A Review of Recent Advances on the Effects of Microstructural Refinement and Nano-Catalytic Additives on the Hydrogen Storage Properties of Metal and Complex Hydrides
Abstract
:1. Introduction
2. The Effects of Particle and Grain/Crystallite Size
2.1. MgH2
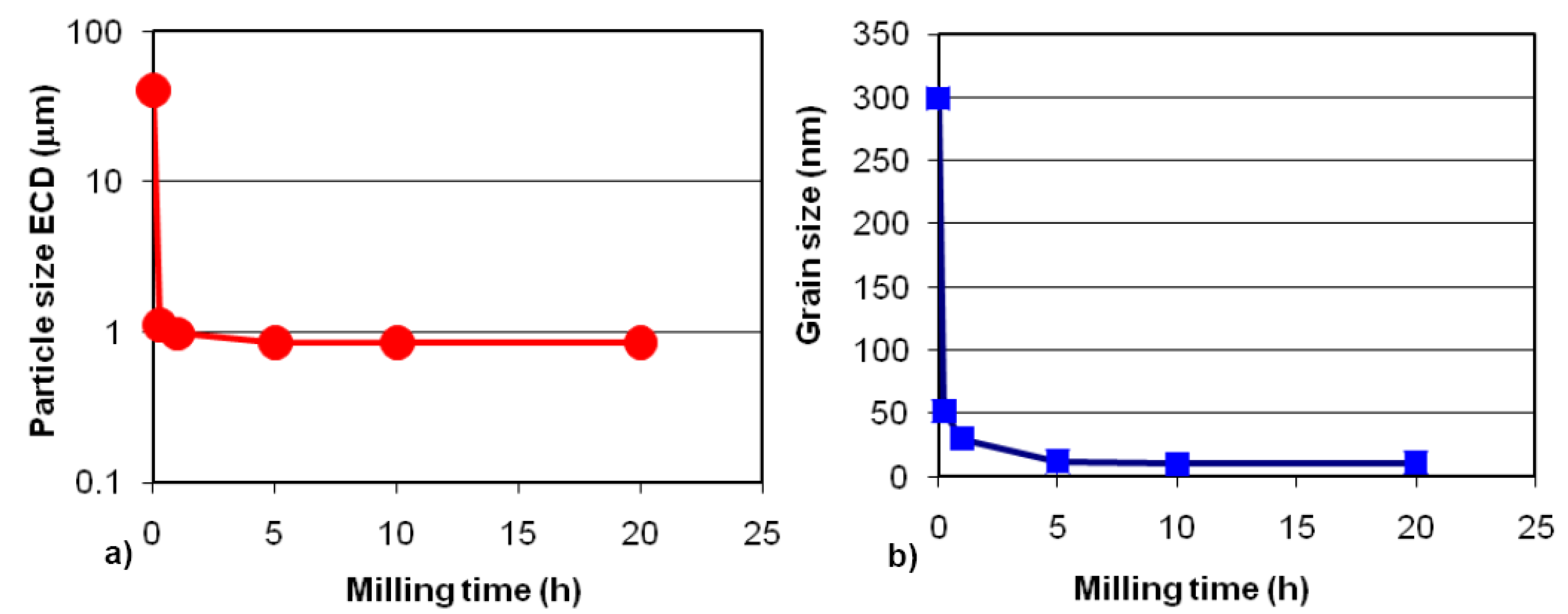
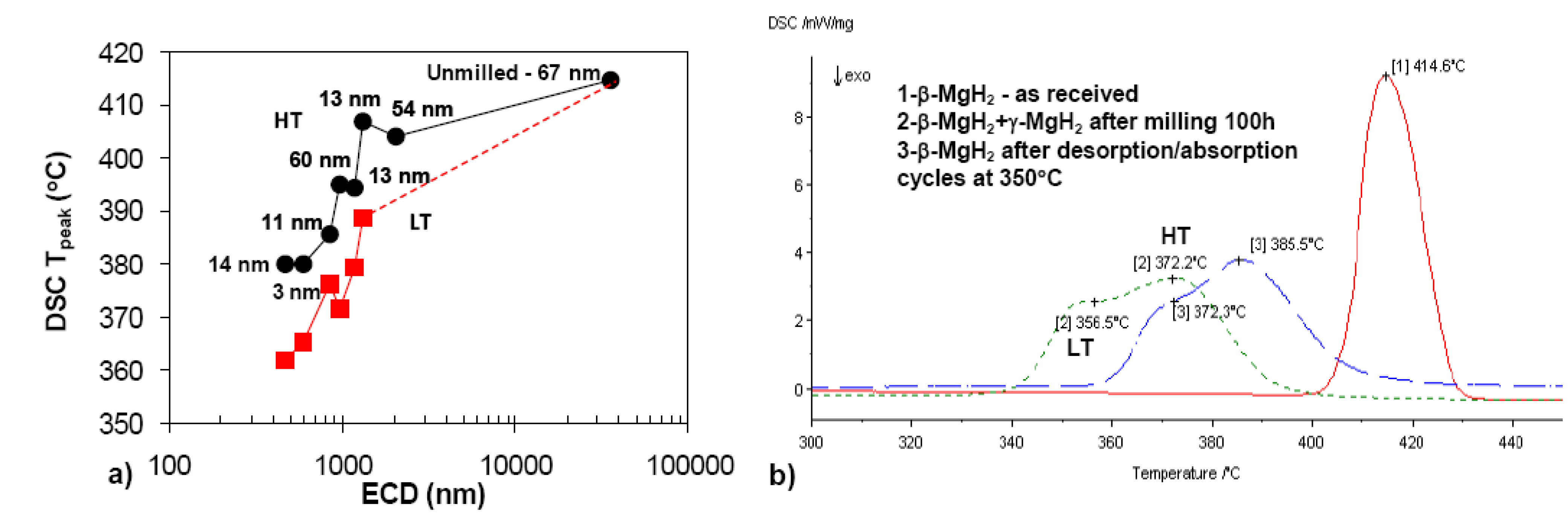

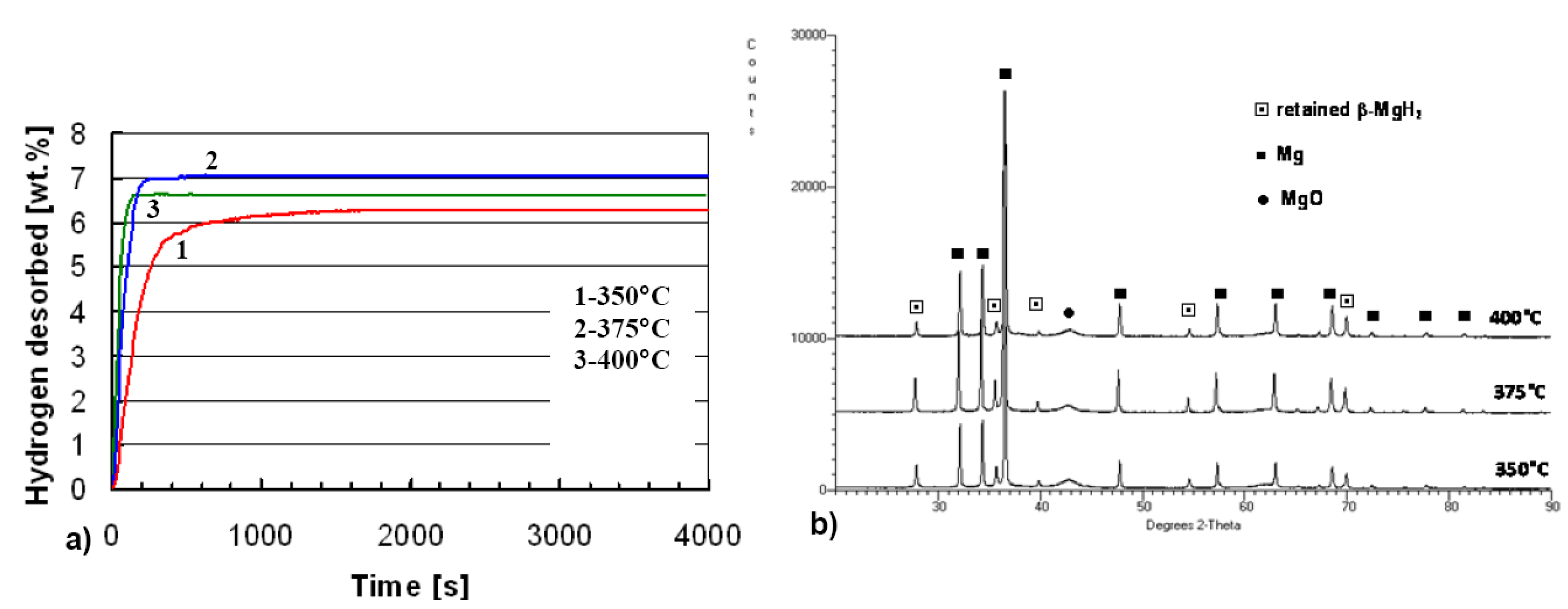
