Treatment of Severely Resorbed Maxilla Due to Peri-Implantitis by Guided Bone Regeneration Using a Customized Allogenic Bone Block: A Case Report
Abstract
:1. Introduction
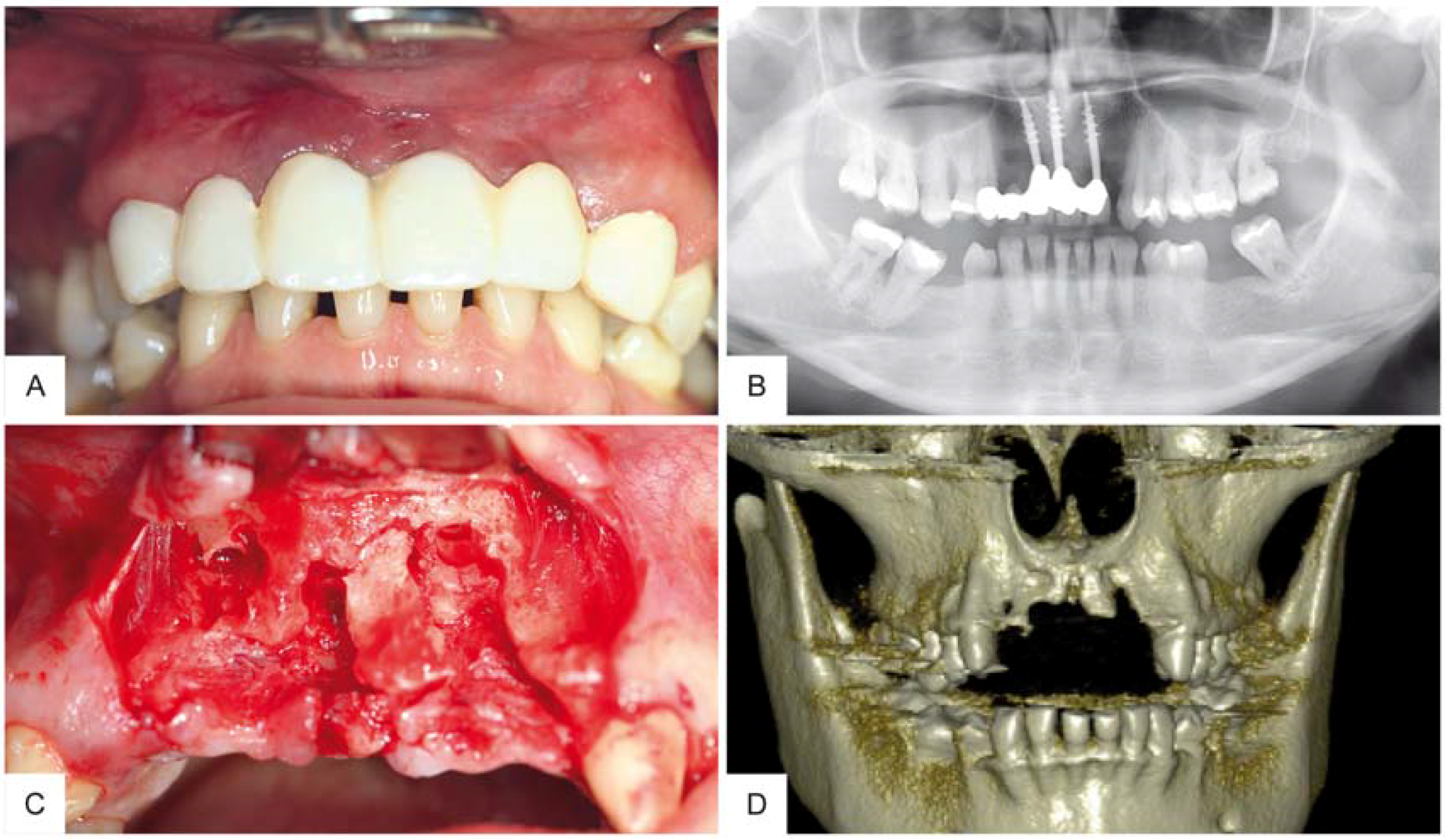
2. Case Report
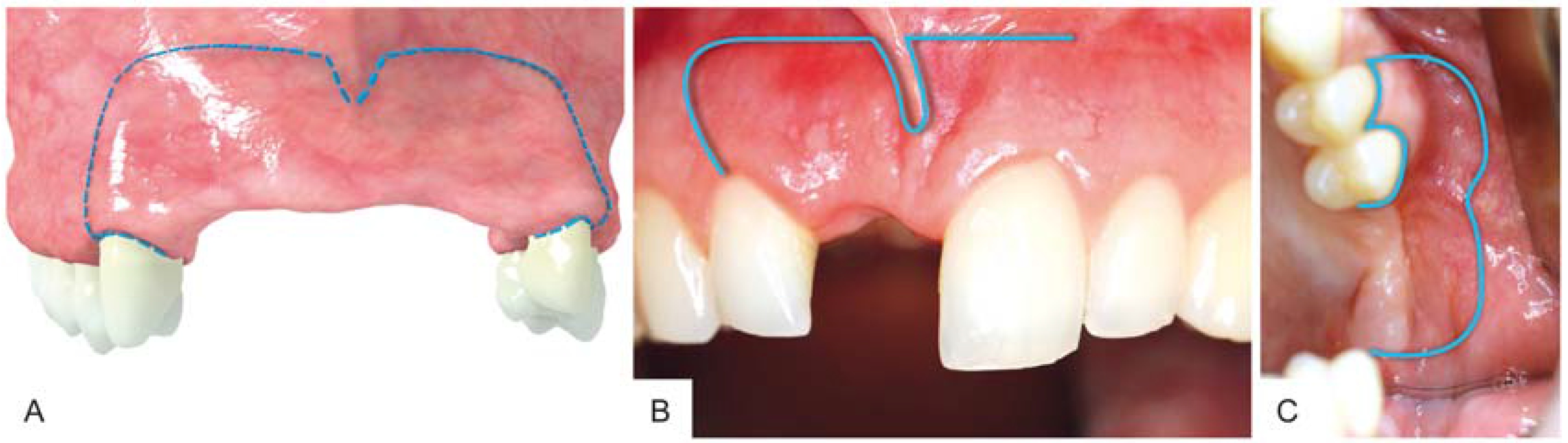
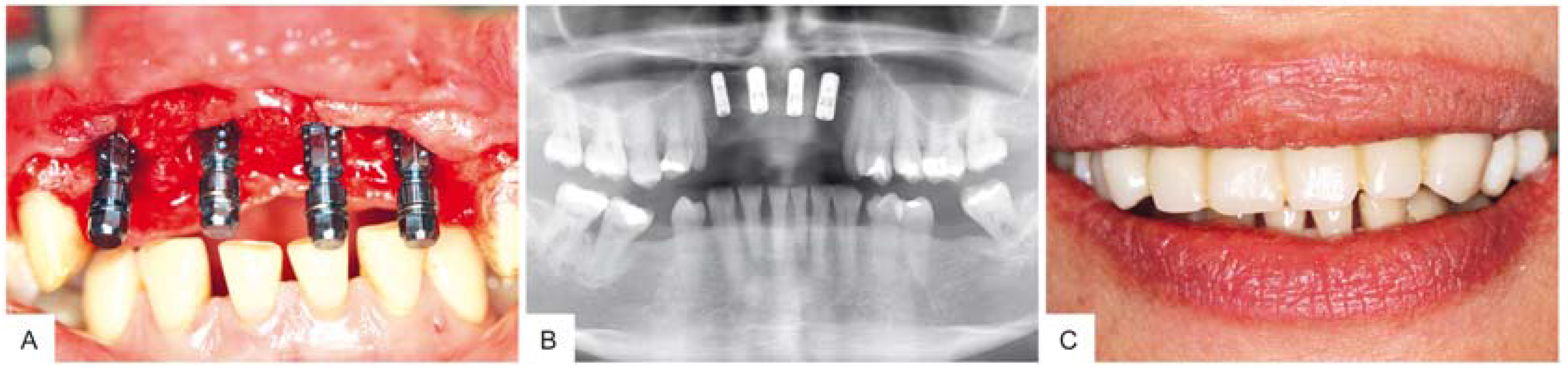
3. Surgical Procedure
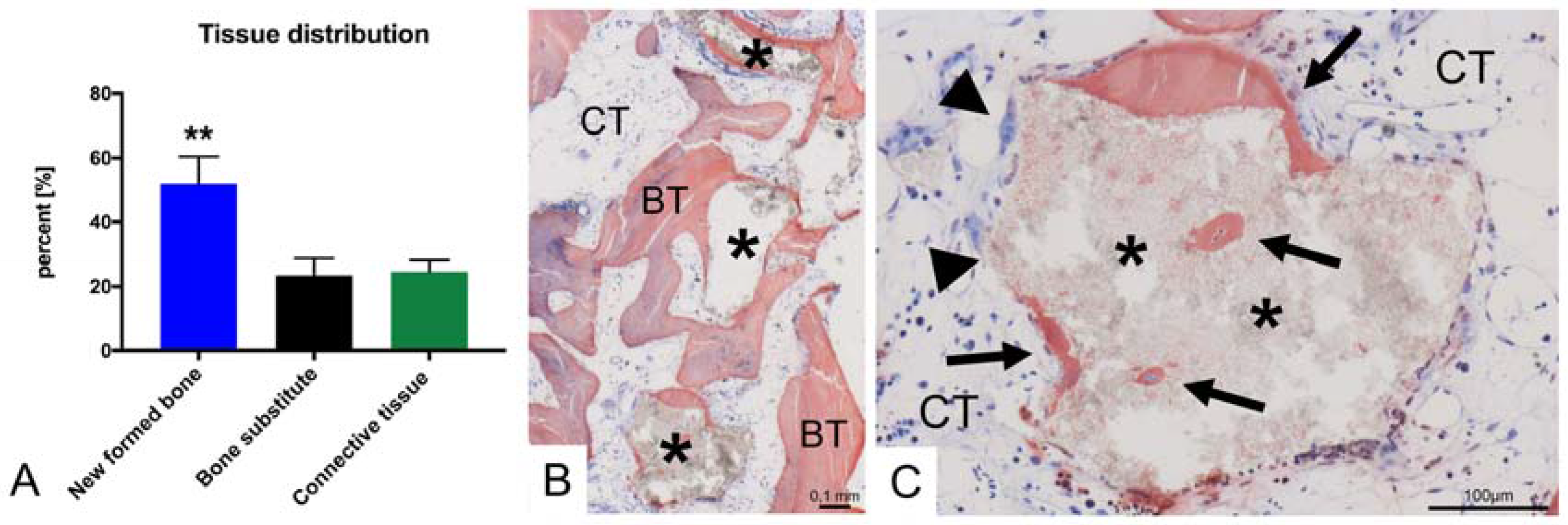
4. Results
5. Discussion
Author Contributions
Conflicts of Interest
References
- Nkenke, E.; Weisbach, V.; Winckler, E.; Kessler, P.; Schultze-Mosqau, S.; Wiltfang, J.; Neukam, F.W. Morbidity of Harvesting of Bone Grafts from the Iliac Crest for Preprosthetic Augmentation Procedures: A Prospective Study. Int. J. Oral Maxillofac. Surg. 2004, 33, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Leonetti, J.A.; Koup, R. Localized Maxillary Ridge Augmentation With a Block Allograft for Dental Implant Placement: Case Reports. Implant Dent. 2003, 12, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Chiapasco, M.; Zaniboni, M. Failures in Jaw Reconstructive Surgery with Autogenous Onlay Bone Grafts for Pre-Implant Purposes: Incidence, Prevention and Management of Complications. Oral Maxillofac. Surg. Clin. N. Am. 2011, 23, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, C.; Johansson, A. Iliac Crest Autogenous Bone Graft Versus Alloplastic Graft And Guided Bone Regeneration in the Reconstruction of Atrophic Maxillae: A 5-Year Retrospective Study on Cost-Effectiveness and Clinical Outcome. Clin. Implant Dent. Relat. Res. 2011, 13, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Truedsson, A.; Hjalte, K.; Sunzel, B.; Warfvinge, G. Maxillary Sinus Augmentation with Iliac Autograft—A Health-Economic Analysis. Clin. Oral Implants Res. 2013, 24, 1088–1093. [Google Scholar] [CrossRef] [PubMed]
