Study of NO and CO Formation Pathways in Jet Flames with CH4/H2 Fuel Blends
Abstract
1. Introduction
2. Numerical Simulation
2.1. Computational Model
2.2. Chemical Reaction Network Model
2.3. NO and CO Mechanisms
3. Results and Discussion
3.1. Effect of Hydrogen Content on NO and CO Production in MILD Flames
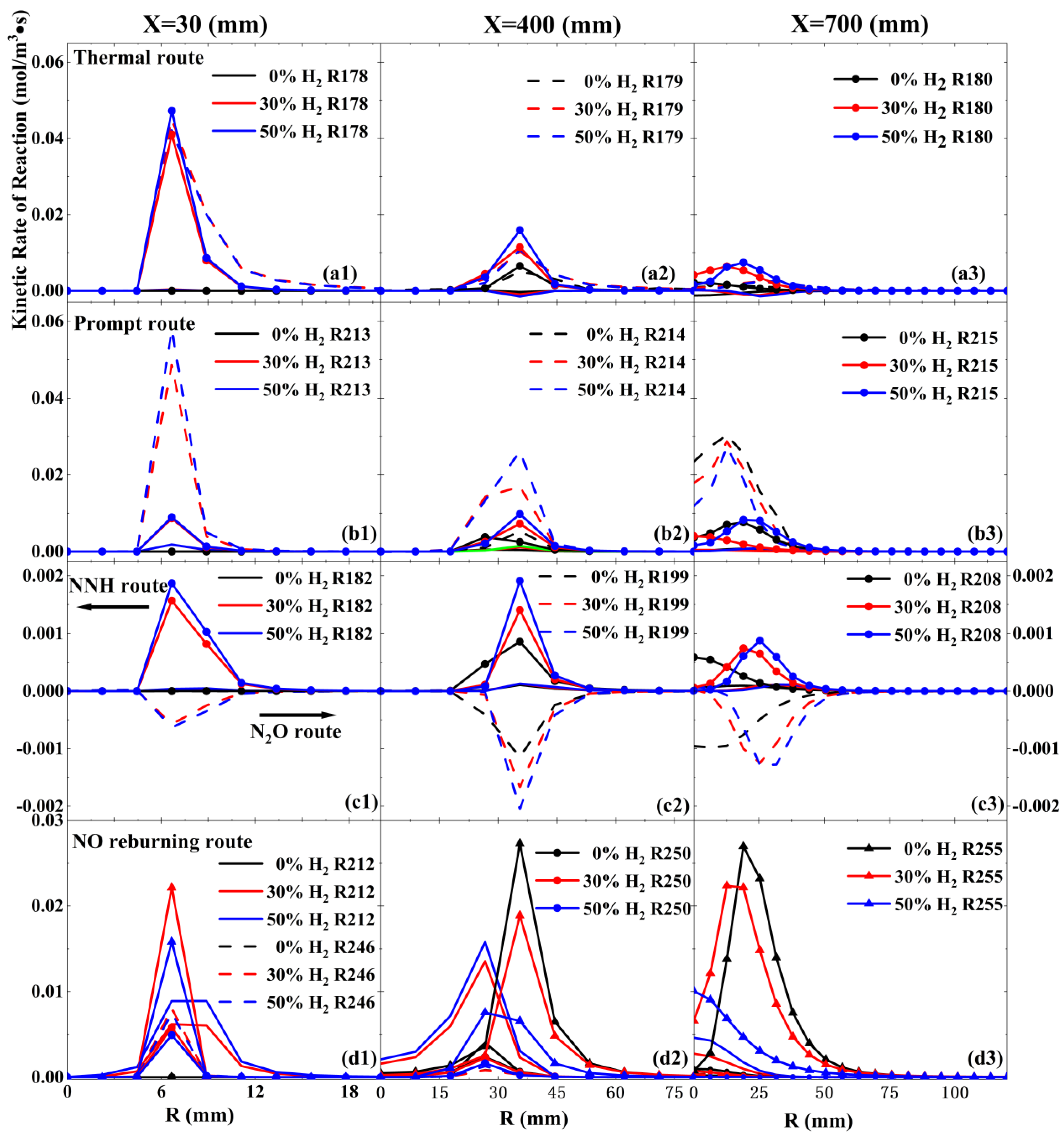
3.2. Effect of Hydrogen Content on the Pathway of NO Production
3.3. Effect of Hydrogen Content on the Pathway of CO Production
4. Conclusions
- (1)
- As the hydrogen content rises, the reaction rate of the NO-reburning pathway via CHi=1–3 tends to decrease. Conversely, the reaction rates for the thermal, NNH, and N2O increase, leading to a larger area for primary NO production and a smaller area for primary NO consumption in the JHC flame. This change results in higher NO production near the burner, with the peak NO concentration increasing from 33.3 ppm to 56.6 ppm.
