Abstract
Biomass fractionation, as an alternative to biomass pretreatment, has gained increasing research attention over the past few years as it provides separate streams of cellulose, hemicellulose, and lignin. These streams can be used separately and can provide a solution for improving the economics of emerging biorefinery technologies. The sugar streams are commonly used in microbial conversions, whereas during recent years lignin has been recognized as a valuable compound as it is the only renewable and abundant source of aromatic chemicals. Successfully converting lignin into valuable chemicals and products is key in achieving both environmental and economic sustainability of future biorefineries. In this work, lignin retrieved from beechwood sawdust delignification pretreatment via an organosolv process was depolymerized with thermal and catalytic pyrolysis. ZSM-5 commercial catalyst was used in situ to upgrade the lignin bio-oil vapors. Lignins retrieved from different modes of organosolv pretreatment were tested in order to evaluate the effect that upstream pretreatment has on the lignin fraction. Both thermal and catalytic pyrolysis yielded oils rich in phenols and aromatic hydrocarbons. Use of ZSM-5 catalyst assisted in overall deoxygenation of the bio-oils and enhanced aromatic hydrocarbons production. The oxygen content of the bio-oils was reduced at the expense of their yield. Organosolv lignins were successfully depolymerized towards phenols and aromatic hydrocarbons via thermal and catalytic pyrolysis. Hence, lignin pyrolysis can be an effective manner for lignin upgrading towards high added value products.
1. Introduction
Utilization of lignocellulosic waste streams and residues derived from the forest and agricultural sectors has attracted a lot of interest for the production of renewable biofuels, such as ethanol, since they do not compete with food and feed production [1,2]. Moreover, the need for renewable carbon in fuels, chemicals, and materials has spurred the development of the biorefinery technology that aims at replacing the conventional refinery technology. Chemical, thermochemical, and biochemical valorization routes are all being investigated in an effort to develop efficient technologies. The biochemical route comprises the following steps: pretreatment, enzymatic saccharification, and microbial conversion of sugars to biofuels. Pretreatment aims at making the cellulosic part of the biomass more amenable to enzymatic hydrolysis and biochemical conversion by reducing the complexity and recalcitrance of lignocellulosic biomass [3]. To achieve this, removal of lignin from the biomass structure must take place in order to liberate the holocellulose sugars. This delignification step is a very challenging and high cost process step of the lignocellulose conversion scheme. Lignin, a polyphenolic polymer which surrounds the biomass holocellulose, acts as protection barrier by making biomass extremely recalcitrant to pathogens, microorganisms, and enzymes [4]. Several different strategies have been investigated for biomass pretreatment, the aim is to allow the hydrolytic enzymes to gain access to the carbohydrates by deconstructing the carbohydrate–lignin complex [5,6,7]. Hydrothermal pretreatment, employing only water, has been shown to efficiently degrade hemicelluloses and increase the biomass porosity which results in more efficient enzymatic hydrolysis of the pretreated biomass [8]. However, lignin which is mostly not removed, partly rearranges on the surface of the biomass particles and acts as an inhibitor for downstream enzymatic hydrolysis [9].
One of the most interesting pretreatment technologies is organosolv delignification. It employs a mixture of water and organic solvent, typically 30–70 vol.%, and pretreats the biomass at relatively mild temperatures (100–220 °C). One of its key benefits is the isolation of a pure lignin fraction which is sulfur free and has very low ash and hydrocarbon content [10]. This allows downstream upgrading of the lignin to take place, which can significantly improve the economics and the environmental footprint of the overall process.
To this day, the main use of lignin is that of a low value fuel burned on site in pulp and paper mills to provide energy consumed in the process or utilized as heat [11]. Lignin however has been identified as an abundant and renewable source of aromatic chemicals, therefore depolymerizing it towards aromatic monomers is potentially an extremely promising valorization route. Although there is significant research interest in lignin valorization, it remains an extremely challenging task as treating it thermochemically promotes condensation reactions towards low value char and coke products [12,13], in addition lignin is extremely heterogeneous and its significantly variable composition depends on its origin and the type of treatment that was used to isolate it [14].
Thermochemical depolymerization via pyrolysis is a very interesting pathway that can potentially address the above challenges. Lignin pyrolysis has been found to produce bio-oils rich in phenols and aromatic hydrocarbons [15,16,17]. The oils produced are complex having up to 300 different compounds, mostly oligomers, dimers, trimers and monomers of phenolic compounds [18]. In situ catalytic upgrading of the bio-oil vapors is a means of selectively enhancing product yields, increasing bio-oil deoxygenation and simplifying the lignin bio-oil composition. Our previous work on Kraft lignin has shown that catalysis with ZSM-5 can increase lignin depolymerization towards monomers and increase the production of alkyl-phenols, while minimizing oxygenated side groups such as methoxy and ethoxy side chains [19]. Other researchers have reported similar findings in the literature [20,21].