- Motamedian, S.R.; Khojaste, M.; Khojasteh, A. Success Rate of Implants Placed in Autogenous Bone Blocks Versus Allogenic Bone Blocks: A Systematic Literature Review. Ann. Maxillofac. Surg. 2016, 6, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Kolk, A.; Handschel, J.; Drescher, W.; Rothamel, D.; Kloss, F.; Blessmann, M.; Heiland, M.; Wolff, K.D.; Smeets, R. Current Trends and Future Perspectives of Bone Substitute Materials—From Space Holders to Innovative Biomaterials. J. Craniomaxillofac. Surg. 2012, 40, 706–718. [Google Scholar] [CrossRef] [PubMed]
- Monje, A.; Pikos, M.A.; Chan, H.-L.; Suarez, F.; Gargallo-Albiol, J.; Hernández-Alfaro, F.; Galindo-Moreno, P.; Wang, H.-L. On the Feasibility of Utilizing Allogeneic Bone Blocks for Atrophic Maxillary Augmentation. BioMed Res. Int. 2014, 2014, 814578. [Google Scholar] [CrossRef] [PubMed]
- Novell, J.; Novell-Costa, F.; Ivorra, C.; Fariñas, O.; Munilla, A.; Martinez, C. Five-Year Results of Implants Inserted into Freeze-Dried Block Allografts. Implant Dent. 2012, 21, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Nissan, J.; Mardinger, O.; Calderon, S.; Romanos, G.E.; Chaushu, G. Cancellous Bone Block Allografts for the Augmentation of the Anterior Atrophic Maxilla. Clin. Implant Dent. Relat. Res. 2011, 13, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Barbeck, M.; Udeabor, S.; Lorenz, J.; Schlee, M.; Holthaus, M.G.; Raetscho, N.; Choukroun, J.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. High-Temperature Sintering of Xenogeneic Bone Substitutes Leads to Increased Multinucleated Giant Cell Formation: In Vivo and Preliminary Clinical Results. J. Oral Implantol. 2015, 41, E212–E222. [Google Scholar] [CrossRef] [PubMed]