- (2)
- In JHC flames, the reaction rate related to CO production varies across different areas and is not significant. The reaction OH + CO ⇔ H + CO2 is the primary one, and its rate increases with higher hydrogen levels. This boost in reaction rate speeds up the transformation of CO into CO2, resulting in reduced CO production.
- (3)
- The addition of hydrogen reduces the influence of the prompt pathway, the primary route for NO production, while boosting the roles of the thermal pathway and the N2O pathway in NO production. Even though the reaction rate of the NO-reburning pathway via H radicals tripled with 50% hydrogen content, However, the rate of the NO-reburning pathway dominated by the via CHi=1–3 decreases, ultimately leading to a decrease in the amount of NO consumed through NO-reburning.
- (4)
- In MILD combustion, the primary source of CO is the reaction CH2(S) + CO2 ⇔ CO + CH2O. The introduction of hydrogen reduces all reactions that produce CO while simultaneously increasing the CO oxidation reaction OH + CO ⇔ H + CO2. This inhibition of CO production and enhancement of CO oxidation is the key factor contributing to the reduction of CO levels in hydrogen-doped MILD combustion.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| Acronyms | |
| MILD | Moderate or intense low oxygen dilution |
| JHC | Jet in a hot coflow |
| CFD | Computational fluid dynamics |
| CRN | Chemical reaction network |
| PSR | Perfectly stirred reactor |
| Da | Damköhler number |
| EDC | Eddy dissipation concept |
| Re | Reynolds number |
| CHBR | Closed Homogeneous Batch Reactor |
| EINO | NO emissions index |
| Symbols | |
| C1ε | Constant of k-ε model |
| Cτ | Timescale constant of EDC model |
| Yi | Mass fraction of species i |
| Tf | Temperature of fuel jet |
| Tc | Temperature of coflow jet |
| Vc | Coflow jet velocity |
| Vf | Fuel jet velocity |
| XH2 | Hydrogen volume fraction in the fuel |
| XCH4 | Methane volume fraction in the fuel |
| Tin | Inlet temperature |
| Tsi | Self-ignition temperature of reactants |
| Tmax | Maximum temperature |
| ∆Tmax | Maximum temperature rise, ∆Tmax = Tmax − Tin |
| MWj | Molecular weigh |
| Greek letters | |
| τf | Turbulent flow time scale |
| τr | Reaction time scale |
| ξ | Characteristic length fraction in EDC model |
References
- Reed, R.J. North American Combustion Handbook; North American Manufacturing Company: Scranton, PA, USA, 2014. [Google Scholar]
- El-Ghafour, S.A.A.; El-dein, A.H.E.; Aref, A.A.R. Combustion Characteristics of Natural Gas–Hydrogen Hybrid Fuel Turbulent Diffusion Flame. Int. J. Hydrogen Energy 2010, 35, 2556–2565. [Google Scholar] [CrossRef]