In our previous work [22] we investigated the efficiency of organosolv pretreatment on beechwood biomass and the effect of different delignification process conditions such as type of solvent, lack or presence of homogeneous catalyst, and type of homogeneous acid catalyst. The pulps that were produced from the delignification of beechwood were evaluated via enzymatic hydrolysis and subsequent fermentation to ethanol. Two of the pulps were selected for high gravity saccharification and fermentation, achieving almost 8 w/v % ethanol in the aqueous solution. In this work, lignins derived from beechwood sawdust pretreated via organosolv delignification were firstly characterized for elemental analysis and via FTIR and then pyrolyzed thermally and catalytically in a lab-scale fast pyrolysis fixed bed reactor. The aim was to maximize bio-oil yield and to evaluate the effect that upstream organosolv delignification has on the lignin pyrolysis process. The produced bio-oils were analyzed for water content and elemental composition and also by gas chromatography-mass spectrometry (GCMS) to understand what types of compounds can be produced via thermal and catalytic pyrolysis. In this way we complete a process scheme that utilizes all parts of the biomass towards either fuels (ethanol via sugars fermentation) and chemicals and aromatic fuels additives derived from the pyrolysis of leftover lignin.
2. Results and Discussion
2.1. Lignin Origin and Characterisation
The lignins tested in this work were derived by organosolv delignification of beechwood sawdust under different conditions. Details about the delignification work that took place, the delignification efficiency, and its effect on cellulose recovery, saccharification, and fermentation towards bioethanol can be found elsewhere [22]. Table 1 summarizes the experimental conditions under which beechwood sawdust was delignified and the corresponding lignins were retrieved, along with their elemental analyses.

Table 1.
Experimental conditions for the organosolv pretreatment and lignin residues characterization.
Lignins L1 and L2 were retrieved from delignification of beechwood sawdust in a semi-pilot-scale hybrid organosolv–steam explosion reactor at 200 °C and 15 min reaction time. The remaining samples were retrieved from organosolv delignification that took place in autoclave reactors at 175 °C and 60 min reaction time. Details about the hybrid organosolv delignification semi-pilot unit can be found elsewhere [23]. The O content of the lignins was calculated by difference. Beechwood sawdust was found to contain 0.6 wt.% inorganic ash on a dry biomass basis, this has been presented in detail elsewhere [24]. In almost all cases the ash content of the lignins was quite low, typically from 0.0 wt.% up to a maximum of 0.5 wt.%. The residues that were recovered after fermentation, named L12enz and L14enz, had significantly higher ash content due to the salts and nutrients that were added in the fermentation broth and which are typically salts such as MgSO4·(NH4)2HPO4 and due to the ash found in the yeast cells that were retrieved along with the residue. As seen from Table 1, lignins revtrieved under harsher conditions, for example lignins L1 and L2 retrieved from the steam explosion reactor or lignins L6 and L12 which were retrieved after organosolv pretreatment assisted by H2SO4 had lower cellulose and hemicellulose contents.
Samples L12 and L14 have two types of lignin. One type is the initially removed lignin via organosolv delignification. The second type, corresponding to samples L12enz and L14enz, is the residue of the high solids hydrolysis, saccharification, and fermentation runs that the resulting pulps underwent to convert biochemically to bioethanol [22]. In these runs an ethanol production yield of 75% and 83% was achieved, leaving behind a solid residue containing the remaining unconverted cellulose and hemicellulose and the lignin that was not initially removed (4.2 and 4.6 wt.% in the pulps produced from runs L12 and L14, respectively), as well as the yeast cells. Figure S1 in the Supplementary Materials presents the FTIR characterization of all retrieved lignins. Absorbance peaks corresponding to characteristic bonds and chemical groups commonly found in lignin were detected indicating that the lignins were not degraded. Samples L12enz and L14enz which also contained leftover culture cells, unreacted cellulose, and hemicellulose yielded FTIR spectra that were unclear. Details about the FTIR analysis and the corresponding lignin peaks have been thoroughly provided elsewhere [22].
2.2. Lignin Valorization via Pyrolysis
The retrieved lignins were pyrolyzed both thermally, employing silica sand as inert heat carrier, and catalytically with a commercially available ZSM-5 catalyst in a fixed bed bench scale reactor. Figure 1 and Figure 2 present the product yields and the elemental analysis of the dry bio-oils for thermal and catalytic pyrolysis of all lignins, respectively.
