The Use of Plant Biomass Pellets for Energy Production by Combustion in Dedicated Furnaces
Abstract
:1. Introduction
2. Materials and Methods
- 955 g of ash + 45 g of bentonite,
- 910 g of ash + 90 g of bentonite,
- 775 g of ash + 225 g of bentonite.
3. Results and Discussion
3.1. Biomass Properties
3.2. Specifications for Biomass Storage Conditions
3.3. Specifications for Optimum Biomass Combustion Conditions
- -
- weight of the biomass used, ranging from 3–769 kg;
- -
- primary energy of biomass, ranging from 58–14,902 MJ (16–4140 kWh);
- -
- energy transferred to the external tank of 1500 L, ranging from 6–2368 kWh;
- -
- energy transferred to the container in the furnace, ranging from 4–92 kWh.
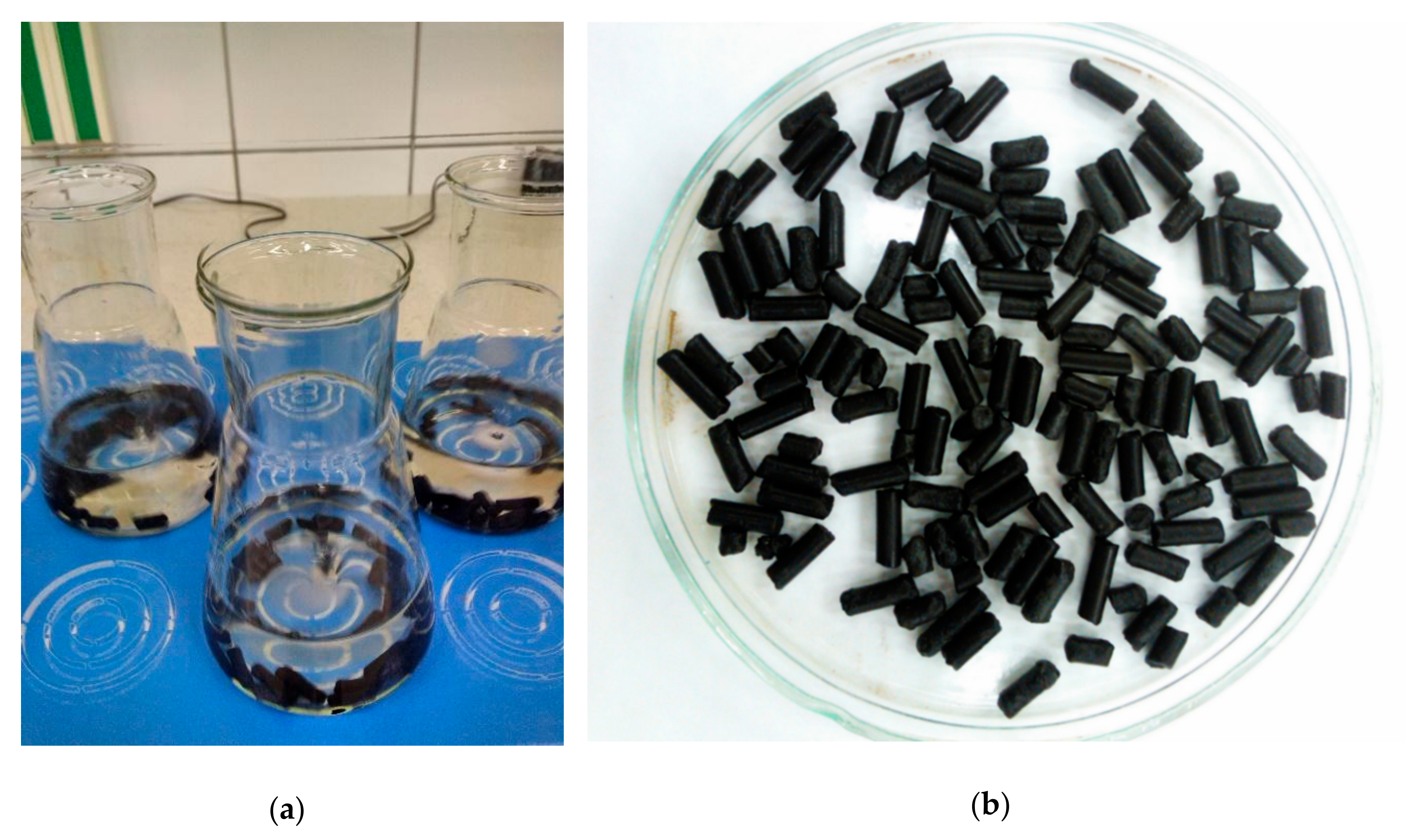
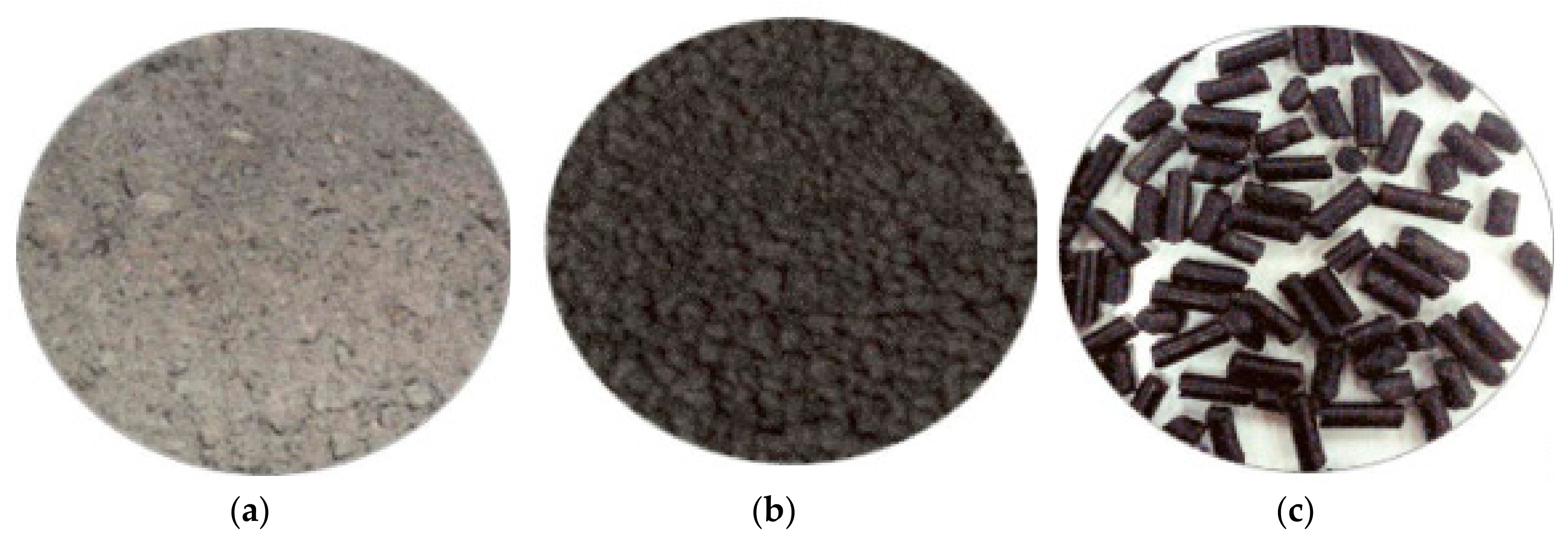
3.4. Preparation of Ash for Further Use
- softening point—1197 °C,
- melting point—1221 °C,
- pour point—1358 °C.
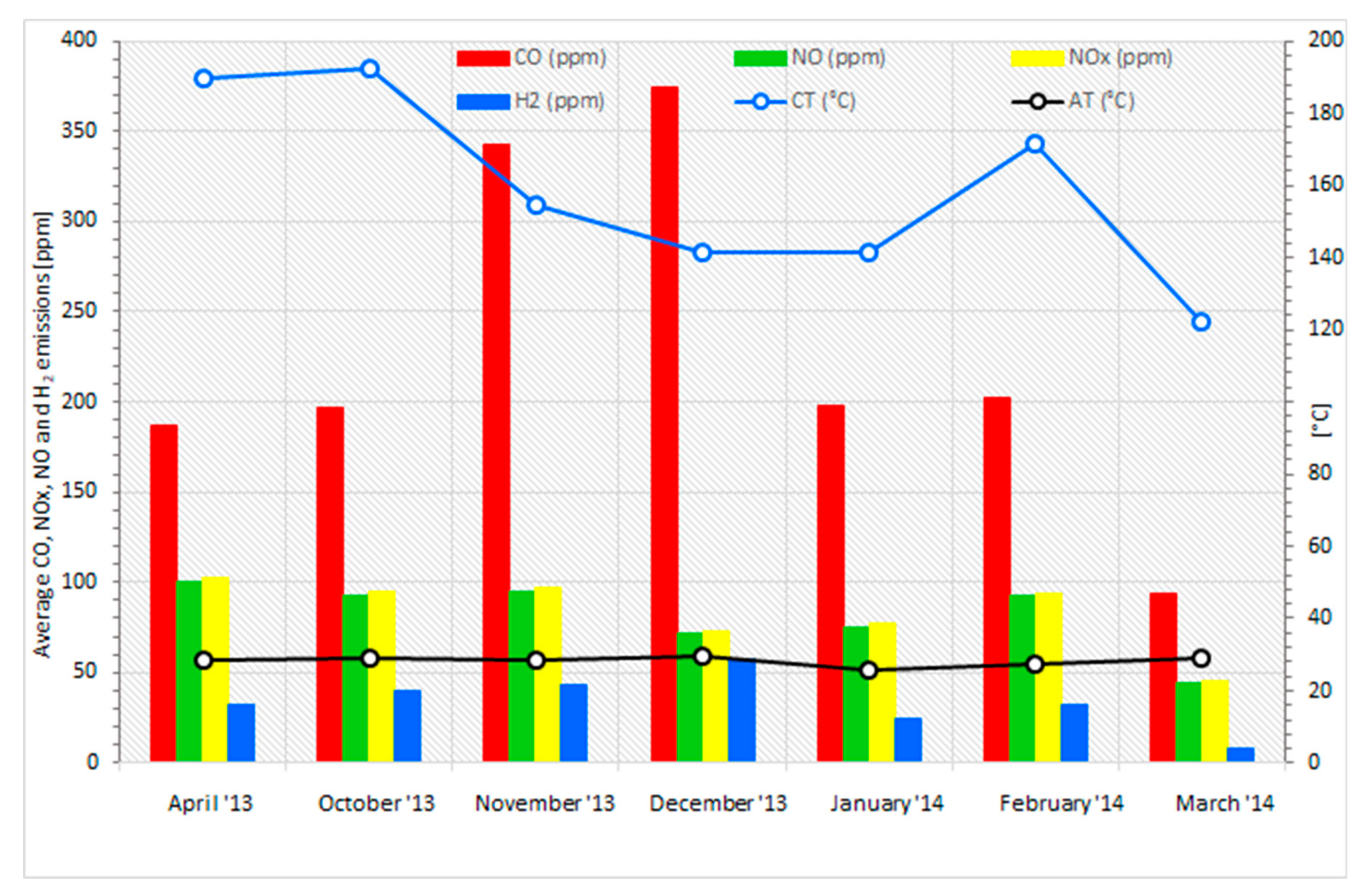