- Schefer, R.W.; Wicksall, D.M.; Agrawal, A.K. Combustion of Hydrogen-Enriched Methane in a Lean Premixed Swirl-Stabilized Burner. Proc. Combust. Inst. 2002, 29, 843–851. [Google Scholar] [CrossRef]
- Mardani, A.; Tabejamaat, S. Effect of Hydrogen on Hydrogen–Methane Turbulent Non-Premixed Flame under MILD Condition. Int. J. Hydrogen Energy 2010, 35, 11324–11331. [Google Scholar] [CrossRef]
- Wünning, J.A.; Wünning, J.G. Flameless Oxidation to Reduce Thermal No-Formation. Prog. Energy Combust. 1997, 23, 81–94. [Google Scholar] [CrossRef]
- Dally, B.B.; Shim, S.H.; Craig, R.A.; Ashman, P.J.; Szegö, G. On the Burning of Sawdust in a MILD Combustion Furnace. Energy Fuels 2010, 24, 3462–3470. [Google Scholar] [CrossRef]
- Szegö, G.G.; Dally, B.B.; Nathan, G.J. Operational Characteristics of a Parallel Jet MILD Combustion Burner System. Combust. Flame 2009, 156, 429–438. [Google Scholar] [CrossRef]
- Li, P.F.; Mi, J.C.; Dally, B.B.; Wang, F.F.; Wang, L.; Liu, Z.H.; Chen, S.; Zheng, C.G. Progress and Recent Trend in MILD Combustion. Sci. China Technol. Sci. 2011, 54, 255–269. [Google Scholar] [CrossRef]
- Tsuji, H.; Gupta, A.; Hasegawa, T.; Katsuki, M.; Kishimoto, K.; Morita, M. High Temperature Air Combustion: From Energy Conservation to Pollution Reduction; CRC Press: Boca Raton, FL, USA, 2002; p. 402. [Google Scholar] [CrossRef]
- Cavaliere, A.; Joannon, M. Mild Combustion. Prog. Energy Combust. 2004, 30, 329–366. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, G.F.; Lin, Q.Z.; Ma, C.B.; Xing, X.J. Flameless combustion for hydrogen containing fuels. Int. J. Hydrogen Energy 2010, 35, 2694–2697. [Google Scholar] [CrossRef]
- Ayoub, M.; Rottier, C.; Carpentier, S.; Villermaux, C.; Boukhalfa, A.M.; Honoré, D. An experimental study of mild flameless combustion of methane/hydrogen mixtures. Int. J. Hydrogen Energy 2012, 37, 6912–6921. [Google Scholar] [CrossRef]
- Fabián, E.C.A.; Julian, E.O.A.; Andrés, A.A.A. Emissions and dynamic stability of the flameless combustion regime using hydrogen blends with natural gas. Int. J. Hydrogen Energy 2021, 46, 1246–1258. [Google Scholar] [CrossRef]
- Marco, D.; Alessandro, V.; Renato, R. Sustainability of mild combustion of hydrogen-containing hybrid fuels. Proc. Combust. Inst. 2007, 31, 3393–3400. [Google Scholar] [CrossRef]
- Markus, M.; Michael, K.; Peter, S.; Hadi, B.; Rene, P.; Christoph, H. MILD combustion of hydrogen and air—An efficient modelling approach in CFD validated by experimental data. Int. J. Hydrogen Energy 2022, 47, 6349–6364. [Google Scholar] [CrossRef]
- Amin, K.; Ali, S.Z.; Mobin, M.; Seyyed, B.N.N.; Vahid, E. Effect of Hydrogen Enrichment on Pollutant and Greenhouse Gases Formation and Exergy Efficiency of Methane MILD Combustion. In Environmentally-Benign Energy Solutions; Springer: Cham, Switzerland, 2019; pp. 403–429. [Google Scholar] [CrossRef]