2.2. Complex Hydrides
3. The Effects of Catalytic Nano-Additives
3.1. Elemental Nanometric Metal Catalysts
3.1.1. MgH2
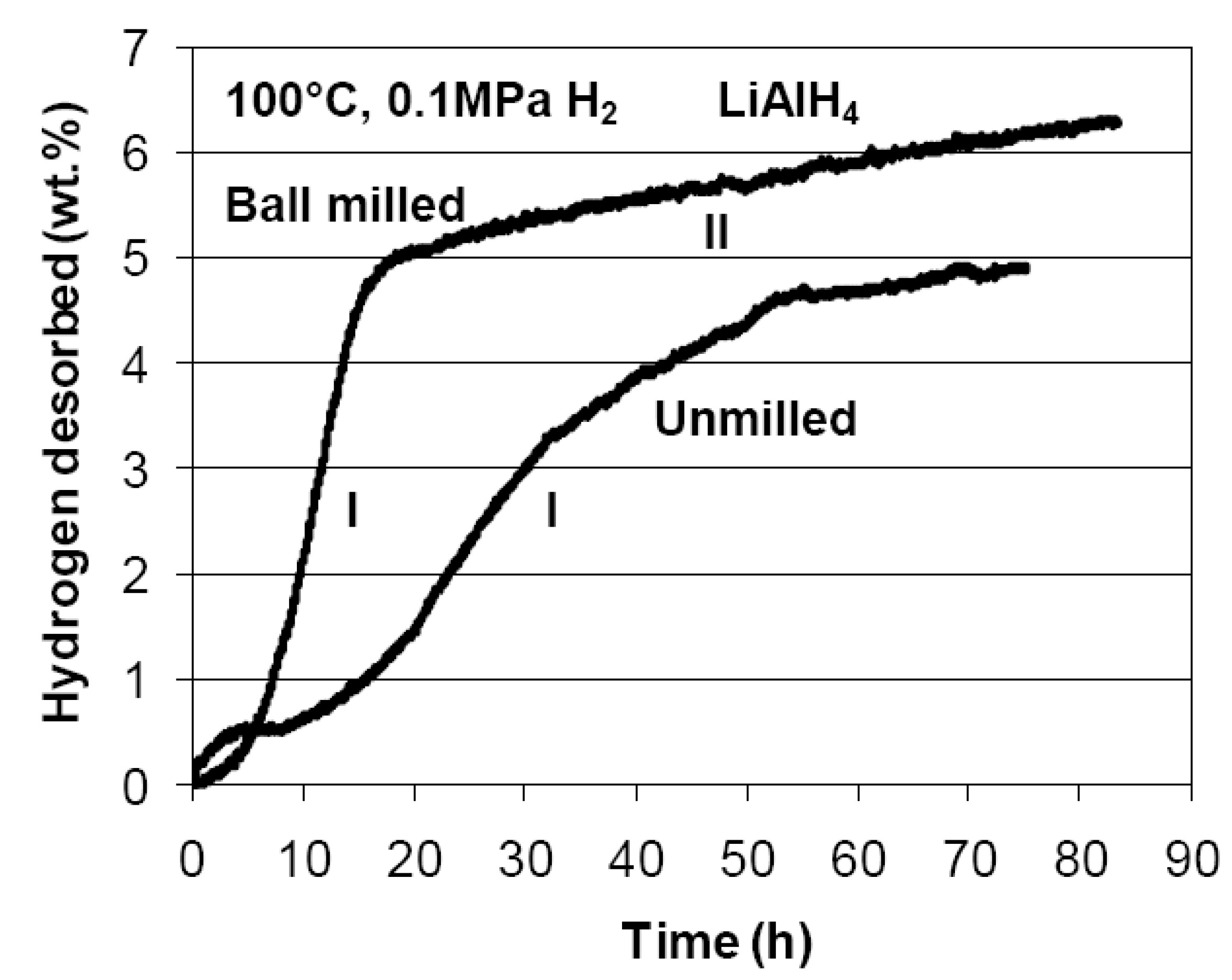
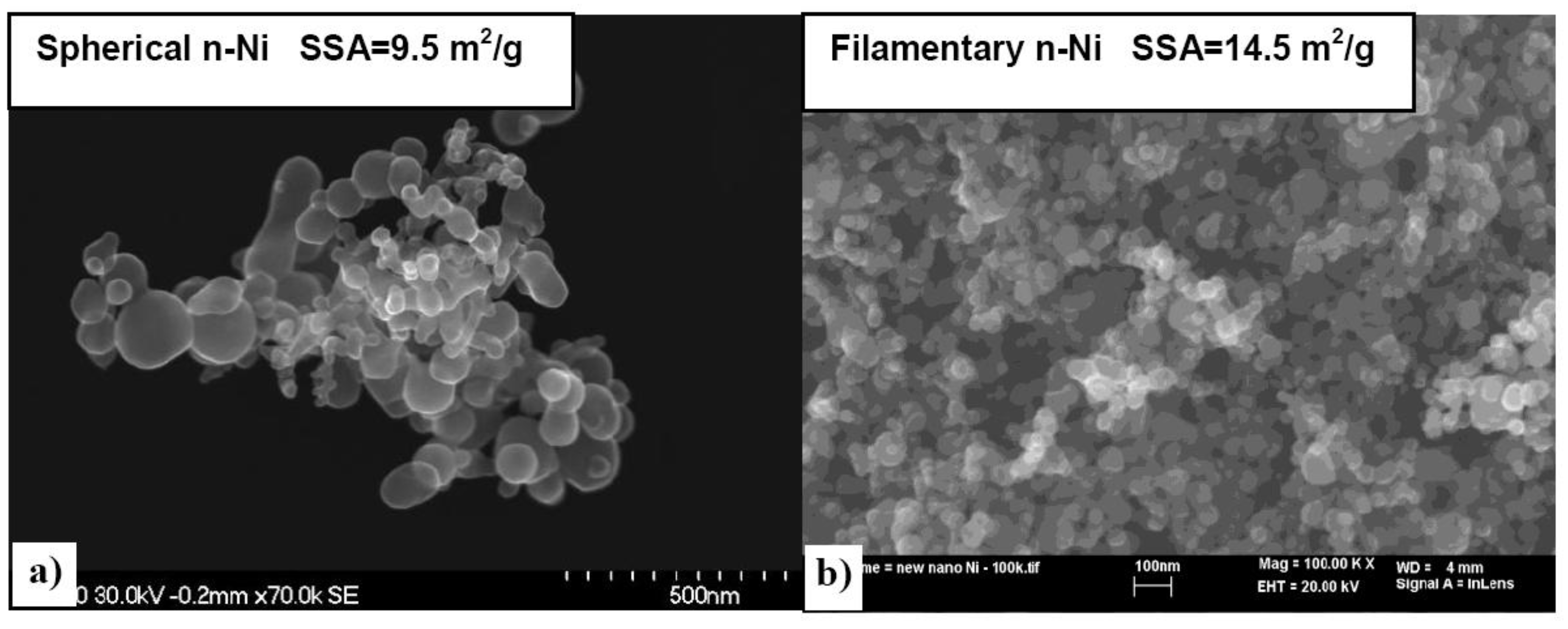