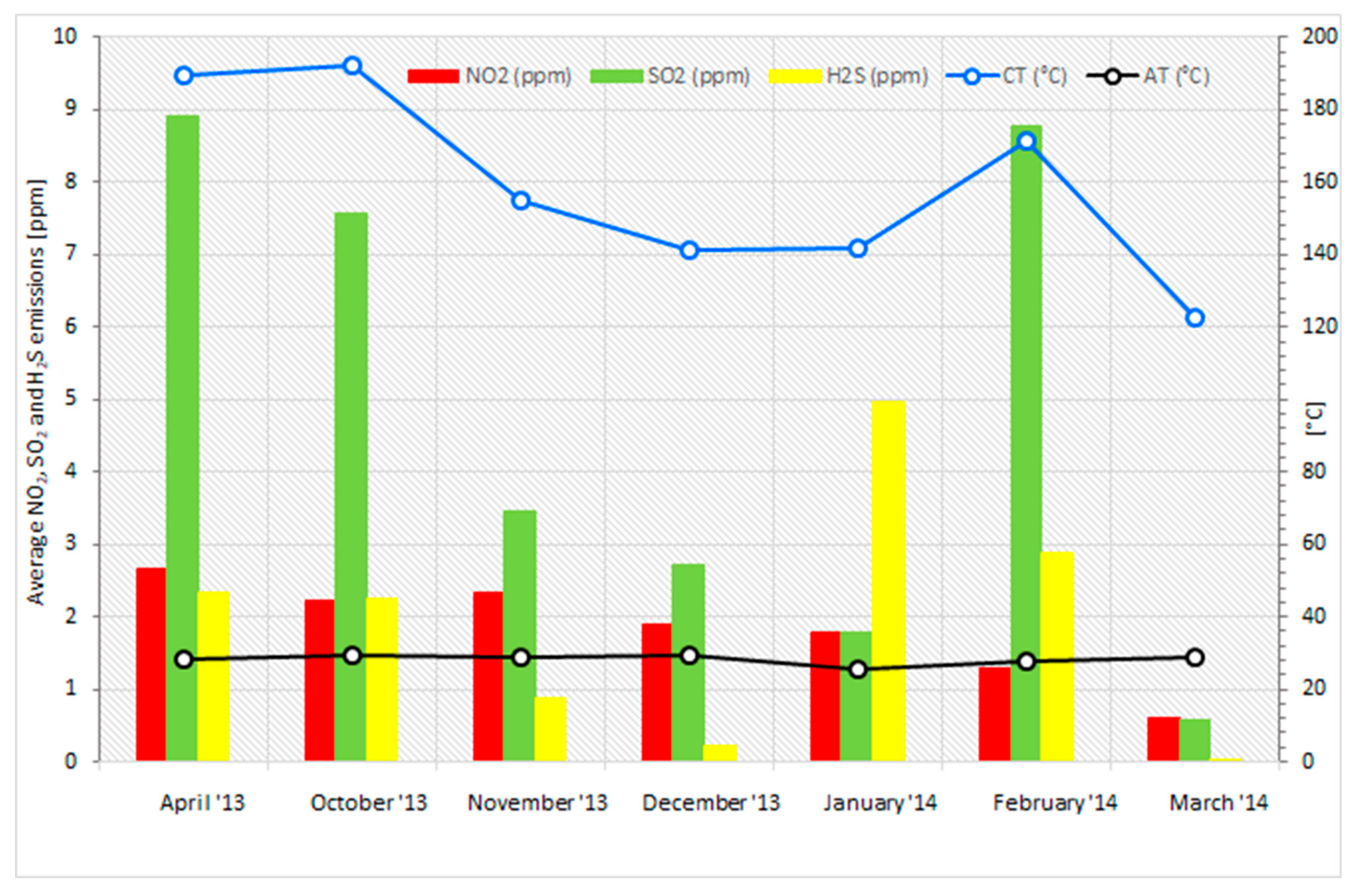
3.5. Analysis of the Combustion Process for Emissions
4. Conclusions and Perspectives for Further Research
- Studies showed the presence of many elements disturbing the combustion process in the biomass pellet composition. Even when the fuel material has a good, promising calorific value ranging from 17.3 to 20.1 MJ·kg−1, irregularities of burning parameters were noted.
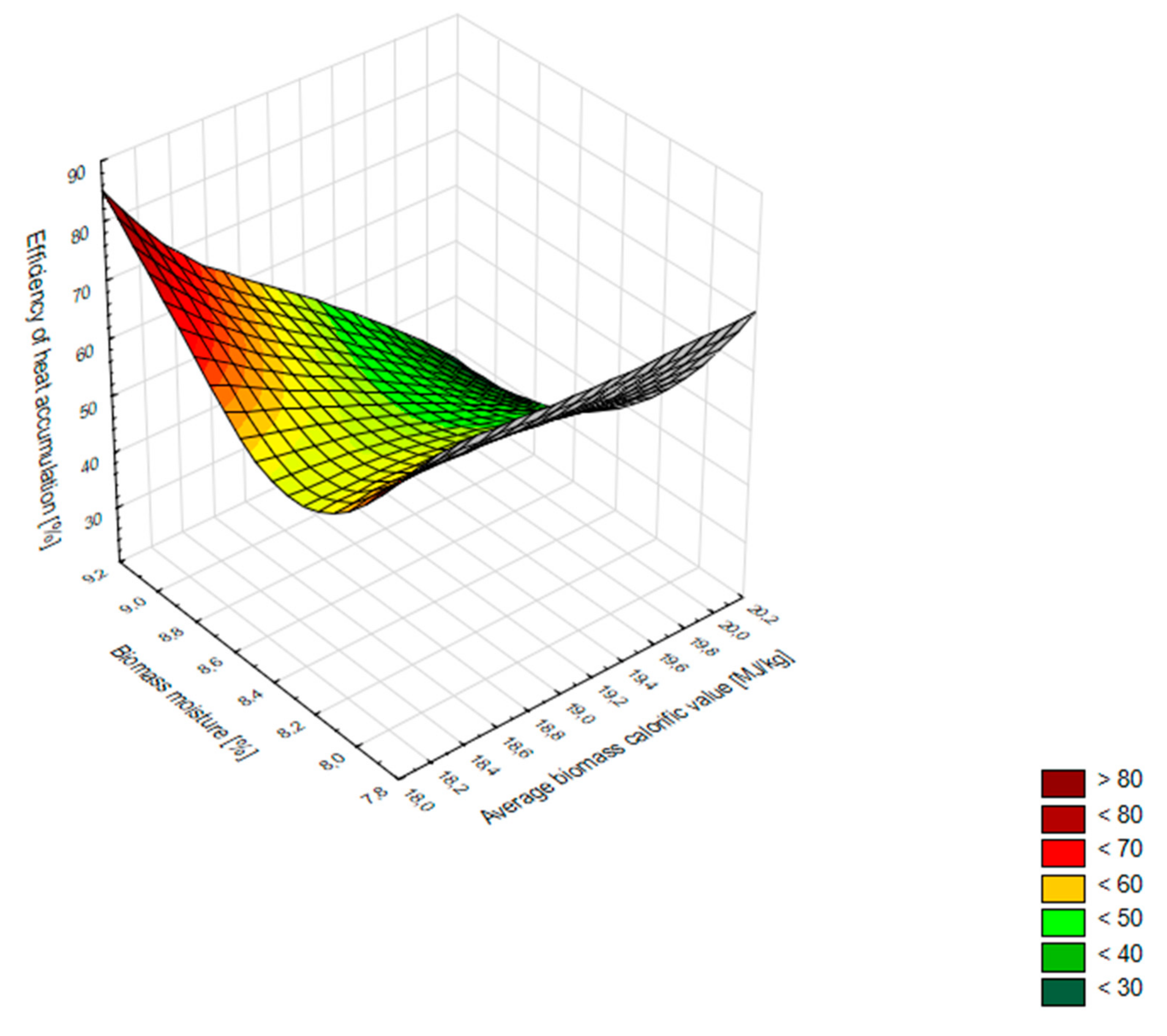
- Biomass moisture should be maintained at a level of 6–8%. In this way, both the calorific value and the combustion process remain at an optimum level. Proper moisture parameters of biomass can be maintained during the heating season by storing it in a room with a controlled microclimate. In the course of this study, it was observed that an increase in the humidity of pellets resulted in a decrease in the average calorific value of biomass and a simultaneous increase in the efficiency of heat accumulation.