- Dally, B.B.; Karpetis, A.N.; Barlow, R.S. Structure of Turbulent Non-Premixed Jet Flames in a Diluted Hot Coflow. Proc. Combust. Inst. 2002, 29, 1147–1154. [Google Scholar] [CrossRef]
- Paul, R.M.; Dally, B.B. Effect of fuel composition on jet flames in a heated and diluted oxidant stream. Combust. Flame 2012, 159, 3138–3145. [Google Scholar] [CrossRef]
- Mendez, L.D.A.; Tummers, M.J.; Veen, E.H.; Roekaerts, D.J.E.M. Effect of hydrogen addition on the structure of natural-gas jet-in-hot-coflow flames. Proc. Combust. Inst. 2015, 35, 3557–3564. [Google Scholar] [CrossRef]
- Gao, X.; Duan, F.; Lim, S.C.; Yip, M.S. NOx formation in hydrogen–methane turbulent diffusion flame under the moderate or intense low-oxygen dilution conditions. Energy 2013, 59, 559–569. [Google Scholar] [CrossRef]
- Liao, H.H.; Hu, F.; Wu, X.Y.; Li, P.F.; Ding, C.J.; Yang, C.; Zhang, T.; Liu, Z.H. Effects of H2 addition on the characteristics of the reaction zone and NOx mechanisms in MILD combustion of H2-rich fuels. Int. J. Hydrogen Energy 2024, 58, 174–189. [Google Scholar] [CrossRef]
- Tu, Y.J.; Liu, O.H.; Yang, W.M. Flame Characteristics of CH4/H2 on a Jet-in-Hot-Coflow Burner Diluted by N2, CO2, and H2O. Energy Fuels 2017, 31, 3270–3280. [Google Scholar] [CrossRef]
- Jiang, X.D.; Li, P.F.; Guo, J.J.; Hu, F.; Wang, F.; Mi, J.; Liu, Z. Detailed investigation of NO mechanism in non-premixed oxy-fuel jet flames with CH4/H2 fuel blends. Int. J. Hydrogen Energy 2018, 43, 8534–8557. [Google Scholar] [CrossRef]
- Amir, M.; Hamed, K.M.M. Hydrogen enrichment of methane and syngas for MILD combustion. Int. J. Hydrogen Energy 2019, 44, 9423–9437. [Google Scholar] [CrossRef]
- Zhao, Z.H.; Zhang, Z.W.; Zha, X.J.; Gao, G.; Mao, W.C.; Wu, F.; Li, X.S.; Luo, C.; Zhang, L.Q. Fuel-NO formation mechanism in MILD-oxy combustion of CH4/NH3 fuel blend. Fuel 2023, 331, 125817. [Google Scholar] [CrossRef]
- Li, P.; Wang, F.; Mi, J.; Dally, B.B.; Mei, Z.; Zhang, J.; Parente, A. Mechanisms of NO formation in MILD combustion of CH4/H2 fuel blends. Int. J. Hydrogen Energy 2014, 39, 19187–19203. [Google Scholar] [CrossRef]
- Ali, G.; Zhang, T.Y.; Wu, W.D.; Zhou, Y.G. Effect of hydrogen addition on NOx formation mechanism and pathways in MILD combustion of H2-rich low calorific value fuels. Int. J. Hydrogen Energy 2020, 45, 9200–9210. [Google Scholar] [CrossRef]
- Pan, H.J.T.; Geng, S.J.; Yang, H.; Zhang, G.H.; Bian, H.; Liu, Y.H. Influence of H2 blending on NOx production in natural gas combustion: Mechanism comparison and reaction routes. Int. J. Hydrogen Energy 2023, 48, 784–797. [Google Scholar] [CrossRef]