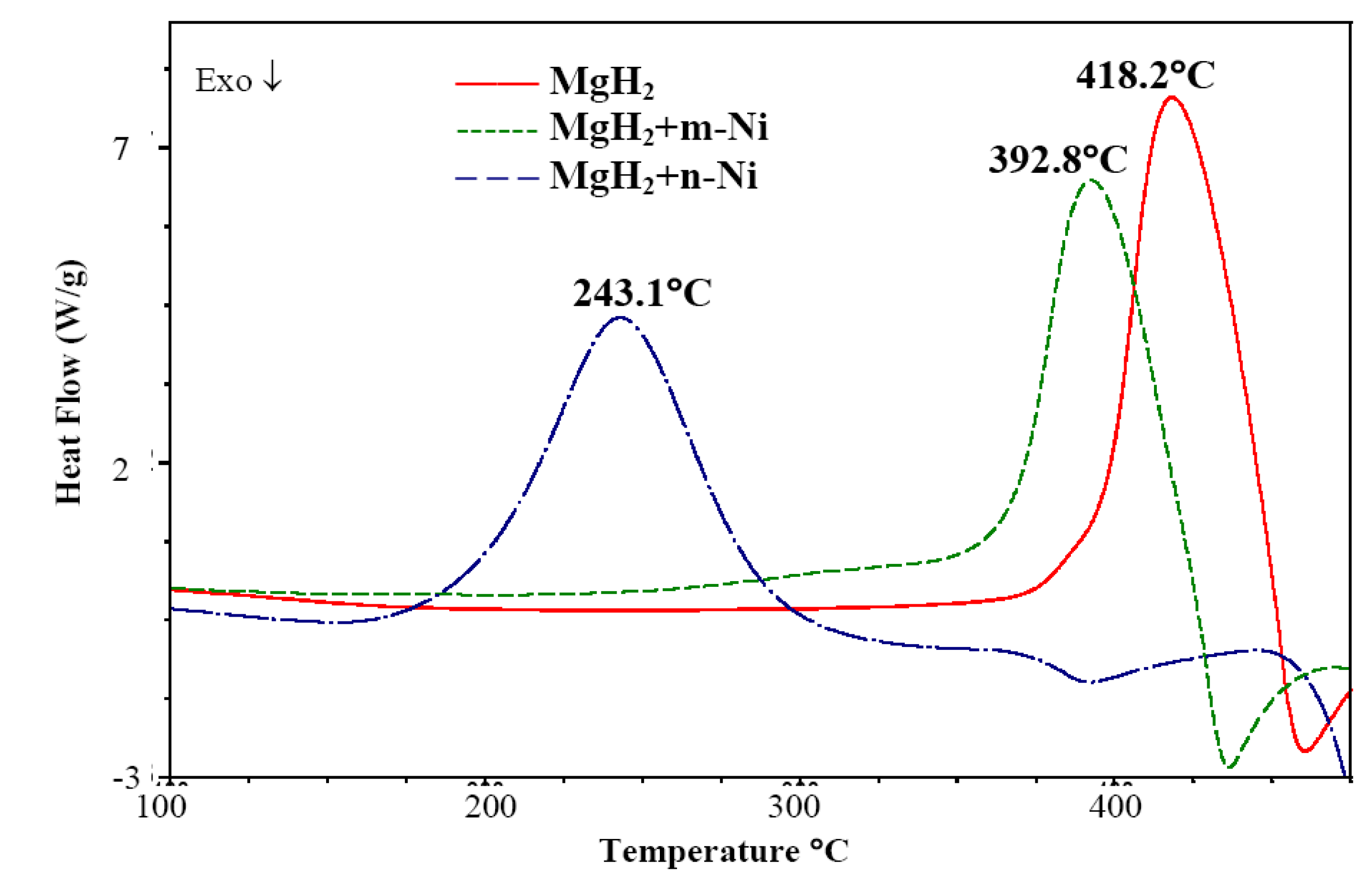
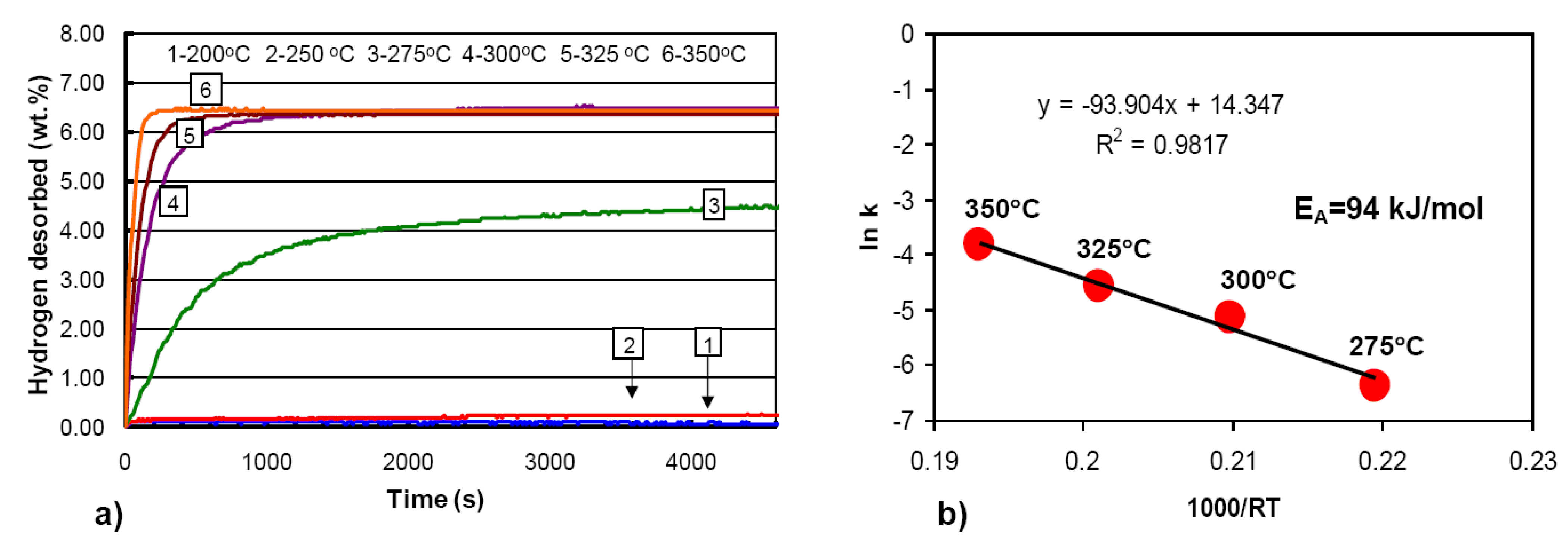
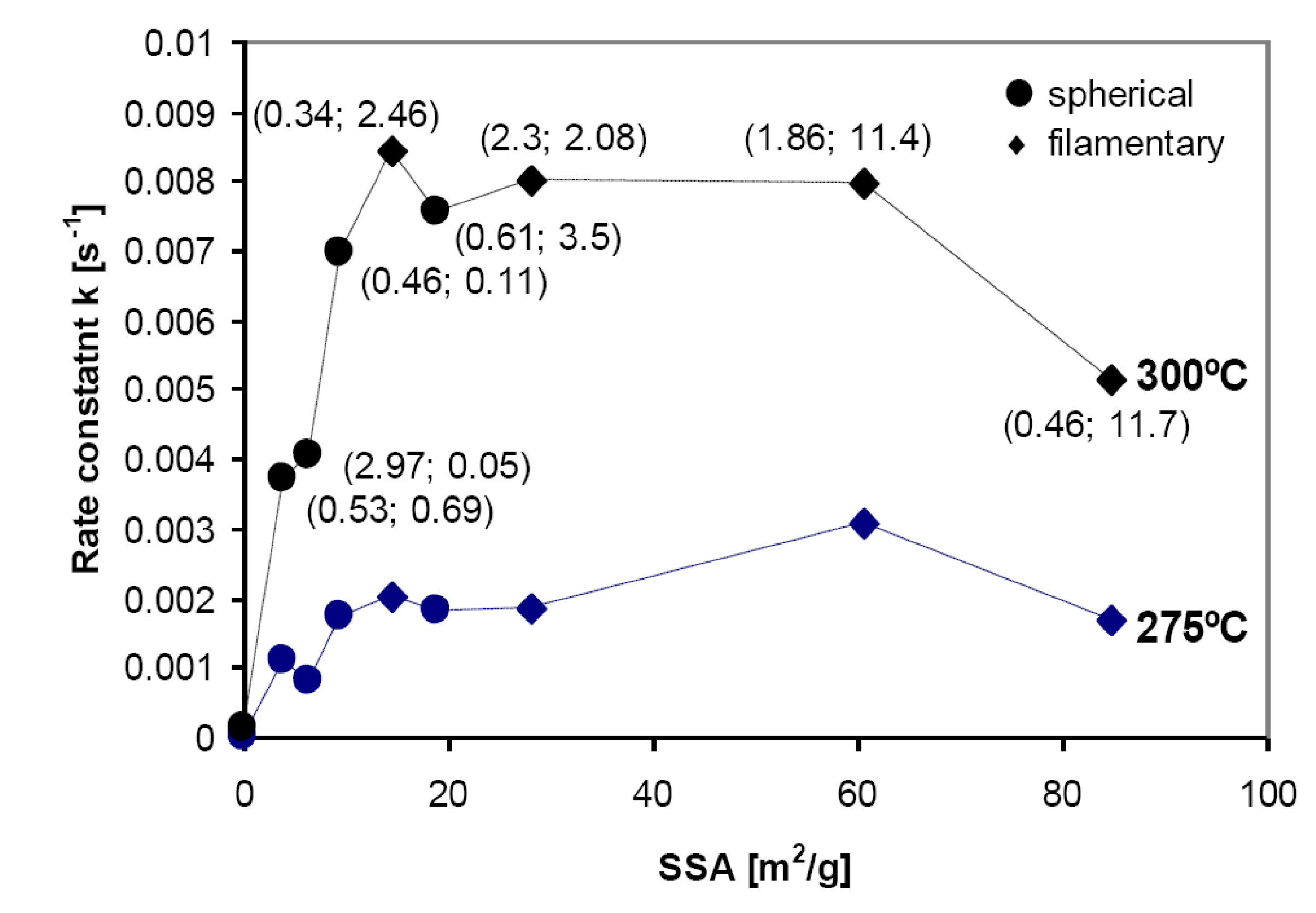
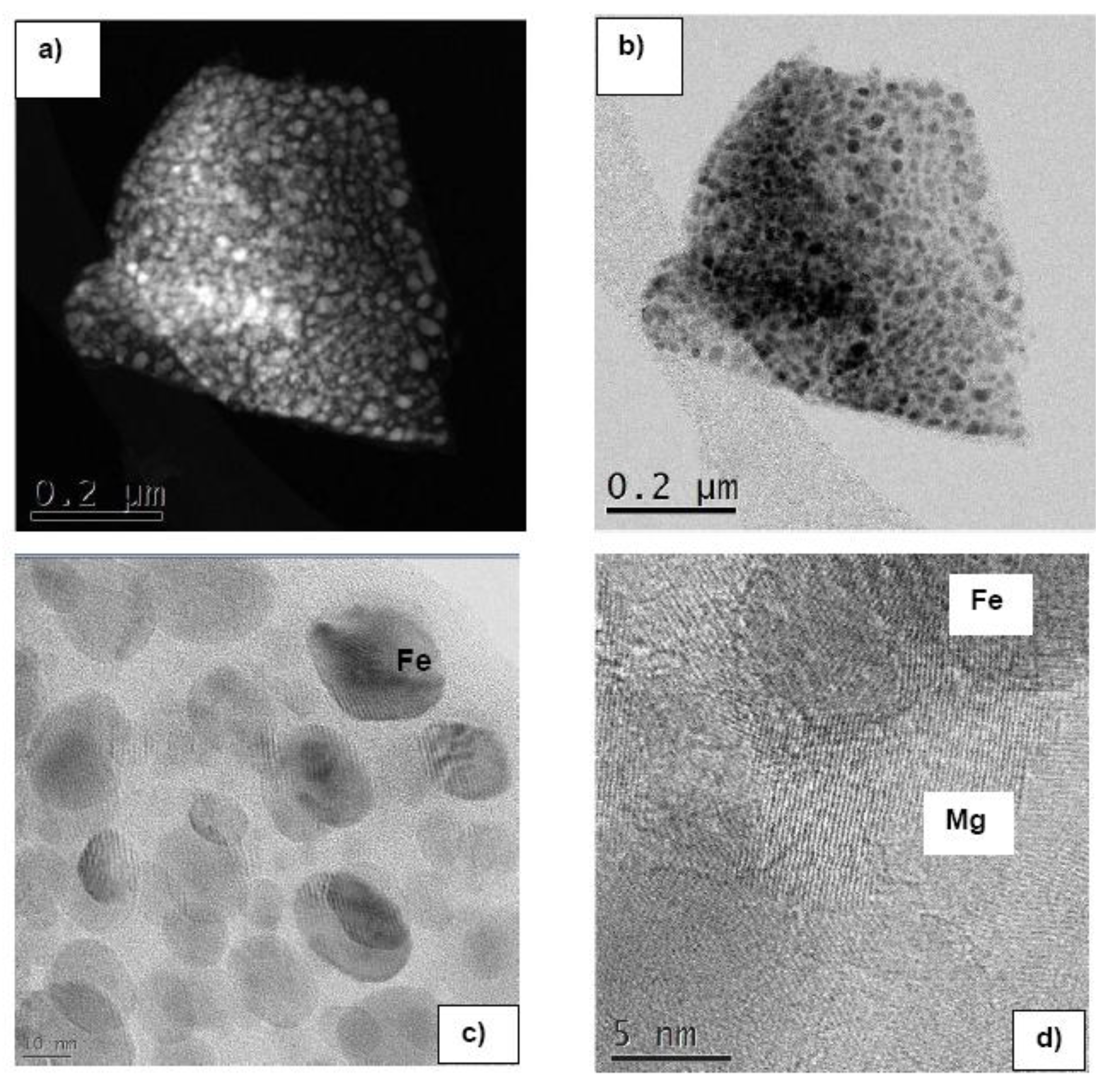
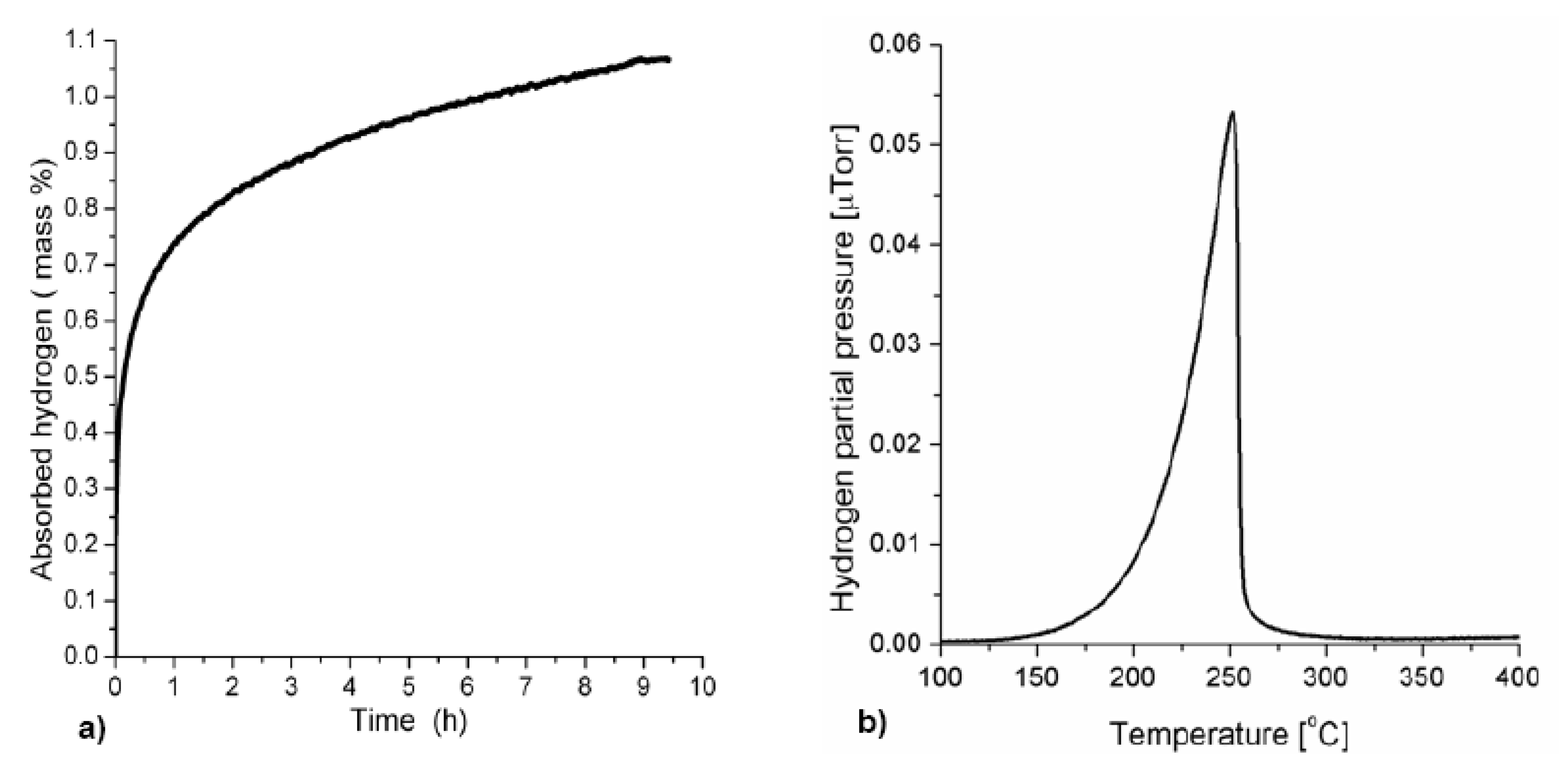