- During the combustion of biomass, there is a technological problem related to the formation of a large amount of bottom ash, which, due to its partial melting, leaves residue on the boiler grate, walls, and equipment, which leads to boiler damage. At the current stage of research, it is difficult to indicate one prescription for removing this defect. In this respect, it seems to be of the utmost importance to avoid significant fluctuations in the combustion temperature by means of continuous regulation of boiler parameters and changes in the pellet composition.
- Bottom ash discharged from the furnace can be disposed of in a variety of ways. At this point, there is a problem with the transport of this material. In order to minimize secondary dust during transport, it was proposed to granulate or pelletize ash with the addition of a binder consisting of bentonite and bran. During the pelletization process, it is important to ensure the correct composition of pellets (ash, bentonite, bran, and water). Even small deviations from the optimum formula can result in a material that will be too loose or too plastic or will tend to form irregular lumps.
Author Contributions
Funding
Conflicts of Interest
References
- Zheng, Y.; Jensen, P.A.; Jensen, A.D.; Sander, B.; Junker, H. Ash transformation during co-firing coal and straw. Fuel 2006, 86, 1008–1020. [Google Scholar] [CrossRef]
- Arce, M.E.; Saavedra, Á.; Míguez, J.L.; Granada, E.; Cacabelos, A. Biomass Fuel and Combustion Conditions Selection in a Fixed Bed Combustor. Energies 2013, 6, 5973–5989. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Chang, S.; Yuan, Z.; Jiang, Y.; Liu, S.; Li, W.; Ma, L. Regionalized Techno—Economic Assessment and Policy Analysis for Biomass Molded Fuel in China. Energies 2015, 8, 13846–13863. [Google Scholar] [CrossRef] [Green Version]
- Łączak, A.; Bazan-Krzywoszańska, A.; Mrówczyńska, M.; Skiba, M. Renewable energy sources in the Lubusz voivodship (Poland). The present conditions and perspectives for development. Civ. Environ. Eng. Rep. 2018, 2, 31–67. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Q.; Bai, Z.; Lei, J.; Jin, H. Thermodynamic investigations of the supercritical CO2 system with solar energy and biomass. Appl. Energy 2018, 227, 108–118. [Google Scholar] [CrossRef]
- Tchapda, A.H.; Pisupati, S.V. A Review of Thermal Co-Conversion of Coal and Biomass/Waste. Energies 2014, 7, 1098–1148. [Google Scholar] [CrossRef] [Green Version]
- Ahmaruzzaman, M. A review on the utilization of fly ash. Prog. Energy Combust. Sci. 2010, 36, 327–363. [Google Scholar] [CrossRef]
- Amaral, S.S.; de Carvalho, J.A., Jr.; Costa, M.A.M.; Pinheiro, C. Particulate Matter Emission Factors for Biomass Combustion. Atmosphere 2016, 7, 141. [Google Scholar] [CrossRef] [Green Version]
- Grams, J.; Ryczkowski, R.; Chałupka, K.; Sobczak, I.; Rzeźnicka, I.; Przybysz, K. Impact of support (MCF, ZrO2, ZSM-5) on the efficiency of Ni catalyst in high-temperature conversion of lignocellulosic biomass to hydrogen-rich gas. Materials 2019, 12, 3792. [Google Scholar] [CrossRef] [Green Version]
- Cuellar, A.D.; Herzog, H. A Path Forward for Low Carbon Power from Biomass. Energies 2015, 8, 1701–1715. [Google Scholar] [CrossRef] [Green Version]
- Michalik, M. Biomasa zagrożona. Nowa Energia 2013, 4, 35–37. [Google Scholar]
- Pudełko, R. Ocena potencjału biomasy ubocznej w rolnictwie. Nowa Energia 2013, 4, 44–47. [Google Scholar]
- Monteiro, E.; Mantha, V.; Rouboa, A. Portuguese pellets market: Analysis of the production and utilization constrains. Energy Policy 2012, 42, 129–135. [Google Scholar] [CrossRef]
- Szlachta, J.; Jakubowska, J. Analiza procesu peletowania słomy zbożowej oraz zasadności dodawania otrąb zbożowych na przykładzie wybranego zakładu produkcyjnego. Inżynieria Rol. 2013, 4, 365–374. [Google Scholar]
- Obernberger, I.; Thek, G. Physical characterisation and chemical composition of densified biomass fuels with regard to their combustion behaviour. Biomass Bioenergy 2004, 27, 653–669. [Google Scholar] [CrossRef]
- Statistical Yearbook of Forestry 2018. Statistical Analyses. Statistics Poland, Warsaw. Available online: https://stat.gov.pl/en/topics/statistical-yearbooks/statistical-yearbooks/statistical-yearbook-of-forestry-2018.12.1.html (accessed on 9 December 2018).