- Park, J.; Kim, D.; Lee, Y. Experimental study on flameless combustion and NO emission with hydrogen-containing fuels. Int. J. Energy Res. 2022, 46, 2512–2528. [Google Scholar] [CrossRef]
- Mehmet, S.C. Flameless combustion investigation of CH4/H2 in the laboratory-scaled furnace. Int. J. Hydrogen Energy 2020, 45, 35208–35222. [Google Scholar] [CrossRef]
- Esmaeil, E.F.; Fateme, A.B. NO emission characteristics for the HTC and MILD combustion regimes with N2, N2/CO2 and CO2 diluents: Effect of H2 addition to CH4. Int. J. Hydrogen Energy 2023, 48, 36185–36210. [Google Scholar] [CrossRef]
- Xu, S.T.; Tong, Y.H.; Jin, S.C.; Ren, H.; Tu, Y.J.; Zhang, S.H.; Liu, H. NO formation and reduction during methane/hydrogen MILD combustion over a wide range of hydrogen-blending ratios in a well-stirred reactor. Fuel 2023, 346, 128324. [Google Scholar] [CrossRef]
- Xu, S.T.; Tian, Z.Y.; Tian, S.J.; Xi, L.Y.; Tu, Y.J.; Liu, H. Sustainability of hydrogen-enriched methane MILD combustion over a wide range of hydrogen-blending ratios in a strongly heat-extracted well-stirred reactor. Fuel 2024, 357, 130035. [Google Scholar] [CrossRef]
- Xu, S.T.; Tian, Z.Y.; Chen, Y.X.; Liang, S.Y.; Tu, Y.J.; Liu, H. Effect of hydrogen-blending ratio and wall temperature on establishment, NO formation, and heat transfer of hydrogen-enriched methane MILD combustion. Fuel 2024, 369, 131787. [Google Scholar] [CrossRef]
- Wang, H.T.; Tong, Z.; Zhou, G.J.; Zhang, C.; Zhou, H.Y.; Wang, Y.; Zheng, W.Y. Research and demonstration on hydrogen compatibility of pipelines: A review of current status and challenges. Int. J. Hydrogen Energy 2022, 47, 28585–28604. [Google Scholar] [CrossRef]
- Cheong, K.P.; Li, P.F.; Wang, F.F.; Mi, J.C. Emissions of NO and CO from counterflow combustion of CH4 under MILD and oxyfuel conditions. Energy 2017, 124, 652–664. [Google Scholar] [CrossRef]
- Zhu, Z.R.; Xiong, Y.; Zheng, X.L.; Chen, W.J.; Ren, B.H.; Xiao, Y.H. Experimental and Numerical Study of the Effect of Fuel/Air Mixing Modes on NOx and CO Emissions of MILD Combustion in a Boiler Burner. J. Therm. Sci. 2021, 30, 656–667. [Google Scholar] [CrossRef]
- He, Y.Z.; Zou, C.; Song, Y.; Luo, J.H.; Jia, H.Q.; Chen, W.Z.; Zheng, J.M.; Zheng, C.G. Comparison of the characteristics and mechanism of CO formation in O2/N2, O2/CO2 and O2/H2O atmospheres. Energy 2017, 141, 1429–1438. [Google Scholar] [CrossRef]
- Zou, C.; Cao, S.Y.; Song, Y.; He, Y.Z.; Guo, F.; Zheng, C.G. Characteristics and mechanistic analysis of CO formation in MILD regime with simultaneously diluted and preheated oxidant and fuel. Fuel 2014, 130, 10–18. [Google Scholar] [CrossRef]