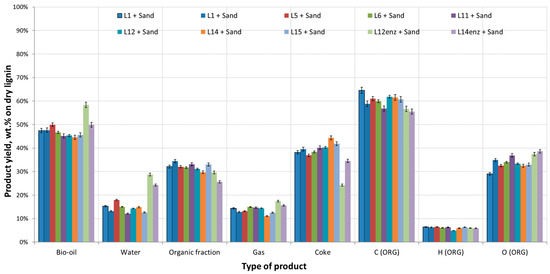
Figure 1.
Product yields and bio-oils elemental analysis (dry basis) from thermal pyrolysis of organosolv-derived beechwood lignins.
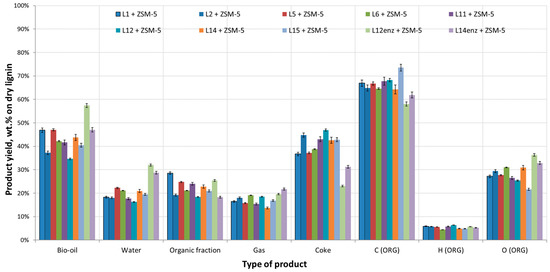
Figure 2.
Product yields and bio-oils elemental analysis (dry basis) from catalytic pyrolysis of organosolv-derived beechwood lignins.
Typical standard deviation (sd) for the mass balances was between 1.5%–2.5%. In the case of individual gas yields the sd was negligible at 0.2%, hence it cannot be depicted in Figure 3 and Figure 4 as error bars. Overall the organic fraction was between 26 and 34 wt.% in the case of thermal pyrolysis and between 18 and 28 wt.% in the case of catalytic pyrolysis. Water production was around 12–17 wt.% for thermal pyrolysis and 17–22 wt.% for catalytic pyrolysis for the lignin rich residues. Residues L12enz and L14enz produced significantly more water, reaching up to 30 wt.%. Gases production were 12–16 wt.% and 14–22 wt.% for thermal and catalytic pyrolysis, respectively. As expected in lignin pyrolysis, coke production was rather high, around 40 wt.%, increasing slightly when ZSM-5 was used due to the catalytically produced coke. The main deoxygenation pathway was through dehydration reactions producing more H2O. The addition of ZSM-5 resulted in the reduction of the oxygen content of the bio-oil, along with the reduction of its yield due to the loss of C, H, and O to other pyrolysis products. Thermal pyrolysis bio-oils had O contents of 28–38 wt.% on dry bio-oil which dropped to 22–35 wt.% with the use of ZSM-5. Overall, use of ZSM-5 resulted in an increase of gases, coke, and water formation, and in the decrease of the organic fraction yield and bio-oil O content.
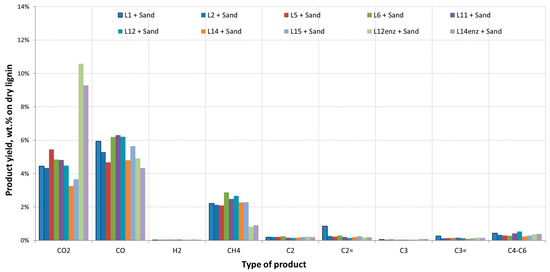
Figure 3.
Individual gas yields (dry basis) from thermal pyrolysis of organosolv-derived beechwood lignins.
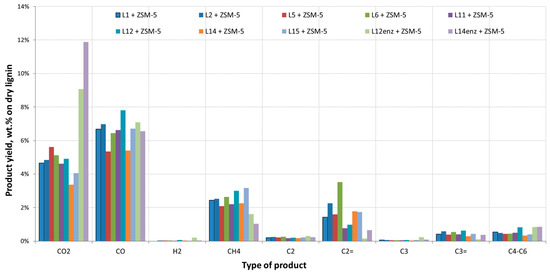
Figure 4.
Individual gas yields (dry basis) from catalytic pyrolysis of organosolv-derived beechwood lignins.
The two lignins received after the enzymatic hydrolysis of pulps from the runs L12 and L14 and the consequent fermentation of the produced sugars had a significantly different behavior. Gas and water production was higher while coke yield was lower when compared to the other lignin samples. In addition, the O content of the produced bio-oils both from thermal and catalytic pyrolysis was higher. This can be attributed to the lower lignin content of these samples. As can be seen in Table 1, lignin content was 23.5% and 49% for samples L12enz and L14enz, respectively, indicating the presence of other species in the form of oligosaccharides and proteins retrieved from the yeast cells that were recovered along with the solid residue of the fermentation process. The lower lignin content of these samples translates into a higher O content (Table 1) of the feedstocks which also results in a higher O content in the produced bio-oils. Figure 3 and Figure 4 present in detail the gases produced during the pyrolysis of all lignin residues.