- Monedero, E.; Portero, H.; Lapuerta, M. Combustion of Poplar and Pine Pellet Blends in a 50 kW Domestic Boiler: Emissions and Combustion Efficiency. Energies 2018, 11, 1580. [Google Scholar] [CrossRef] [Green Version]
- Antizar-Ladislao, B.; Turrion-Gomez, J.L. Decentralized Energy from Waste Systems. Energies 2010, 3, 194–205. [Google Scholar] [CrossRef] [Green Version]
- Falemara, B.C.; Joshua, V.I.; Aina, O.O.; Nuhu, R.D. Performance Evaluation of the Physical and Combustion Properties of Briquettes Produced from Agro-Wastes and Wood Residues. Recycling 2018, 3, 37. [Google Scholar] [CrossRef] [Green Version]
- Lehtikangas, P. Quality properties of pelletised sawdust, logging residues and bark. Biomass Bioenergy 2001, 20, 351–360. [Google Scholar] [CrossRef]
- García-Maraver, A.; Popov, V.; Zamorano, M. A review of European standards for pellet quality. Renew. Energy 2011, 36, 3537–3540. [Google Scholar] [CrossRef]
- Chen, W.S.; Chang, F.C.; Shen, Y.H.; Tsai, M.S. The characteristics of organic sludge/sawdust derived fuel. Bioresour. Technol. 2011, 102, 5406–5410. [Google Scholar] [CrossRef] [PubMed]
- Houshfar, E.; Løvås, T.; Skreiberg, Ø. Experimental Investigation on NOx Reduction by Primary Measures in Biomass Combustion: Straw, Peat, Sewage Sludge, Forest Residues and Wood Pellets. Energies 2012, 5, 270–290. [Google Scholar] [CrossRef] [Green Version]
- Shao, Y.; Wang, J.; Preto, F.; Zhu, J.; Xu, C. Ash Deposition in Biomass Combustion or Co-Firing for Power/Heat Generation. Energies 2012, 5, 5171–5189. [Google Scholar] [CrossRef]
- Sander, B. Properties of Danish biofuels and the requirements for power production. Biomass Bioenergy 1997, 12, 177–183. [Google Scholar] [CrossRef]
- Carroll, J.P.; Finnan, J. Physical and chemical properties of pellets from energy crops and cereal straws. Biosyst. Eng. 2012, 112, 151–159. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, J.; Han, K.; Wang, J.; Lu, C. Influence of BaCO3 on chlorine fixation, combustion characteristics and KCl conversion during biomass combustion. Fuel 2017, 208, 82–90. [Google Scholar] [CrossRef]
- Obernberger, I.; Brunner, T.; Bärnthaler, G. Chemical properties of solid biofuels—Significance and impact. Biomass Bioenergy 2006, 30, 973–982. [Google Scholar] [CrossRef]
- Febrero, L.; Granada, E.; Regueiro, A.; Míguez, J.L. Influence of Combustion Parameters on Fouling Composition after Wood Pellet Burning in a Lab-Scale Low-Power Boiler. Energies 2015, 8, 9794–9816. [Google Scholar] [CrossRef] [Green Version]
- Sarenbo, S.L.; Claesson, T. Limestone and dolomite powder as binders for wood ash agglomeration. Bull. Eng. Geol. Environ. 2004, 63, 191–207. [Google Scholar] [CrossRef]
- Greinert, A.; Mrówczyńska, M.; Szefner, W. Study on the Possibilities of Natural Use of Ash Granulate Obtained from the Combustion of Pellets from Plant Biomass. Energies 2019, 12, 2569. [Google Scholar] [CrossRef] [Green Version]
- Greinert, A.; Mrówczyńska, M.; Szefner, W. The Use of Waste Biomass from the Wood Industry and Municipal Sources for Energy Production. Sustainability 2019, 11, 3083. [Google Scholar] [CrossRef] [Green Version]