- Amir, M.; Sadegh, T.; Shahla, H. Numerical study of CO and CO2 formation in CH4/H2 blended flame under MILD condition. Combust. Flame 2013, 160, 1636–1649. [Google Scholar] [CrossRef]
- Wang, F.; Mi, J.; Li, P.; Zheng, C. Diffusion Flame of a CH4/H2 Jet in Hot Low-Oxygen Coflow. Int. J. Hydrogen Energy 2011, 36, 9267–9277. [Google Scholar] [CrossRef]
- Mei, Z.; Mi, J.; Wang, F.; Zheng, C. Dimensions of CH4-Jet Flame in Hot O2/CO2 Coflow. Energy Fuels 2012, 26, 3257–3266. [Google Scholar] [CrossRef]
- Christo, F.C.; Dally, B.B. Modelling turbulent reacting jets issuing into a hot and diluted coflow. Combust. Flame 2005, 142, 117–129. [Google Scholar] [CrossRef]
- Li, P.; Wang, F.; Mi, J.; Dally, B.B.; Mei, Z. Mild combustion under different premixing patterns and characteristics of the reaction regime. Energy Fuel 2014, 28, 2211–2226. [Google Scholar] [CrossRef]
- Aanjaneya, K.; Cao, W.; Chen, Y.; Borgnakke, C.; Atreya, A. A numerical study of confined turbulent jets for homogeneous combustion with oxygen enrichment. Fuel 2020, 261, 116449. [Google Scholar] [CrossRef]
- Azarinia, A.; Mahdavy-Moghaddam, H. Comprehensive numerical study of molecular diffusion effects and eddy dissipation concept model in MILD combustion. Int. J. Hydrogen Energy 2021, 46, 2952–2965. [Google Scholar] [CrossRef]
- Mardani, A. Optimization of the Eddy Dissipation Concept (EDC) Model for Turbulence-Chemistry Interactions under Hot Diluted Combustion of CH4/H2. Fuel 2017, 191, 114–129. [Google Scholar] [CrossRef]
- Parente, A.; Malik, M.R.; Contino, F.; Cuoci, A.; Dally, B.B. Extension of the eddy dissipation concept for turbulence/chemistry interactions to MILD combustion. Fuel 2016, 163, 98–111. [Google Scholar] [CrossRef]
- Gran, I.R.; Magnussen, B.F. A numerical study of a bluff-body stabilized diffusion flame. Part 2. Influence of combustion modeling and finite-rate chemistry. Combust. Sci. Technol. 1996, 119, 191–217. [Google Scholar] [CrossRef]
- Guo, J.; Li, X.; Huang, X.; Liu, Z.; Zheng, C. A Full Spectrum K-Distribution Based Weighted-Sum-of-Gray-Gases Model for Oxy-Fuel Combustion. Int. J. Heat Mass Transf. 2015, 90, 218–226. [Google Scholar] [CrossRef]
- Li, P.F.; Mi, J.C. Influence of Inlet Dilution of Reactants on Premixed Combustion in a Recuperative Furnace. Flow Turbul. Combust. 2011, 87, 617–638. [Google Scholar] [CrossRef]
- Shu, Z.; Dai, C.; Li, P.; Mi, J. Nitric Oxide of MILD Combustion of a Methane Jet Flame in Hot Oxidizer Coflow: Its Formations and Emissions under H2O, CO2, and N2 Dilutions. Fuel 2018, 234, 567–580. [Google Scholar] [CrossRef]