A few general conclusions may be drawn from the gas yields. Residues L12enz and L14enz produce much higher CO2 and lower CH4 yields in both thermal and catalytic pyrolysis. This can be attributed to their composition and the fact they contain proteins and leftover oligosaccharides, which have a higher O2 content and which tend to deoxygenate via decarboxylation reactions, especially in the case of hemicellulose. In addition, the lower lignin content in these residues is confirmed by the lower CH4 yield presented in Figure 3 and Figure 4. As previously reported, high lignin residues typically have low gas yields in thermal pyrolysis, which increase slightly when ZSM-5 is introduced. A slight increase is noted mostly in CO production and in light hydrocarbons up to C6. The increased CO yield contributes to the deoxygenation of the produced bio-oil, however as noted before it was the dehydration reactions producing H2O that were mostly responsible for the bio-oil O reduction. The char retrieved from lignin was analyzed and as expected its C content was very high ranging between 80 to 90 wt.%, H was around 3.2 wt.%, S content was negligible below 0.1 wt.% even in the cases where H2SO4 was used, and O was typically around 10 wt.%. The high C content of the char makes it an interesting alternative fuel or a precursor for activated carbon.
2.3. Effect of Use of Acids during Delignification on Lignin Pyrolysis
Figure 1 and Figure 2 follow some common trends. Apparently, in all cases the use of H2SO4 has some specific effects. Comparing lignins L1 to L2, L5 to L6, and L11 to L12, in which cases delignification took place with and without the use of H2SO4 catalysis, runs L1, L5, L11 (no H2SO4) produced more organic fraction and less coke compared to the lignins retrieved from H2SO4-assisted delignification. This was valid for both thermal and catalytic pyrolysis. In addition, elemental analysis of the produced bio-oils showed that lignins retrieved from delignification conditions where no H2SO4 was used, produced bio-oils with higher C and lower O contents with the exception of the pair L11 and L12 where the reverse was observed. A possible explanation for this is the partial degradation of the retrieved lignin. Use of H2SO4 created more severe conditions which led to enhancement of recondensation reactions of lignin. The more degraded condensed lignins formed more coke, therefore less C moved to the desirable products, which is the organic fraction of the bio-oil, and this is especially true in the case of catalytic pyrolysis where use of H2SO4 resulted in higher coke production. When the less severe catalysts H3PO4 and oxalic acid are used, the effects were negligible due to their lower severity. In all cases where an acid was used during the organosolv pretreatment, the bio-oils produced, whether thermal or catalytic, had a lower O content. It should be noted at this point that although both H3PO4 and oxalic acid were much less severe compared to H2SO4, the achieved delignification degrees (DD) were very high, while the produced pulps were found to be extremely suitable for saccharification and subsequent fermentation [22]. This means that the lignin can be removed from lignocellulosic biomass without causing too much degradation and chemical modification which is essential in its downstream valorization, as seen from this work. On the other hand, the use of different solvents in this work, acetone and ethanol, during the delignification step, did not seem to have any particular effect on the pyrolysis products. This would suggest that the organosolv process can be more flexible in terms of use of solvents since the further degradation of lignin can be avoided with different types of solvents.
2.4. GCMS Semiquantitative Bio-Oils Analysis
GCMS analysis was done on all bio-oil samples. Table 2 presents the grouping of all chemical substances detected in the bio-oils. The grouping is as follows: AR = aromatic hydrocarbons, PH = phenols, AC = acids, EST = esters, ALD = aldehydes, KET = ketones, PAH = polyaromatic hydrocarbons, OxyAR = aromatic rings with an oxygenated substituent, OxyPH = phenols with an oxygenated substituent excluding extra hydroxyl groups.

Table 2.
GCMS semiquantitative representation of thermal and catalytic pyrolysis of lignin biooils.