- Bindig, R.; Butt, S.; Hartmann, I.; Matthes, M.; Thiel, C. Application of Heterogeneous Catalysis in Small-Scale Biomass Combustion Systems. Catalysts 2012, 2, 223–243. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhang, Z.; Zhou, Y.; Dong, R. The Influences of Various Testing Conditions on the Evaluation of Household Biomass Pellet Fuel Combustion. Energies 2018, 11, 1131. [Google Scholar] [CrossRef] [Green Version]
- Polonini, L.F.; Petrocelli, D.; Parmigiani, S.P.; Lezzi, A.M. Influence on CO and PM Emissions of an Innovative Burner Pot for Pellet Stoves: An Experimental Study. Energies 2019, 12, 590. [Google Scholar] [CrossRef] [Green Version]
- Qin, X.; Keefe, R.F.; Daugaard, D.E. Small Landowner Production of Pellets from Green, Beetle-Killed, and Burned Lodgepole Pine. Energies 2018, 11, 648. [Google Scholar] [CrossRef] [Green Version]
- Zając, G.; Szyszlak-Bargłowicz, J.; Gołębiowski, W.; Szczepanik, M. Chemical Characteristics of Biomass Ashes. Energies 2018, 11, 2885. [Google Scholar] [CrossRef] [Green Version]
- Toscano, G.; Alfano, V.; Scarfone, A.; Pari, L. Pelleting Vineyard Pruning at Low Cost with a Mobile Technology. Energies 2018, 11, 2477. [Google Scholar] [CrossRef] [Green Version]
- Huhtinen, M. Wood Biomass as a Fuel. In Material for 5EURES Training Sessions; Huhtinen, M., Ed.; European Commission under the Intelligent Energy—Europe Programme: Brussels, Belgium, 2005; pp. 1–7. [Google Scholar]
- Moskalik, T.; Gendek, A. Production of Chips from Logging Residues and Their Quality for Energy: A Review of European Literature. Forests 2019, 10, 262. [Google Scholar] [CrossRef] [Green Version]
- Olanders, B.; Steenari, B. Characterization of ashes from wood and straw. Biomass Bioenergy 1994, 8, 105–115. [Google Scholar] [CrossRef]
- Obernberger, I.; Biedermann, F.; Widmann, W.; Riedl, R. Concentrations of inorganic elements in biomass fuels and recovery in the different ash fractions. Biomass Bioenergy 1997, 12, 211–224. [Google Scholar] [CrossRef]
- James, A.K.; Thring, R.W.; Helle, S.; Ghuman, H.S. Ash Management Review—Applications of Biomass Bottom Ash. Energies 2012, 5, 3856–3873. [Google Scholar] [CrossRef]
- Papandreou, A.D.; Stournaras, C.J.; Panias, D.; Paspaliaris, I. Adsorption of Pb(II), Zn(II) and Cr(III) on coal fly ash porous pellets. Miner. Eng. 2011, 24, 1495–1501. [Google Scholar] [CrossRef]
- Kessler, F.; Knechtle, N.; Frischknecht, R. Heizenergie aus Heizöl, Erdgas oder Holz? Swiss Federal Office of Environment (BUWAL). Umwelt Schrift 2000, 315, 163. [Google Scholar]



| (% Dry Matter (d.m.)) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Ash | C | S | N | H | O | Cl | K | Ca |
| 2.74 | 51.64 | 0.14 | 0.28 | 6.30 | 41.64 | 0.21 | 0.542 | 0.246 |
| (mg·kg−1 d.m.) | ||||||||
| Mg | Na | Fe | Cd | Cr | Cu | Ni | Pb | Zn |
| 700 | 100 | 120 | 0.299 | 0.996 | 2.017 | nd | nd | 30 |
| Measurement Period | Weight of the Biomass Used | Average Calorific Value of Biomass | Primary Energy of Biomass | Energy Transferred to the External Tank 1500 L | Energy Transferred to the Container in the Furnace | Efficiency of Heat Accumulation | |
|---|---|---|---|---|---|---|---|
| (kg) | (MJ·kg−1) | MJ | (kWh) | (kWh) | (kWh) | (%) | |
| 10 April 2013–12 April 2013 | 99 | 20.01 | 1976 | 549 | 307 | 9 | 57.57 |
| 12 April 2013–16 April 2013 | 43 | 18.40 | 787 | 219 | 116 | 21 | 62.60 |