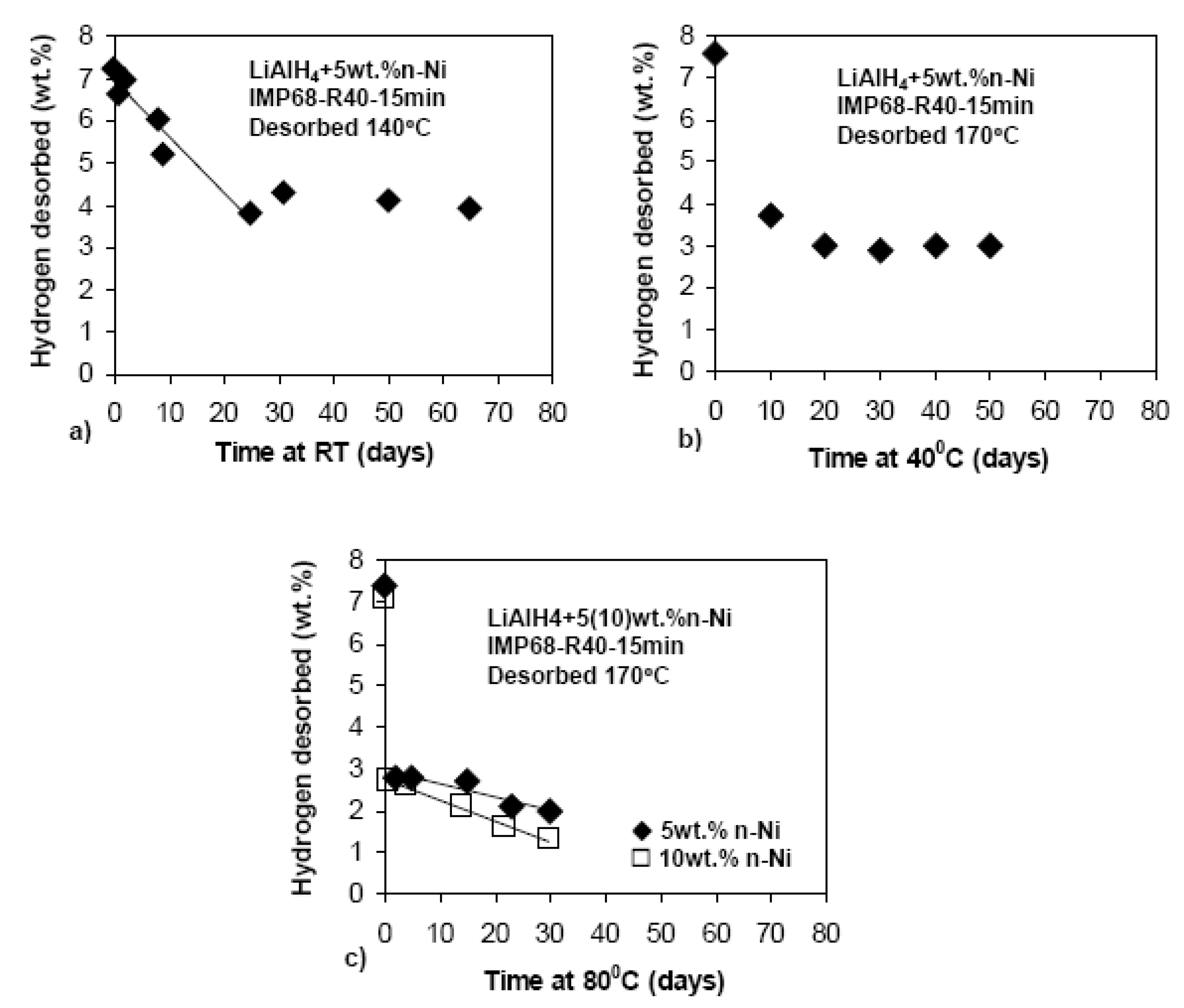
3.1.2. Complex Hydrides
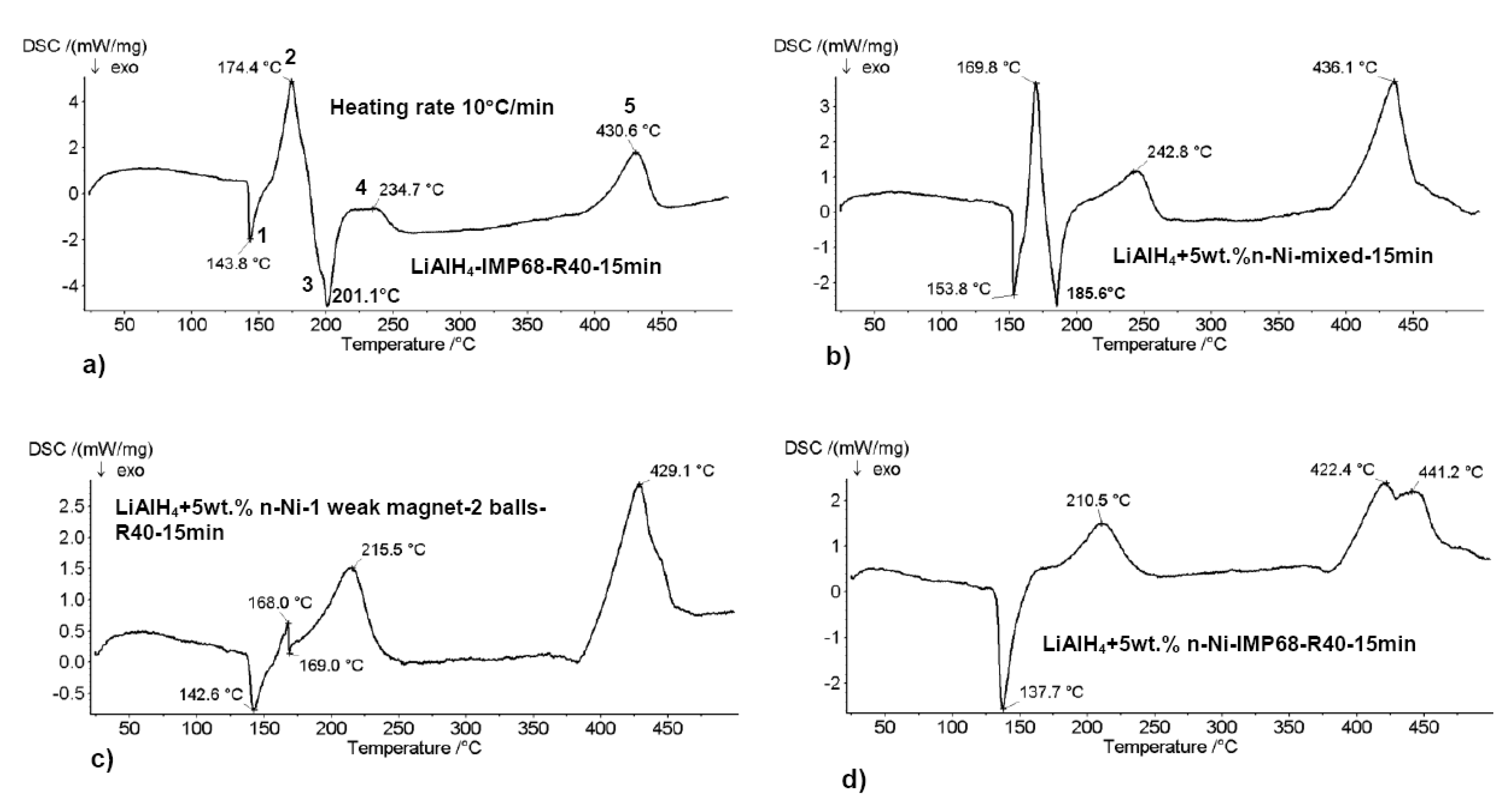
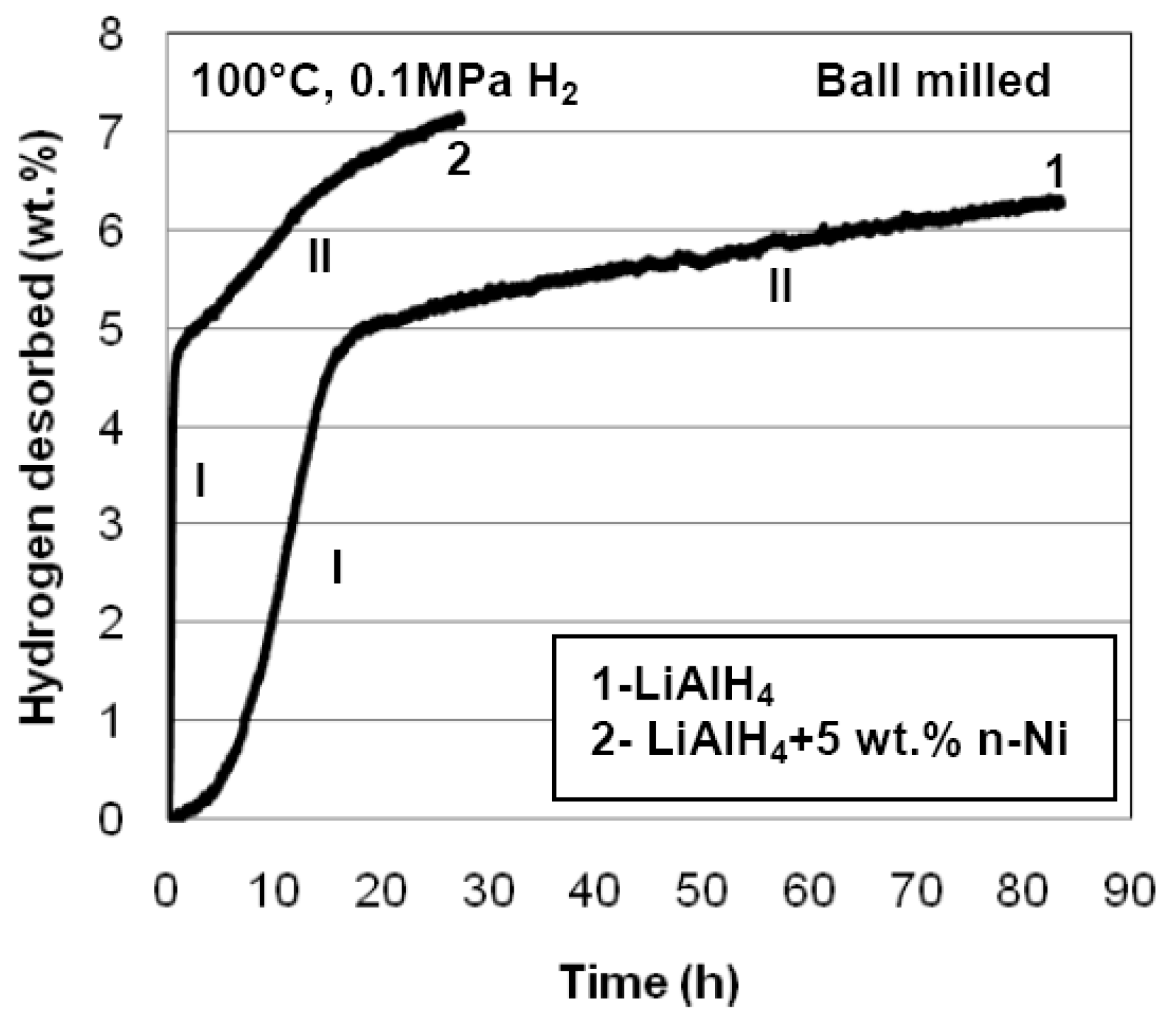
3.2. Nanometric Metal Oxides in MgH2