The chemical compounds detected in the bio-oils were assigned to specific chemical groups. Unidentified peaks of the chromatogram or peaks with a low similarity (< 70%) were not assigned to chemical groups to ascertain the validity of the results. It should be noted that the data presented in Table 2 are based on chromatogram area and is therefore representative of tendencies rather than absolute values regarding the products found in different chemical groups. Chemical compounds produced from the pyrolysis of the lignins residues belonged mostly to the groups PH and OxyPH, as expected. The thermal depolymerization of the high lignin feedstocks resulted in bio-oils rich in the corresponding oligomers and monomers. GCMS analysis was not able to identify the entire samples due to the existence of heavier lignin oligomers. The part of the bio-oils that was analyzed was very rich in phenolic compounds with substitute groups that either included or not oxygen, such as methoxy and ethoxy groups or methyl and ethyl groups, respectively. Switching from thermal to catalytic pyrolysis resulted in most cases in a decrease of the OxyPH and an increase of the PH, indicating the effect of enhanced deoxygenation as methoxy and ethoxy are converted to methyl and ethyl groups. These observations are in agreement with our previous work where ZSM-5 catalytic upgrading resulted in an increase in the production of alkyl phenols [22]. In addition, ZSM-5 also resulted in the increase of aromatic hydrocarbons, although this increase was not significant since phenol oligomers and monomers are refractory to zeolite catalysis [23].
Comparing thermal to catalytic bio-oils, it is observed that the ZSM-5 catalyst, owing to its increased Brønsted acidity, can further facilitate the cracking of lignin oligomers, according to a mechanism proposed by Fogassy et al. [25]. This is documented by the increased gases and water content and the decreased O content of the produced lignin oils, presented in Figure 1 and Figure 2, which are products and effects of increased cracking of the bio-oil vapors [19]. This observation has been thoroughly documented in the literature where Py-GC/MS experiments showed an increase in the GC detectable compounds when ZSM-5 catalysts were used or when higher pyrolysis temperatures were employed [26,27]. Hardwood lignins have both syringyl- and guaiacyl-type nuclei, resulting in the detection of both types of phenols in the bio-oils [28]. Guaiacol originates from the thermal decomposition of softwood lignin and then forms other products due to secondary reactions. Catechols are formed via the homolysis of the O-CH3 bond and alkylated phenols are formed via radical induced rearrangement reactions [28,29]. Carbon coupling of carbon-centered radicals which are formed as products of the O-CH3 homolysis lead to the production of alkylated catechols and guaiacols [28]. In addition, demethoxylation of syringol results in guaiacol formation as well [28]. It was found that guaiacol (Phenol, 2-methoxy), catechol (1,2-benzenediol), and their alkyl derivatives were the primary products found in the thermal and catalytic bio-oils Employing ZSM-5 led to the increase of guaiacols, catechols, and alkyl substituted phenols, because of the increased cracking of the lignin oligomers, as mentioned above. The most pronounced increase was observed in the catechols. This confirms findings in the literature [30], which proposed a mechanism for the conversion of guaiacols to catechols on the strong brønsted acid sites of the zeolite.
3. Materials and Methods
3.1. Raw Materials
Lignins were produced by organosolv pretreatment of Lignocel® HBS 150–500, a commercially available beechwood sawdust with particle size 150–500 μm and moisture content 8 wt.%. Details of the organosolv pretreatment can be found elsewhere [22,31]. In summary, the L5, L6, L11, L12, L14, and L15 lignins were isolated from the organosolv pretreatment of Lignocel that was performed in 2.5 L stainless steel cylinders in an air-heated multidigester apparatus at 175 °C for 60 min. Treatment took place by using 110 g biomass and 1.1 L of 60% v/v ethanol or acetone with the addition or absence of an acidic catalyst (Table 1). The L1 and L2 were isolated from the pretreatment of lignocel in a hybrid organosolv–steam explosion reactor as previously described [22]. Pretreatment temperature was 200 °C and treatment time was 15 min in a 60% v/v ethanol solution with or without the addition of 1% w/wbiomass of H2SO4. At the end of the pretreatment, the liquor was collected after solids removal by vacuum filtration. The solvent was recovered by evaporating it under vacuum and the lignin was retrieved from the aqueous fraction that was left by centrifugation (14,000 rpm, 29,416 × g, at 4 °C for 15 min). The recovered lignin was afterwards air-dried [23]. All the experimental conditions where lignins were produced are presented in Table 1 along with their elemental analyses and their purity as measured by the NREL protocol (see Section 3.3).
3.2. Bed Material
For thermal pyrolysis, silica sand with particle size in the range 90–500 μm was used as inert material. The commercial catalyst used in the catalytic pyrolysis runs was a commercial sample which was composed of 30 wt.% ZSM-5 on a silica-alumina matrix with particle size which ranged between 60 to 90 μm. Specific surface area was 138 m2·g−1, mean pore diameter was 10−10 m, micro- and meso- porosities were 0.037 and 0.071 cm3·g−1, respectively. More details about these materials and their characterization can be found in previous work [32]. Regarding the pyrolysis runs, the experimental setup and procedure followed has been described elsewhere in detail [19]. In short, 1.5 g of lignin were introduced in the hot reactor which led to fast pyrolysis of the solid sample. The oil vapors passed through 0.7 g of silica sand or catalyst fixed bed. A constant stream of 100 ml·min−1 N2 introduced at the top of the reactor removed the products and maintained an inert atmosphere during pyrolysis. The bio-oil vapors were condensed in a glass receiver which was cooled at −17 °C and the non-condensable gases were collected in a gas collection system. Pyrolysis temperature was 500 °C in all thermal and catalytic runs. The values presented in this work are average values of runs that were repeated three times.