| 16 April 2013–19 April 2013 | 55 | 18.39 | 1003 | 279 | 153 | 22 | 62.62 |
| 19 April 2013–23 April 2013 | 3 | 18.08 | 58 | 16 | 6 | 4 | 62.07 |
| 23 April 2013–26 April 2013 | 42 | 17.47 | 734 | 204 | 131 | 3 | 65.94 |
| 26 April 2013–30 April 2013 | 48 | 17.27 | 826 | 229 | 149 | 4 | 66.69 |
| 1 October 2013–6 October 2013 | 275 | 19.64 | 5402 | 1501 | 832 | 48 | 58.64 |
| 7 October 2013–13 October 2013 | 77 | 19.68 | 1519 | 422 | 223 | 24 | 58.55 |
| 14 October 2013–20 October 2013 | 499 | 19.41 | 9692 | 2692 | 1552 | 46 | 59.35 |
| 21 October 2013–27 October 2013 | 267 | 20.07 | 5362 | 1489 | 783 | 72 | 57.41 |
| 28 October 2013–31 October 2013 | 316 | 19.50 | 6160 | 1711 | 987 | 24 | 59.08 |
| 4 November 2013–10 November 2013 | 769 | 19.38 | 14,902 | 4140 | 2368 | 92 | 59.43 |
| 11 November 2013–17 November 2013 | 249 | 19.35 | 4826 | 1341 | 730 | 68 | 59.53 |
| 18 November 2013–24 November 2013 | 378 | 18.84 | 7116 | 1977 | 1141 | 68 | 61.16 |
| 25 November 2013–30 November 2013 | 358 | 18.62 | 6669 | 1853 | 1102 | 44 | 61.86 |
| 11 December 2013–23 December 2013 | 428 | 18.56 | 7943 | 2206 | 1249 | 98 | 61.05 |
| 23 December 2013–09 January 2014 | 377 | 18.34 | 6921 | 1922 | 1149 | 71 | 63.46 |
| 04 February 2014–17 February 2014 | 667 | 18.49 | 12,332 | 3426 | 2135 | 47 | 63.70 |
| 17 February 2014–27 February 2014 | 663 | 18.39 | 12,200 | 3389 | 2112 | 91 | 65.00 |
| 27 February 2014–06 March 2014 | 577 | 18.14 | 10,469 | 2908 | 1931 | 61 | 68.50 |
| 6 March 2014–18 March 2014 | 675 | 18.49 | 12,472 | 3465 | 1770 | 194 | 56.69 |
| 18 March 2014–04 April 2014 | 456 | 18.20 | 8306 | 2307 | 975 | 191 | 50.53 |
| Minimum | 17.27 | 50.53 | |||||
| Maximum | 20.07 | 68.50 | |||||
| Mean value | 18.76 | 60.97 | |||||
| SD | 0.75 | 3.84 | |||||
| SE | 0.03 | 0.17 | |||||
| Bentonite | Ash | Bran | Water | State | |
|---|---|---|---|---|---|
| (g) | |||||
| 1 | 45 | 955 | 20 | 150 | − |
| 2 | 45 | 955 | 22 | 170 | − |
| 3 | 45 | 955 | 23 | 180 | + |
| 4 | 45 | 955 | 25 | 190 | ++ |
| 5 | 45 | 955 | 30 | 200 | + |
| 6 | 45 | 955 | 35 | 210 | − |
| 7 | 45 | 955 | 40 | 220 | − |
| 8 | 90 | 910 | 50 | 120 | − |
| 9 | 90 | 910 | 60 | 130 | − |
| 10 | 90 | 910 | 70 | 130 | − |
| 11 | 90 | 910 | 80 | 140 | − |
| 12 | 90 | 910 | 90 | 140 | − |
| 13 | 90 | 910 | 100 | 150 | ++ |
| 14 | 90 | 910 | 110 | 160 | + |
| 15 | 225 | 775 | 150 | 40 | − |
| 16 | 225 | 775 | 170 | 45 | − |
| 17 | 225 | 775 | 190 | 45 | + |
| 18 | 225 | 775 | 200 | 50 | ++ |
| 19 | 225 | 775 | 210 | 55 | + |
| 20 | 225 | 775 | 220 | 60 | − |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greinert, A.; Mrówczyńska, M.; Grech, R.; Szefner, W. The Use of Plant Biomass Pellets for Energy Production by Combustion in Dedicated Furnaces. Energies 2020, 13, 463. https://doi.org/10.3390/en13020463
Greinert A, Mrówczyńska M, Grech R, Szefner W. The Use of Plant Biomass Pellets for Energy Production by Combustion in Dedicated Furnaces. Energies. 2020; 13(2):463. https://doi.org/10.3390/en13020463
Chicago/Turabian StyleGreinert, Andrzej, Maria Mrówczyńska, Radosław Grech, and Wojciech Szefner. 2020. "The Use of Plant Biomass Pellets for Energy Production by Combustion in Dedicated Furnaces" Energies 13, no. 2: 463. https://doi.org/10.3390/en13020463