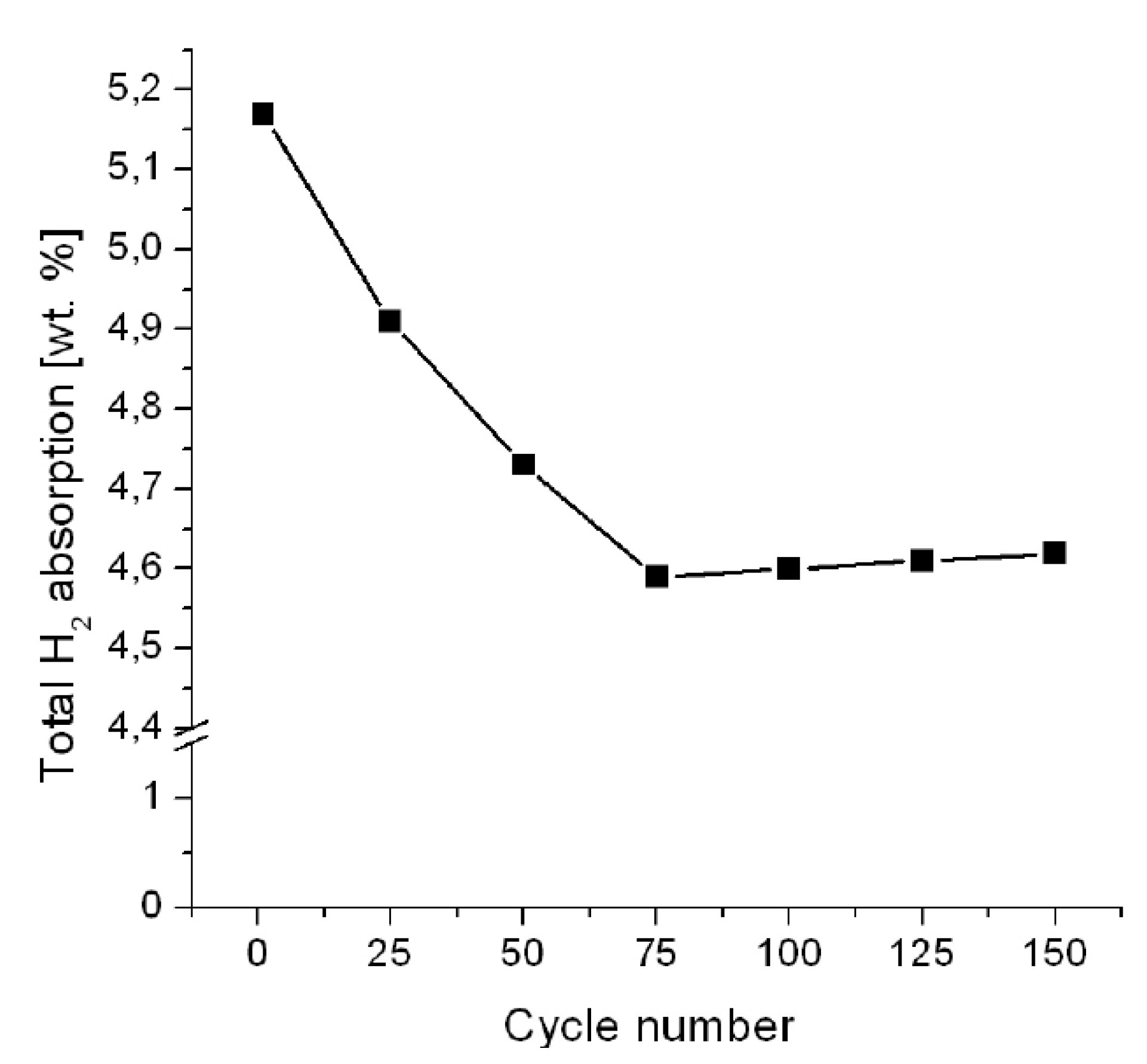
3.3. Metal Halides Catalytic Precursors
3.3.1. MgH2

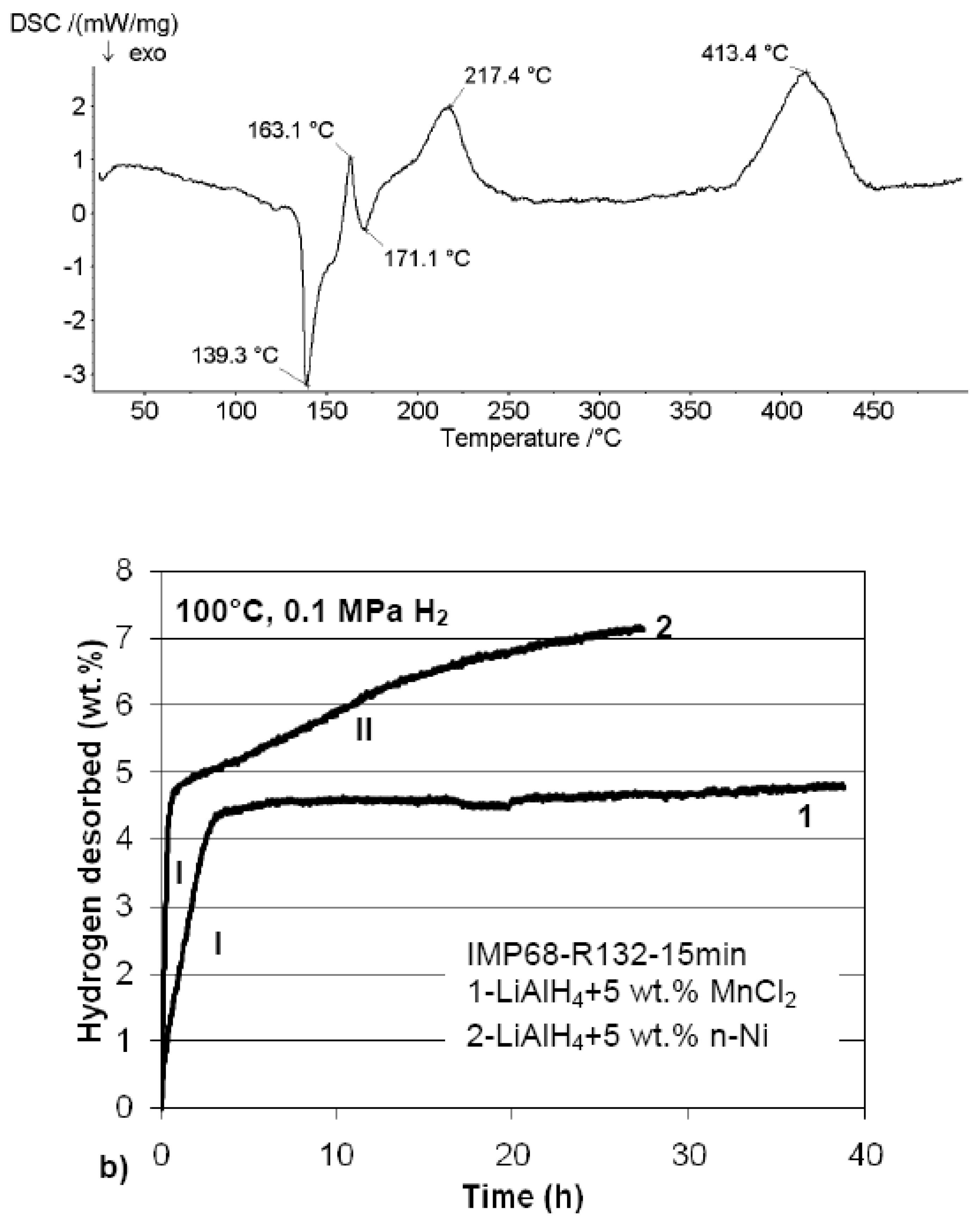
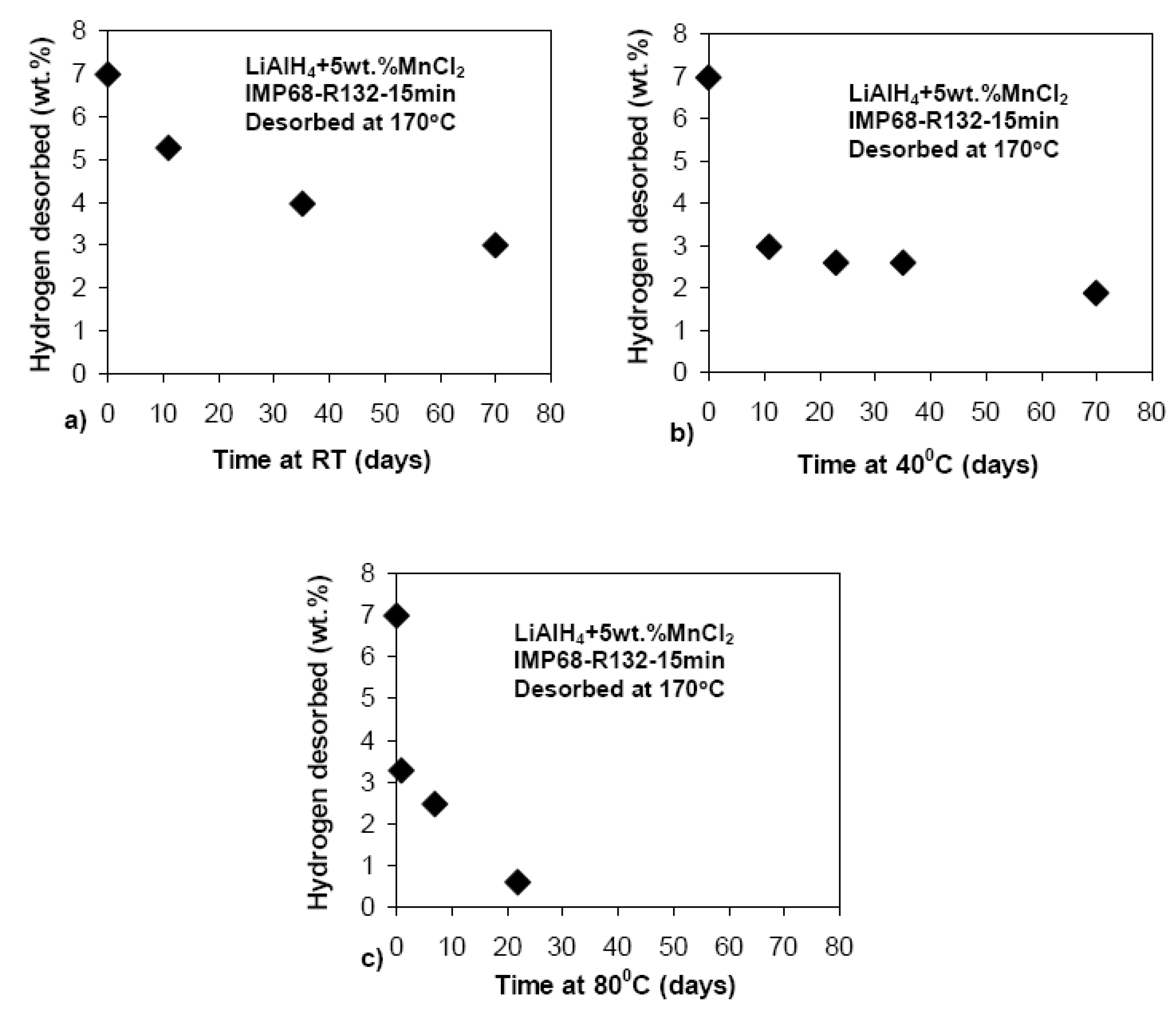
3.3.2. Complex Hydrides
4. Summary and Conclusions
Acknowledgements
References
- Homepage of Nuvera Fuel Cells. Available online: http://www.nuvera.com/products/androm.php (Accessed on 20 December 2010).