- Jacotti, M.; Wang, H.L.; Fu, J.H.; Zamboni, G.; Bernardello, F. Ridge Augmentation With Mineralized Block Allografts: Clinical and Histological Evaluation of 8 Cases Treated with the 3-Dimensional Block Technique. Implant Dent. 2012, 21, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Eskow, A.J.; Mealey, B.L. Evaluation of Healing Following Tooth Extraction with Ridge Preservation Using Cortical Versus Cancellous Freeze-Dried Bone Allograft. J. Periodontol. 2014, 85, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Jamjoom, A.; Cohen, R.E. Grafts for Ridge Preservation. J. Funct. Biomater. 2015, 6, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Chaushu, G.; Mardinger, O.; Peleg, M.; Ghelfan, O.; Nissan, J. Analysis of Complications Following Augmentation with Cancellous Block Allografts. J. Periodontol. 2010, 81, 1759–1764. [Google Scholar] [CrossRef] [PubMed]
- Jacotti, M.; Barausse, C.; Felice, P. Posterior Atrophic Mandible Rehabilitation with Onlay Allograft Created with CAD-CAM Procedure: A Case Report. Implant Dent. 2014, 23, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Le, B.; Rohrer, M.D. Screw “ Tent-Pole ” Grafting Technique for Reconstruction of Large Vertical Alveolar Ridge Defects Using Human Mineralized Allograft for Implant Site Preparation. YJOMS 2010, 68, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Nissan, J.; Marilena, V.; Gross, O.; Mardinger, O.; Chaushu, G. Histomorphometric Analysis Following Augmentation of the Anterior Atrophic Maxilla with Cancellous Bone Block Allograft. Int. J. Oral Maxillofac. Implants 2012, 27, 84–89. [Google Scholar] [PubMed]