3.3. Analytical Methods
The cellulose, hemicellulose, and lignin contents of the lignins were determined according to a method developed by National Renewable Energy Laboratory ((NREL/TP-510-42618 and NREL/TP-510-42619) [33]. Carbon and hydrogen of the lignin samples and bio-oils were determined by a CHN LECO-800 elemental analyzer from Leco Corporation (USA), while oxygen was determined by difference. The moisture content was measured by drying a pre-weighed sample at 105 °C for 4 h. Description of the analysis method of the Fourier Transform Infrared Spectroscopy (FTIR) can be found elsewhere [22]. The Karl–Fischer method (ASTM E203-08) was used for the water content of the bio-oil. The organic phase of the bio-oil was analyzed by GCMS using an Agilent 7890A/5975C gas chromatograph-mass spectrometer (Electron energy 70eV; Emission 300V; Helium flow rate: 0.7 cm3·min−1; Column: HP-5MS 30 m × 0.25 mm ID × 0.25 μm). Internal libraries were used for the identification of the compounds found in the bio-oil and their categorization into main functional groups according to previous work [19]. For the determination of the surface area (BET method), pore volume, and pore size distribution (BJH method) of the catalyst samples, N2 adsorption/desorption measurements were carried out at −196 °C, using an Automatic Volumetric Sorption Analyzer (Autosorb-1MP, Quantachrome); details about the analyses and sample preparation and characterization are provided elsewhere [34].
4. Conclusions
In the present work, lignins retrieved from organosolv delignification of beechwood were tested in thermal and catalytic (ZSM-5) pyrolysis. All lignin pyrolysis tests were conducted at 500 °C. The organosolv lignins were produced under a variety of delignification conditions where either different solvents (ethanol and acetone) or different acids (sulfuric, phosphoric, and oxalic) were used in order to induce lignin removal from the biomass. The retrieved lignins were analyzed via elemental analysis and FTIR and were found to be of good quality without high degradation in all cases. The lignins that were retrieved from runs where no H2SO4 was used produced more organic fraction and less coke compared to the lignins retrieved from H2SO4-assisted delignification. This was noted for both thermal and catalytic pyrolysis. The elemental analysis of the produced bio-oils showed that lignins retrieved from delignification conditions without use of H2SO4 produced bio-oils with higher C and lower O contents, with the exception of the pair L11 and L12 where the reverse was observed. It was assumed that use of H2SO4 led to enhanced condensation reactions of lignin, which under pyrolysis conditions formed more coke and less bio-oil. The less severe phosphoric and oxalic acids had negligible effects due to their lower severity. Use of different types of solvent did not have any effect on the pyrolysis products. GCMS analysis revealed that all lignin bio-oils consisted mostly of aromatic chemical compounds, mostly phenolic rings with alkyl or alkoxy side groups. Use of ZSM-5 led overall to an increase of alkyl-phenols over alkoxy-phenols or to more aromatic hydrocarbon molecules. The high phenols concentration in the lignin bio-oils makes lignin pyrolysis an attractive process for retrieving high added value aromatic chemicals from what is considered a low value residue of the biomass pretreatment process.
Supplementary Materials
The following are available online at https://www.mdpi.com/1996-1073/12/9/1606/s1, Figure S1: FTIR spectra of organosolv retrieved beechwood lignins.
Author Contributions
Conceptualization, K.G.K. and L.M.; Methodology, K.G.K. and L.M.; Investigation, K.G.K. and L.M.; Resources, P.C. and U.R.; Data Curation, K.G.K., L.M., P.C. and A.A.L.; Writing-Original Draft Preparation, K.G.K.; Writing-Review & Editing, L.M., P.C., U.R. and A.A.L.; Funding Acquisition, K.G.K., L.M., P.C., U.R. and A.A.L.
Funding
The work was supported by the STSM program of COST Action FP1306 that funded a short-term scientific mission of K. Kalogiannis at LTU.