- Varin, R.A.; Czujko, T.; Wronski, Z.S. Nanomaterials for Solid State Hydrogen Storage; Springer Science + Business Media: New York, NY, USA, 2009; Chapter 1–3. [Google Scholar]
- Bogdanović, B.; Bohmhammel, K.; Christ, B.; A. Reiser, A.; Schlichte, K.; Vehlen, R.; Wolf, U. Thermodynamic investigation of the magnesium–hydrogen system. J. Alloys Compd. 1999, 282, 84–92. [Google Scholar] [CrossRef]
- Bogdanović, B.; Hofmann, H.; Neuy, A.; Reiser, A.; Schlichte, K.; Spliethoff, B.; Wessel, S. Ni-doped versus undoped Mg–MgH materials for high temperature heat or hydrogen storage. J. Alloys Compd. 1999, 292, 57–71. [Google Scholar] [CrossRef]
- Zaluski, L.; Zaluska, A.; Ström-Olsen, J.O. Nanocrystalline metal hydrides. J. Alloys Compd. 1997, 253–254, 70–79. [Google Scholar]
- Zaluska, A.; Zaluski, L.; Ström-Olsen, J.O. Nanocrystalline magnesium for hydrogen storage. J. Alloys Compd. 1999, 288, 217–225. [Google Scholar] [CrossRef]
- Zaluska, A.; Zaluski, L.; Ström-Olsen, J.O. Structure, catalysis and atomic reactions on the nano-scale: A systematic approach to metal hydrides for hydrogen storage. Appl. Phys. A 2001, 72, 157–165. [Google Scholar] [CrossRef]
- Huot, J.; Liang, G.; Boily, S.; Van Neste, A.; Schulz, R. Structural study and hydrogen sorption kinetics of ball-milled magnesium hydride. J. Alloys Compd. 1999, 293–295, 495–500. [Google Scholar]
- Schulz, R.; Huot, J.; Liang, G.; Boily, S.; Van Neste, A. Structure and hydrogen sorption properties of ball milled Mg dihydride. Mater. Sci. Forum 1999, 312–314, 615–622. [Google Scholar]
- Schulz, R.; Huot, J.; Liang, G.; Boily, S.; Lalande, G.; Denis, M.C.; Dodelet, J.P. Recent developments in the application of nanocrystalline materials to hydrogen technologies. Mater. Sci. Eng. A 1999, 267, 240–245. [Google Scholar] [CrossRef]
- Dornheim, M.; Doppiu, S.; Barkhordarian, G.; Boesenberg, U.; Klassen, T.; Gutfleisch, O.; Bormann, R. Hydrogen storage in magnesium-based hydrides and hydride composites. Scr. Mater. 2007, 56, 841–846. [Google Scholar] [CrossRef]
- Varin, R.A.; Czujko, T.; Wronski, Z. Particle size, grain size and γ-MgH2 effects on the desorption properties of nanocrystalline commercial magnesium hydride processed by controlled mechanical milling. Nanotechnology 2006, 17, 3856–3865. [Google Scholar] [CrossRef]
- Bastide, J.-P.; Bonnetot, B.; Létoffé, J.-M.; Claudy, P. Polymorphisme de l’hydrure de magnesium sous haute pression. Mat. Res. Bull. 1980, 15, 1779–1787. [Google Scholar] [CrossRef]
- Gennari, F.C.; Castro, F.J.; Urretavizcaya, G. Hydrogen desorption behavior from magnesium hydrides synthesized by reactive mechanical alloying. J. Alloys Compd. 2001, 321, 46–53. [Google Scholar] [CrossRef]
- Selvam, P.; Viswanathan, B.; Swamy, C.S.; Srinivasan, V. Magnesium and magnesium alloy hydrides. Int. J. Hyd. Energy 1986, 11, 169–192. [Google Scholar] [CrossRef]
- Barkhordarian, G.; Klassen, T.; Bormann, R. Kinetic investigation of the effect of milling time on the hydrogen sorption reaction of magnesium catalyzed with different Nb2O5 contents. J. Alloys Compd. 2006, 407, 249–255. [Google Scholar] [CrossRef]
- Varin, R.A.; Czujko, T.; Chiu, C.; Wronski, Z. Particle size effects on the desorption properties of nanostructured magnesium dihydride (MgH2) synthesized by controlled reactive mechanical milling (CRMM). J. Alloys Compd. 2006, 424, 356–364. [Google Scholar] [CrossRef]
- Polanski, M.; Bystrzycki, J.; Plocinski, T. The effect of milling conditions on microstructure and hydrogen absorption/desorption properties of magnesium hydride (MgH2) without and with Cr2O3 nanoparticles. Int. J. Hyd. Energy 2008, 33, 1859–1867. [Google Scholar] [CrossRef]
- Varin, R.A.; Jang, M.; Czujko, T.; Wronski, Z.S. The effect of ball milling under hydrogen and argon on the desorption properties of MgH2 covered with a layer of Mg(OH)2. J. Alloys Compd. 2010, 493, L29–L32. [Google Scholar] [CrossRef]
- Varin, R.A.; Zbroniec, L. Decomposition behavior of unmilled and ball milled lithium alanate (LiAlH4) including long-term storage and moisture effects. J. Alloys Compd. 2010, 504, 89–101. [Google Scholar] [CrossRef]
- Varin, R.A.; Zbroniec, L. The effects of nanometric nickel (n-Ni) catalyst on the dehydrogenation and rehydrogenation behavior of ball milled lithium alanate (LiAlH4). J. Alloys Compd. 2010, 506, 928–939. [Google Scholar] [CrossRef]
- Paserin, V.; Baksa, S.; Zaitsev, A.; Shu, J.; Shojai, F.; Nowosiadly, W. Potential for mass production of nickel-based nanomaterials by carbonyl process. J. Nanosci. Nanotechnol. 2008, 8, 4049–4055. [Google Scholar] [CrossRef] [PubMed]
- Varin, R.A.; Czujko, T.; Wronski, Z.S. Thermal stability of Vale Inco nanonometric nickel as a catalytic additive for magnesium hydride (MgH2). Int. J. Hydrogen Ener. 2009, 34, 8603–8610. [Google Scholar] [CrossRef]
- Polanski, M.; Plocinski, T.; Kunce, I.; Bystrzycki, J. Dynamic synthesis of ternary Mg2FeH6. Int. J. Hydrogen Energy 2010, 35, 1257–1266. [Google Scholar] [CrossRef]
- Polanski, M.; Bystrzycki, J.; Varin, R.A.; Plocinski, T. Rapid hydrogenation at 30 °C of magnesium (Mg) and iron (Fe) nanocomposite obtained through a decomposition of Mg2FeH6 precursor. Int. J. Hydrogen Energy 2010, 8. [Google Scholar] [CrossRef]
- Hanada, N.; Ichikawa, T.; Hino, S.; Fujii, H. Remarkable improvement of hydrogen sorption kinetics in magnesium catalyzed with Nb2O5. Int. J. Hydrogen Energy 2006, 420, 46–49. [Google Scholar]
- Varin, R.A.; Li, S.; Wronski, Z.; Morozova, O.; Khomenko, T. The effect of sequential and continuous high-energy impact mode on the mechano-chemical synthesis of nanostructured complex hydride Mg2FeH6. J. Alloys Compd. 2005, 390, 282–96. [Google Scholar] [CrossRef]
- He, Y.; Zhao, Y. Hydrogen storage and cycling properties of a vanadium decorated Mg nanoblade array on a Ti coated Si substrate. Nanotechnology 2009, 20, 204008. [Google Scholar] [CrossRef] [PubMed]
- Block, J.; Gray, A.P. The thermal decomposition of lithium aluminum hydride. Inorg. Chem. 1965, 4, 304–305. [Google Scholar] [CrossRef]
- Polanski, M.; Bystrzycki, J. Comparative studies of the influence of different nano-sized metal oxides on the hydrogen sorption properties of magnesium hydride. J. Alloys Compd. 2009, 486, 697–701. [Google Scholar] [CrossRef]
- Polanski, M.; Bystrzycki, J.; Robert, A.; Varin, R.A.; Plocinski, T.; Pisarek, M. The effect of chromium (III) oxide (Cr2O3) nanopowder on the microstructure and cyclic hydrogen storage behavior of magnesium hydride (MgH2). J. Alloys Compd. 2010, 11. in press. [Google Scholar] [CrossRef]
- Malka, I.E.; Czujko, T.; Bystrzycki, J. Catalytic effect of halide additives ball milled with magnesium hydride. Int. J. Hydrogen Energy 2010, 35, 1706–1712. [Google Scholar] [CrossRef]
- Malka, I.E.; Bystrzycki, J.; Plocinski, T. Microstructure and hydrogen storage capacity of magnesium hydride with zirconium and niobium fluoride additives after cyclic loading. J. Alloys Compd. 2010, 10. in press. [Google Scholar] [CrossRef]
- Varin, R.A.; Zbroniec, L. Fast and slow dehydrogenation of ball milled lithium alanate (LiAlH4) catalyzed with manganese chloride (MnCl2) as compared to nanometric nickel catalyst. J. Alloys Compd. 2010, 09. in press. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Varin, R.A.; Zbroniec, L.; Polanski, M.; Bystrzycki, J. A Review of Recent Advances on the Effects of Microstructural Refinement and Nano-Catalytic Additives on the Hydrogen Storage Properties of Metal and Complex Hydrides. Energies 2011, 4, 1-25. https://doi.org/10.3390/en4010001
Varin RA, Zbroniec L, Polanski M, Bystrzycki J. A Review of Recent Advances on the Effects of Microstructural Refinement and Nano-Catalytic Additives on the Hydrogen Storage Properties of Metal and Complex Hydrides. Energies. 2011; 4(1):1-25. https://doi.org/10.3390/en4010001
Chicago/Turabian StyleVarin, Robert A., Leszek Zbroniec, Marek Polanski, and Jerzy Bystrzycki. 2011. "A Review of Recent Advances on the Effects of Microstructural Refinement and Nano-Catalytic Additives on the Hydrogen Storage Properties of Metal and Complex Hydrides" Energies 4, no. 1: 1-25. https://doi.org/10.3390/en4010001



