo, J.; Gao, Q.; Li, J. “Green” bio-thermoset resins derived from soy protein isolate and condensed tannins. Ind. Crops Prod. 2017, 108, 363–370. [Google Scholar] [CrossRef]
- Zhang, F.; Sautter, K.; Larsen, A.M.; Davis, D.A.; Samha, H.; Linford, M.R. Chemical vapor deposition of three aminosilanes on silicon dioxide: Surface characterization, stability, effects of silane concentration, and cyanine dye adsorption. Langmuir 2010, 26, 14648–14654. [Google Scholar] [CrossRef] [PubMed]
- Karout, A.; Pierre, A.C. Porous texture of silica aerogels made with ionic liquids as gelation catalysts. J. Sol–Gel Sci. Technol. 2009, 49, 364–372. [Google Scholar] [CrossRef]
- Vigil, G.; Xu, Z.; Steinberg, S.; Israelachvili, J. Interactions of silica surfaces. J. Colloid Interface Sci. 1994, 165, 367–385. [Google Scholar] [CrossRef]
- Bandi, S.; Schiraldi, D.A. Glass transition behavior of clay aerogel/poly (vinyl alcohol) composites. Macromolecules 2006, 39, 6537–6545. [Google Scholar] [CrossRef]
- Zhuravlev, L.Z. The surface chemistry of amorphous silica. Zhuravlev model. Colloids Surf. A Physicochem. Eng. Asp. 2000, 173, 1–38. [Google Scholar] [CrossRef] [Green Version]
- Gun’ko, V.M.; Zarko, V.I.; Leboda, R.; Chibowski, E. Aqueous suspension of fumed oxides: Particle size distribution and zeta potential. Adv. Colloid Interface Sci. 2001, 91, 1–112. [Google Scholar] [CrossRef]
- Pope, E.J.A.; Mackenzie, J.D. Sol-gel processing of silica: II. The role of the catalyst. J. Non-Cryst. Solids 1986, 87, 185–198. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Provisional). Pure Appl. Chem. 1982, 54, 2201–2218. [Google Scholar] [CrossRef] [Green Version]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Commission on colloid and surface chemistry including catalysis. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Jiang, J.; Cao, J.; Wang, W.; Xue, J. How silanization influences aggregation and moisture sorption behaviours of silanized silica: Analysis of porosity and multilayer moisture adsorption. R. Soc. Open Sci. 2018, 5, 180206. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Feng, D.; Wang, X.; Zhu, Y. A thermochemical approach to enhance hydrophobicity of SiC/SiO2 powder using γ-methacryloxypropyl trimethoxy silane and octylphenol polyoxyethylene ether (7). Appl. Surf. Sci. 2016, 360, 45–51. [Google Scholar] [CrossRef]
- Pantoja, M.; Díaz-Benito, B.; Velasco, F.; Abenojar, J.; Del Real, J.C. Analysis of hydrolysis process of γ-methacryloxypropyltrimethoxysilane and its influence on the formation of silane coatings on 6063 aluminum alloy. Appl. Surf. Sci. 2009, 255, 6386–6390. [Google Scholar] [CrossRef]
- Zerda, T.W.; Artaki, I.; Jonas, J. Study of polymerization processes in acid and base catalyzed silica sol-gels. J. Non-Cryst. Solids 1986, 81, 365–379. [Google Scholar] [CrossRef]


| Samples | BET Surface Area (m2·g−1) | Average Pore Width (nm) |
|---|---|---|
| MMT | 73 | 4.79 |
| SMC-10 | 308 | 3.49 |
| SMC-20 | 474 | 2.62 |
| SMC-40 | 338 | 3.43 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, J.; Cao, J.; Wang, W.; Mei, C. Analysis on the Influence of Component Ratio on Properties of Silica/Montmorillonite Nanocomposites. Materials 2018, 11, 2074. https://doi.org/10.3390/ma11112074
Jiang J, Cao J, Wang W, Mei C. Analysis on the Influence of Component Ratio on Properties of Silica/Montmorillonite Nanocomposites. Materials. 2018; 11(11):2074. https://doi.org/10.3390/ma11112074
Chicago/Turabian StyleJiang, Jun, Jinzhen Cao, Wang Wang, and Changtong Mei. 2018. "Analysis on the Influence of Component Ratio on Properties of Silica/Montmorillonite Nanocomposites" Materials 11, no. 11: 2074. https://doi.org/10.3390/ma11112074