- Beér, J.M. Minimizing NOx Emissions from Stationary Combustion; Reaction Engineering Methodology. Chem. Eng. Sci. 1994, 49, 4067–4083. [Google Scholar] [CrossRef]
- Skottene, M.; Rian, K.E. A Study of NOx Formation in Hydrogen Flames. Int. J. Hydrogen Energy 2007, 32, 3572–3585. [Google Scholar] [CrossRef]
- Turns, S.R. An Introduction to Combustion; McGraw-Hill: New York, NY, USA, 1996. [Google Scholar]
- Bozzelli, J.W.; Dean, A.M. O + NNH: A Possible New Route for NOX Formation in Flames. Int. J. Chem. Kinet. 1995, 27, 1097–1109. [Google Scholar] [CrossRef]
- Myerson, A.L.; Taylor, F.R.; Faunce, B.G. Ignition limits and products of the multistage flames of propane-nitrogen dioxide mixtures. Symp. (Int.) Combust. 1957, 6, 154–163. [Google Scholar] [CrossRef]
- Dagaut, P.; Lecomte, F. Experiments and Kinetic Modeling Study of NO-Reburning by Gases from Biomass Pyrolysis in a JSR. Energy Fuels 2003, 17, 608–613. [Google Scholar] [CrossRef]
- Glarborg, P.; Kristensen, P.G.; Dam-Johansen, K.; Alzueta, M.; Millera, A.; Bilbao, R. Nitric oxide reduction by non-hydrocarbon fuels. Implications for reburning with gasification gases. Energy Fuels 2000, 14, 828–838. [Google Scholar] [CrossRef]
- Xu, S.T.; Jin, S.C.; Tong, Y.H.; Shi, B.; Tu, Y.J.; Liu, H. Quantitative Evaluation of NO Formation and Destruction Routes during Methane MILD Combustion Using an Improved Calculation Method. Fuel 2022, 324, 124593. [Google Scholar] [CrossRef]
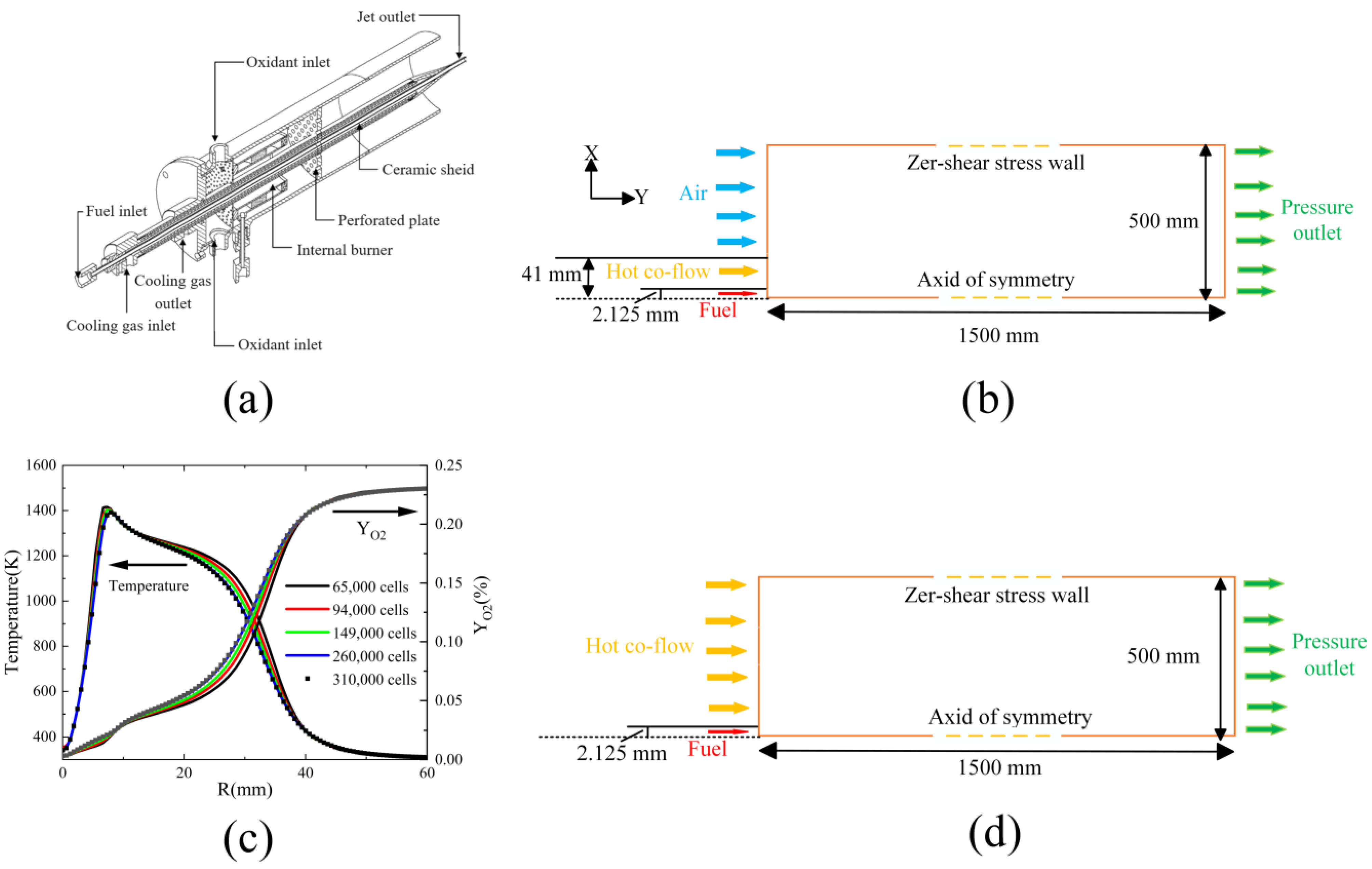










| Case | Fuel Jet | Hot Coflow | ||||||
|---|---|---|---|---|---|---|---|---|
| Re | Tf(K) | Tc(k) | Vc(m/s) | YO2 | YCO2 | YH2O | YN2 | |
| HM1 | 9482 | 305 | 1300 | 3.2 | 3 | 5.5 | 6.5 | 85 |
| HM2 | 9482 | 305 | 1300 | 3.2 | 6 | 5.5 | 6.5 | 82 |
| HM3 | 9482 | 305 | 1300 | 3.2 | 9 | 5.5 | 6.5 | 79 |
| Case | Fuel Inlet (Composition Vol%) | Hot Coflow (Composition Mass%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Re | Vf(m/s) | Tf(K) | XH2 | XCH4 | Vc(m/s) | Tc(k) | YO2 | YCO2 | YH2O | YN2 | |
| 1 | 9482 | 36.1 | 305 | 0 | 100 | 3.2 | 1300 | 9 | 5.5 | 6.5 | 79 |
| 2 | 9482 | 39.5 | 305 | 10 | 90 | 3.2 | 1300 | 9 | 5.5 | 6.5 | 79 |
| 3 | 9482 | 43.2 | 305 | 20 | 80 | 3.2 | 1300 | 9 | 5.5 | 6.5 | 79 |
| 4 | 9482 | 47.8 | 305 | 30 | 70 | 3.2 | 1300 | 9 | 5.5 | 6.5 | 79 |
| 5 | 9482 | 53.5 | 305 | 40 | 60 | 3.2 | 1300 | 9 | 5.5 | 6.5 | 79 |
| 6 | 9482 | 61 | 305 | 50 | 50 | 3.2 | 1300 | 9 | 5.5 | 6.5 | 79 |
| Route | Reaction | ||
|---|---|---|---|
| Thermal | R178: N + NO ⇔ N2 + O | R179: N + O2 ⇔ NO + O | R180: N + OH ⇔ NO + H |
| Prompt | R240: CH + N2 ⇔ HCN + N | R242: CH2 + N2 ⇔ HCN + NH | R243: CH2(S) + N2 ⇔ NH + HCN |
| N2O-intermediate | R181: N2O + O ⇔ N2 + O2 | R182: N2O + O ⇔ 2NO | R183: N2O + H ⇔ N2 + OH |