Acknowledgments
L.M., U.R. and P.C. would like to thank Bio4Energy, a strategic research environment appointed by the Swedish government, for supporting this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Puri, M.; Abraham, R.E.; Barrow, C.J. Biofuel production: Prospects, challenges and feedstock in Australia. Renew. Sustain. Energy Rev. 2012, 16, 6022–6031. [Google Scholar] [CrossRef]
- Hahn-Hägerdal, B.; Galbe, M.; Gorwa-Grauslund, M.F.; Lidén, G.; Zacchi, G. Bio-ethanol—The fuel of tomorrow from the residues of today. Trends Biotechnol. 2006, 24, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Cheng, K.; Liu, D. Organosolv pretreatment of lignocellulosic biomass for enzymatic hydrolysis. Appl. Microbiol. Biotechnol. 2009, 82, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Studer, M.H.; DeMartini, J.D.; Davis, M.F.; Sykes, R.W.; Davison, B.; Keller, M.; Tuskan, G.A.; Wyman, C.E. Lignin content in natural Populus variants affects sugar release. Proc. Natl. Acad. Sci. USA 2011, 108, 6300–6305. [Google Scholar] [CrossRef]
- Zhang, B.; Shahbazi, A. Recent Developments in Pretreatment Technologies for Production of Lignocellulosic Biofuels. J Pet. Env. Biotechnol. 2011. [Google Scholar] [CrossRef]
- Yang, B.; Wyman, C.E. Pretreatment: The key to unlocking low-cost cellulosic ethanol. Biofuels Bioprod. Biorefining 2008, 2, 26–40. [Google Scholar] [CrossRef]
- Wyman, C.E.; Dale, B.E.; Elander, R.T.; Holtzapple, M.; Ladisch, M.R.; Lee, Y.Y. Coordinated development of leading biomass pretreatment technologies. Bioresour. Technol. 2005, 96, 1959–1966. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, M.; Viikari, L. Xylans inhibit enzymatic hydrolysis of lignocellulosic materials by cellulases. Bioresour. Technol. 2012, 121, 8–12. [Google Scholar] [CrossRef]
- Kristensen, J.B.; Thygesen, L.G.; Felby, C.; Jorgensen, H.; Elder, T. Cell wall structural changes in wheat straw pretreated for bioethanol production. Biotechnol. Biofuels 2008. [Google Scholar] [CrossRef] [PubMed]
- Constant, S.; Wienk, H.L.J.; Frissen, A.E.; de Peinder, P.; Boelens, R.; van Es, D.S.; Grisel, R.J.H.; Weckhuysen, B.M.; Huijgen, W.J.J.; Gosselink, R.J.A.; et al. New insights into the structure and composition of technical lignins: A comparative characterisation study. Green Chem. 2016, 18, 2651–2665. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of Transportation Fuels from Biomass: Chemistry, Catalysts, and Engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Stefanidis, S.D.; Kalogiannis, K.G.; Iliopoulou, E.F.; Michailof, C.M.; Pilavachi, P.A.; Lappas, A.A. A study of lignocellulosic biomass pyrolysis via the pyrolysis of cellulose, hemicellulose and lignin. J. Anal. Appl. Pyrolysis 2014, 105, 143–150. [Google Scholar] [CrossRef]
- Rinaldi, R.; Jastrzebski, R.; Clough, M.T.; Ralph, J.; Kennema, M.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Paving the Way for Lignin Valorisation: Recent Advances in Bioengineering, Biorefining and Catalysis. Angew. Chemie Int. Ed. 2016, 55, 8164–8215. [Google Scholar] [CrossRef]
- Nowakowski, D.J.; Bridgwater, A.V.; Elliott, D.C.; Meier, D.; de Wild, P. Lignin fast pyrolysis: Results from an international collaboration. J. Anal. Appl. Pyrolysis 2010, 88, 53–72. [Google Scholar] [CrossRef]
- Azadi, P.; Inderwildi, O.R.; Farnood, R.; King, D.A. Liquid fuels, hydrogen and chemicals from lignin: A critical review. Renew. Sustain. Energy Rev. 2013, 21, 506–523. [Google Scholar] [CrossRef]
- De Wild, P.; Reith, H.; Heeres, E. Biomass pyrolysis for chemicals. Biofuels 2011, 2, 185–208. [Google Scholar] [CrossRef]
- De Wild, P.J.; Huijgen, W.J.J.; Gosselink, R.J.A. Lignin pyrolysis for profitable lignocellulosic biorefineries. Biofuels Bioprod. Biorefining 2014, 8, 645–657. [Google Scholar] [CrossRef]