- Kleinheinz, J.; Büchter, A.; Kruse-Lösler, B.; Weingart, D.; Joos, U. Incision Design in Implant Dentistry Based on Vascularization of the Mucosa. Clin. Oral Implants Res. 2005, 16, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Farzad, M.; Mohammadi, M. Guided Bone Regeneration: A Literature Review. J. Oral Health Oral Epidemiol. 2012, 83, 111–122. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blume, O.; Hoffmann, L.; Donkiewicz, P.; Wenisch, S.; Back, M.; Franke, J.; Schnettler, R.; Barbeck, M. Treatment of Severely Resorbed Maxilla Due to Peri-Implantitis by Guided Bone Regeneration Using a Customized Allogenic Bone Block: A Case Report. Materials 2017, 10, 1213. https://doi.org/10.3390/ma10101213
Blume O, Hoffmann L, Donkiewicz P, Wenisch S, Back M, Franke J, Schnettler R, Barbeck M. Treatment of Severely Resorbed Maxilla Due to Peri-Implantitis by Guided Bone Regeneration Using a Customized Allogenic Bone Block: A Case Report. Materials. 2017; 10(10):1213. https://doi.org/10.3390/ma10101213
Chicago/Turabian StyleBlume, Oliver, Lisa Hoffmann, Phil Donkiewicz, Sabine Wenisch, Michael Back, Jörg Franke, Reinhard Schnettler, and Mike Barbeck. 2017. "Treatment of Severely Resorbed Maxilla Due to Peri-Implantitis by Guided Bone Regeneration Using a Customized Allogenic Bone Block: A Case Report" Materials 10, no. 10: 1213. https://doi.org/10.3390/ma10101213