| R185: N2O( + M) ⇔ N2 + O( + M) | R199: NH + NO ⇔ N2O + H | ||
| NNH | R204: NNH ⇔ N2 + H | R205: N2 + H + M ⇔ NNH + M | R206: NNH + O2 ⇔ HO2 + N2 |
| R207: NNH + O ⇔ OH + N2 | R208: NNH + O ⇔ NH + NO | ||
| NO-reburning | R212: H + NO + M ⇔ HNO + M | R244: C + NO ⇔ CN + O | R245: C + NO ⇔ CO + N |
| R246: CH + NO ⇔ HCN + O | R247: CH + NO ⇔ H + NCO | R248: CH + NO ⇔ N + HCO | |
| R249: CH2 + NO ⇔ H + HNCO | R250: CH2 + NO ⇔ HCN + OH | R251: CH2 + NO ⇔ H + HCNO | |
| R252: CH2(S) + NO ⇔ H + HNCO | R253: CH2(S) + NO ⇔ HCN + OH | R254: CH2(S) + NO ⇔ H + HCNO | |
| R255: CH3 + NO ⇔ HCN + H2O | R256: CH3 + NO ⇔ H2CN + OH | R274: HCCO + NO ⇔ HCNO + CO |
| R10: O + CH3 ⇔ H + CH2O | R11: C + CH4 ⇔ OH + CH3 | R15: O + CH2O ⇔ OH + HCO |
| RR53: H + CH4 ⇔ CH3 + H2 | R56: H + CH2O( + M) ⇔ CH2OH( + M) | R57: H + CH2O( + M) ⇔ CH3O( + M) |
| R58: H + CH2O ⇔ HCO + H2 | R61: H + CH2OH ⇔ OH + CH3 | R92: OH + CH2 ⇔ H + CH2O |
| R93: OH + CH2 ⇔ CH + H2O | R95: OH + CH3( + M) ⇔ CH3OH( + M) | R97: OH + CH3 ⇔ CH2(S) + H2O |
| R98: OH + CH4 ⇔ CH3 + H2O | R99: OH + CO ⇔ H + CO2 | R100: OH + HCO ⇔ H2O + CO |
| R101: OH + CH2O ⇔ HCO + H2O | R104: OH + CH3OH ⇔ CH2OH + H2O | R105: OH + CH3OH ⇔ CH3O + H2O |
| R125: CH + O2 ⇔ O + HCO | R127: CH + H2O ⇔ H + CH2O | R135: CH2 + O2 ⇔ OH + H + CO |
| R142: CH2(S) + N2 ⇔ CH2 + N2 | R144: CH2(S) + O2 ⇔ H + OH + CO | R148: CH2(S) + H2O ⇔ CH2 + H2O |
| R152: CH2(S) + CO2 ⇔ CH2 + CO2 | R153: CH2(S) + CO2 ⇔ CO + CH2O | R156: CH3 + O2 ⇔ OH + CH2O |
| R166: HCO + H2O ⇔ H + CO + H2O | R167: HCO + M ⇔ H + CO + M | R168: HCO + O2 ⇔ HO2 + CO |
| R169: CH2OH + O2 ⇔ HO2 + CH2O | R288: OH + CH3 ⇔ H2 + CH2O | R291: CH2 + O2 ⇔ O + CH2O |
| Step | Mechanism Processing Method |
|---|---|
| 1 | Full mechanism |
| 2 | Removing the NO-reburning route |
| 3 | Removing the NO-reburning and prompt routes |
| 4 | Removing the NO-reburning, prompt, and NNH routes |
| 5 | Removing the NO-reburning, prompt, NNH, and N2Ointermediate routes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, L.; Jiang, H. Study of NO and CO Formation Pathways in Jet Flames with CH4/H2 Fuel Blends. Energies 2024, 17, 4382. https://doi.org/10.3390/en17174382
Lu L, Jiang H. Study of NO and CO Formation Pathways in Jet Flames with CH4/H2 Fuel Blends. Energies. 2024; 17(17):4382. https://doi.org/10.3390/en17174382
Chicago/Turabian StyleLu, Lin, and Haoyuan Jiang. 2024. "Study of NO and CO Formation Pathways in Jet Flames with CH4/H2 Fuel Blends" Energies 17, no. 17: 4382. https://doi.org/10.3390/en17174382
APA StyleLu, L., & Jiang, H. (2024). Study of NO and CO Formation Pathways in Jet Flames with CH4/H2 Fuel Blends. Energies, 17(17), 4382. https://doi.org/10.3390/en17174382