- Kalogiannis, K.G.; Stefanidis, S.D.; Michailof, C.M.; Lappas, A.A.; Sjöholm, E. Pyrolysis of lignin with 2DGC quantification of lignin oil: Effect of lignin type, process temperature and ZSM-5 in situ upgrading. J. Anal. Appl. Pyrolysis 2015, 115, 410–418. [Google Scholar] [CrossRef]
- Sharma, R.K.; Bakhshi, N.N. Catalytic upgrading of pyrolysis oil. Energy Fuels 1993, 7, 306–314. [Google Scholar] [CrossRef]
- Huber, G.W.; Corma, A. Synergies between Bio- and Oil Refineries for the Production of Fuels from Biomass. Angew. Chemie Int. Ed. 2007, 46, 7184–7201. [Google Scholar] [CrossRef]
- Kalogiannis, K.; Matsakas, L.; Aspden, J.; Lappas, A.; Rova, U.; Christakopoulos, P. Acid Assisted Organosolv Delignification of Beechwood and Pulp Conversion towards High Concentrated Cellulosic Ethanol via High Gravity Enzymatic Hydrolysis and Fermentation. Molecules 2018, 23, 1647. [Google Scholar] [CrossRef]
- Matsakas, L.; Nitsos, C.; Raghavendran, V.; Yakimenko, O.; Persson, G.; Olsson, E.; Rova, U.; Olsson, L.; Christakopoulos, P. A novel hybrid organosolv: Steam explosion method for the efficient fractionation and pretreatment of birch biomass. Biotechnol. Biofuels 2018, 11, 160. [Google Scholar] [CrossRef]
- Kalogiannis, K.G.; Stefanidis, S.D.; Lappas, A.A. Catalyst deactivation, ash accumulation and bio-oil deoxygenation during ex situ catalytic fast pyrolysis of biomass in a cascade thermal-catalytic reactor system. Fuel Process. Technol. 2019, 186, 99–109. [Google Scholar] [CrossRef]
- Fogassy, G.; Thegarid, N.; Schuurman, Y.; Mirodatos, C. From biomass to bio-gasoline by FCC co-processing: Effect of feed composition and catalyst structure on product quality. Energy Environ. Sci. 2011, 4. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Lee, J.H.; Park, J.; Kim, J.K.; An, D.; Song, I.K.; Choi, J.W. Catalytic pyrolysis of lignin over HZSM-5 catalysts: Effect of various parameters on the production of aromatic hydrocarbon. J. Anal. Appl. Pyrolysis 2015, 114, 273–280. [Google Scholar] [CrossRef]
- Ma, Z.; Troussard, E.; Van Bokhoven, J.A. Controlling the selectivity to chemicals from lignin via catalytic fast pyrolysis. Appl. Catal. A Gen. 2012, 423–424, 130–136. [Google Scholar] [CrossRef]
- Asmadi, M.; Kawamoto, H.; Saka, S. Thermal reactions of guaiacol and syringol as lignin model aromatic nuclei. J. Anal. Appl. Pyrolysis 2011, 92, 88–98. [Google Scholar] [CrossRef]
- Britt, P.F.; Buchanan, A.C.; Malcolm, E.A. Impact of Restricted Mass Transport on Pyrolysis Pathways for Aryl Ether Containing Lignin Model Compounds. Energy Fuels 2000, 14, 1314–1322. [Google Scholar] [CrossRef]
- Ben, H.; Ragauskas, A.J. One step thermal conversion of lignin to the gasoline range liquid products by using zeolites as additives. RSC Adv. 2012, 2, 12892. [Google Scholar] [CrossRef]
- Matsakas, L.; Raghavendran, V.; Yakimenko, O.; Persson, G.; Olsson, E.; Rova, U.; Olsson, L.; Christakopoulos, P. Lignin-first biomass fractionation using a hybrid organosolv – Steam explosion pretreatment technology improves the saccharification and fermentability of spruce biomass. Bioresour. Technol. 2019, 273, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Kalogiannis, K.G.; Stefanidis, S.D.; Karakoulia, S.A.; Triantafyllidis, K.S.; Yiannoulakis, H.; Michailof, C.; Lappas, A.A. First pilot scale study of basic vs acidic catalysts in biomass pyrolysis: Deoxygenation mechanisms and catalyst deactivation. Appl. Catal. B Environ. 2018, 238, 346–357. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass: Laboratory Analytical Procedure (LAP) (Revised July 2011); NREL: Golden, CO, USA, 2008. [Google Scholar]
- Kalogiannis, K.G.; Stefanidis, S.D.; Michailof, C.M.; Lappas, A.A. Castor bean cake residues upgrading towards high added value products via fast catalytic pyrolysis. Biomass Bioenergy 2016, 95, 405–415. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).